Introduction
As a dynamic organ, the bone undergoes continuous
resorption and generation process, of which the process is called
remodeling. Under normal circumstances, there is a balance between
osteoclast-mediated resorption and osteoblast-mediated formation,
which maintains metabolism and homeostasis. However, the loss of
balance may lead to skeletal diseases (1). Osteoclasts are the only cells that
are responsible for bone resorption. Overexpression of osteoclasts
leads to excessive bone resorption and bone destruction, thus
causing osteoporosis, osteoarthritis and other common joint
diseases (2–4).
Berberine hydrochloride, as an isoquinoline
alkaloid, with molecular formula [C20H18NO4]+, also known as
berberine, presents in many plants of the Berberidaceae families.
Berberine can be precipitated in ether as yellow needle-like
crystals, with melting point at 145°C, soluble in water,
antibacterial to hemolytic Streptococcus, Staphylococcus aureus,
Neisseria gonorrhoeae, and Freund's Shigella, and also can enhance
leukocyte phagocytosis. Berberine hydrochloride has been widely
used in the treatment of gastroenteritis, bacillary dysentery,
pulmonary tuberculosis, scarlet fever, acute tonsillitis and
respiratory infections. However, it has been reported neither
whether the drug can affect osteoclastogenesis, nor
osteoclast-associated bone destruction can be improved (5). Therefore, it is of certain
significance to be investigated (6).
Materials and methods
RAW264.7 cell culture and the effect
of berberine on lipopolysaccharide (LPS)-induced
osteoclastogenesis
The 3rd generation passaged RAW264.7 cells (from Sun
Yat-Sen University) were inoculated into 96-well plates at a
density of 1×104/well and cultured in DMEM medium
containing 10% FBS and 1% double antibody, 200 µl/well, and then
put in an incubator at 37°C with 5% CO2. RAW264.7 cells
were treated with LPS (100 ng/ml) except control group; normal
saline was added to the model group, and Berberine hydrochloride
(5, 10 and 20 µM; Manst Biological Technology Co., Ltd.) was added
into the Drug group simultaneously. Each group was set with three
multiple holes. Tartrate-resistant acid phosphatase (TRAP) staining
was performed after 5 days' culture. The staining method was
carried out according to the kit instructions. The cells were
observed under an inverted microscope.
ELISA method to detect tumor necrosis
factor-α (TNF-α) levels
After 24 h culture, the cells were put in the ELISA
plate and added with diluted samples or standardized samples. The
Control group holes were set at 100 µl. Biotinylated antibody were
added into each hole at 50 µl, mixed, and sealed with closure
plates. The cells were incubated at 37°C for 90 min, washed for 4
times, then added with streptavidin-HRP, with 100 µl per hole.
Subsequently, the cells were incubated at 37°C for 30 min, and
washed for 4 times. Color reagent TMB was added at 100 µl per hole.
The cells were colored at 37°C for 10 min. Stop solution was added
at 50 µl per hole. Absorbance at a wavelength of 450 nm was read by
a microplate reader, and TNF-α density was calculated.
Quantitative polymerase chain reaction
(PCR) assay to detect the mRNA expressions of fos-related antigen 2
(Fra-2), TRAP, β3-integrin, cathepsin K, dendritic cell-specific
transmembrane protein (DC-STAMP), V-type proton ATPase subunit d 2
(Atp6v0d2) and NFATcl
The cells were incubated for 24 h and added with 1
ml TRIzol reagent, then agitated sufficiently. A total of 0.2 ml
chloroform was added, mixed, agitated, and then the cells were
ice-bathed standing for 5 min. The cells were centrifuged at 12,000
rpm for 20 min under 4°C. The supernatant was replaced with an
equal volume of isopropanol, then ice-bathed standing for 5 min.
The cells were centrifuged at 12,000 rpm for 20 min under 4°C. The
supernatant was discarded and a total of 1 ml ethanol at 75% was
added. The solution was agitated to dissolve the RNA thoroughly.
The cells were centrifuged at 10,000 rpm for 5 min under 4°C. The
supernatant was carefully removed, and the EP tube was turned
upside down, then dried under room temperature for 15 min. A total
of 20 µl RNase-free water was added to dissolve the precipitate,
and 1 µl solution was suctioned into an Eppendorf, and the rest was
stored in-70°C refrigerator. The 1 µl solution was diluted to 80
µl. OD260 and OD280 ratio was measured by a spectrophotometer, then
total RNAs were calculated.
The cDNA synthesis and reverse transcription were
carried out using the PrimeScript RT reagent kit according to the
instructions, as follows: 5 times PrimeScript buffer 2 µl,
PrimeScript RT Enzyme Mix 0.5 µl, Total RNA 2 µl, RNase Free
dH2O 5 µl, and Total 10 µl. The reaction was carried out
in water bath at 37°C for 15 min, and then at 85°C for 15 sec.
After the reaction, an appropriate amount of cDNA was quantified by
real-time PCR, and the remaining samples were stored under-20°C.
Fluorescence real-time PCR was performed according to the
instructions of the SYBR Premix Ex Taq™ II (Perfect Real Time) kit:
SYBR Premix Ex Taq 12.5 µl, PCR Forward Primer 1 µl, PCR Reverse
Primer 1 µl, DNA template 2 µl, d H2O 8.5 µl, and Total
25 µl. Fluorescence real-time PCR was performed as follows:
Amplification curve: 5 min pre-denaturation at 95°C; 20 sec
denaturation at 95°C; 30 sec annealing at 60°C; and 20 sec
extending at 72°C. This stage was used for fluorescence signal
acquisition, 40 cycles in total. The dissolution curve: 60°C-95°C,
0.5°C in each incremental, for 20 sec. This stage is for
fluorescence signal acquisition, a total of 71 cycles. The size of
PCR product was verified and a total of 10 µl was obtained to
perform electrophoresis in 1% agar gel.
PCR primers were as follows: Fra-2,
5′-CCAACACGTAGTTTGAAGAC-3′ and 5′-TCCTGCCGCAGTTACACCG-3′; TRAP,
5′-TGACATCGAGCAGGTGAAAG-3′ and 5′-GAGTAGCAAGGAATGAGC-3′;
β3-integrin, 5′-AGCGGACATTCTGGAAATG-3′ and
5′-TCGTTCATGCACTGCTGA-3′; cathepsin K, 5′-ACCGCACAACAGCAGCATT-3′
and 5′-AGCTTGCTGTGCTTCAGT-3′; DC-STAMP, 5′-AACTGTTGTGGCCTGAATC-3′
and 5′-CGGTAAATGCAGGCGTAT-3′; Atp6v0d2, 5′-CATCCCATCACCATCTTCC-3′
and 5′-TCACACATGACGAAGGCA-3′; NFATcl, 5′-TCCTCCATGAACAAACAG-3′ and
5′-AGACGTGGTTTAGGAATGCAG-3′; and GAPDH, 5′-AACTTTGGCATGTGGAAGG-3′
and 5′-ACACATTGGGGGTAGGAAC-3′.
Western blotting to detect the
expressions of calcineurin in PLCyl, toll like receptor 4 (TLR4),
TNF receptor associated factor 6 (TRAF6) and nuclear factor of
activated T-cell 1 (NFATc1) signaling pathways
The above cells were cultured for 24 h, harvested,
and washed with PBS twice. Each bottle was added with 400 µl of
cell lysate, followed by 40 µl PMSF, agitated. The cells were
placed on ice 10 min for sufficient lysis. After being repeatedly
aspirated by a sterile syringe, the cells were put into an EP tube,
which was ice-bathed for 30 min, and subsequently centrifuged at
12,000 × g for 15 min. After the supernatant was transferred to a
new EP tube, 20 µl of protein sample buffer was added into each 100
µl tube, boiled for 5 min, mixed, and then stored under −80°C. The
protein from the above samples was separated by 12% SDS-PAGE
electrophoresis. The separated protein bands were transferred to a
PVDF membrane by wet method and closed under room temperature for 1
h. Subsequently, the primary antibody was added (concentration
1:1,000) for 4°C overnight incubation. The primary antibody was
discarded, and the secondary antibody (BA1026) was added
(concentration 1:1,000) for 1 h incubation. The secondary antibody
was abandoned, and color development and fixation were carried out
after chemiluminescence. The expressions of calcineurin in PLCyl,
TLR4, TRAF6 and NFATc1 signaling pathways were measured.
Confocal assay to detect the effect of
berberine hydrochloride on intracellular Ca2+
concentration
After 24 h culture, the supernatant was discarded.
The cells were washed with HESS, following this, HBSS containing
5Flou-3/AM and 0.05% Pluronic F127 was added. The cells were
incubated for 30 min under 37°C. The supernatant was abandoned, and
the cells were washed with HEPES buffer saline for 3 times.
Subsequently, a sum of 2 ml HEPES buffer saline was added. At the
excitation wavelength of 488 nm and the emission wavelength of
500–530 nm, the intracellular Ca2+ concentration was
measured according to the intensity changes of FIuo-3/AM by using
A1R laser scanning confocal microscopy.
Statistical methods
All data were expressed as mean ± SD. Comparisons
between the two groups were carried out using the t-test, and
multiple sets of data (>2) comparison using the one-way ANOVA,
where P<0.05 denoted statistical significance. The data were
analyzed by GraphPad Prism 5.0 (GraphPad Software Inc., San Diego,
CA, USA).
Results
Effects of berberine on LPS-induced
osteoclastogenesis
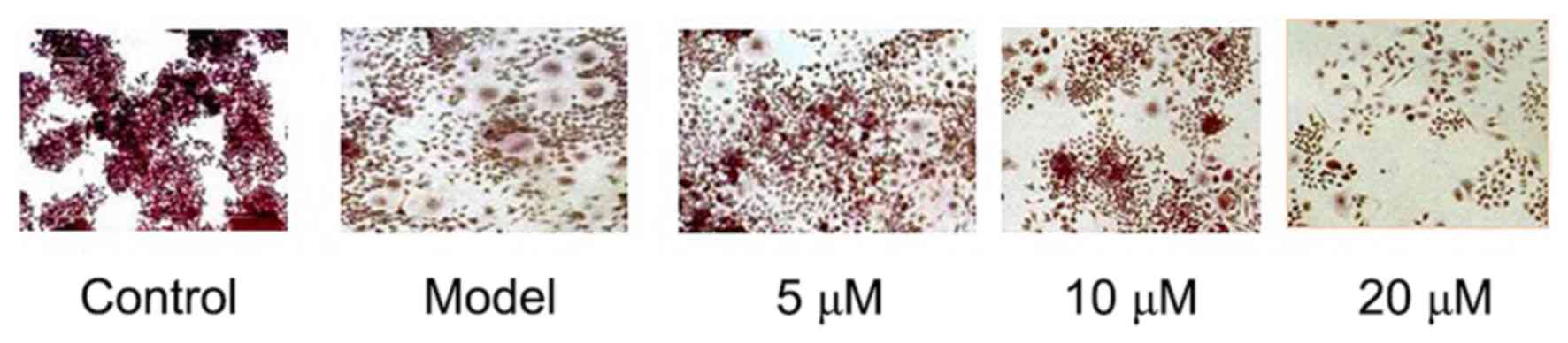
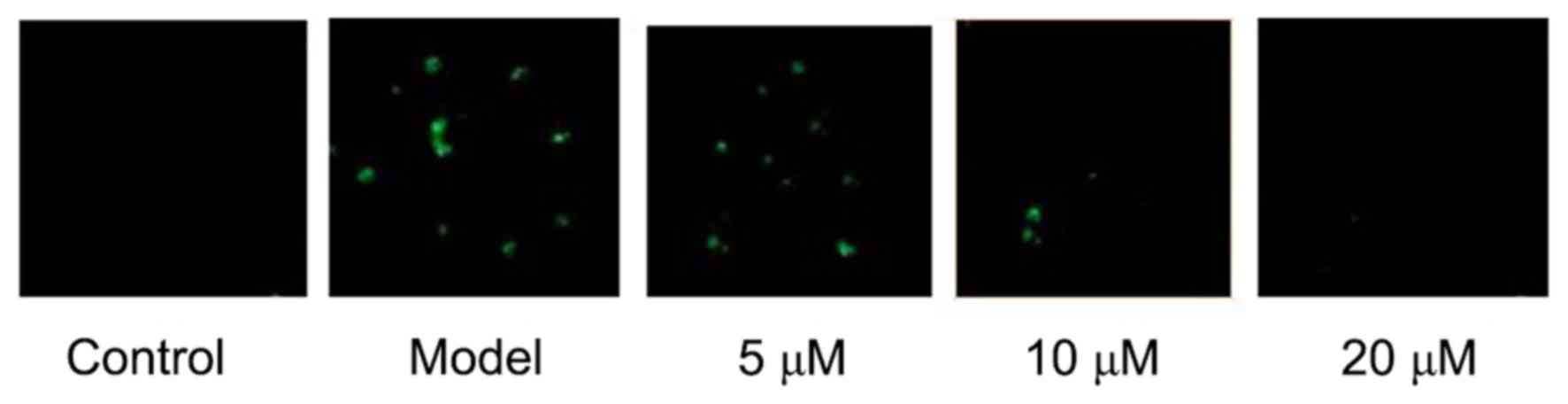
TRAP staining showed that RAW264.7 could be
differentiated into mature osteoclasts after 5 days of LPS
induction, while osteoclastogenesis was not seen in RAW264.7 cells
in the negative Control group. The results showed that LPS-induced
RAW264.7 cells' differentiation into osteoclasts can be
significantly inhibited by Berberine hydrochloride of high
concentration. See Fig. 1.
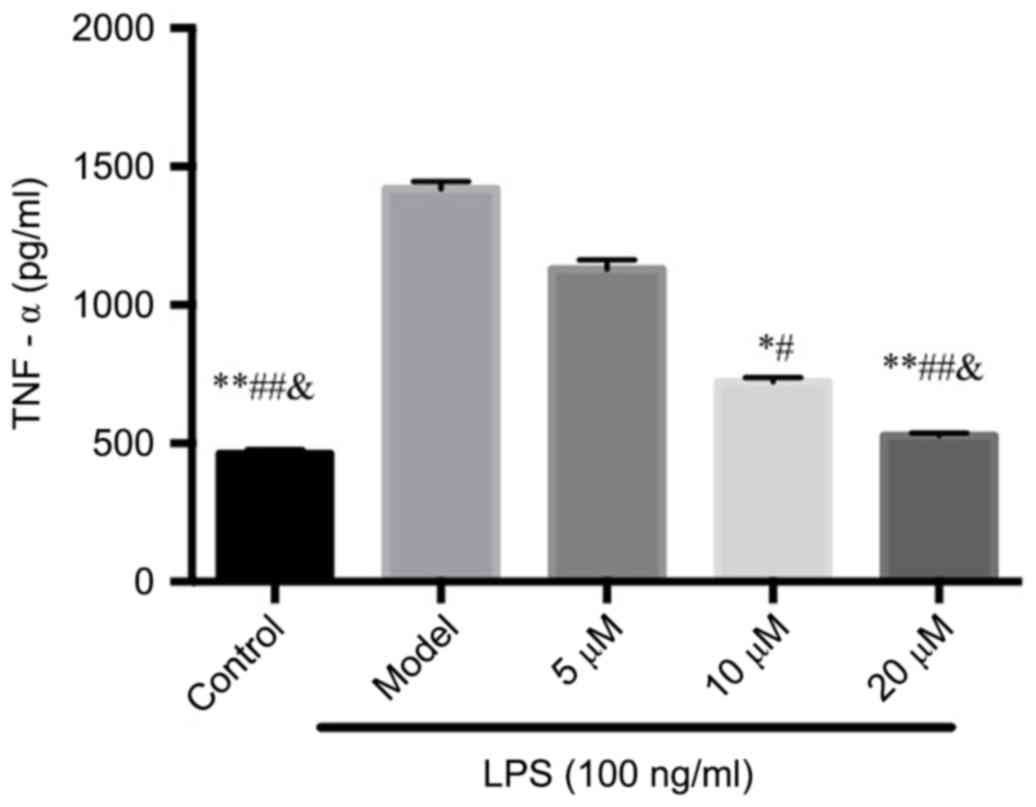
The inhibition of berberine on
LPS-induced osteoclast TNF-α secretion
In the supernatant, LPS significantly induced TNF-α
secretion, while the inhibition of TNF-α by berberine exhibited in
a dose-dependent manner compared with that of the Control group,
suggesting that berberine may inhibit osteoclastogenesis through
inhibiting TNF-α production. See Fig.
2.
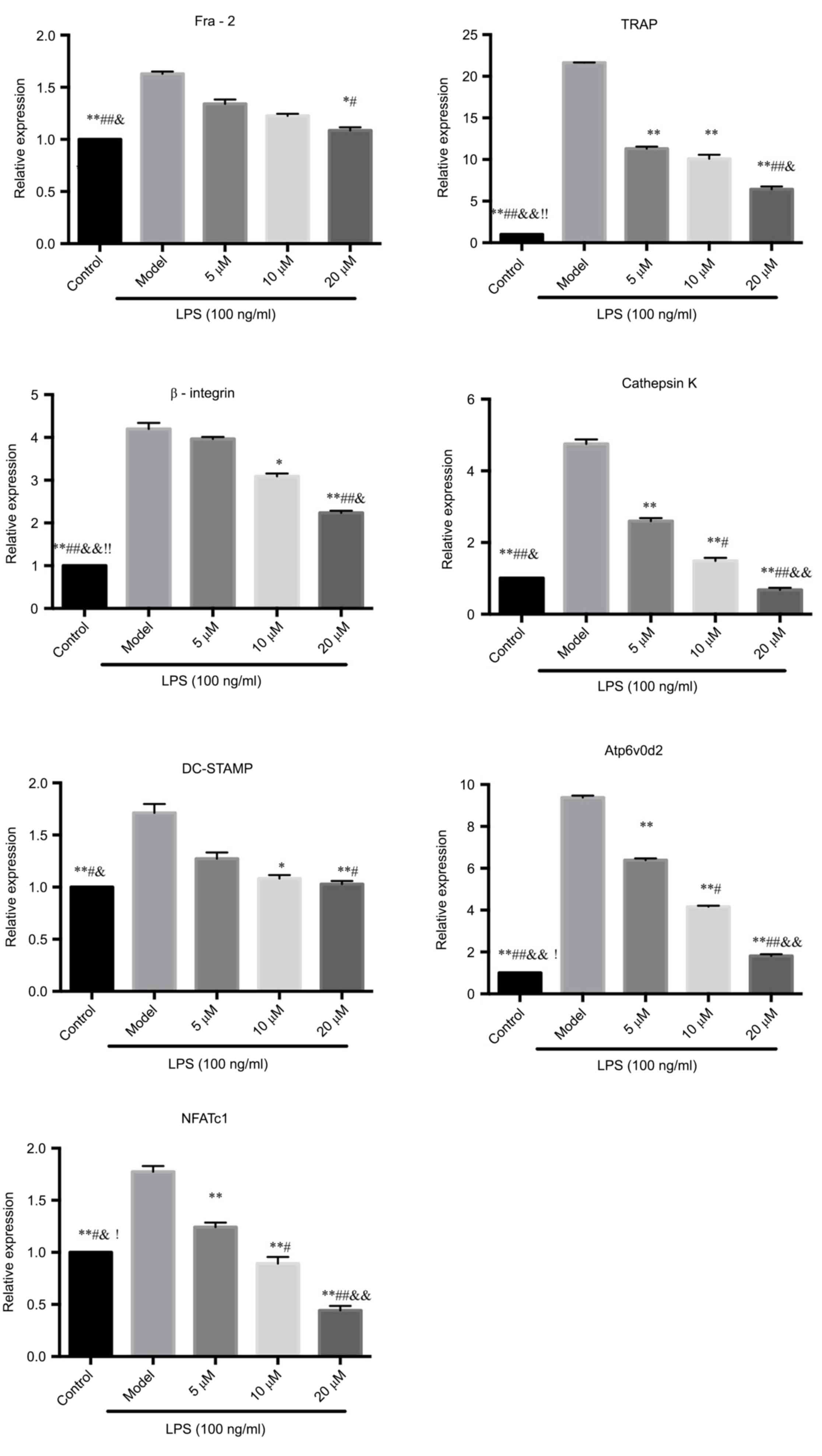
PCR assay to detect the mRNA
expressions of Fra-2, TRAP, β3-integrin, cathepsin K, DC-STAMP,
Atp6v0d2 and NFATcl
The mRNA expressions of Fra-2, TRAP, DC-STAMP and
Atp6v0d2 in RAW264.7 cells induced by LPS were significantly
inhibited by berberine hydrochloride, suggesting that berberine
could inhibit LPS-induced osteoclasts-related gene expression,
thereby inhibiting the differentiation of RAW264.7 cells to
osteoclasts.
During the osteoclastogenesis, NFATcl was amplified
and NFATcl mRNA was upregulated. The results showed that berberine
can inhibit the increase of NFATcl gene expression induced by LPS
in a concentration-dependent manner. The experimental results
confirmed the hypothesis that the inhibition of osteoclastogenesis
by berberine may be related to the activation of nuclear
transcription factor NFATcl. See Fig.
3.
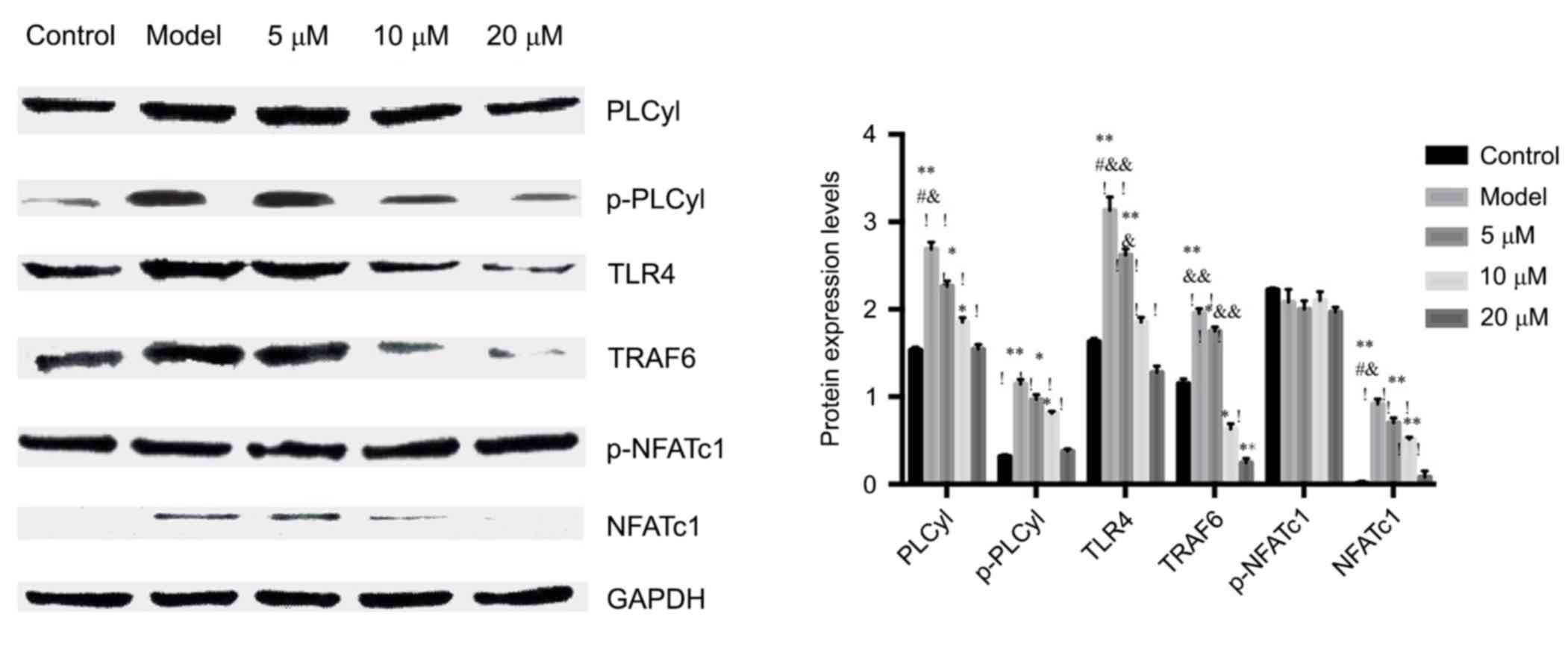
Western blot to detect the calcineurin
expressions of PLCyl, p-PLCyl, TLR4, TRAF6 and NFATc1, p-NFATc1
signaling pathways
WB results showed that berberine hydrochloride
significantly inhibited the expression of NFATcl in the nucleus.
The results further confirmed the hypothesis that berberine
inhibited the osteoclastogenesis possibly by inhibiting the
activation of nuclear transcription factor NFATcl.
After stimulated by LPS, PLCyl was activated and
increased intracellular Ca2+ concentration. The results
showed that the activation of PLCyl, or the p-PLCyl protein, was
significantly upregulated by LPS which was inhibited by berberine,
suggesting that berberine could inhibit the activation of PLCyl,
thereby inhibiting Ca2+ influx, which reduced
intracellular Ca2+ concentration.
In order to further confirm the effect of berberine
hydrochloride on LPS signal transduction pathway, the effect of
berberine on the expression of LPS-induced TRAF6 protein was
investigated. The expression of TRAF6 protein was significantly
increased in RAW264.7 cells after LPS stimulation, but was
significantly inhibited by berberine. The results were consistent
with the detection of PCR. See Fig.
4.
Berberine to reduce the intracellular
Ca2+ concentration
The intracellular Ca2+ concentration was
measured by the Confocal technique. The results showed that LPS
significantly increased intracellular Ca2+
concentration. However, berberine significantly reduced
intracellular Ca2+ concentration, in particular, the
high concentration group reduced intracellular Ca2+
concentration the most, even close to the normal group, suggesting
that berberine hydrochloride can strongly inhibit Ca2+
influx. As shown in Fig. 5.
Discussion
‘Bone immunology’ is more and more a hot topic, in
which the osteoclast is the only cell responsible for bone
resorption. Under the category of ‘Bone immunology’, there are
excessive activations of osteoclasts in degenerative diseases such
as osteoporosis, inflammatory arthritis, and tumor-induced bone
destruction (7). Activated
osteoclasts can absorb bones, resulting in bone loss, causing
fractures, reactive bone proliferation, and subsequent pain and
disability. Drugs that whip the osteoclast differentiation can be
applied to the treatment of osteoporosis, and in recent years
expanding to the diseases such as tumor bone metastasis and
rheumatoid arthritis (8).
LPS is a component of the G-cell wall, which can
induce bone destruction through chronic infection. In vitro
TRAP staining showed that berberine inhibited LPS-induced
osteoclastogenesis. Studies have shown that inflammatory factors
such as TNF-α, IL-1, and IL-6 can promote osteoclast-mediated bone
resorption, posing a key regulatory factor in osteoclast activity
(9,10). These inflammatory factors
contribute to the formation of strong osteoclastogenesis
stimulating molecules PGE2 by increasing the expression of COX-2 in
osteoblasts and stromal cells. Increased TNF-α and other
inflammatory factors are associated with the fracture of the
femoral head in older women (11,12).
The present study found that berberine can reduce
the TNF-α levels in LPS stimulated supernatant, suggesting that
berberine may have the potential anti-inflammatory and
anti-osteoclastogenesis effect by downregulating the production of
TNF-α. In addition, it is found that berberine significantly
inhibited the expression of TRAP gene in RAW264.7 cells. Inhibition
of TNF-α activity may be the reason for the inhibition of TRAP
activity.
Intracellular Ca2+ influx can activate
calcineurin, which can de-phosphorylate the inactivated p-NFATcl,
and promote NFATcl into the nucleus, thereby activating
osteoclast-specific gene expression. The present study found that
berberine inhibited calcineurin expression, thereby inhibiting
cytoplasmic p-NFATcl de-phosphorylation and NFATcl nuclear
translocation, indicating that drugs can inhibit the activation of
NFATcl, and thus inhibit osteoclast-associated gene expression. And
molecules such as DC-STAMP, Atp6v0d2 and Fra-2 are all related to
osteoclastogenesis. During the osteoclastogenesis, the osteoclast
precursors fuse into large multinucleated macrophages under the
intervention of DC-STAMP and Atp6v0d2 (13,14).
Fra-2 can regulate the size and survival of
osteoclasts (15). β3-integrin,
cathepsin K and MMP-9 are necessary for osteoclasts to carry out
bone resorption. Both β3-integrin and Cathepsin K played important
roles in the bone resorption by mediating migration and adsorption
of osteoclasts (16,17). Cathepsin K exhibited high
expression in osteoclasts, making a key enzyme in degradation of
organic bone matrix, thus facilitated the bone resorption of
osteoclasts (18–20).
In conclusion, berberine hydrochloride may target
through the TRAF6 and NFATcl, and inhibitosteoclastogenesis and
bone destruction probably through inhibiting
TRAF6-Ca2+-calcineurin-NFATcl signaling pathways.
References
|
1
|
Geusens P and Lems WF: Osteoimmimology and
osteoporosis. Arthritis Res Ther. 13:2422011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deal C: Bone loss in rheumatoid arthritis:
Systemic, periarticular, and focal. Curr Rheumatol Rep. 14:231–237.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McClung M, Harris ST, Miller PD, Bauer DC,
Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK and Lewiecki EM:
Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug
holiday. Am J Med. 126:13–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schett G and Gravallese E: Bone erosion in
rheumatoid arthritis: Mechanisms, diagnosis and treatmentJ. Nat Rev
Rheumatol. 8:656–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao K, Xiao D, Weng J, Xiong A, Kang B and
Zeng H: Berberine promotes bone marrow-derived mesenchymal stem
cells osteogenic differentiation via canonical Wnt/β-catenin
signaling pathway. Toxicol Lett. 240:68–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banks L and Takayanagi H: Immunology and
bone. J Biochem. 154:29–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stickeler E and Fehm T: Targeted and
osteo-oncologic: Treatment in early breast cancer: What is state of
the art and what might become so within the next 5 years. Breast
Care (Basel). 9:161–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Amico AV: US Food and drug
administration approval of drugs for the treatment of prostate
cancer: A new era has begun. J Clin Oncol. 32:362–364. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manolagas SC and Jilka RL: Bone marrow,
cytokines, and bone remodeling. Emerging insights into the
pathophysiology of osteoporosis. N Engl J Med. 332:305–311. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Braun T and Schett G: Pathways for bone
loss in inflammatory disease. Curr Osteoporos Rep. 10:101–108.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okada Y, Lorenzo JA, Freeman AM, Tomita M,
Morham SG, Raisz LG and Pilbeam CC: Prostaglandin G/H synthase-2 is
required for maximal formation of osteoclast-like cells in cultur.
J Clin Invest. 105:823–832. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barbour KE, Boudreau R, Danielson ME, Youk
AO, Wactawski-Wende J, Greep NC, LaCroix AZ, Jackson RD, Wallace
RB, Bauer DC, et al: Inflammatory markeirs and the risk of hip
fracture: The women's health initiative. J Bone Miner Res.
27:1167–1176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim K, Lee SH, Ha Kim J, Choi Y and Kim N:
NFATcl induces osteoclast fusion via up-regulation of Atp6v0d2 and
the dendritic cell specific transmembrane protein (DC-STAMP). Mol
Endocrinol. 22:H6–85. 2008. View Article : Google Scholar
|
|
14
|
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K,
Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K,
et al: DC-STAMP is essential for cell-cell fusion in osteoclasts
and foreign body giant cells. J Exp Med. 202:345–351. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bozec A, Bakiri L, Hoebertz A, Eferl R,
Schilling AF, Komnenovic V, Scheuch H, Priemel M, Stewart CL,
Amling M and Wagner EF: Osteoclast size is controlled by Fra-2
through LIF/LIF-receptor signalling and hypoxia. Nature.
454:221–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song I, Kim R, Kim K, Jin HM, Youn BU and
Kim N: Regulatory mechanism of NFATcl in RANKL induced osteoclast
activation. FEBS Lett. 583:2435–2440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei ZF, Tong B, Xia YF, Lu Q, Chou GX,
Wang ZT and Dai Y: Norisoboldine suppresses osteoclast
differentiation through preventing the accumulation of TRAF6-TAK1
complexes and activation of MAPKs/NF-κB/c-Fos/NFATc1 pathways. PLoS
One. 8:e591712013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa AG, Cusano NE, Silva BC, Cremers S
and Bilezikian JP: Cathepsin K: Its skeletal actions and role as a
therapeutic target in osteoporosis. Nat Rev Rheumatol. 7:447–456.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Broadhead ML, Clark JC, Dass CR, Choong PF
and Myers DE: The apeutic targeting of osteoclast fimction and
pathways. Expert Opin Ther Targets. 15:169–181. 2011. View Article : Google Scholar : PubMed/NCBI
|