Introduction
Microbial keratitis is a common ocular infection
caused by bacteria, fungi, viruses or parasites and is the second
most significant cause of monocular blindness, particularly in
certain developing countries and, generally, in the tropics
(1). Clinically, this infection
requires aggressive antimicrobial management to eliminate the
causative organisms, suppress destructive reactions, and restore
normal ocular structure and vision (2,3).
However, despite timely and correct therapeutic strategies,
infective keratitis remains clinically challenging, in which
approximately 50% of eyes have poor visual outcomes (4,5),
because conventional therapies, such as antibiotic treatment, often
fail to control the tissue damage caused by excessive local
inflammation, even if viable bacteria are cleared from the cornea
(6). Hence, in addition to
antibiotic treatment, it is also important to develop new
therapeutic modalities to control the inflammatory response in
microbial keratitis.
The innate immune response is the first line of host
defence that invading pathogens rely on for innate immune
recognition through pattern recognition receptors (PRRs) (7). The activation of PRRs initiates a
variety of inflammatory events, including the infiltration of
inflammatory cells (e.g., polymorphnuclear neutrophils [PMNs] and
monocytes/macrophages), the production of pro- and
anti-inflammatory cytokines (7),
the interplay of cellular apoptosis vs. necrosis (8), and causes immune cells to become
refractory to subsequent endotoxin challenge a state known as
endotoxin tolerance (9). These
PRR-mediated inflammatory responses are necessary for microbial
clearance; however, if uncontrolled, excessive host inflammation
also leads to immunopathological tissue damage (7). Therefore, it is important to
precisely balance pro- and anti-inflammatory responses in ocular
immune defence.
Tacrolimus (FK506), a macrolide molecule that
suppresses the activation of T cells, T helper cell-mediated B-cell
proliferation and the formation of cytokines (10,11),
the reports of its role on non-T cells is comparatively few but it
has been reported that FK506 cannot control the cytokine production
in non-T cells in cornea transplantation (12). It has been widely used clinically
in preventing organ transplant rejection and autoimmune diseases
(13–15) and has also been effective in
preventing corneal transplant rejection (16), dry eye (18), and steroid-resistant refractory VKC
(17) in ophthalmology. Recently,
Erdal demonstrated that FK506 causes a significant decrease in
inflammatory cells accompanied by reduced corneal vascularisation
in the experimental herpetic stromal keratitis corneal edema model
(18). Moreover, our previous
study demonstrated that tacrolimus can inhibit the inflammation
induced by fungi and alleviate the severity of corneal damage at an
early stage of fungal keratitis (19); and Wang et al (20) showed that a high dose of FK506
promoted HEV infection. These studies together suggest that
tacrolimus is a good immunoregulator in viral and fungal
inflammatory responses. Furthermore, it has been reported that
FK506 cannot control the cytokine production in non-T cells in
cornea transplantation (12) while
its role on non-T cells in bacterial keratitis remains unknown;
however, its role in bacterial keratitis has few reports,
especially its role on non-T cells, and many aspects remain
unknown.
Microbial keratitis induced by lipopolysaccharide
(LPS), a well-characterized pathogen-associated molecular pattern
found in the outer leaflet of the outer membrane of the bacteria,
is a rapidly progressive infectious ocular disease (21). Upon recognition of invading
microbes by PRRs, inflammatory cells are recruited to the cornea to
produce various pro-inflammatory cytokines (22) (e.g., IL-6 and IL-1β) and modulate
anti-inflammatory cytokines (23)
(e.g., IL-10 and TGF-β) to regulate antibacterial immunity
(24). However, if uncontrolled,
these inflammatory mediators often elicit an overly robust
response, resulting in bystander tissue damage. Thus, tight
regulation of innate immune response, especially pro-inflammatory
and anti-inflammatory responses, is critical for the resolution of
LPS-induced bacterial keratitis (21).
With this background, the present study was aimed to
explore the role of tacrolimus in LPS-induced bacterial keratitis.
An in vitro study was designed to explore the
histocompatibility and immunoregulation of tacrolimus in human
corneal epithelial cells, PMN, and monocytes after LPS
stimulation.
Materials and methods
Cell culture and isolation of human
PMN
Human monocytic THP-1 cells (ATCC-TIB-202, Manassas,
VA, USA) were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin-streptomycin
(Invitrogen, Carlsbad, CA, USA). A human corneal epithelial cell
(HCEC) line was obtained from American Type Culture Collection
(ATCC-CRL-11515; ATCC, Manassas, VA, USA). The cells were
maintained in keratinocyte serum-free medium (GIBCO-BRL 17005-042;
Gibco, Grand Island, NY, USA) supplemented with 0.05 mg/ml bovine
pituitary extract, 5 ng/ml human recombinant epidermal growth
factor, 0.005 mg/ml insulin, 500 ng/ml hydrocortisone, 100 IU/ml
penicillin, 100 lg/ml streptomycin, and 0.125 lg/ml amphotericin
B.
The fresh isolation of human PMN, using blood of 3
healthy male and 3 healthy female volunteers (ages 30–45), was
performed as previously described with minor modifications
(25). In short, blood was diluted
with cold HBSS (1:1), after which, gradient centrifugation was used
to remove the lymphocytes. The remaining lowest layer containing
PMN and erythrocytes was then suspended in cold lysis buffer to
lyse the erythrocytes. The remaining pellet containing only PMN was
suspended in HBSS, and the cells were counted using a Bürker
chamber. Viability was evaluated using trypan blue dye exclusion
(0.4%). Keeping all solutions and PMN on ice to prevent premature
activation, this isolation method consistently yielded PMN with a
viability >95%. The cells were cultured at 37°C in a humidified
atmosphere with 5% carbon dioxide. The culture medium was changed
daily.
FK506 was firstly dissolved in Dimethylsulfoxide
(DMSO) with a concentration of 10 mg/ml (1%), and then was
incubated with culture medium containing FK506 at a final
concentration of 0.1%.
Flow cytometry
Tacrolimus' role on the apoptosis of THP-1, HCEC and
PMN was determined by flow cytometry, and each cell was divided
into two groups: Group I was the control group and received no
treatment; and group II received 0.1% FK506 (Sigma-Aldrich, St.
Louis, MO, USA) for 24 h.
Cells were seeded at 5×105 cells/well in
12-well plates, and the Annexin V-fluorescein isothiocyanate
Apoptosis Detection kit (BD Bioscience, CA, USA) was used according
to the manufacturer's instructions. Briefly, cells were pooled,
washed, and resuspended in 500 µl of binding buffer, followed by
the addition of 5 µl of Annexin V-fluorescein isothiocyanate and 5
µl of PI. Then, the cells were incubated at room temperature in the
dark for 15 min and analysed by flow cytometry (EPICS XL/MCL;
Beckman Coulter Miami, FL, USA). Viable cells did not exhibit
Annexin V or PI staining, early-apoptotic cells showed Annexin V
but not PI staining, and late-apoptotic cells exhibited both
Annexin V and PI staining.
Cytotoxicity assay
The cytotoxicity of tacrolimus was evaluated using
the MTT assay in THP-1, HCEC and PMN, which were each grouped into
four groups: Group I was the control group and received no
treatment; group II received 0.1% tacrolimus for 8 h; group III
received 0.1% tacrolimus for 24 h; and group IV received 0.1%
tacrolimus for 48 h. THP-1, HCEC and PMN were plated at
concentrations of 5×103 cells/well in 96-well plates.
After 24 h, the medium was replaced with 200 µl of serum-free
medium. The remaining cells were either untreated or incubated with
30 µl of 0.1% FK506 for 8, 24 or 48 h. Next, the medium in each
well was replaced with 200 µl of fresh serum-free medium. To each
well, 20 µl of 5 mg/ml MTT solution in PBS (pH 7.4) was added.
Following incubation for 3 h at 37°C, the medium was aspirated, and
the formazan crystals that had formed in the well were dissolved in
200 µl of DMSO. The absorbance of the dissolved formazan crystals
in DMSO was recorded at 570 nm and used to estimate cell viability
relative to control cells.
Real-time PCR
We verified tacrolimus' role on LPS-induced
inflammatory responses in THP-1, HCEC and PMN, in which each cell
type was divided into three groups with a seeding density of
5×106 cells/well in 6-well plates: Group I was the
control group that received PBS; group II received 5 µg/ml LPS
(Sigma-Aldrich) for 8 h; and group III was incubated with 0.1%
FK506 for 24 h before being treated with 5 µg/ml LPS for 8 h. Total
RNA was isolated from cultured cells using TRIzol (Invitrogen)
according to the manufacturer's recommendations and was quantified
using a NanoDrop 2000C spectrophotometer (Thermo Scientific, West
Palm Beach, FL, USA). One microgram of total RNA was reverse
transcribed to produce cDNA, and the cDNA was amplified using
SYBR-Green Master Mix (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) according to the manufacturer's instructions. Primers for
human pro-inflammatory cytokines, such as IL-1β, IL-6 and MMP9,
human anti-inflammatory cytokines, such as IL-10 and TGF-β, and
human angiogenesis factors, such as VEGF and TNF-α were purchased
from SABiosciences (Frederick, MD, USA), and the primer sequences
are listed in Table I.
Quantitative real-time PCR was performed using a CFX96 Real-Time
PCR System (Bio-Rad Laboratories, Inc.). Relative gene expression
levels were calculated after normalization to the internal control
β-actin.
 | Table I.Nucleotide sequences of the specific
primers used for PCR amplification. |
Table I.
Nucleotide sequences of the specific
primers used for PCR amplification.
| Gene | Primer Sequence
(5′-3′) |
|---|
| IL-1β | F:
TGTATGTGACTGCCCAAGAT |
|
| R:
GCACACCCAGTAGTCTTGCT |
| IL6 | F:
GCACTGGCAGAAAACAACCT |
|
| R:
GCTCTGGCTTGTTCCTCACTAC |
| MMP9 | F:
GAAAGCCTATTTCTGCCAGG |
|
| R:
TGCAGGATGTCATAGGTCAC |
| IL-10 | F:
AGCTGGACAACATACTGCTAACCGAC |
|
| R:
CTTGATTTCTGGGCCATGCTTCTCTG |
| TGF-β | F:
GCAGCTGTACATTGACTTC |
|
| R:
GTTATGCTGGTTGTACAGGG |
| VEGF | F:
GCAGATTATGCGGATCAAACC |
|
| R:
TTTCGTTTTTGCCCCTTTCC |
| TNF-α | F:
GGTATGAGCCCATCTATC TG |
|
| R:
GCAATGATCCCAAAGTAGAC |
| β-actin | F:
GCTCCTCCTGAGCGCAAG |
|
| R:
CATCTGCTGGAAGGTGGACA |
Statistical analyses
Unpaired, two-tailed Student's t test was used to
determine the significance of the flow cytometry and MTT assays.
Differences in the RNA expression levels of pro-inflammatory,
anti-inflammatory and angiogenesis cytokines in the three groups
were determined using the Mann-Whitney U test. The data were
considered significant at P<0.05.
Results
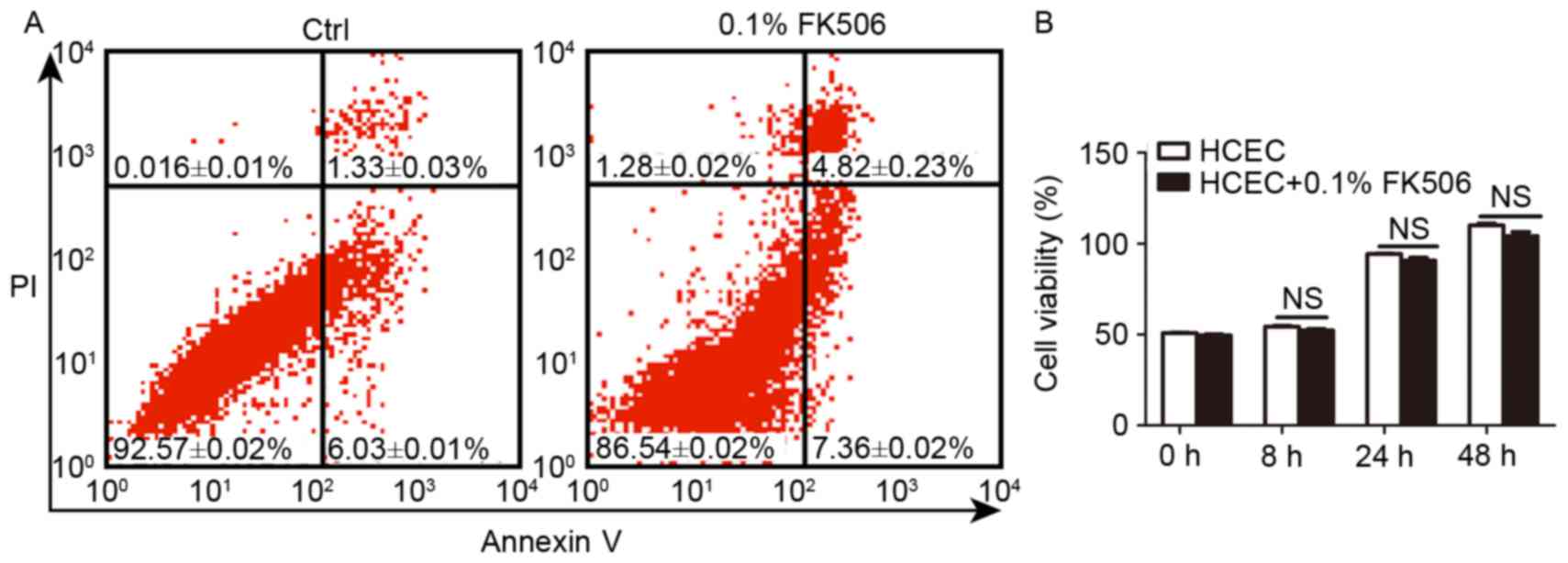
The cytotoxicity of tacrolimus on
HCEC
To confirm the safety of tacrolimus used in the
in vitro experiment, HCEC were used to determine the
biocompatibility using flow cytometry and MTT assays. The results
demonstrated that tacrolimus had no obvious influence on apoptosis;
early and late apoptosis in the control and tacrolimus groups
showed no significant differences (P>0.05) (Fig. 1A). After incubation for 8, 24 and
48 h, HCEC viability showed a slight reduction, with no significant
difference (P>0.05) (Fig. 1B).
The results demonstrated that tacrolimus (FK506) had good
biocompatibility in HCEC.
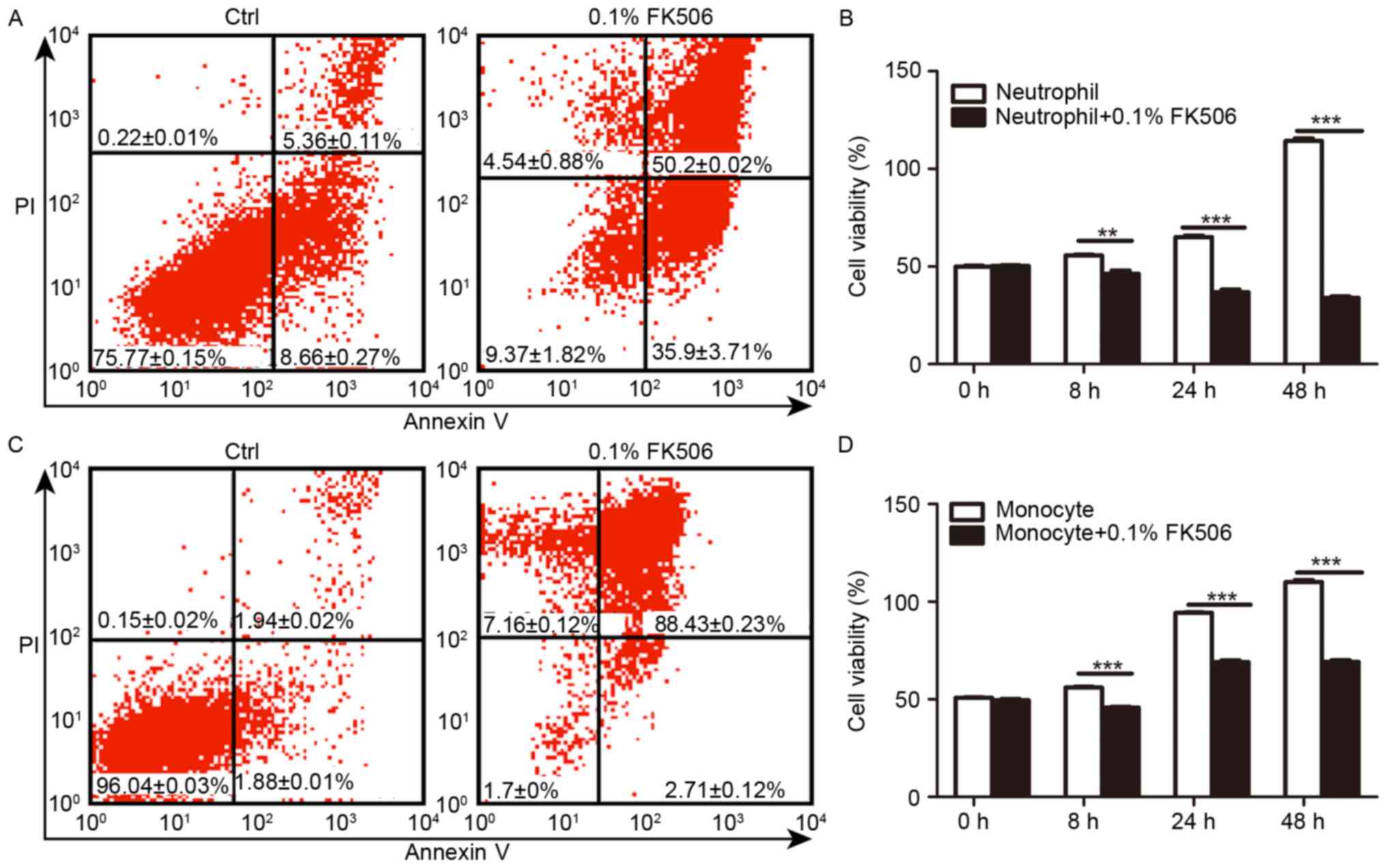
Tacrolimus increased apoptosis and
decreased the viability of THP-1 and PMN cells
To explore the role of tacrolimus on the
proliferation of two main human inflammatory cells, 0.1% FK506 was
added to THP-1 and PMN to analyse the apoptosis rate and cell
viability compared with that of the control group. In PMN, flow
cytometry (Fig. 2A) showed that
the early and the late-apoptosis rate was 50.2±1.02 and 35.9±3.71%,
respectively, in the FK506 group after 24 h, which was
significantly higher than that of the control group with 5.36±0.11%
(P<0.05) and 18.66±0.27% (P<0.05). Moreover, we incubated
cells without or with FK506 for 8, 24 and 48 h, and evaluation of
cell viability revealed that FK506 had a significant inhibitory
effect on cell viability, which correlated positively with the
stimulation time (Fig. 2B). In
THP-1, for both early or late apoptosis, the rates in the FK506
group after 24 h, 88.43±0.23 and 2.71±0.12%, respectively, were
greater than the rates in the control group, which had a high
fraction of normal cells 96.04±0.03% and a low fraction of early
(1.94±0.02%) (P<0.01) and late (1.88±0.01%) apoptotic cells
(P<0.01) (Fig. 2C). The control
group displayed significantly higher cell viability at 8, 24 and 48
h than the FK506 group (P<0.001) (Fig. 2D). Conclusively, FK506 raise the
apoptosis's rates and reduced cell viability in THP-1 and PMN.
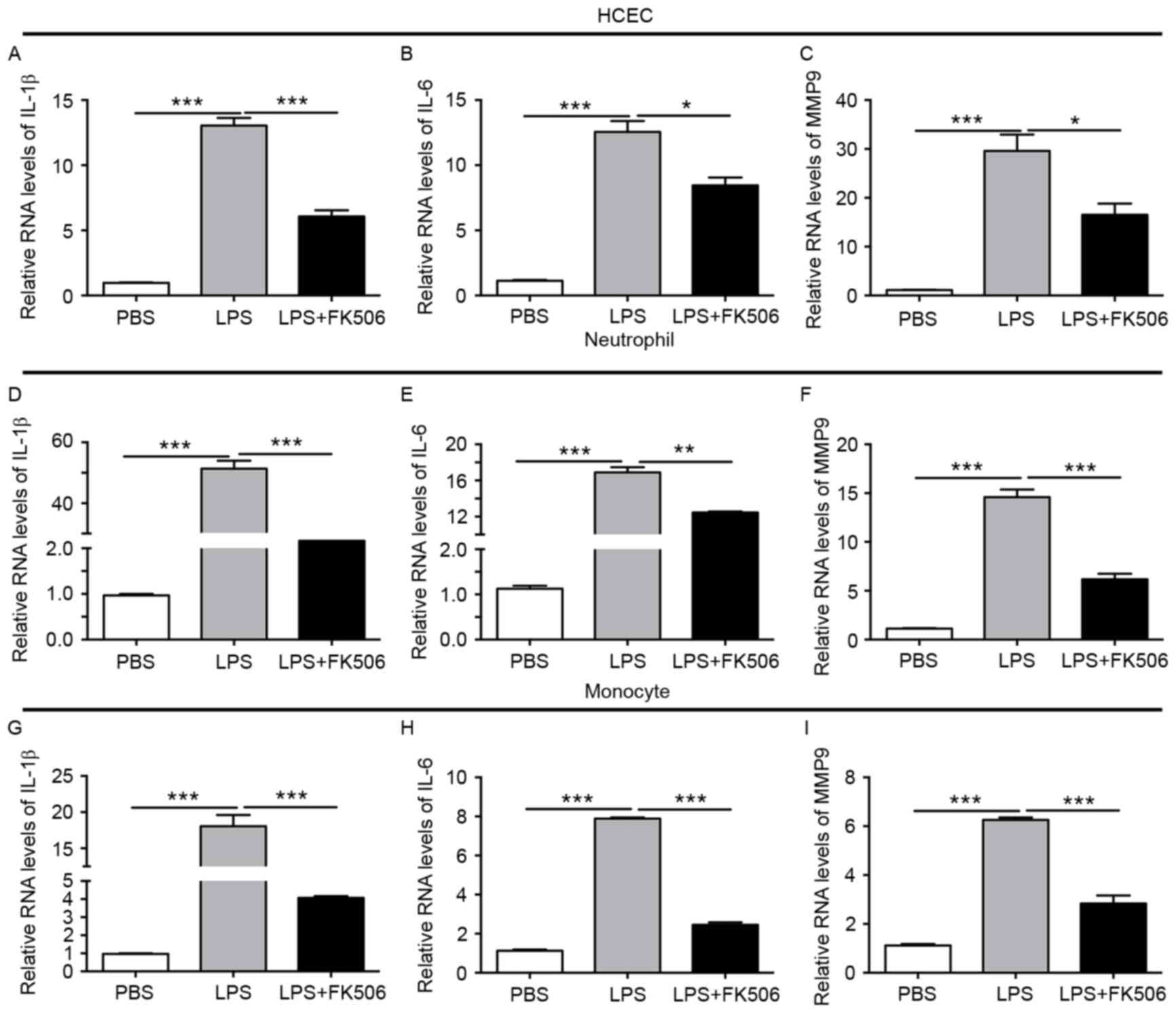
Tacrolimus reduced pro-inflammatory
cytokines in LPS-induced inflammatory responses
Based on the decreased apoptosis and cell viability
in HCEC, THP-1 and PMN, we further evaluated tacrolimus' role on
the pro-inflammatory cytokines in LPS-induced inflammatory
responses. In HCEC with LPS, IL-1β, and IL-6 increased up to
approximately 10 times, and MMP-9 to 20 times more than the control
group (both P<0.001), while after the FK506 intervention, both
expression levels obviously decreased (both P<0.05) (Fig. 3A-C). In PMN, after incubation with
LPS for 8 h, IL-1β, IL-6 and MMP9 showed a clear elevation (both
P<0.001), while in the LPS + FK506 group, IL-1β reduced its
expression to approximately one thirtieth of the LPS group
(P<0.001), IL-6 was reduced by one third (P<0.01), and MMP9
was also lower than the LPS group (P<0.001) (Fig. 3D-F). In monocytes, compared with
the control group, pro-inflammatory cytokines also displayed a
similar tendency to increase after stimulation with LPS; moreover,
FK506 depressed the expression of IL-1β, IL-6 and MMP9 (both
P<0.001) (Fig. 3G-I).
Therefore, we concluded that FK506 alleviated the expression of
pro-inflammatory cytokines IL-1β, IL-6 and MMP9 induced by LPS.
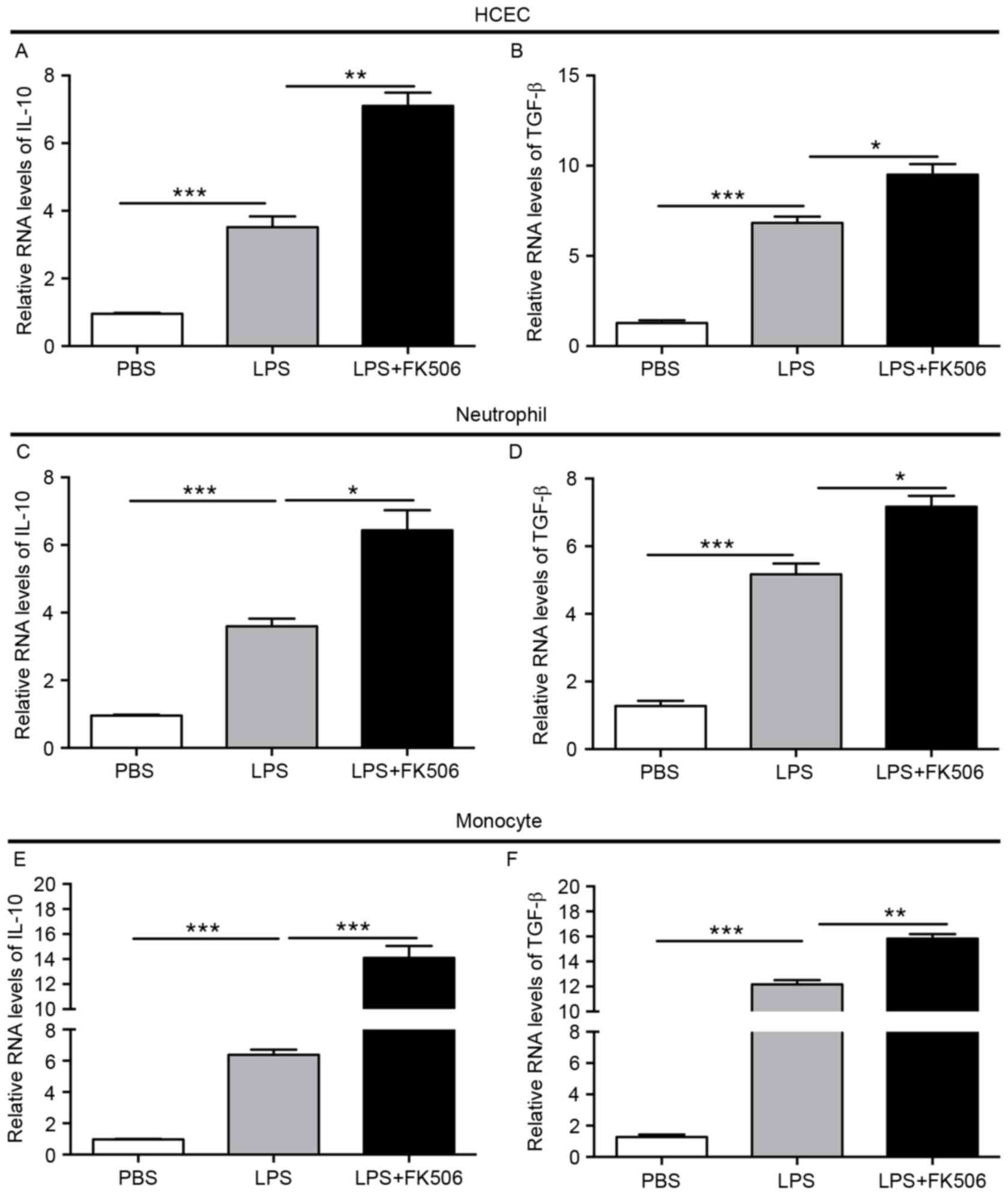
Tacrolimus increased anti-inflammatory
cytokines in LPS-induced inflammatory responses
Furthermore, we detected the levels of the
anti-inflammatory cytokines IL-10 and TGF-β in LPS-induced
inflammatory responses. In HCEC, IL-10 and TGF-β showed mild
elevation after LPS stimulation, and a more obvious significant
upregulation could be observed in the LPS + FK506 group (both
P<0.05) (Fig. 4A-B). Compared
with the control group, IL-10 and TGF-β levels gradually increased
4 to 5 times in the neutrophil LPS group (both P<0.001), then
showed a continuous increase after treatment with FK506 (both
P<0.05) (Fig. 4C-D). For
monocytes, LPS also improved the expression of IL-10 and TGF-β, and
FK506 significantly enhanced their levels, where IL-10 was almost 2
times more in the LPS + FK506 group than the LPS group
(P<0.001), and TGF-β was slightly higher than the LPS group
(P<0.01) (Fig. 4E-F). These
results suggest that FK506 partially improved anti-inflammatory
cytokine levels after the inflammatory cytokines were induced by
LPS.
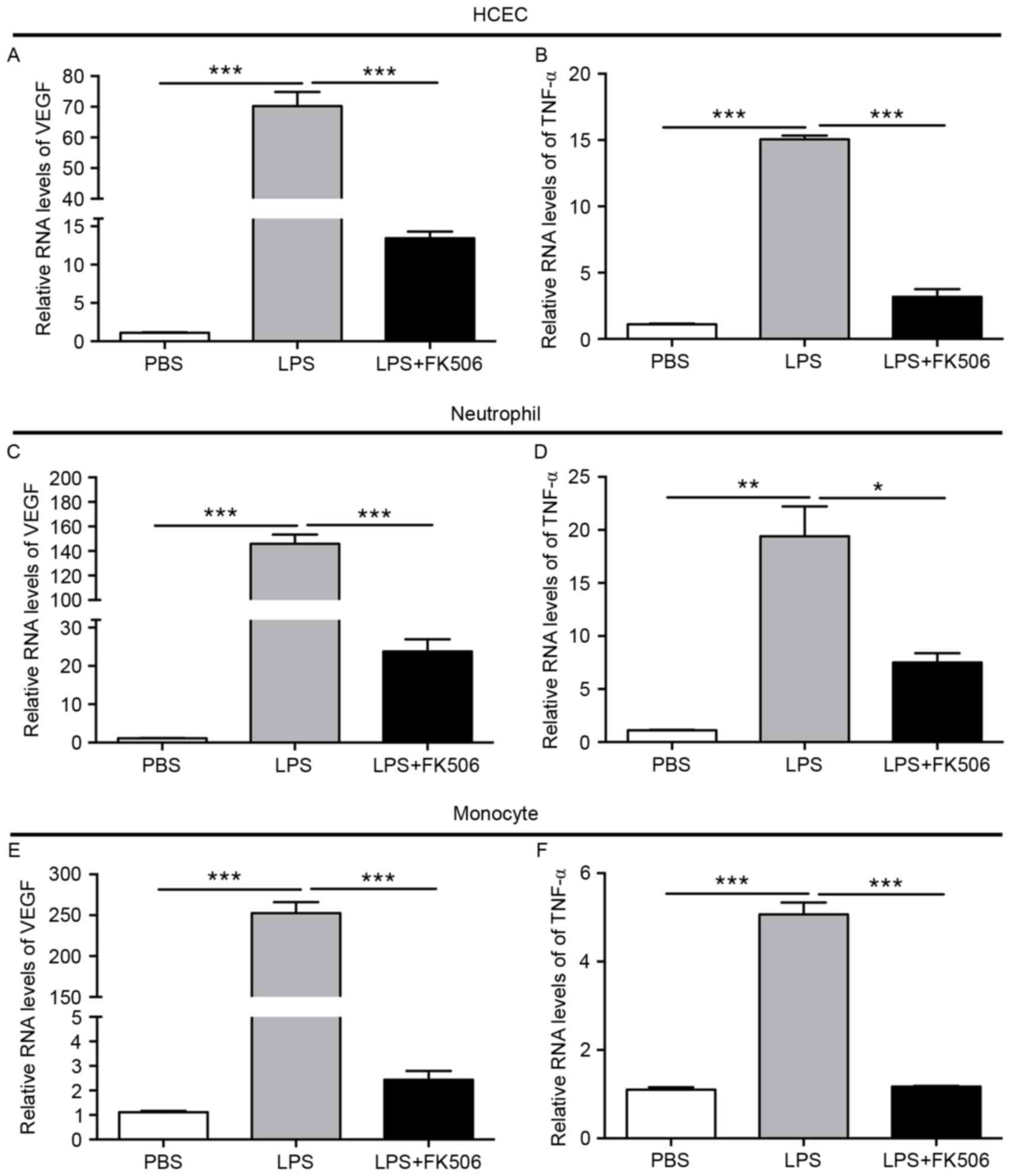
Tacrolimus lessened proangiogenic
factors in LPS-induced inflammatory responses
VEGF and TNF-α are important angiogenesis factors.
In HCEC with LPS, compared with the control group, VEGF increased
approximately 70 times (P<0.001), which was then reduced to
approximately 10 times after FK506 (P<0.001). TNF-α was slightly
elevated in the LPS group (P<0.001), and then declined
drastically in the LPS + FK506 group (P<0.001) (Fig. 5A-B). Incredibly, compared with the
control group, in PMN, VEGF levels increased rapidly by
approximately 150 times in the LPS group (P<0.001), then
significantly decreased to approximately 20 times by FK506
(P<0.001); in addition, TNF-α levels were also increased in the
LPS group and decreased by FK506 (Fig.
5C-D). With regard to monocytes, VEGF also exhibited an
apparent upregulation after LPS (P<0.001) and doubled that of
the control group. A slight enhancement was observed in TNF-α in
the LPS group (P<0.001), which lessened to almost the same
levels as the control group in the LPS + FK group (Fig. 5E-F). Taken together, these results
clearly demonstrated that the angiogenesis factors VEGF and TNF-α
were markedly increased after LPS induction in HCEC, PMN and THP1
and that FK506 was partly effective in reducing these levels.
Discussion
In microbial keratitis, there is activation of the
immune system, in which inflammatory cells are involved in the
cornea's inflammatory response to microbial proliferation and
invasion (26); these host
reactions account for much of the oedematous, infiltrative, and
necrotizing changes (27). Both
pro-inflammatory and anti-inflammatory processes involve
chemoattractants, adhesion molecules, and other mediators (27). However, this inflammatory response
of the immune system is a double-edged sword in the host defence
mechanism. On one hand, this response is the main approach that the
immune system uses to eliminate pathogens, which is beneficial in
protecting against microbial infection. On the other hand,
overenthusiastic inflammation can be damaging, and persistent
inflammation can lead to degeneration or tissue necrosis (28). In fungal keratitis, a type of
severe microbial keratitis, excessive inflammation is an important
contributor to corneal damage during fungal infection. Our previous
study demonstrated that tacrolimus exerted an obvious
anti-inflammatory effect by not only reducing the infiltration of
inflammatory cells but also suppressing the expression of
pro-inflammatory cytokines in innate immune responses at an early
stage of corneal fungal infection (21).
In LPS-induced bacterial keratitis, the innate
immune responses are important, not just for protecting the host
during the period that adaptive responses are required to develop
but also because they can influence the nature of the adaptive
response (24,29). The interplay of these two systems
also appears to participate in the host response to bacterial
corneal infection (30).
Clinically, topical antibiotics are mandatory for the treatment of
bacterial keratitis; moreover, the most widely used
anti-inflammatory agents are corticosteroids, which can regulate
the innate and adaptive immune responses by inhibiting leukocytes,
downregulating pro-inflammatory cytokines, affecting
metalloproteinase production, and modifying wound healing in the
inflamed cornea (31). However,
topical corticosteroids have consistently been avoided by many
clinicians for fear of causing immunosuppression, which would lead
to neutrophil inhibition, accelerated bacterial replication and an
exacerbated infection in acute inflammation (32,33).
While clinically, even if the causative organisms are eradicated by
antibiotics, the excessive inflammation would also deteriorate the
clinical features; therefore, except for corticosteroids, exploring
other adjuvant therapies for bacterial keratitis is important. In
the present study, we studied the topical immunosuppressant
tacrolimus to evaluate its role in modulating
pro-/anti-inflammatory responses in LPS-induced bacterial
keratitis.
Although the inhibitory mechanisms of tacrolimus
have been extensively studied in T cells, little is known regarding
the precise suppressive mechanisms of FK506 in non-T cells. We
found that tacrolimus induced the apoptosis of PMN and monocytes,
which was mainly early apoptosis. Among these two cell types, the
early- and late-apoptosis rate of monocytes was consistent with
Yoshino's report, which also determined that tacrolimus suppresses
the function of macrophages and promotes their apoptosis (34). Moreover, the results showed that
both pro- and anti-inflammatory cytokines were activated, and
demonstrated that the dynamic balance of pro- and anti-inflammatory
responses proposed by Morris et al (35) was disrupted by LPS stimulation in
innate immune responses. Then, tacrolimus exhibited its strong
regulatory role on reducing pro-inflammatory cytokines and
increasing anti-inflammatory cytokines. However, there is evidence
that FK506 inhibits T-cell proliferation and T-cell-derived
cytokines (36), then modulates
lymphocytes, producing less TNF-α and other cytokines, and finally
inflammatory responses (18,37).
Based on the results of our study, we speculate that tacrolimus may
act not only on T cells but also on PMN and monocytes to modulate
their proliferation and their production of inflammatory cytokines
and inflammatory responses in LPS-induced inflammation or bacterial
keratitis. It is interesting to find that only part and not all of
the expression levels of pro- and anti-inflammatory cytokines were
modulated by tacrolimus in LPS-induced keratitis, which hints at
other pathways other than only innate immune responses
participating in this inflammatory activity. Therefore, future
research should focus on simultaneously modulating the innate and
adaptive immune systems to determine the inflammatory responses in
LPS-induced keratitis.
Another important finding in our study is that
tacrolimus can reduce LPS-induced angiogenesis in vitro.
Published reports have demonstrated that LPS leads to the
upregulation of VEGF and promotes angiogenesis (38) and that systemic and topical
administration of tacrolimus may be beneficial in the prevention of
corneal neovascularization because of its effect on VEGF (39,40).
Our study first verified that VEGF and TNF-α were significantly
elevated after LPS stimulation in HCEC, PNM and THP-1 and that
tacrolimus effectively reduced the expression of VEGF and TNF-α in
LPS-induced inflammation or bacterial keratitis. As LPS stimulate
macrophages mediators and increase the levels of TNFα through
attenuate both FAK and Src activation in osteoblast, which
Calcineurin FAK and Src may have been functionally formed a complex
(41,42). A possible mechanism in the
prevention of VEGF by FK506 in bacterial keratitis might be via the
suppression of inflammatory cells and/or a decrease in the levels
of the cytokines regulating inflammation (39).
Furthermore, as tacrolimus has not only been
regarded as systematic administration (43), but also has been used to suppress
immune reactions in chronic disorders, including corneal and limbal
transplants, allergic eye disease and uveitis as a long-term use
medicine (17), it is thus
necessary to confirm the safety of tacrolimus. In our study, 0.1%
FK506 has been demonstrated to be safe in vitro, and no
negative influence on apoptosis and cell viability in HCEC was
observed. Cell viability was evaluated along with apoptosis, and
the results revealed that 0.1% FK506 had good biocompatibility,
with no toxic effects on HCEC. Therefore, ophthalmic tacrolimus is
a welcome addition to the therapeutic armamentarium for these
corneal and ocular surface diseases, particularly in light of its
excellent efficiency and safety profile.
Conclusively, tacrolimus appears to be a safe and
effective treatment to suppress the activity of PMN and monocytes,
modulate the balance of pro-/anti-inflammatory cytokines, and
reduce the inflammatory response and angiogenic activity in
LPS-induced bacterial keratitis. Further studies should focus on
the specific mechanism of tacrolimus's role in modulating
LPS-induced keratitis, and animal models should be used to explore
the effects of antibacterial drugs combined with tacrolimus.
Further, our study provided references and evidence that FK506
might be a promising adjuvant alternative, together with
antibiotics, in treating LPS-induced or bacterial keratitis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China to J.Y. (81270972), Guangdong
Province Science and Technology Projects to J.Y. (303090100502039)
and Jiangxi Province Natural Science Foundation to YF.Y.
(20161BAB205270).
References
|
1
|
Chan TC, Agarwal T, Vajpayee RB and Jhanji
V: Cross-linking for microbial keratitis. Curr Opin Ophthalmol.
27:348–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keay L, Edwards K, Naduvilath T, Taylor
HR, Snibson GR, Forde K and Stapleton F: Microbial keratitis
predisposing factors and morbidity. Ophthalmology. 113:109–116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong T, Ormonde S, Gamble G and McGhee CN:
Severe infective keratitis leading to hospital admission in New
Zealand. Br J Ophthalmol. 87:1103–1108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schaefer F, Bruttin O, Zografos L and
Guex-Crosier Y: Bacterial keratitis: A prospective clinical and
microbiological study. Br J Ophthalmol. 85:842–847. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levey SB, Katz HR, Abrams DA, Hirschbein
MJ and Marsh MJ: The role of cultures in the management of
ulcerative keratitis. Cornea. 16:383–386. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engel LS, Callegan MC, Hobden JA, Reidy
JJ, Hill JM and O'Callaghan RJ: Effectiveness of specific
antibiotic/steroid combinations for therapy of experimental
Pseudomonas aeruginosa keratitis. Curr Eye Res. 14:229–234. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hazlett LD: Corneal response to
Pseudomonas aeruginosa infection. Prog Retin Eye Res. 23:1–30.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Z, Barrett RP, McClellan SA, Zhang Y,
Szliter EA, van Rooijen N and Hazlett LD: Substance P delays
apoptosis, enhancing keratitis after Pseudomonas aeruginosa
infection. Invest Ophthalmol Vis Sci. 49:4458–4467. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Biswas SK and Lopez-Collazo E: Endotoxin
tolerance: New mechanisms, molecules and clinical significance.
Trends Immunol. 30:475–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alaiti S, Kang S, Fiedler VC, Ellis CN,
Spurlin DV, Fader D, Ulyanov G, Gadgil SD, Tanase A, Lawrence I, et
al: Tacrolimus (FK506) ointment for atopic dermatitis: A phase I
study in adults and children. J Am Acad Dermatol. 38:69–76. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kino T, Hatanaka H, Hashimoto M, Nishiyama
M, Goto T, Okuhara M, Kohsaka M, Aoki H and Imanaka H: FK-506, a
novel immunosuppressant isolated from a Streptomyces. I.
Fermentation, isolation, and physico-chemical and biological
characteristics. J Antibiot (Tokyo). 40:1249–1255. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao K, Lu Y, Wang J, Chen X, Zhang Z,
Wang X, Wang X, Yang H and Liu G: Different effects of tacrolimus
on innate and adaptive immune cells in the allograft
transplantation. Scand J Immunol. 83:119–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hannah J, Casian A and D'Cruz D:
Tacrolimus use in lupus nephritis: A systematic review and
meta-analysis. Autoimmun Rev. 15:93–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge Y, Zhou H, Shi J, Ye B, Peng Q, Lu X
and Wang G: The efficacy of tacrolimus in patients with refractory
dermatomyositis/polymyositis: A systematic review. Clin Rheumatol.
34:2097–2103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moscovici BK, Holzchuh R,
Sakassegawa-Naves FE, Hoshino-Ruiz DR, Albers MB, Santo RM and Hida
RY: Treatment of Sjogren's syndrome dry eye using 0.03% tacrolimus
eye drop: Prospective double-blind randomized study. Cont Lens
Anterior Eye. 38:373–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fei WL, Chen JQ, Yuan J, Quan DP and Zhou
SY: Preliminary study of the effect of FK506 nanospheric-suspension
eye drops on rejection of penetrating keratoplasty. J Ocul
Pharmacol Ther. 24:235–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kheirkhah A, Zavareh MK, Farzbod F, Mahbod
M and Behrouz MJ: Topical 0.005% tacrolimus eye drop for refractory
vernal keratoconjunctivitis. Eye (Lond). 25:872–880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eris E, Yüksel N, Pirhan D, Karadenizli A,
Aslan M, Gacar G, Erman G, Subaş C, Uzuner H, Yldz DK and Karaöz E:
Evaluation of effect of topical Tacrolimus treatment on herpetic
stromal keratitis in a rat model. Eye Contact Lens. 42:163–170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang W, Ling S, Jia X, Lin B, Huang X,
Zhong J, Li W, Lin X, Sun Y and Yuan J: Tacrolimus (FK506)
suppresses TREM-1 expression at an early but not at a late stage in
a murine model of fungal keratitis. PLoS One. 9:e1143862014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Zhou X, Debing Y, Chen K, Van Der
Laan LJ, Neyts J, Janssen HL, Metselaar HJ, Peppelenbosch MP and
Pan Q: Calcineurin inhibitors stimulate and mycophenolic acid
inhibits replication of hepatitis E virus. Gastroenterology.
146:1775–1783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen K, Wu Y, Zhu M, Deng Q, Nie X, Li M,
Wu M and Huang X: Lithium chloride promotes host resistance against
Pseudomonas aeruginosa keratitis. Mol Vis. 19:1502–1514.
2013.PubMed/NCBI
|
|
22
|
Rocksen D, Koch B, Sandström T and Bucht
A: Lung effects during a generalized Shwartzman reaction and
therapeutic intervention with dexamethasone or vitamin E. Shock.
22:482–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei H, Yin L, Feng S, Wang X, Yang K,
Zhang A and Zhou H: Dual-parallel inhibition of IL-10 and TGF-b1
controls LPS-induced inflammatory response via NF-κB signaling in
grass carp monocytes/macrophages. Fish Shellfish Immunol.
44:445–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kerber-Momot T, Leemhuis D, Lührmann A,
Munder A, Tümmler B, Pabst R and Tschernig T: Beneficial effects of
TLR-2/6 ligation in pulmonary bacterial infection and immunization
with Pseudomonas aeruginosa. Inflammation. 33:58–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knaapen AM, Seiler F, Schilderman PA,
Nehls P, Bruch J, Schins RP and Borm PJ: Neutrophils cause
oxidative DNA damage in alveolar epithelial cells. Free Radic Biol
Med. 27:234–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akpek EK and Gottsch JD: Immune defense at
the ocular surface. Eye (Lond). 17:949–956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang JH, McCluskey PJ and Wakefield D:
Toll-like receptors in ocular immunity and the immunopathogenesis
of inflammatory eye disease. Br J Ophthalmol. 90:103–108. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee HN, Na HK and Surh YJ: Resolution of
inflammation as a novel chemopreventive strategy. Semin
Immunopathol. 35:151–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taube MA, del Mar Cendra M, Elsahn A,
Christodoulides M and Hossain P: Pattern recognition receptors in
microbial keratitis. Eye (Lond). 29:1399–1415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hazlett LD: Role of innate and adaptive
immunity in the pathogenesis of keratitis. Ocul Immunol Inflamm.
13:133–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hindman HB, Patel SB and Jun AS: Rationale
for adjunctive topical corticosteroids in bacterial keratitis. Arch
Ophthalmol. 127:97–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herretes S, Wang X and Reyes JM: Topical
corticosteroids as adjunctive therapy for bacterial keratitis.
Cochrane Database Syst Rev: CD005430. 2014. View Article : Google Scholar
|
|
33
|
Wilhelmus KR: Indecision about
corticosteroids for bacterial keratitis: An evidence-based update.
Ophthalmology. 109:835–843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshino T, Nakase H, Honzawa Y, Matsumura
K, Yamamoto S, Takeda Y, Ueno S, Uza N, Masuda S, Inui K and Chiba
T: Immunosuppressive effects of tacrolimus on macrophages
ameliorate experimental colitis. Inflamm Bowel Dis. 16:2022–2033.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morris MC, Gilliam EA, Button J and Li L:
Dynamic modulation of innate immune response by varying dosages of
lipopolysaccharide (LPS) in human monocytic cells. J Biol Chem.
289:21584–21590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shihab F, Christians U, Smith L, Wellen JR
and Kaplan B: Focus on mTOR inhibitors and tacrolimus in renal
transplantation: Pharmacokinetics, exposure-response relationships,
and clinical outcomes. Transpl Immunol. 31:22–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aomatsu T, Imaeda H, Takahashi K, Fujimoto
T, Kasumi E, Yoden A, Tamai H, Fujiyama Y and Andoh A: Tacrolimus
(FK506) suppresses TNF-α-induced CCL2 (MCP-1) and CXCL10 (IP-10)
expression via the inhibition of p38 MAP kinase activation in human
colonic myofibroblasts. Int J Mol Med. 30:1152–1158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shin MR, Kang SK, Kim YS, Lee SY, Hong SC
and Kim EC: TNF-α and LPS activate angiogenesis via VEGF and SIRT1
signalling in human dental pulp cells. Int Endod J. 48:705–716.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Turgut B, Guler M, Akpolat N, Demir T and
Celiker U: The impact of tacrolimus on vascular endothelial growth
factor in experimental corneal neovascularization. Curr Eye Res.
36:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park JH, Joo CK and Chung SK: Comparative
study of tacrolimus and bevacizumab on corneal neovascularization
in rabbits. Cornea. 34:449–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cavagis A, Takamori E, Granjeiro J,
Oliveira R, Ferreira C, Peppelenbosch M and Zambuzzi W: TNFα
contributes for attenuating both Y397FAK and Y416Src
phosphorylations in osteoblasts. Oral Dis. 20:780–786.
2014.PubMed/NCBI
|
|
42
|
Karginov AV, Tsygankov D, Berginski M, Chu
PH, Trudeau ED, Yi JJ, Gomez S, Elston TC and Hahn KM: Dissecting
motility signaling through activation of specific Src-effector
complexes. Nat Chem Biol. 10:286–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fischer L, Saliba F, Kaiser GM, De Carlis
L, Metselaar HJ, De Simone P, Duvoux C, Nevens F, Fung JJ, Dong G,
et al: Three-year outcomes in de novo liver transplant patients
receiving everolimus with reduced Tacrolimus: Follow-up results
from a randomized, multicenter study. Transplantation.
99:1455–1462. 2015. View Article : Google Scholar : PubMed/NCBI
|