Introduction
Preeclampsia is a pregnancy-specific disorder which
is a leading cause of maternal and perinatal mortality and
morbidity (1). It is characterised
by systemic endothelial cell activation and exaggerated
inflammation. Although the pathogenesis of preeclampsia remains
unclear, it is well documented that deficient placentation is
associated with this disease; this starts from 6–18 weeks of
gestation. Disruption to extravillous cytotrophoblast invasion and
spiral artery remodelling, subsequently induces placental
dysfunction and placental oxidative stress, which in turn
contribute to the pathogenesis of the disease (2–4).
During pregnancy, levels of estrogen and
progesterone are increased. In animal models, inhibition of
estrogen production results in pregnancy loss, suggesting that
estrogen may have a role in the maintenance of a healthy pregnancy
(5,6). 17β-estradiol (E2) is the common form
of estrogen in women and the levels of E2 are increased during
pregnancy due to placental production (7). There is evidence to suggest that
reduced levels of estrogen and increased levels of progesterone are
associated with the pathogenesis of preeclampsia (8–10).
The level of E2 has been also suggested to be reduced in
preeclampsia (11). In addition, a
previous study suggested that estrogen deficiency affects
endothelial cell function (12).
The biological effects of estrogen are commonly
mediated by estrogen receptors α (ERα) and β (ERβ) (13,14).
However, a previous study suggested that factors other than ERα and
ERβ are involved in the functions of estrogen in pregnancy
(15). The G-protein-coupled
receptor 30 (GPR30) was reported as a novel estrogen receptor in
2005 and is able to mediate estrogen action (16,17).
Unlike ERα and ERβ that function as estrogen-activated
transcription factors in the nucleus and do not influence gene
transcription (18), GPR30 is a
transmembrane estrogen receptor (19). The most potent estrogen produced in
the body is E2 (7) and GPR30 is a
specific receptor for E2 (19).
GPR30 can additionally promote the ability of estrogen to inhibit
apoptosis induced by oxidative stress in keratinocytes (20) and GPR30 mediates the proliferative
effects of estrogen in endometrial cancer (21).
GPR30 mRNA is expressed in the placenta (19), however, the role of GPR30 in
pregnancy or in pregnancy complications such as preeclampsia and
fetal growth restriction (FGR) remains to be investigated. It has
been previously reported that hypoxia-reoxygenation is a potential
inducer of placental oxidative stress and thus the development of
preeclampsia (22). Therefore, the
present study aimed to investigate whether the expression of GPR30
in the placenta is altered in preeclampsia and whether
hypoxia-reoxygenation conditions affects the expression of GPR30
in vitro. In addition, the potential function of GPR30 in
pregnancy was investigated in vitro.
Materials and methods
Ethical approval and consent
The present study was approved by the Ethics
Committee of Human Experimentation of the First Affiliated Hospital
of Chongqing Medical University (Chongqing, China). For placentae
collection, all patients gave written informed consent.
Study population for placenta
collection
Placentae from women with preeclampsia (n=21, three
with early onset <34 weeks and 18 with late on-set >34 weeks
gestation) and from women whose pregnancies were uncomplicated
(n=21) were collected after delivery between January 2013 and May
2014 at the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China). All women underwent caesarean
section. The clinical details of the 42 women for placentae
collection are summarised in Table
I.
 | Table I.Summary of clinical characteristics
of patients with preeclampsia and normotensive controls. |
Table I.
Summary of clinical characteristics
of patients with preeclampsia and normotensive controls.
|
| Preeclampsia
(n=21) | Normotensive
control (n=21) | P-value |
|---|
| Maternal age
[median age in years (range)] | 28 (23–33) | 28 (17–33) | P>0.05 |
| BMI [median BMI in
kg/m2 (range)] | 23.9 (17–27.7) | 24 (18.4–28.1) | P>0.05 |
| Gestation weeks (+
days) at sampling [median (range)] | 35+3 (31–38+6) | 35+3 (33–37+6) | P>0.05 |
| Systolic blood
pressure [median value in mmHg (range)] | 167 (149–178) | 121 (101–137) | P=0.0001 |
| Diastolic blood
pressure [median value in mmHg (range)] | 97 (88–115) | 77 (66–89) | P=0.0001 |
| Birth weight
[median value in g (range)] | 2,398
(1,985–2,958) | 3,097
(2,534–3,684) | P=0.0001 |
Preeclampsia was defined as a maternal systolic
blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg
measured on two occasions separated by at least 6 h following 20
weeks of gestation in accordance with the guidelines of the
American College of Obstetricians and Gynaecologists (23). Three of the preeclampsia
pregnancies were also complicated by FGR.
Reagents
E2, a general agonist for estrogen receptors, was
purchased from Abcam (ab120657; Cambridge, UK). The selective GPR30
agonist G1 and the selective GPR30 antagonist G15 were purchased
from Sigma-Aldrich; Merck Millipore (G6798 and G6748; Darmstadt,
Germany).
Tissue preparation
Placentae were collected within 10 min of delivery
and placental explants (collected from the maternal side of the
placenta) and decidua were then harvested. All the tissues were
snap frozen and stored at −80°C for western blotting or fixed in 4%
formaldehyde (Shanghai Aladdin Bio-Chem Technology Co., Ltd.,
Shanghai, China) prior to embedding in paraffin for
immunohistochemical analysis (IHC).
Cell culture and treatment
The human-transformed primary extravillous
trophoblast cell line, HTR8/SVneo cell line was donated by Dr C.H.
Graham (Queen's University, Kingston, ON, Canada). HTR8/SVneo cells
were cultured were cultured at 20% O2 in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; 10099; Gibco; Invitrogen).
HTR-8/SVneo cells were growth in 12 well plates until confluent.
Cells were then pretreated with E2 (100 nM) or G1 (1 µM) or G15 (2
µM) for 1 h under normal conditions. Pretreated cells were randomly
divided into 2 groups: Cells in one group were cultured under
normal conditions, whereas cells in the other were treated in
hypoxia (1% O2) for 4 h and followed by 18 h of
reoxygenation (20% O2). Cells were then harvested for
further studies.
Placental explants culture and
treatment
Placental explants were cultured on matrigel (10%)
in 48-well plates. Explants were pre-treated with E2 (100 nM) or G1
(1 µM) or G15 (2 µM) for 1 h in normoxic conditions (20%
O2). Explants were subsequently maintained in normoxic
conditions for 24 h or subjected to hypoxia (1% O2) for
4 h followed by 18 h of reoxygenation (20% O2).
Extravillous trophoblast sprouting and migration from the distal
end of the villous tips was recorded. The extent of migration
(i.e., the distance from the cell column base to the tip of the
outgrowth) was measured at defined positions with Adobe Fireworks
software version CS5 (Adobe Systems, Inc., San Jose, CA, USA).
Western blotting
HTR-8/SVneo cells that had been treated were lysed
with RIPA buffer (P0013; Beyotime Institute of Biotechnology,
Haimen, China). Protein concentration was determined by the BCA
Protein Assay kit (P0010; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Protein samples (30
µg) were loaded in 10% SDS-polyacrylamide gels, resolved by
electrophoresis and transferred to polyvinylidene difluoride
membranes (PVDF; 0.22 mm; EMD Millipore, Billerica, MA, USA).
Immunoblotting was performed using primary antibodies against GPR30
(1:1,000, ab154069; Abcam) and β-actin (1:1,000, TA-09; OriGene
Technologies, Inc., Rockville, MD, USA). Following incubation with
the goat anti-rabbit (1:1,000, A0208; Beyotime Institute of
Biotechnology) or goat anti-mouse (1:1,000, A0192; Beyotime
Institute of Biotechnology) horseradish peroxidase-conjugated
secondary antibodies, the bands of specific proteins on the
membranes were developed with BeyoECL Plus (P0018; Beyotime
Institute of Biotechnology). The levels of proteins were quantified
by a ChemiDoc image analyzer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The expression of β-actin was used as a loading
control.
IHC
IHC was performed as previously described (15). Briefly, 5 µm paraffin-embedded
tissue sections were deparaffinized in xylene, then rehydrated in a
serial gradient of ethanol and washed in PBS. Tissue sections were
further quenched sequentially by the use of 3% hydrogen peroxide
for 15 min and incubated in 10% normal goat serum (Sigma-Aldrich;
Merck Millipore) for 45 min at room temperature. The slides were
then incubated at 4°C overnight with polyclonal rabbit anti-GPR30
antibody (1:150, ab39742; Abcam) or polyclonal rabbit anti-CD31
antibody (1:100, cat. no. GTX110602; GeneTex, Inc., Irvine, CA,
USA). The slides were rinsed with PBS, and then incubated with a
horseradish peroxidase-conjugated goat anti-rabbit IgG for 30 min
at 37°C. 3,3′-Diaminobenzidine (chromogenic reagent; OriGene
Technologies, Inc.) was used as the chromogen, and hematoxylin
(Sigma-Aldrich; Merck Millipore) was used for nuclear
counterstaining. For the negative controls, the primary antibodies
were omitted. Experiments were repeated at least three times.
Statistical analysis
Data are presented as the mean ± standard
deviations. The statistical significance of the results was
assessed by a Mann-Whitney U test or a Kruskal-Wallis test using
Graphpad Prism software (version 5.0; GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
GPR30 expression was significantly
lower in placenta and decidua from preeclampsia pregnancies
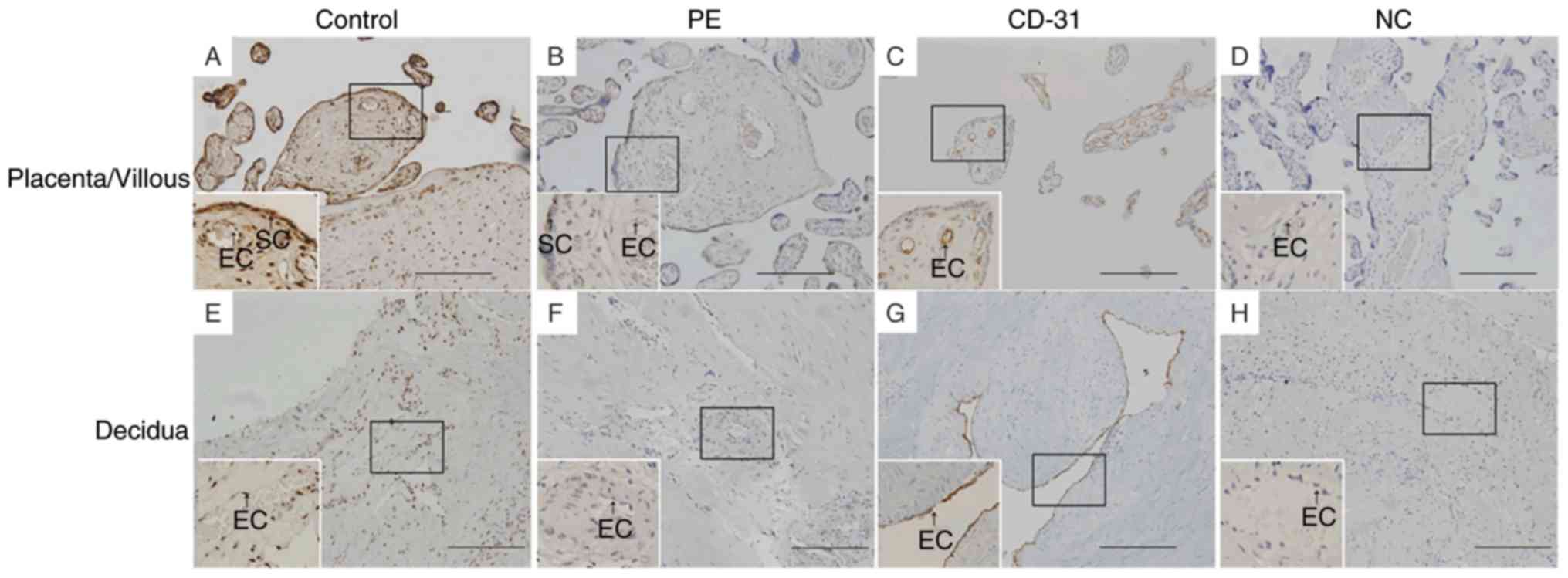
IHC analysis demonstrated that GPR30 was expressed
in vascular endothelial cells and syncytiotrophoblasts in placentae
from uncomplicated pregnancies (Fig.
1). GPR30 was also expressed in vascular endothelial cells and
in the stroma in decidua from uncomplicated pregnancies (Fig. 1). However, GPR30 expression was
reduced in the placenta and decidua from pregnancies complicated by
preeclampsia (Fig. 1). To quantify
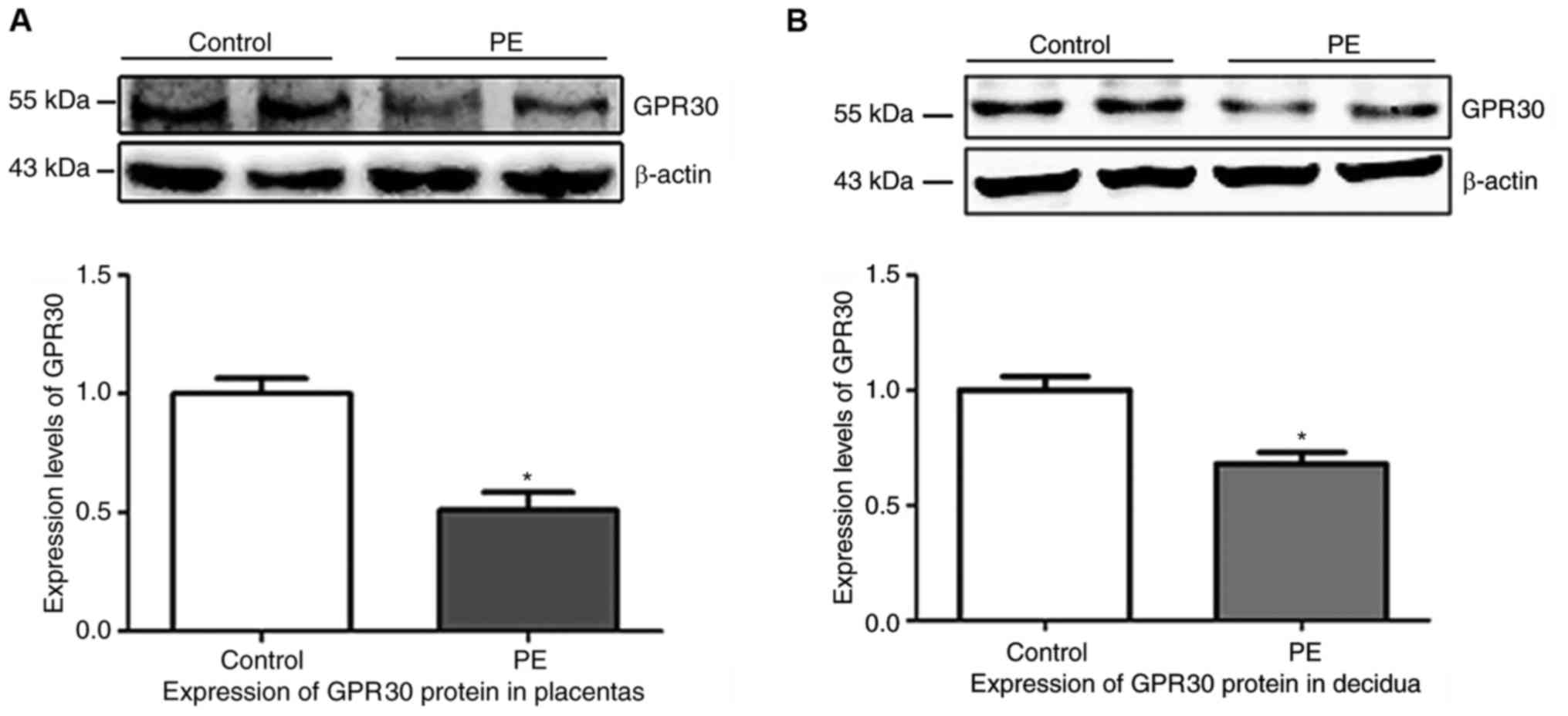
the IHC results, semi-quantitative analysis of the western blots
was performed. The protein levels of GPR30 relative to β-actin were
significantly lower in preeclampsia placentas (Fig. 2A; P=0.0078) and decidua (Fig. 2B; P=0.0163), as compared with
tissues from uncomplicated pregnancies.
Expression of GPR 30 in HTR-8/SVneo
was increased by E2
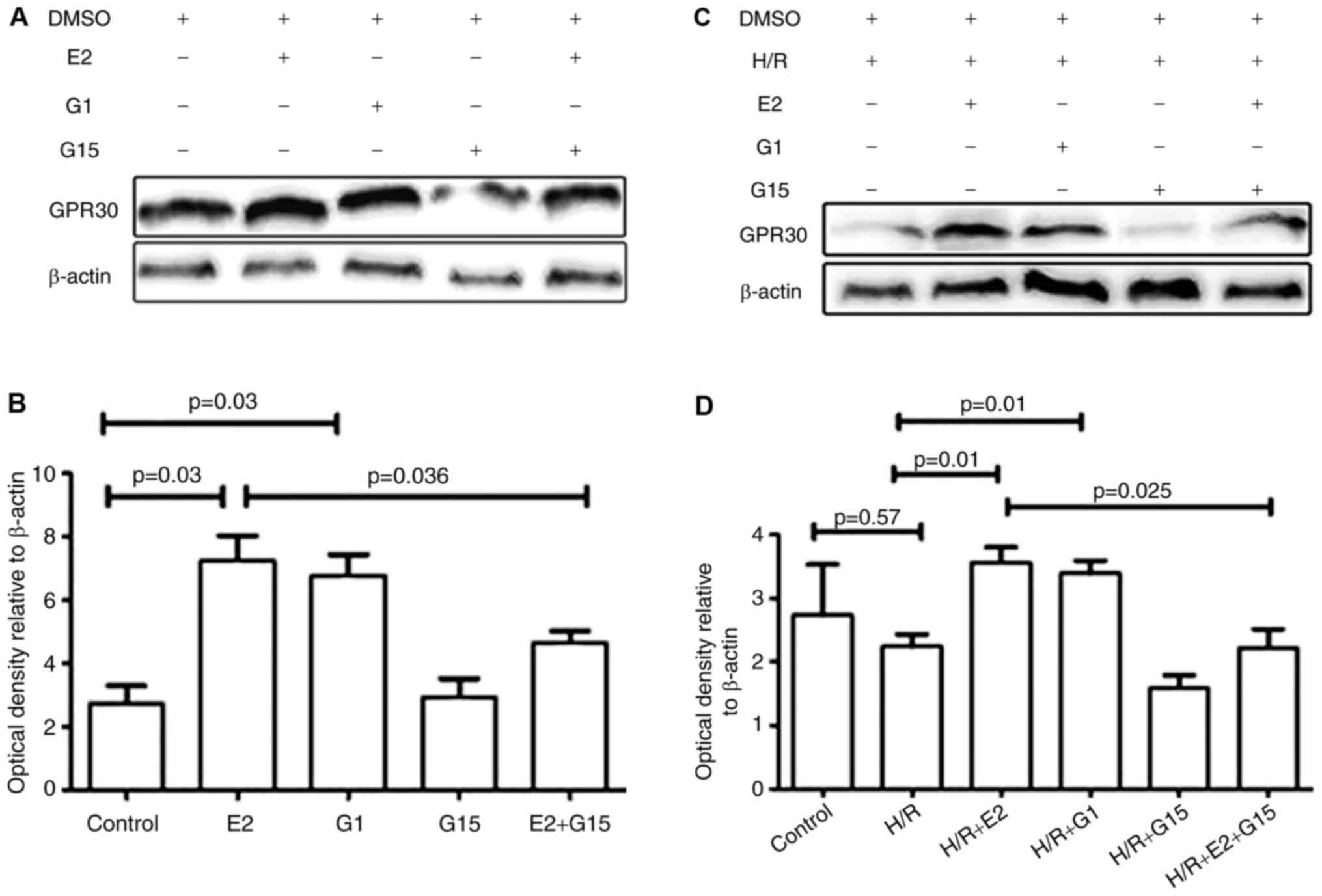
Whether E2 could enhance the in vitro
expression of GPR30 was investigated. GPR30 expression was
significantly increased by treatment with E2 in HTR-8/SVneo cells
(Fig. 3; P=0.03). The expression
of GPR30 in HTR8/SVneo cells was additionally significantly
increased by pre-treatment with the GPR30 agonist G1 (Fig. 3; P=0.03). The increased expression
of GPR30 induced by E2 was inhibited by pre-treatment with G15, a
GPR30 selective antagonist (Fig.
3; P=0.04). Treatment of HTR-8/SVneo cells with G15 did not
change GPR30 expression (Fig. 3;
P=0.87).
There were no significant differences in GPR30
expression under hypoxia/reoxygenation conditions compared with
normoxic conditions (Fig. 3;
P=0.57). However, GPR30 expression was significantly increased by
E2 treatment under hypoxia/reoxygenation conditions (Fig. 3; P=0.01) and this increase was
inhibited by pre-treatment with G15 (Fig. 3; P=0.025). When HTR-8/SVneo cells
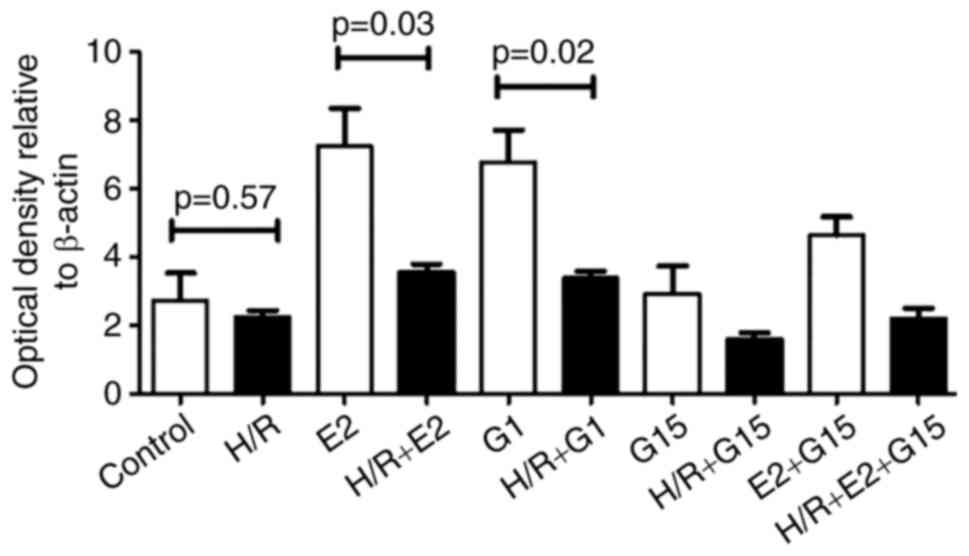
were pre-treated with E2 or G1, the expression of GPR30 was
significantly reduced in hypoxia/reoxygenation conditions compared
with normoxic conditions (Fig. 4;
P=0.03 or P=0.02). There was no significant difference in GPR30
expression in normoxic compared with hypoxia/reoxygenation
conditions in HTR-8/SVneo cells that were treated with G15
(P=0.18).
Treatment with E2 or G1 enhances
placental explants outgrowth
In order to investigate whether GPR30 is involved in
extravillous trophoblast invasion, the outgrowth of placental
explants in hypoxia/reoxygenation conditions. Notably, it was
identified that the outgrowth of placental explants was
significantly reduced in hypoxia/reoxygenation compared with
normoxic conditions (Fig. 5). A
trend towards an E2-induced increase in the reduced outgrowth did
not reach statistical significance (P=0.08); in contrast, the
reduced outgrowth was significantly increased by treatment with G1
(Fig. 5; P=0.038).
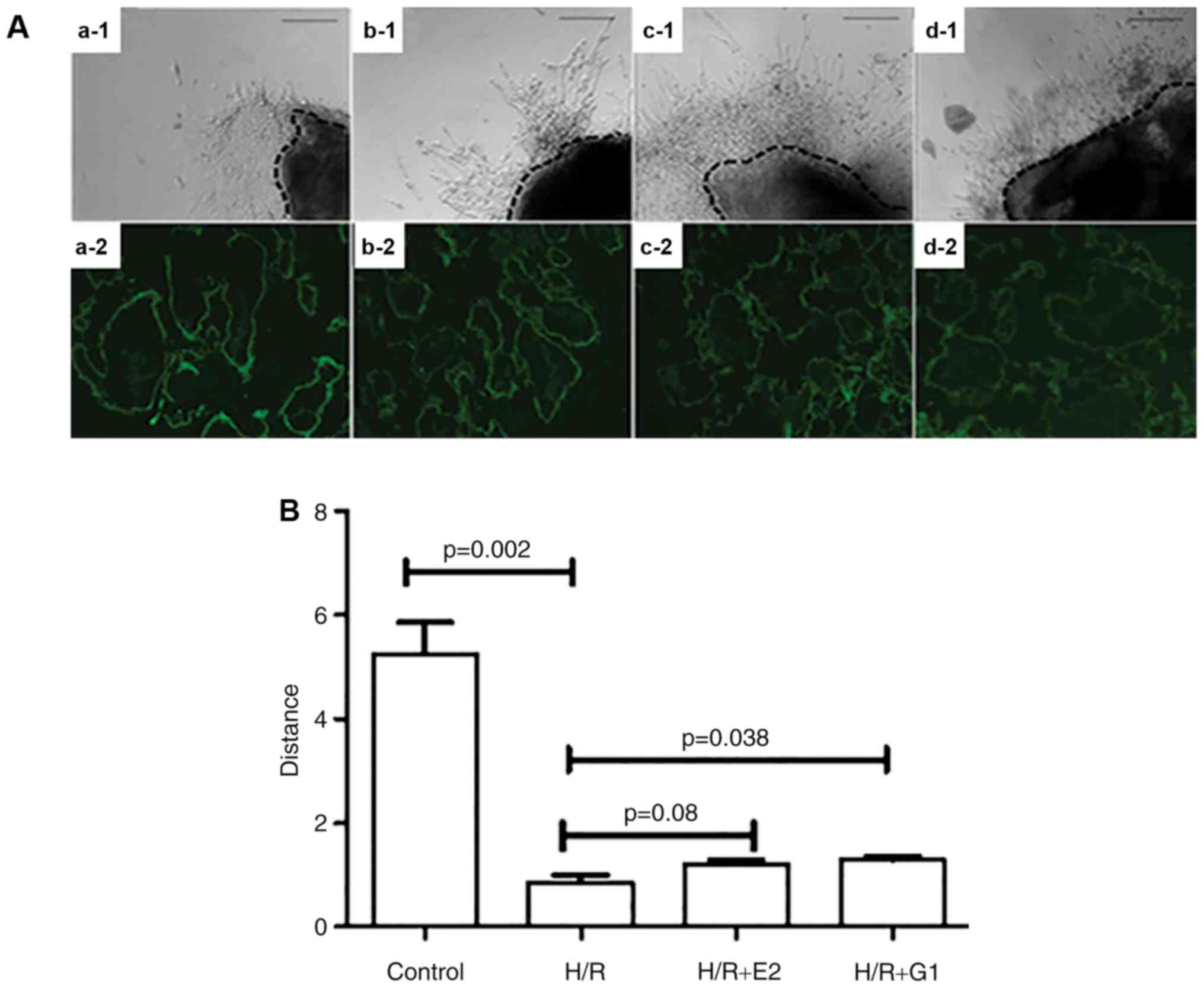 | Figure 5.Placental explants were cultured on
Matrigel under various culture conditions. (A) The growth of
placental explants was captured under a light microscope (a-1, b-1,
c-1, d-1). Scale bar, 100 µm. GPR30 expression was detected using
immunohistochemical staining in placental explants (a-2, b-2, c-2,
d-2). Scale bar, 100 µm. (a-1 and a-2) Control; (b-1 and b-2) H/R;
(c-1 and c-2) H/R+E2; (d-1 and d-2) H/R+G1. (B) When compared with
normoxic conditions (control), the outgrowth of placental explants
was significantly reduced in H/R conditions. Treatment with G1
significantly increased the outgrowth of placental explants in H/R
conditions. H/R, hypoxia/reoxygenation; GPR30, G-protein coupled
receptor 30; E2, 17β-estrogen. |
Discussion
The present study was, to the best of our knowledge,
the first to report that the expression of GPR30 is reduced in
preeclamptic placentae. The in vitro study further
demonstrated that supplementation of E2 enhanced the GPR30
expression in trophoblasts in normal and hypoxia/reperfusion
conditions, in addition to enhancing trophoblast outgrowth under
conditions of hypoxia/reperfusion.
Although the pathogenesis of preeclampsia is
unclear, placental hypoxia/reperfusion injury may be involved in
the development of preeclampsia. During pregnancy, both maternal
estrogen and progesterone is rapidly increased in order to maintain
a successful pregnancy. Estrogen is a critical hormone during
pregnancy, exerting its effect both at the transcriptional level
and the level of intracellular signaling through secondary
messengers (24). The effect of
estrogens is mediated by the classical receptors, ERα and ERβ
(13,14). However, in 2005, studies identified
that estrogen also binds to GPR30 (16,17).
GPR30 is widely expressed in a number of tissues
including the placenta and ovary (25,26),
and promotes estrogen-mediated inhibition of oxidative stress
induced apoptosis (20), which is
one of the mechanisms implicated in the development of
preeclampsia. However the expression of GPR30 in preeclampsia has
not previously been investigated. The present study indicated, to
the best of our knowledge for the first time, that expression of
GPR30 was significantly reduced in trophoblasts and vascular
endothelial cells from placentae of preeclampsia as compared with
uncomplicated pregnancies using IHC and western blotting.
Consistent with earlier reports (15), it was observed that GPR30 was
expressed in decidual stroma and vascular endothelial cells. GPR30
expression in decidual stroma and vascular endothelial cells was
also significantly reduced in preeclampsia.
Higher levels of estrogen are suggested to exert
anti-inflammatory effects and the reduction of estrogen has been
reported in women with preeclampsia (11). Estrogen such as E2, the most common
form of estrogen, exerts its action either by binding to ERα/ERβ
receptors of estrogen or by activating GPR30 (27,28).
Therefore, it was investigated whether exogenous estrogen could
alter the expression of GPR30 in trophoblasts. In the present study
it was identified that treatment with E2 significantly increased
the expression of GPR30 in trophoblasts in vitro. The
increased expression of GPR30 in trophoblasts induced by E2 was
blocked by the specific antagonist of GPR30, G15. The in
vitro study demonstrated that E2 supplementation enhanced
trophoblast GPR30 expression in normoxic and hypoxia/reperfusion
conditions, in addition to trophoblast outgrowth in
hypoxia/reperfusion conditions.
There is much evidence for increased placental
oxidative stress in preeclampsia and for hypoxia-reoxygenation as a
possible mechanism for inducing the oxidative stress (22). Hypoxia-reoxygenation induces
apoptotic changes in syncytiotrophoblasts (22). Increasing evidence suggests that
hypoxia-reoxygenation within the placenta leads to production of
reactive oxidative species (ROS) (29,30)
and G-protein coupled pathways are involved in ROS production
(31). Increased levels of ROS may
result in reduced GPR expression. The in vitro study using
1% O2 as a hypoxic condition and 20% O2 as a
normoxic condition, the data demonstrated that treatment with E2
increased trophoblast GPR30 expression in hypoxia-reoxygenation
(Fig. 3), although there was no
statistical difference between trophoblasts that were cultured in
normal conditions and in hypoxia-reoxygenation conditions. Notably,
it was identified that compared with trophoblasts that were treated
with E2 under normoxic conditions, trophoblast GPR30 expression
following E2 treatment was significantly reduced under
hypoxia-reoxygenation conditions (Fig.
4). This suggests that hypoxia-reoxygenation may alter the
positive regulation of GPR30 expression by E2. It is well
documented that reduced trophoblast invasion is associated with the
pathogenesis of preeclampsia and that hypoxia-reoxygenation is
involved in the inhibition of trophoblast invasion (29), however the potential mechanism is
unclear. In the present study using 1% O2 as a hypoxic
condition and 20% O2 as a normoxic condition for
placental explants culture, it was confirmed that extravillous
trophoblast invasion was significantly reduced in
hypoxia-reoxygenation condition. However, E2 treatment did not
significantly increase trophoblast invasion in the
hypoxia-reoxygenation conditions. This may be due to the fact that
E2 treatment in hypoxia-reoxygenation conditions was unable to
increase GPR30 expression to the levels that result from E2
treatment in normoxic conditions (Fig.
4). Therefore, the data suggest that the action of GPR30 in
trophoblasts may not promote estrogen-mediated inhibition of
apoptosis induced by oxidative stress alone (20), however may additionally be involved
in trophoblast invasion during placentation.
Previous studies indicate that there are distinct
vascular adaptation profiles in early onset and late onset
preeclampsia, and that the placental pathology in early onset
preeclampsia is significantly different from that in late onset
preeclampsia (32,33). Due to the sample size in the
present study (n=21), it was not possible to investigate GPR30
expression differences in severe and mild preeclampsia or early
onset and late onset preeclampsia. Future studies to investigate
whether the expression of GPR30 is associated with the severity or
time of onset in preeclampsia is warranted. In addition, numerous
women with preeclampsia additionally present with growth restricted
fetuses. There were three cases of FGR in the present study, and it
was not possible to investigate associations between GPR30 and FGR.
However, preeclampsia and FGR share common mechanisms (deficient
placentation) thus there may be also an association between GPR30
and FGR.
It remains unclear whether the low levels of
estrogen cause the reduction of GPR30 expression in preeclampsia or
whether the lower expression of GPR30 affect the reduction of
estrogen action in preeclampsia. Further study is required.
In conclusion, it was demonstrated that placental
GPR30 levels were significantly reduced in preeclampsia. Treatment
with one of the common forms of estrogen, E2, increased GPR30 in
conditions of normoxia and hypoxia/reoxygenation. However,
treatment with E2 in hypoxia-reoxygenation conditions did not
increase GPR30 levels to those in normoxic conditions. It was
further identified that GPR30 may also be involved in extravillous
trophoblast invasion. The present study may suggest that the
promotion of placental development through increased levels of
estrogen in normal pregnancy is at least partially mediated by
GPR30, and that aberrant levels of E2 in preeclampsia contribute to
the pathogenesis of the disease through this mechanism.
Acknowledgements
The authors would like to thank Dr. Joanna Stanley,
from The University of Auckland (Auckland, New Zealand) for editing
this manuscript. The present study was supported by National
Natural Science Foundation of China (grant nos. 81370732 and
81571453).
References
|
1
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hubel CA: Oxidative stress in the
pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 222:222–235.
1999; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberts JM and Hubel CA: Is oxidative
stress the link in the two-stage model of pre-eclampsia? Lancet.
354:788–789. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonney EA: Demystifying animal models of
adverse pregnancy outcomes: Touching bench and bedside. Am J Reprod
Immunol. 69:567–584. 2013.PubMed/NCBI
|
|
6
|
Jukic AM, Weinberg CR, Wilcox AJ and Baird
DD: Effects of early pregnancy loss on hormone levels in the
subsequent menstrual cycle. Gynecol Endocrinol. 26:897–901. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heldring N, Pike A, Andersson S, Matthews
J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M and
Gustafsson JA: Estrogen receptors: How do they signal and what are
their targets. Physiol Rev. 87:905–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jobe SO, Tyler CT and Magness RR: Aberrant
synthesis, metabolism, and plasma accumulation of circulating
estrogens and estrogen metabolites in preeclampsia implications for
vascular dysfunction. Hypertension. 61:480–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamimi R, Lagiou P, Vatten LJ, Mucci L,
Trichopoulos D, Hellerstein S, Ekbom A, Adami HO and Hsieh CC:
Pregnancy hormones, pre-eclampsia, and implications for breast
cancer risk in the offspring. Cancer Epidemiol Biomarkers Prev.
12:647–650. 2003.PubMed/NCBI
|
|
10
|
Zeisler H, Jirecek S, Hohlagschwandtner M,
Knöfler M, Tempfer C and Livingston JC: Concentrations of estrogens
in patients with preeclampsia. Wien Klin Wochenschr. 114:458–461.
2002.PubMed/NCBI
|
|
11
|
Hertig A, Liere P, Chabbert-Buffet N, Fort
J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N,
Lefevre G, et al: Steroid profiling in preeclamptic women: Evidence
for aromatase deficiency. Am J Obstet Gynecol. 203:477.e1–e9. 2010.
View Article : Google Scholar
|
|
12
|
Lee SJ, Lee DW, Kim KS and Lee IK: Effect
of estrogen on endothelial dysfunction in postmenopausal women with
diabetes. Diabetes Res Clin Pract. 54 Suppl 2:S81–S92. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maruyama A, Nakayama T, Sato N, Mizutani
Y, Furuya K and Yamamoto T: Association study using single
nucleotide polymorphisms in the estrogen receptor beta (ESR2) gene
for preeclampsia. Hypertens Res. 27:903–909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molvarec A, Vér A, Fekete A, Rosta K,
Derzbach L, Derzsy Z, Karádi I and Rigó J Jr: Association between
estrogen receptor alpha (ESR1) gene polymorphisms and severe
preeclampsia. Hypertens Res. 30:205–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kolkova Z, Noskova V, Ehinger A, Hansson S
and Casslén B: G protein-coupled estrogen receptor 1 (GPER, GPR 30)
in normal human endometrium and early pregnancy decidua. Mol Hum
Reprod. 16:743–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Revankar CM, Cimino DF, Sklar LA,
Arterburn JB and Prossnitz ER: A transmembrane intracellular
estrogen receptor mediates rapid cell signaling. Science.
307:1625–1630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas P, Pang Y, Filardo EJ and Dong J:
Identity of an estrogen membrane receptor coupled to a G protein in
human breast cancer cells. Endocrinology. 146:624–632. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szego CM and Davis JS: Adenosine
3′,5′-monophosphate in rat uterus: Acute elevation by estrogen.
Proc Natl Acad Sci USA. 58:1711–1718. 1967; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prossnitz ER, Arterburn JB and Sklar LA:
GPR30: A G protein-coupled receptor for estrogen. Mol Cell
Endocrinol 265–266. 1–142. 2007.
|
|
20
|
Kanda N and Watanabe S: 17beta-estradiol
inhibits oxidative stress-induced apoptosis in keratinocytes by
promoting Bcl-2 expression. J Invest Dermatol. 121:1500–1509. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vivacqua A, Bonofiglio D, Recchia AG,
Musti AM, Picard D, Andò S and Maggiolini M: The G protein-coupled
receptor GPR30 mediates the proliferative effects induced by
17beta-estradiol and hydroxytamoxifen in endometrial cancer cells.
Mol Endocrinol. 20:631–646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hung TH, Skepper JN, Charnock-Jones DS and
Burton GJ: Hypoxia-reoxygenation: A potent inducer of apoptotic
changes in the human placenta and possible etiological factor in
preeclampsia. Circ Res. 90:1274–1281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
American College of Obstetricians and
Gynecologists; Task Force on Hypertension in Pregnancy:
Hypertension in pregnancy. report of the american college of
obstetricians and gynecologists' task force on hypertension in
pregnancy. Obstet Gynecol. 122:1122–1131. 2013.PubMed/NCBI
|
|
24
|
Prossnitz ER, Oprea TI, Sklar LA and
Arterburn JB: The ins and outs of GPR30: A transmembrane estrogen
receptor. J Steroid Biochem Mol Biol. 109:350–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takada Y, Kato C, Kondo S, Korenaga R and
Ando J: Cloning of cDNAs encoding G protein-coupled receptor
expressed in human endothelial cells exposed to fluid shear stress.
Biochem Biophys Res Commun. 240:737–741. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carmeci C, Thompson DA, Ring HZ, Francke U
and Weigel RJ: Identification of a gene (GPR30) with homology to
the G-protein-coupled receptor superfamily associated with estrogen
receptor expression in breast cancer. Genomics. 45:607–617. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mårtensson UE, Salehi SA, Windahl S, Gomez
MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N,
Hellstrand P, Grände PO, et al: Deletion of the G protein-coupled
receptor 30 impairs glucose tolerance, reduces bone growth,
increases blood pressure, and eliminates estradiol-stimulated
insulin release in female mice. Endocrinology. 150:687–698. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar R, Balhuizen A, Amisten S, Lundquist
I and Salehi A: Insulinotropic and antidiabetic effects of
17β-estradiol and the GPR30 agonist G-1 on human pancreatic islets.
Endocrinology. 152:2568–2579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hung TH and Burton GJ: Hypoxia and
reoxygenation: A possible mechanism for placental oxidative stress
in preeclampsia. Taiwan J Obstet Gynecol. 45:189–200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hung TH, Skepper JN and Burton GJ: In
vitro ischemia-reperfusion injury in term human placenta as a model
for oxidative stress in pathological pregnancies. Am J Pathol.
159:1031–1043. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burton GJ, Hempstock J and Jauniaux E:
Oxygen, early embryonic metabolism and free radical-mediated
embryopathies. Reprod Biomed Online. 6:84–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nelson DB, Ziadie MS, McIntire DD, Rogers
BB and Leveno KJ: Placental pathology suggesting that preeclampsia
is more than one disease. Am J Obstet Gynecol. 210:66.e1–e7. 2014.
View Article : Google Scholar
|
|
33
|
Stergiotou I, Crispi F, Valenzuela-Alcaraz
B, Bijnens B and Gratacos E: Patterns of maternal vascular
remodeling and responsiveness in early-versus late-onset
preeclampsia. Am J Obstet Gynecol. 209:558.e1–558.e14. 2013.
View Article : Google Scholar
|