Introduction
Approximately one third of the world's population
has serological evidence of past or present infection with
hepatitis B virus (HBV), and >350 million people are chronic HBV
surface antigen (HBsAg) carriers at high risk of developing liver
cirrhosis and hepatocellular carcinoma (1). Currently, treatment options for
chronic hepatitis B (CHB) mainly depend on interferon-α or
nucleoside/nucleotide analogs, which control virus replication
efficiently but rarely clear the virus completely (2). Hence, various novel vaccine
approaches, including DNA vaccines, have been developed for better
therapeutic effect of hepatitis B (2,3).
Therefore, the principal therapeutic goal for
treating patients chronically infected with HBV is to successfully
stimulate an immune response, with the aim of leading to effective
viral clearance. Due to an increasing capacity to elicit T-cell
immune response, a DNA vaccine may be a more effictive technique to
stimulate specific cellular immune responses compared with a
recombinant protein-based vaccine (4–6).
Dendritic cells (DCs) are the most significant
antigen presenting cells (APCs), and serve a central role in
antiviral immunity with a unique capacity to bridge innate and
adaptive immunity (7). However,
DCs are functionally impaired in patients with CHB (8). As the interaction between the virus
and the host determines the magnitude of the virus-specific immune
response (9), defective processing
and presentation of the HBV by antigen presenting DCs may be
responsible for the impaired HBV-specific immune responses in CHB
carriers. Therefore, enhancing DC function and inducing a specific
immune response may be effective for viral clearance.
The CD40 ligand (CD40L), a member of the tumor
necrosis factor (TNF) gene family, is preferentially expressed on
activated CD4+ T cells (10,11).
The receptor of CD40L is CD40, which is expressed on APCs,
including on DCs. Previous studies have demonstrated that CD40 and
CD40L interactions are prominent in the maturation of dendritic
cells and additionally enhance the ability of DCs to induce a
T-cell response (12–14).
Our previous study successfully constructed plasmids
that contain the HBV S-ecdCD40L fusion gene (pcDNA3.1-S-ecdCD40L),
which may promote DC activation and enhance their function, with
the aim to develop a novel type of hepatitis B vaccine (15). In the present study, it was
hypothesized that HBV S-ecdCD40L therapeutic vaccines will promote
the maturation and activation of DCs and thus, may enhance the
antigen-specific T-cell generation of HBV transgenic mice to
control and eliminate the HBV virus.
Materials and methods
Reagents and materials
RNAiso reagent, a BCaBEST RNA PCR kit, restriction
endonucleases (KpnI, EcoRI and XhoI), a T4 DNA ligase and ExTaq
polymerase were purchased from Takara Biotechnology Co., Ltd.
(Dalian, China). Polymerase chain reaction (PCR) primers were
synthesized by Generay Biotech Co., Ltd. (Shanghai, China). DNA
sequencing was performed by Generay Biotech Co., Ltd. RPMI-1640
medium was purchased from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The recombinant plasmid (pcDNA3.1-S), the
expression vector [pcDNA3.1 (+)] and Escherichia coli DH5α
were maintained in the authors' laboratory at The First Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China).
Recombinant murine granulocyte/macrophage colony-stimulating factor
(GM-CSF), recombinant murine interleukin (IL)-4 were purchased from
PeproTech, Inc. (Rocky Hill, NJ, USA). The mouse CD11c magnetic
bead sorting kit and CD4+ T isolation kit were purchased from
Miltenyi Biotech (Bergisch Gladbach, Germany).
Allophycocyanin-conjugated anti-CD11c monoclonal and corresponding
homotype antibodies [Phycoerythrin (PE)-conjugated anti-CD86 (cat.
no. 12-0869-41); R at IgG2b K Isotype Control PE/Rat IgG2b K
Isotype Control FITC; cat. no. 12-4031/11-4031; APC anti-human
CD11c antibody; cat. no. 301613; (MHC)-II antibody cat. no. 561107]
were purchased from BioLegend (USA). Phycoerythrin (PE)/fluorescein
isothiocyanate (FITC)-conjugated anti-CD11c, anti-CD86 and
anti-major histocompatility complex (MHC)-II for
fluorescence-activated cell sorting (FACS) analysis were purchased
from eBioscience, Inc. (San Diego, CA, USA). Cell Counting kit-8
(CCK-8) was purchased from Beyotime Institute of Biotechnology
(Haimen, China). ELISA kits (cat. no. MAB611) specific for mouse
IL-12p70 were purchased from R&D Systems, Inc. (Minneapolis,
MN, USA). The HBV fluorescence quantitative PCR Diagnostic kit was
purchased from Shenzhen Piji Biotech (Shenzhen, China).
Animals
A total of 20 HBV transgenic (HBV-Tg) BALB/c mice
(age, 8–10 weeks; weight, 18–25 g) were purchased from the
Infectious Disease Center of No.458 Hospital (Guangzhou, China). A
total of 20 C57BL/6 mice (age, 6–8 weeks; weight, 18–25 g) were
purchased from the Shanghai Slac Laboratory Animal Center, Chinese
Academy of Sciences (Shanghai, China). All the mice (n=40) were
maintained under a pathogen-free facility with controlled
temperatures (20–22°C), humidity (45–55%) and a 12-h light/dark
cycle lights on from 08:00 to 20:00. This study was carried out
with ethical approval from of the Institutional Animal Committee of
Wenzhou Medical University (Wenzhou, China) and all mice received
care throughout the experiment in accordance with ‘Guide for the
Care and Use of Laboratory Animals’ (National Institutes of Health,
Bethesda, MD, USA).
Construction of the
pcDNA3.1-S-ecdCD40L
A 650 bp cDNA fragment coding for the full open
reading frame of the murine ecdCD40L gene was cloned from murine
lymphocytes of C57BL/6 mice by PCR using Ex Taq polymerase. The
following primers for the ecdCD40L gene were used: Forward,
5′-TTAGAATTCCTTCATAGAAGATTGGATAAGGTCGAA-3′ and reverse,
5′-GCGCTCGAGTTAGAGTTTGAGTAAGCCAAAAGA-3′. A 670 bp DNA fragment
coding for the HBV S gene was cloned by PCR from a recombinant
plasmid containing two times the length of HBV sequence (PCP10),
which was obtained from Dr Cheng Jun (Infectious Disease Center,
Beijing Ditan Hospital, Beijing, China). There were two primers
specific for the HBV S gene that were used: Forward,
5′-GCGGGTACCATGGAGAACATCACATCAGGATTC-3′ and reverse,
5′-GGAGAATTCGCCGCCGCCGCCAATGTATACCCAAAGACAAAAGAAAAT-3′. Following
this, the pcDNA3.1-S-ecdCD40L vaccine was constructed, as described
previously (15).
Immunization schedule
HBV-Tg mice randomly allocated into four groups (n=5
per group) received intramuscular injections in the tibialis
anterior muscles as follows: Group A, 100 µg pcDNA3.1-S-ecdCD40L in
100 µl 0.9% saline; and groups B, C and D, 100 µg pcDNA3.1-S,
pcDNA3.1 in 100 µl 0.9% saline and 100 µl PBS. Intramuscular
injections for the negative control were performed by injecting PBS
using a 1 ml insulin syringe with a 28-gauge needle. All mice were
immunized on day 0 and boosted on days 14 and 28.
Isolation of dendritic cells
A total of 6 weeks following the first immunization,
liver DCs were isolated from the mouse liver according to
established protocols with minor modifications as
magnetic-activated cell sorting was used (16). The mice were anaesthetized with 10%
chloral hydrate. Following opening of the abdomen, the needle of a
1 ml syringe was inserted into the portal vein and the liver was
perfused with Hanks' Balanced Salt solution containing 1% type IV
collagenase and 0.19 g/l EDTA (Beijing Leagene Biotechnology, Co.,
Ltd., Beijing, China). The inferior vena cava was cut to free
outflow of blood. The perfused liver was removed and disrupted with
ophthalmic forceps. The cell suspensions were passed through a
sterile strainer (pore size, 74 µm), and subsequently resuspended
in 35% percoll solution. Following centrifugation at 450 × g for 20
min at room temperature, non-parenchymal cells (NPCs) of the liver
were collected. In order to isolate liver DCs, NPCs were purified
with a CD11c magnetic bead sorting kit according to the
manufacturer's protocol. CD11+cells of ≥90% purity (often ~95%
purity) were used in experiments. Subsequently, CD11c-enriched
cells were cultured overnight in complete medium.
Flow cytometry
To characterize and compare the expression of
surface molecules on DCs, flow cytometry was performed. DCs were
washed and stained in PBS for 30 min at 4°C with PE- or
FITC-conjugated monoclonal antibodies against CD86 and MHCII
(dilution 1:50). Isotype-matched antibodies (Rat IgG2b K Isotype
Control PE/Rat IgG2b K Isotype Control FITC, cat no.
12-4031/11-4031, eBioscience Inc., USA) served as FITC- or
PE-conjugated controls, incubation at 4°C for 20 min. Stained cells
were centrifuged with PBS (5 min, 300 × g at 4°C), the supernatant
was discarded and 1% paraformaldehyde 200 µl was added every tube
to fix the DCs at 4°C. Stained cells were then analyzed using an
Elite flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Cytokine ELISA
Cytokine levels in the supernatants were measured by
an ELISA kit specific for IL-12p70 (cat. no. MAB611; R&D
Systems, Inc., Minneapolis, MN, USA) according to manufacturer's
instructions, at a wavelength of 450 nm using a microplate
reader.
Allogeneic-mixed lymphocyte reactions
(MLRs)
T cells were purified from the spleen lymphocytes of
C57BL/6 mouse using a CD4+ T isolation kit. Briefly, graded
concentrations of DCs from different groups were co-cultured in
U-bottom 96-well plates with constant number of allogeneic T-cells
(1×105 cells/200 ml) at different stimulator:responder
(DC:T-cell) ratios (1:5, 1:10, 1:20) for 96 h at 37°C. The T-cell
stimulatory activity of DC populations in MLR was expressed as
stimulation index (SI) value and measured using CCK-8 assay in
accordance with the manufacturer's protocol.
HBV DNA quantitation
At weeks 0, 3 and 6 following immunization, blood
was collected from the mice orbital sinus and 50 µl sera were
collected for HBV DNA assay. Quantitative PCR (qPCR) was performed
to quantify HBV DNA using an HBV DNA PCR-FIUOTEC kit (Sun Yat-sen
University, Guangzhou, China) with a detection limit of 500
copies/ml. The fluorescent signals were quantified by PRISM 7000
Sequence Detection system version 1.2.3 (Applied Biosystems; Thermo
Fisher Scientific, Inc.).
Serum HBsAg and alanine
aminotransferase (ALT) analyses
Frozen serum samples collected from the immunized
mice were transferred to the reference lab at The First Affiliated
Hospital of Wenzhou Medical University for assays of HBsAg and
serum ALT levels. The Modular E170 analyzer (Roche Diagnostics,
Basel, Switzerland) was employed for HBsAg assay. ALT activity was
quantified by the AU5800 automatic analyzer (Beckman Coulter,
Inc.).
Statistical analysis
Data analysis was performed using SPSS software
(version 18.0; SPSS Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. Statistical analysis was performed
using one-way analysis of variance followed by LSD-t or Dunnett's
T3 post hoc tests for multi group comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction and characterization of
the recombinant plasmid pcDNA3.1-S-ecdCD40L
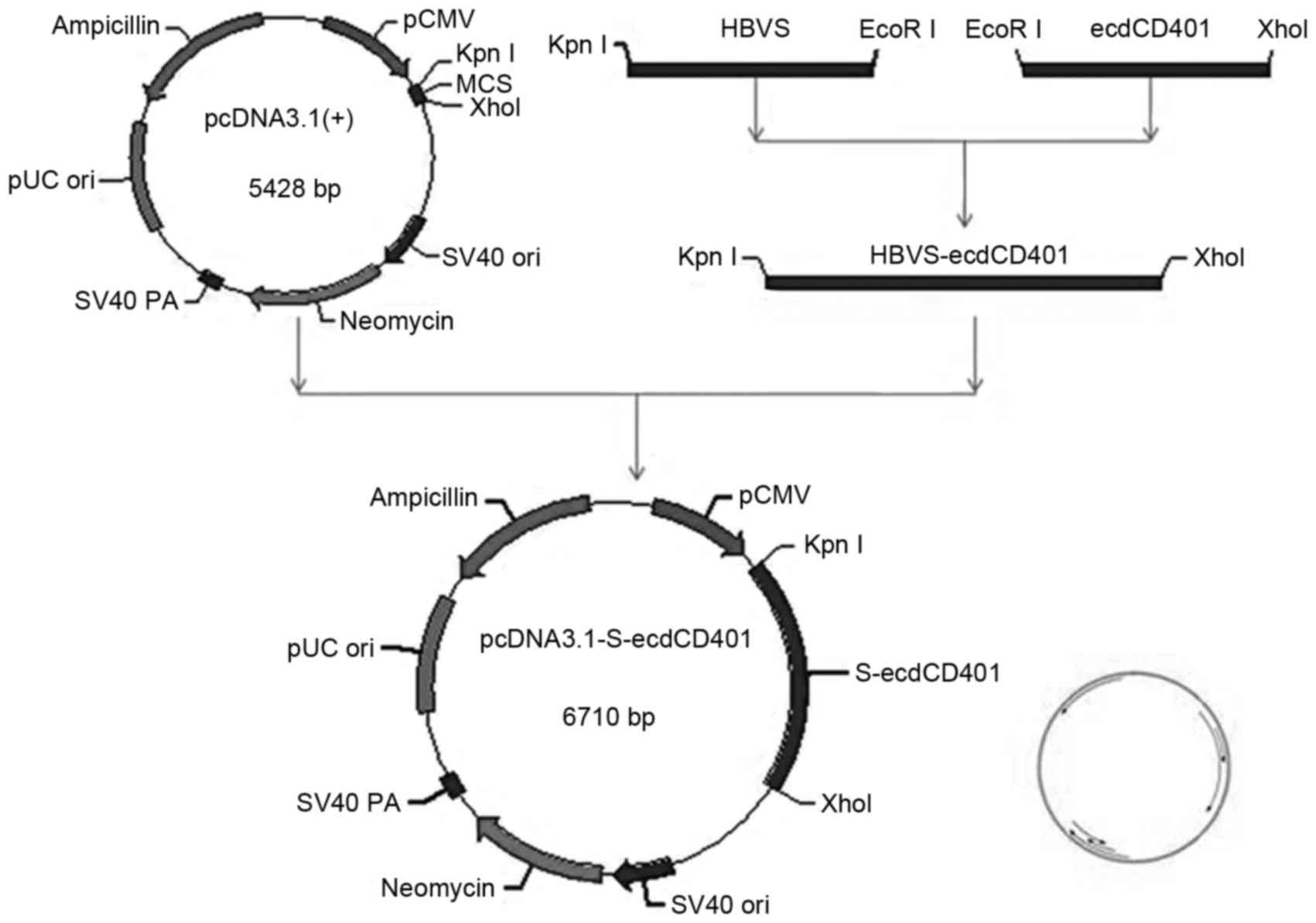
The recombinant plasmid was constructed and used for
DNA vaccination (Fig. 1).
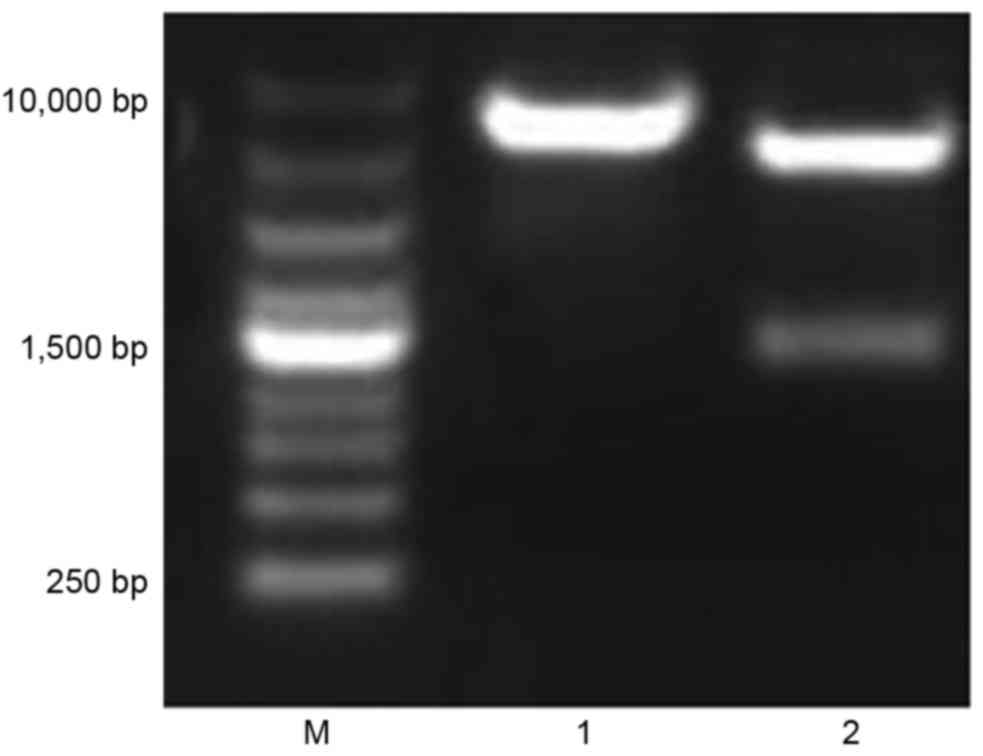
Following construction, the recombinant plasmids were characterized
by digestion with restriction enzymes Xho I/KpnI and EcoRI
(Fig. 2). The fragment digested
from the recombinant plasmids by EcoRI was ~6,700 bp in size, as
expected. The fragment digested from the recombinant plasmids by
Xho I/Kpn I was ~1,500 and 5,200 bp, as expected. The result
confirmed that the inserted HBV S-ecdCD40L fusion gene had the
correct reading frame and length.
Vaccination induces activation of DC
with upregulated expression of CD86 and MHC-II
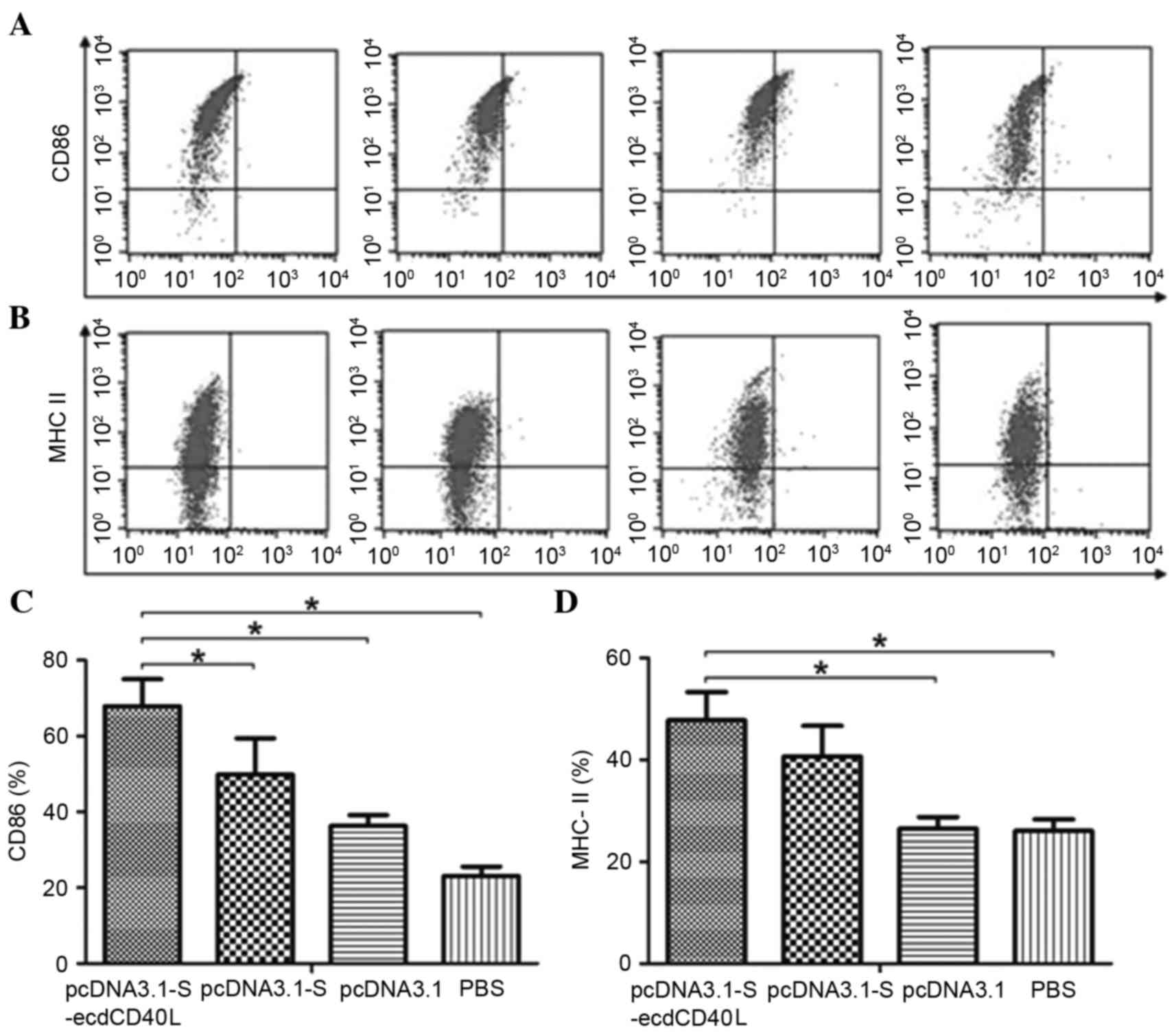
To evaluate the phenotypic alterations induced
within liver DCs, purified DCs from the liver of
pcDNA3.1-S-ecdCD40L-treated transgenic mice, as well as the
controls, were subjected to phenotypic analysis by flow cytometry
(Fig. 3A and B). The expression of
MHC molecules and co-stimulatory molecules serve an essential role
in the maturation of dendritic cells, which interact with T cells
for T cell activation (17). The
results demonstrated an evident upregulated expression of CD86 in
DCs from the pcDNA3.1-S-ecdCD40L-treated transgenic mice compared
with the other three groups (Fig.
3C; P<0.05). In addition, the proportion of DCs that were
positive for MHC-II was increased in the pcDNA3.1-S-ecdCD40L group,
but showed no significant difference when compared with the
pcDNA3.1-S group (Fig. 3D;
P>0.05).
IL-12p70 production by DC
The production of IL-12, which is known to induce
T-cell proliferation, was assessed. Spontaneous IL-12 production of
DCs in the supernatants of four groups is presented in Table I. Compared with the three control
groups, IL-12 secretion by DCs from pcDNA3.1-S-ecdCD40L-treated
transgenic mice was significantly greater (P<0.05).
 | Table I.Spontaneous IL-12p70 production in a
pure DC population. |
Table I.
Spontaneous IL-12p70 production in a
pure DC population.
|
| Pure DC
supernatants |
|---|
|
|
|
|---|
|
| A | B | C | D |
|---|
| IL-12p70 (pg/ml) |
107.16±19.09a |
72.23±23.60 |
23.89±4.68 |
23.19±4.21 |
Vaccination significantly enhances
allogeneic T-cell proliferation in DCs
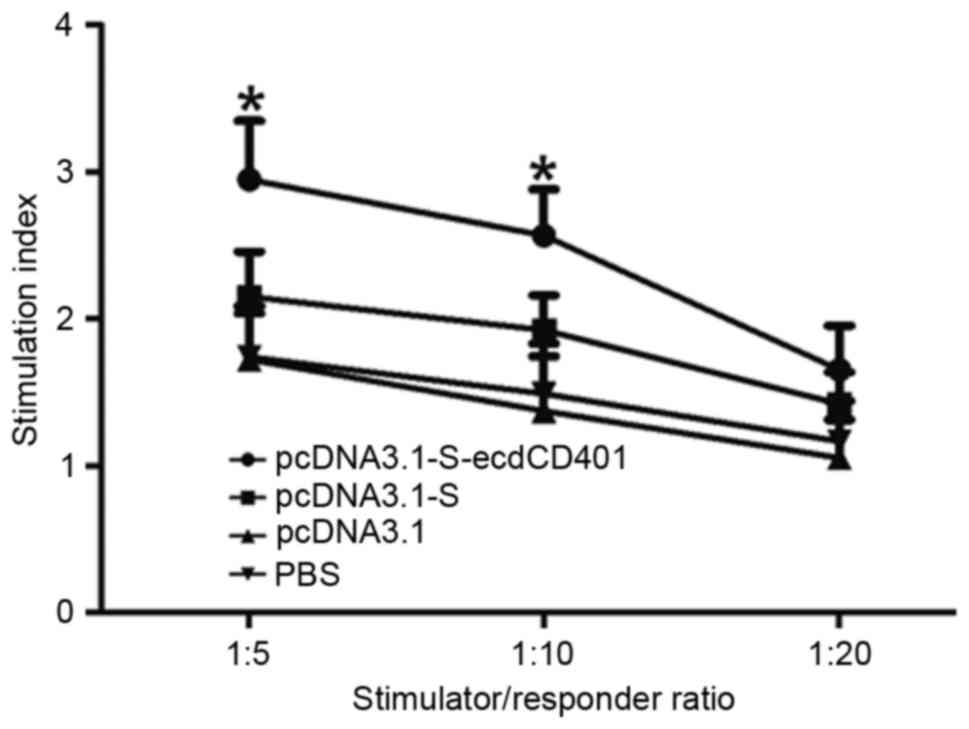
To determine the functional properties of DCs, the
T-cell stimulatory capacity, expressed as a stimulation index (SI)
value, in an MLR was examined. SI is the ratio between the
proliferative response (optical density) of T cells in the presence
and the absence of DCs in the cultures at different DC/T cell
ratio. As presented in Fig. 4,
although the number of T cells was the same in all MLR cultures,
the SI went up significantly according to the increasing DCs
number. The results showed that DCs from HBV-Tg mice immunized with
pcDNA3.1-S-ecdCD40L tended to have significantly increased T-cell
stimulatory activity (P<0.05), when compared with the other
three groups at the DC/T ratios of 1:5 and 1:10. However, when DC/T
was 1:20, there was no significant difference between the four
groups (P>0.05).
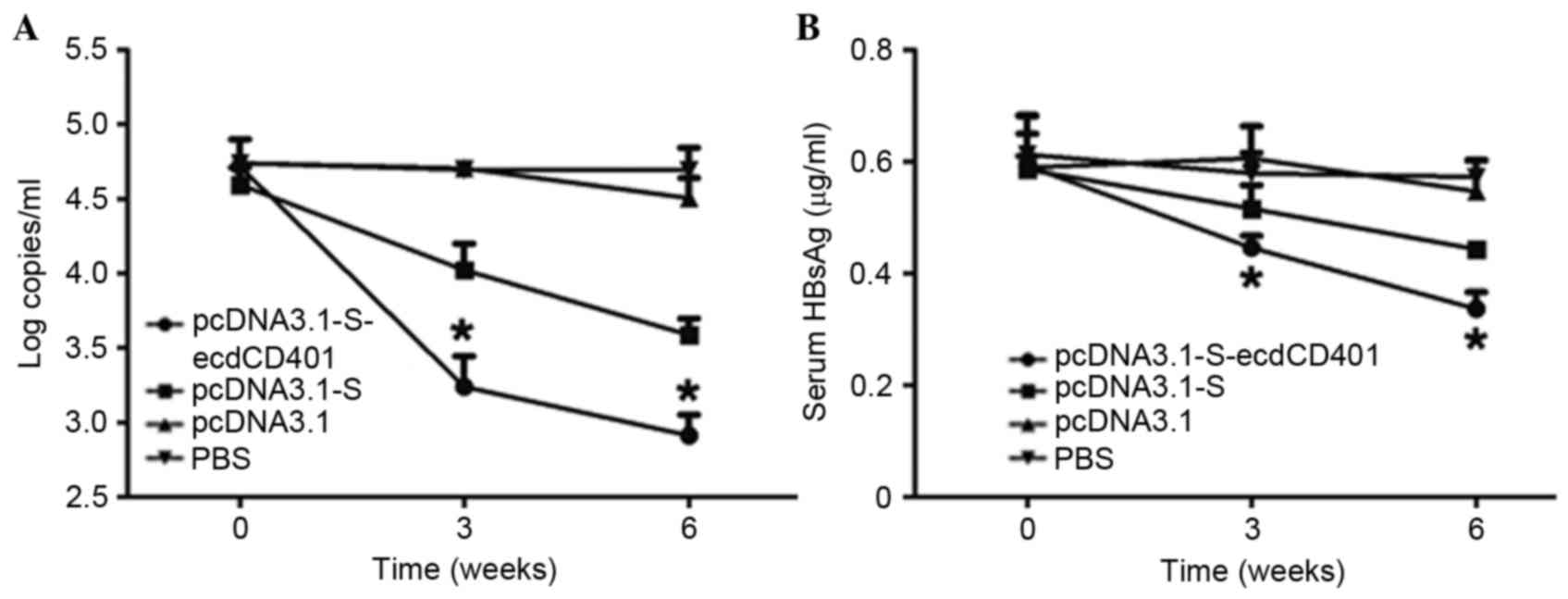
HBV-S-ecdCD40L vaccination results in
the downregulation of HBV-DNA and the clearance of HBsAg in HBV
transgenic mice
The HBV transgenic mice used in the current study
had a high-level titer of HBV DNA in their sera prior to
immunization. qPCR results revealed that pcDNA3.1-S-ecdCD40L may
significantly inhibit HBV DNA replication. Particularly following
the third injection, the decrease was obvious (Fig. 5A; P<0.05). Furthermore, HBsAg
significantly dropped in the sera following the vaccinations
(Fig. 5B; P<0.05). The HBV
S-ecdCD40L vaccine resulted in a more rapid downregulation of
HBsAg. The reduced levels of serum HBV DNA were observed in
pcDNA3.1-S-ecdCD40L or pcDNA3.1-S vaccine-recipients, but not in
pcDNA3.1- and PBS-vaccinated HBV-Tg mice. In addition, reduced
levels of serum HBV DNA and HBsAg were observed in
pcDNA3.1-S-ecdCD40L and pcDNA3.1-S vaccine-recipients, but not in
pcDNA3.1- and PBS-vaccinated HBV-Tg mice.
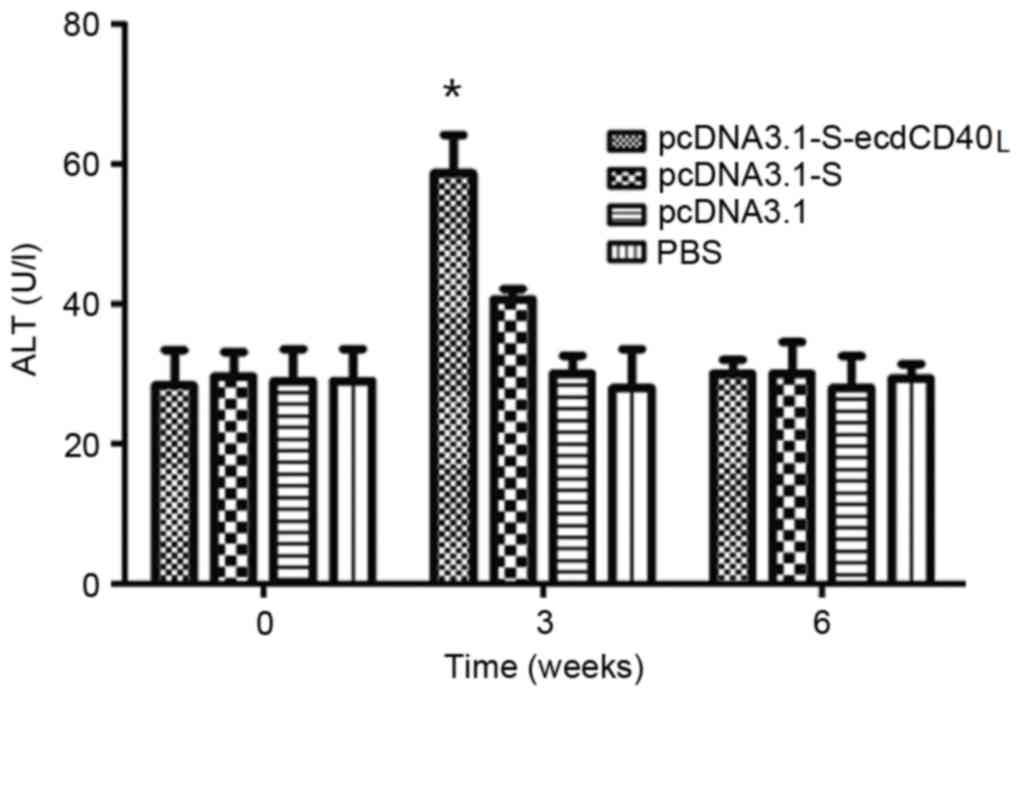
Evaluation of liver injury
An important question was whether an immunization
inducing immune responses in HBV-Tg mice would result in
inflammation or damage of the liver. Liver injury was evaluated by
determining ALT activity, which indicated hepatocyte damage in the
sera of the immunized mice. The results demonstrated that ALT
activity rose following HBV S-ecdCD40L vaccination, and
subsequently returned to the baseline level (Fig. 6). ALT increased to a high level at
week 3 in HBV-Tg mice, then dropped back to the basal level.
Discussion
Despite the presence of an effective prophylactic
vaccine, ~1 million patients with CHB succumb to disease annually
from liver cirrhosis or hepatocellular carcinoma (18).
The ultimate goal of therapy for CHB is the
eradication of HBV or persistent suppression of viral replication,
in order to improve quality of life and survival by preventing
disease progression. However, clinical therapeutic drugs for CHB
infection often do not lead to satisfactory results. Previously,
DNA vaccination has emerged as an attractive approach, and has
demonstrated importance in antiviral application due to its ability
to induce T cell responses (19).
CHB is an immune-mediated disease. There is a
general consensus that a combination of antiviral treatment and
immunomodulation is essential to achieve a sustained control of HBV
infection (20). The classical DNA
vaccination plasmid only encodes for a single viral antigen, the S
or the PreS2/S antigen, and its immunogenicity remains relatively
low (21). Only a few injected DNA
molecules are taken up by APCs, which leads to a minority of the
corresponding proteins being presented (22). Many strategies have been employed
to improve the efficacy of DNA vaccination. In the present study,
eukaryotic expression plasmids of the HBV S-ecdCD40L fusion gene
were constructed to promote DC maturation as well as induce
specific immune responses to HBV. The HBV-Tg mice model was used as
a surrogate model for its persistent HBV infection following
neonatal transmission to validate the HBV S-ecdCD40L vaccine's
antiviral effect. To date, studies have illustrated the usefulness
of HBV-Tg mice in elucidating the viral biology and host/viral
interactions when they are used to address questions that cannot be
otherwise approached by the existing methodology in human HBV
carriers (23,24).
It is important for therapeutic vaccine to be able
to induce potent T-cell responses. However, the induction of
efficient T-cell response requires equally effective
cross-presentation of the exogenous vaccine antigens by
professional APCs (25). It is
widely accepted that DCs, the most potent type of APC, serve
essential roles in priming the T-cell response (26). The structure of CD40L, the ligand
for CD40 on DCs, includes extracellular, transmembrane and
intracellular parts. The extracellular domain of CD40L (ecd40L) may
combine with CD40 and promote the maturation of DC and enhance its
function (27).
A previous report indicated that an
Ad-sig-E7-ecdCD40L vector may activate DCs with a higher level of
IL-12 and interferon-γ than controls, and induce increased CD80+
and CD86+ DCs (27). An additional
report demonstrated that a vaccine created by attaching the
target-associated antigen (TAA) to the ecdCD40L may facilitate the
activation and maturation of DCs by promoting the presentation of
TAA on the DCs, thus resulting in expansion of TAA specific T cells
and B cells (28). Although CD40L
is significantly involved in the activation of DCs and is essential
for the induction of antigen-specific T-cell response, it has not
been investigated as a component of DNA vaccination for chronic HBV
infection. Therefore, the vaccine formulation of the present study
included the S antigen to offer a large epitope repertoire for the
immune system, and murine ecdCD40L to activate maturation of
DCs.
Our previous study indicated that activating CD40 by
pcDNA 3.1-S-ecdCD40L-mediated HBV S-ecdCD40L expression may induce
DC activation with upregulated expression of cytokines (including
IL-12) and immune regulatory molecules (15). In addition, activated DC transfects
with pcDNA 3.1-S-ecdCD40L stimulated enhanced allogeneic T-cell
proliferation (15). Liver DCs, a
particular DC subset, are poorly immunogenic when compared with DCs
in other compartments (29). A
profound impairment in the function of DCs (lack of co-stimulatory
molecule expression and decreased production of pro-inflammatory
cytokines) has been reported in patients with persistent HBV
infection, which may account for HBV ability to escape immune
response (8). To study the effect
of vaccination on DCs, in the present follow-up study, liver DCs
from immunized mice were analyzed by FACS. The results demonstrated
that the HBV-S-ecdCD40L vaccine may increase the expression of
surface molecules and the production of IL-12. In addition, the
allostimulatory activities of DCs were measured in an MLR, and the
results indicated that DCs from HBV-Tg mice immunized with
pcDNA3.1-S-ecdCD40L tended to have significantly enhanced T-cell
stimulatory capacity. These results suggested that the
HBV-S-ecdCD40L vaccine may promote activation of DCs, which was
consistent with a previous study (15). It may represent a novel effective
and promising treatment of chronic HBV infection.
To further evaluate the potential therapeutic
efficacy of the vaccination, serum HBV DNA and HBsAg were detected.
The results demonstrated that serum HBsAg and HBV DNA were markedly
reduced in HBV transgenic mice immunized with the HBV S-ecdCD40L
vaccine. During DNA vaccination alone with a gene of HBV S antigen,
the reduction in serum HBsAg and HBV DNA was less evident.
Furthermore, a potential complication of inducing an immune
response against HBV by the vaccination is that this may generate
liver injury. However, the result of serum ALT detection suggested
that this may not be an issue. The results of liver injury
suggested that HBV-S-ecdCD40L vaccination may break immunotolerance
and elicit effective antiviral immunity in a mouse model without
causing obvious liver injury.
In conclusion, the present study demonstrated that a
HBV S-ecdCD40L vaccine may induce DC activation with upregulated
expression of immune regulatory molecules including MHCII/CD86, and
pro-inflammatory cytokines including IL-12. These DCs are able to
stimulate enhanced allogeneic T-cell proliferation. The effect of
this therapeutic vaccine was evaluated in a mouse model of CHB.
These findings indicated a significant inhibition of HBV DNA
replication and downregulation of HBsAg in HBV-Tg mice, without
obvious liver injury. Therefore, the HBV-S-ecdCD40L vaccine may
offer a novel and effective therapeutic strategy for CHB
sufferers.
Acknowledgements
The present study was supported by the Science and
Technology Bureau of Wenzhou (grant no. 20140628) and the Zhejiang
Provincial Natural Science Foundation of China (grant nos.
LY12H03003 and Y2110768).
References
|
1
|
European Association For The Study Of The
Liver, . EASL clinical practice guidelines: Management of chronic
hepatitis B virus infection. J Hepatol. 57:167–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buchmann P, Dembek C, Kuklick L, Jäger C,
Tedjokusumo R, von Freyend MJ, Drebber U, Janowicz Z, Melber K and
Protzer U: A novel therapeutic hepatitis B vaccine induces cellular
and humoral immune responses and breaks tolerance in hepatitis B
virus (HBV) transgenic mice. Vaccine. 31:1197–1203. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maini MK, Boni C, Lee CK, Larrubia JR,
Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, et al:
The role of virus-specific CD8(+) cells in liver damage and viral
control during persistent hepatitis B virus infection. J Exp Med.
191:1269–1280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michel ML and Loirat D: DNA vaccines for
prophylactic or therapeutic immunization against hepatitis B.
Intervirology. 44:78–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Yang X, Jiang Y and Liu J: A novel
HBV DNA vaccine based on T cell epitopes and its potential
therapeutic effect in HBV transgenic mice. Int Immunol.
17:1293–1302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thermet A, Rollier C, Zoulim F, Trepo C
and Cova L: Progress in DNA vaccine for prophylaxis and therapy of
hepatitis B. Vaccine. 21:659–662. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beckebaum S, Cicinnati VR, Dworacki G,
Müller-Berghaus J, Stolz D, Harnaha J, Whiteside TL, Thomson AW, Lu
L, Fung JJ and Bonham CA: Reduction in the circulating pDC1/pDC2
ratio and impaired function of ex vivo-generated DC1 in chronic
hepatitis B infection. Clin Immunol. 104:138–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kakimi K, Isogawa M, Chung J, Sette A and
Chisari FV: Immunogenicity and tolerogenicity of hepatitis B virus
structural and nonstructural proteins: Implications for
immunotherapy of persistent viral infections. J Virol.
76:8609–8620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Kooten C and Banchereau J: CD40-CD40
ligand. J Leukoc Biol. 67:2–17. 2000.PubMed/NCBI
|
|
11
|
Grewal IS and Flavell RA: CD40 and CD154
in cell-mediated immunity. Annu Rev Immunol. 16:111–135. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Sullivan B and Thomas R: CD40 and
dendritic cell function. Crit Rev Immunol. 23:83–107. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caux C, Massacrier C, Vanbervliet B,
Dubois B, Van Kooten C, Durand I and Banchereau J: Activation of
human dendritic cells through CD40 cross-linking. J Exp Med.
180:1263–1272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cella M, Scheidegger D, Palmer-Lehmann K,
Lane P, Lanzavecchia A and Alber G: Ligation of CD40 on dendritic
cells triggers production of high levels of interleukin-12 and
enhances T cell stimulatory capacity: T-T help via APC activation.
J Exp Med. 184:747–752. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu JM, Lin XF, Huang ZM and Wu JS:
Construction of the HBV S-ecdCD40L fusion gene and effects of HBV
S-ecdCD40L modification on function of dendritic cells. J Viral
Hepat. 18:e461–e467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu L, Woo J, Rao AS, Li Y, Watkins SC,
Qian S, Starzl TE, Demetris AJ and Thomson AW: Propagation of
dendritic cell progenitors from normal mouse liver using
granulocyte/macrophage colony-stimulating factor and their
maturational development in the presence of type-1 collagen. J Exp
Med. 179:1823–1834. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao W, Sun Y, Chen S, Zhang J, Kang J,
Wang Y, Wang H, Xia G, Liu Q and Kang Y: Mushroom lectin enhanced
immunogenicity of HBV DNA vaccine in C57BL/6 and HBsAg-transgenic
mice. Vaccine. 31:2273–2280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lavanchy D: Hepatitis B virus
epidemiology, disease burden, treatment and current, and emerging
prevention and control measures. J Viral Hepat. 11:97–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng S, Zhong Y, Wang S, Liu H, Zou Q, Xie
X, Li C, Yu Q, He Z and Wang B: Amiloride enhances antigen specific
CTL by faciliting HBV DNA vaccine entry into cells. PLoS One.
7:e330152012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kosinska AD, Zhang E, Lu M and Roggendorf
M: Therapeutic vaccination in chronic hepatitis B: Preclinical
studies in the woodchuck. Hepat Res Treat.
2010:8175802010.PubMed/NCBI
|
|
21
|
Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey
LG, Speller S, Culp J, Burkholder JK, Swain WF, Dixon RM, et al:
Induction of antigen-specific CD8+ T cells, T helper cells, and
protective levels of antibody in humans by particle-mediated
administration of a hepatitis B virus DNA vaccine. Vaccine.
19:764–778. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Su WJ, Wang J, Bai XF, Huang CX
and Lian JQ: A fusion DNA vaccine encoding middle version of HBV
envelope protein fused to interleukin-21 did not enhance
HBV-specific immune response in mice. Viral Immunol. 27:430–437.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon SK, Seo YB, Im SJ, Bae SH, Song MJ,
You CR, Jang JW, Yang SH, Suh YS, Song JS, et al: Safety and
immunogenicity of therapeutic DNA vaccine with antiviral drug in
chronic HBV patients and its immunogenicity in mice. Liver Int.
35:805–815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mishra S, Lavelle BJ, Desrosiers J, Ardito
MT, Terry F, Martin WD, De Groot AS and Gregory SH: Dendritic
cell-mediated, DNA-based vaccination against hepatitis C induces
the multi-epitope-specific response of humanized, HLA transgenic
mice. PLoS One. 9:e1046062014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burgdorf S, Kautz A, Böhnert V, Knolle PA
and Kurts C: Distinct pathways of antigen uptake and intracellular
routing in CD4 and CD8 T cell activation. Science. 316:612–616.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato K and Fujita S: Dendritic cells:
Nature and classification. Allergol Int. 56:183–191. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Tang Y, Akbulut H, Zelterman D,
Linton PJ and Deisseroth AB: An adenoviral vector cancer vaccine
that delivers a tumor-associated antigen/CD40-ligand fusion protein
to dendritic cells. Proc Natl Acad Sci USA. 100:15101–15106. 2003;
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deisseroth A, Tang Y, Zhang L, Akbulut H
and Habib N: TAA/ecdCD40L adenoviral prime-protein boost vaccine
for cancer and infectious diseases. Cancer Gene Ther. 20:65–69.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pillarisetty VG, Shah AB, Miller G, Bleier
JI and DeMatteo RP: Liver dendritic cells are less immunogenic than
spleen dendritic cells because of differences in subtype
composition. J Immunol. 172:1009–1017. 2004. View Article : Google Scholar : PubMed/NCBI
|