Introduction
G protein-coupled receptor (GPCR)-associated sorting
protein 2 (GPRASP2), located at the chromosome region Xq22.1, is a
member of the GPCR-associated sorting protein (GASP) family,
comprising 10 members, which were identified by sequence homology
searches (1,2). It exhibits significant functions in
modulating the activity of GPCRs (3), which triggers numerous cellular
events, including the modification of secondary messenger levels
(4), receptor desensitization and
internalization (5), and
modification of gene transcription (6,7). For
example, GASP-1 interacts with cytoplasmic tails of several
GPCRs, including D2 dopamine receptor, δ opioid receptor 1, β-2
adrenergic receptor and D4 dopamine receptor (8), and has been reported as an important
breast cancer tumor and serum biomarker (9). GPRASP2 has been identified as
a non-synonymous rare variant involved in the regulation of neurite
outgrowth and other synaptic functions (10), and is an essential component of the
Hedgehog-induced ciliary targeting complex, which regulates the
translocation of Smoothened into the primary cilia (11). In addition, the knockdown of
GPRASP2 has been shown to enhance hematopoietic stem cell
repopulation (12). However,
previous studies have shown that current understanding of the
association between GPRASP2 and diseases remains
limited.
Armadillo repeat-containing 10 (Armc10), a 343-amino
acid protein, which contains six ARM repeats, is a member of the
Armc10/Armadillo repeat-containing X-linked protein (Armcx) family
of proteins, which exhibit a variety of functions in embryogenesis
and tumorigenesis, including cell migration, cell proliferation,
tissue maintenance, tumorigenesis, signal transduction and
maintenance of overall cell structure (13,14).
The armc10 gene is widely expressed in several species, and
zebrafish armc10 has been found to be a homologous gene of
GPRASP2 in our previous synteny analysis study (15).
To further examine the underlying molecular
pathogenesis of GPRASP2, zebrafish at different embryonic
stages were used in the present study as a model organism to
perform whole mount in situ hybridization (WISH) and reverse
transcription polymerase chain reaction (RT-PCR) analysis of
zebrafish armc10, the homologous gene of human
GPRASP2. The results revealed the spatial and temporal
expression patterns of armc10 in zebrafish during early
embryonic development and assist in further understanding the role
of GPRASP2 in embryogenesis and disease pathogenesis.
Materials and methods
Zebrafish care and maintenance
Zebrafish (Tübingen line) were provided by China
Zebrafish Resource Center (Wuhan, China). The zebrafish care and
experimental procedures were performed in accordance with the
regulations set forth by the Institutional Animal Care and Use
Committee of Nanjing Medical University (Nanjing, China). Zebrafish
were maintained under 14 h light/10 h dark cycles and fed twice
daily in a static water system at 28.5°C. The vessels used for
collecting embryos were placed at the four corners of the
hydrostatic system fish tank 1 day prior to collecting embryos. The
vessels were removed from the water following exposure to light for
30 min the subsequent day. The embryos were then raised at 28.5°C
in an incubator following collection and washing. The embryonic
stages were defined as described previously (16).
RNA purification and cDNA
synthesis
Total RNA was extracted from 80 embryos at 24 h
post-fertilization (hpf) using TRIzol reagent (Sangon Biotech Co.,
Ltd., Shanghai, China). Following extraction, 1 µg of RNA was
reverse transcribed into cDNA using RT Prime mix (Takara Bio, Inc.,
Otsu, Japan) according to the manufacturer's protocol. The primers
were designed based on the sequences of armc10
(ENSDARG00000062960) provided by the Ensembl database (http://asia.ensembl.org/index.html) to clone the
coding sequence of armc10. The primers used were as follows:
armc10 F1, 5′-TGGGAGATGGCAGATGAT-3′ and R1,
5′-AGGAGCCGTCCAGTAAAA-3′; armc10 F2,
5′-CTCTGCTGGGGATTGTGG-3′ and R2, 5′-GAGAGTCCGGTCTCCTCCTC-3′. The RT
product was used as a template for nested-PCR with 10 µl 2X PCR
Mastermix (Beijing TransGen Biotech Co., Ltd., Beijing, China), 1
µl cDNA, 2 µl F/R primers and 7 µl H2O. The conditions
for the nested-PCR were as follows: 95°C for 3 min, and 35 cycles
of 95°C for 30 sec, 56°C for 30 sec and 72°C for 1 min, followed by
incubation for 10 min at 72°C.
Probe synthesis
The cDNAs of the 3′untranslated region (3′UTR) of
zebrafish armc10 was used to amplify templates for the
synthesis of armc10 antisense RNA probes using the following
primer pair: F2-armc10-utr 5′-CTCTGCTGGGGATTGTGG-3′ and
R2-armc10-utr 5′-GAGAGTCCGGTCTCCTCCTC-3′. The sequence was
then cloned into the pGEM-T Easy vector with T7 and SP6 RNA
polymerase promoter sequences for in vitro
transcription.
The templates used for synthesizing armc10
antisense RNA probes were generated by PCR amplification using
pGEMT-armc10 as templates. RNA probes were generated by
in vitro transcription from the T7 RNA promoter,
incorporating DIG-11-UTP (Roche Diagnostics, Indianapolis, IN, USA)
nucleotides, using Sp6 RNA polymerase with the MAXIscript kit
(Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The DNA
template was removed from the synthesized probe by DNaseI treatment
and the probe was purified using LiCl-based precipitation. The
probe was dissolved in DEPC-treated water and stored at −80°C.
Sequence analysis
The full-length sequence of zebrafish was obtained
from the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/). The coding
sequence data of zebrafish armc10 were analyzed using
Jellyfish 1.1 (http://www.jellyfishsoftware.com/) (17). Multiple sequence alignment of the
amino acid sequences was performed using ClustalX2 (http://www.clustal.org/) to identify the
evolutionarily conserved regions of ARMC10 among animals. Mega 6.0
(http://www.megasoftware.net/) was used
to construct a phylogenetic tree of the evolution of ARMC10.
Synteny analysis was performed using the Ensembl database.
Detection of armc10 mRNA using RT-PCR
analysis and WISH
The distribution of the mRNA expression of
armc10 was examined using RT-PCR analysis, as previously
reported (18). The embryos were
staged as previously described (16). The sequences of primers used to
detect the presence of armc10 cDNA during embryogenesis were
armc10, F2 5′-CTCTGCTGGGGATTGTGG-3′ and R2,
5′-GAGAGTCCGGTCTCCTCCTC-3′. PCR analysis was performed using, 10 µl
2X PCR Mastermix (Beijing TransGen Biotech Co., Ltd.), 1 µl cDNA, 2
µl F/R primers and 7 µl H2O and the conditions were as
follows: 95°C for 3 min, and 35 cycles of 95°C for 30 sec, 56°C for
30 sec and 72°C for 1 min, followed by 10 min at 72°C. The
sensitivity of the RT-PCR analysis was controlled by performing
amplification of zebrafish β-actin using the same cDNA as a
template (19). The primers of
β-actin were as follows: β-actin, forward
5′-CCAGACATCAGGGAGTGA-3′ and reverse
5′-GATACCGCAAGATTCCATAC-3′.
WISH was performed as previously described (16,17).
To prevent the development of melanin pigmentation at later stages,
0.003% 1-phenyl-2-thiouera was added at 24 hpf. The concentration
of the probe used in hybridization was 1.0 ng/µl for armc10.
Images were captured using a stereoscopic microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Results
Analysis of the zebrafish armc10
gene
On examining the zebrafish genome (armc10;
ensembl.org), it was found that the zebrafish
armc10 gene (XM_009297973) is located on chromosome 25, has
six exons and encodes a 348 amino acid protein. The Armc10 protein
contains a transmembrane domain at the N-terminus (aa7-29), a
putative cleavage site (aa30-36) and a flanking basic region close
to the transmembrane region, similar to that found in translocase
of outer mitochondria membrane 20 and B-cell lymphoma 2, which
predicts putative targeting to the outer mitochondrial membrane
(20). Full-length Armc10 contains
six Arm domains arranged in a DUF634 domain (aa 85–337), which are
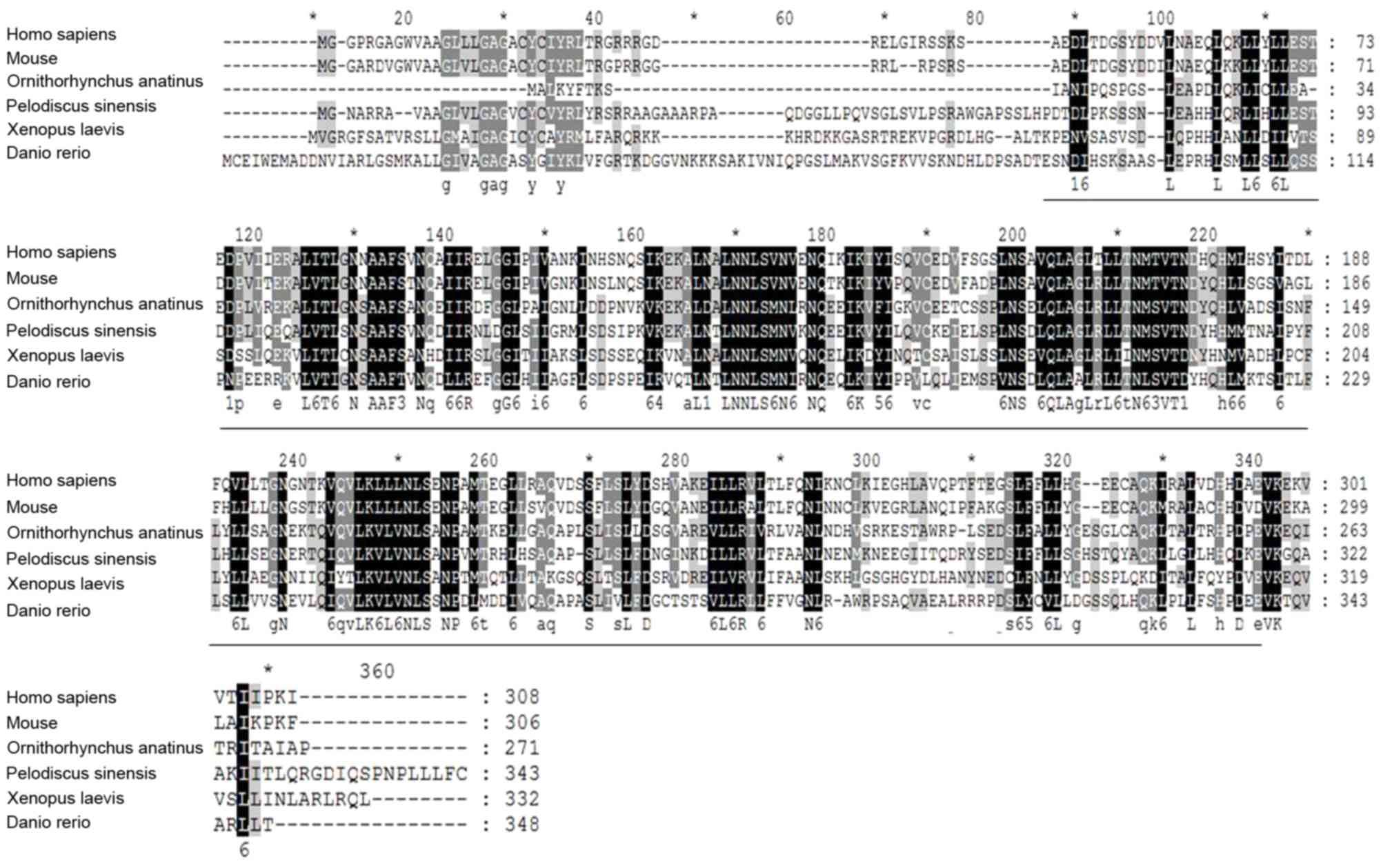
partially deleted in certain isoforms (21). Multiple sequence alignment of the
amino acid sequences of ARMC10 derived from six different species
shows a high level of conservation in the ARMC10 protein sequences
among different species (Fig. 1).
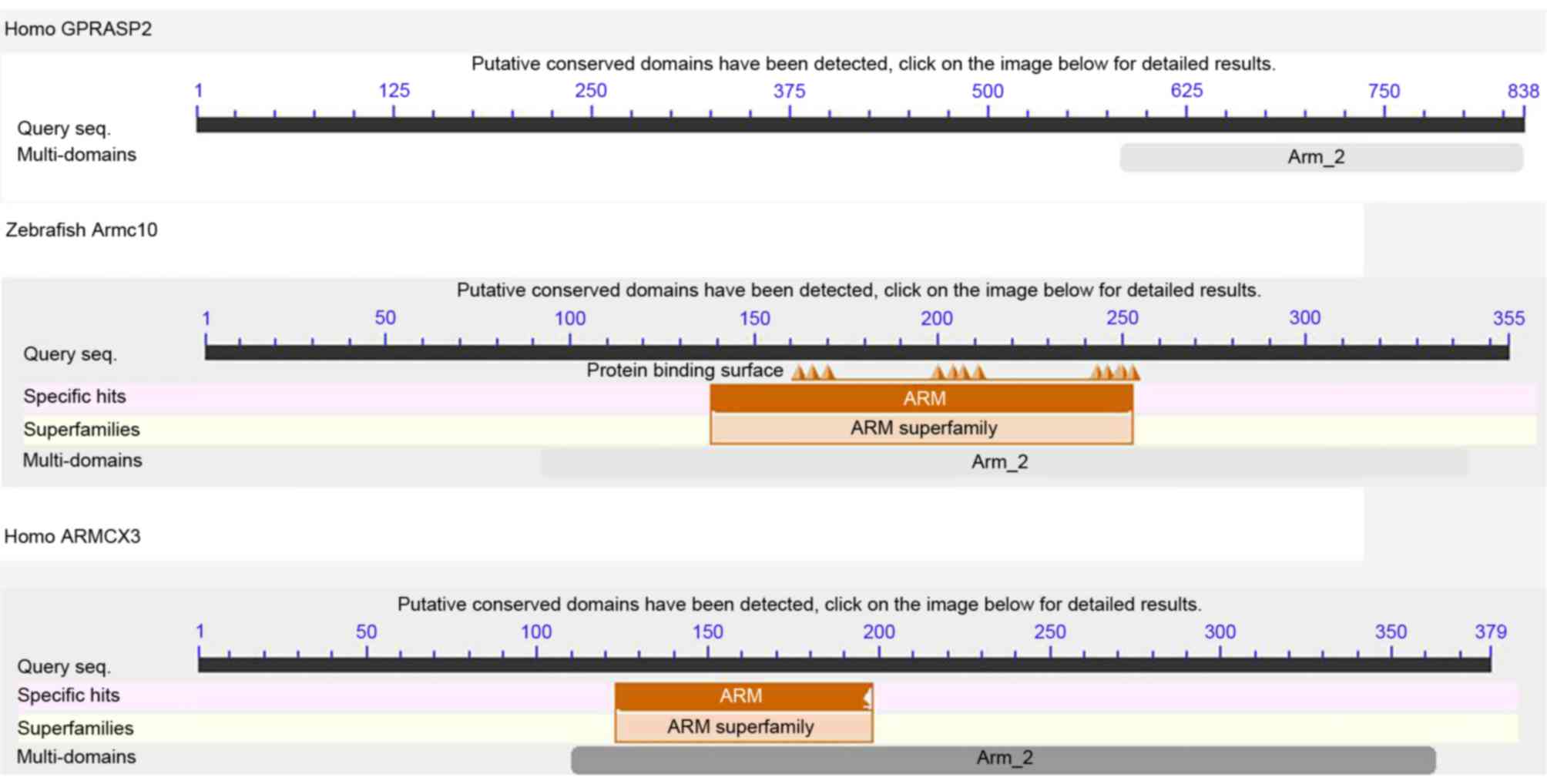
Typically, conserved Arm domains of ~253 amino acids (22) were found to be distributed in
ARMC10 (Fig. 1). The existence of
ARM_2 multi-domains also confirmed the presence of the amino acid
residues (Fig. 2).
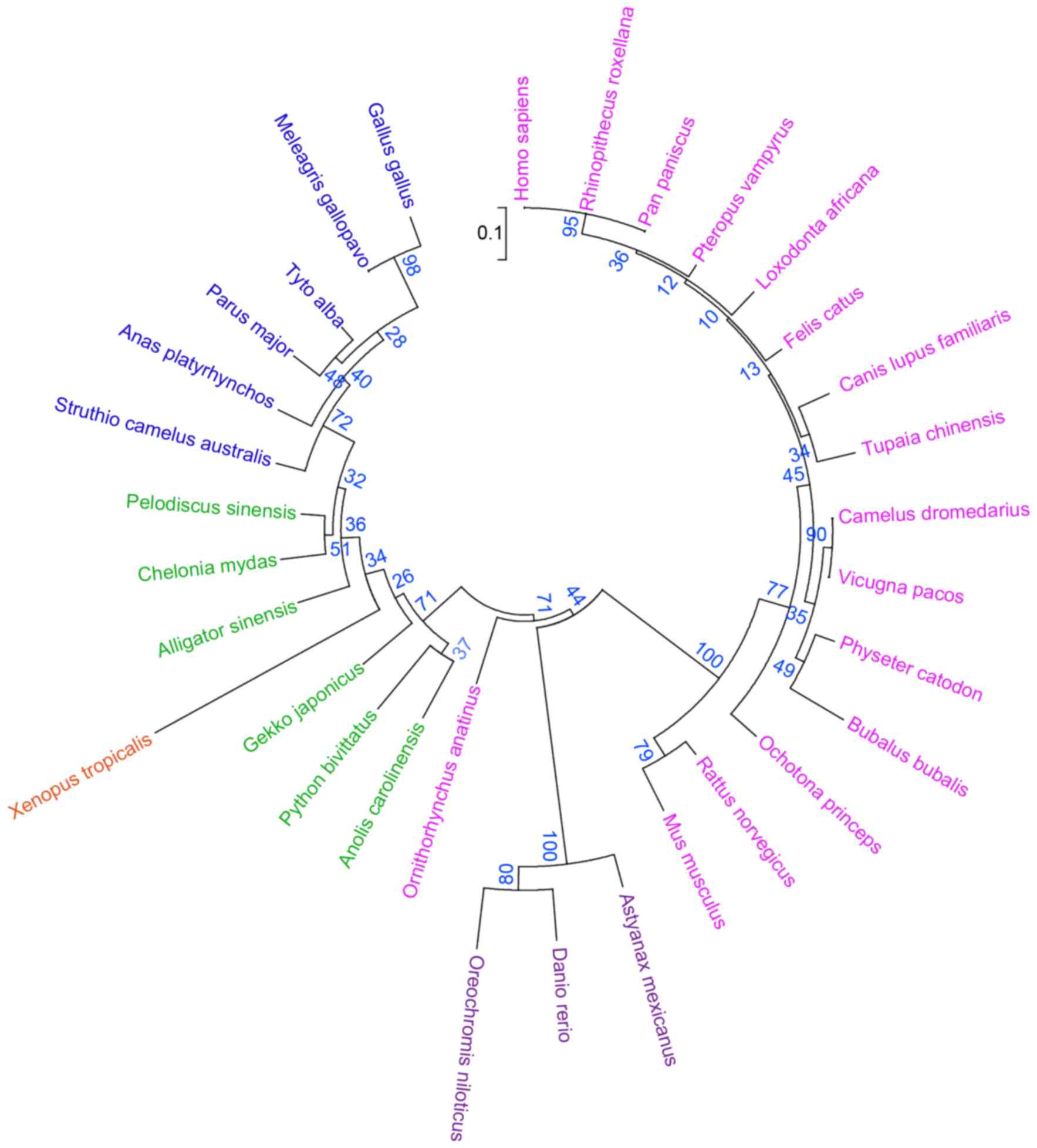
Mega 6.0 was used to construct a phylogenetic tree
of the evolution of ARMC10 using amino acid sequences from 32
species (Fig. 3). The results
showed that sequences belonging to the same family or order were
formed in a cluster. The zebrafish armc10 sequence formed
one clad with that of Oreochromis niloticus (bootstrap value
77). Higher bootstrap values were observed among the mammalians,
including Rattus norvegicus, Ochotona princeps, Camelus
dromedarius and Homo sapiens, Pan paniscus and Rhinopithecus
roxellana). The Tyto alba, Parus major and
Anas platyrhynchos species formed a clad, separating it from
that of reptilia (Pelodiscus sinensis, Chelonia mydas and
Alligator sinensis). The tree indicated that the ARMC10
protein underwent natural selection during evolution in accordance
with the requirements of the environment.
Through blasting of the current zebrafish database
in Ensemble with zebrafish armc10, the present study found
that human GPRASP2 was a homologous gene of zebrafish
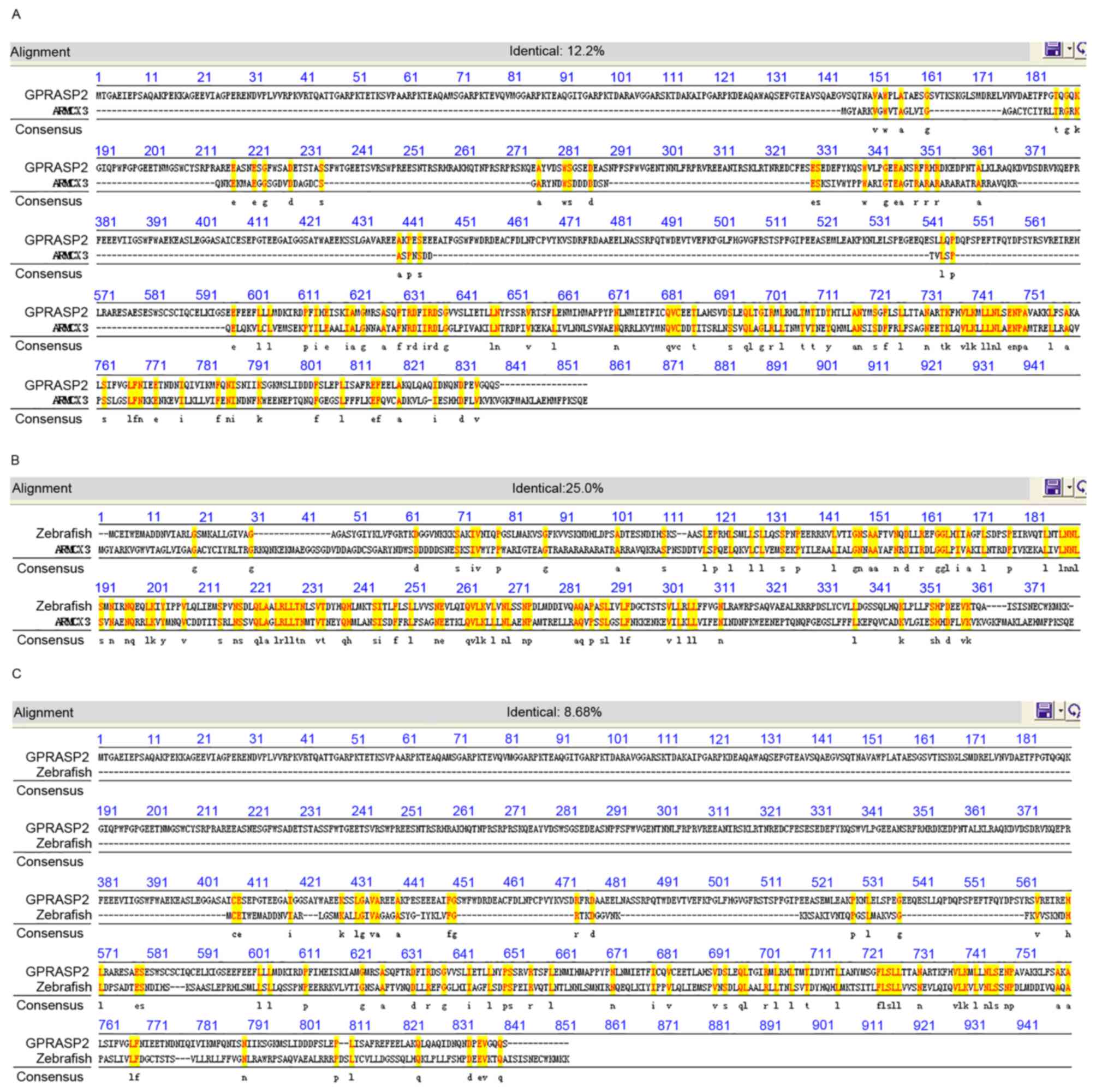
armc10. Synteny analysis indicated that human GPRASP2
(NP_001171805) and human ARMCX3 (NP_775104) were paralogous
genes with 12.2% identity (Fig.
4A), whereas human ARMCX3 (NP_775104) exhibited 25%
amino acid identity with zebrafish armc10 (Fig. 4B). Human GPRASP2 also shared
8.68% identity with zebrafish armc10 (Fig. 4C). Therefore, human GPRASP2
and zebrafish armc10 were considered homologous genes.
Expression of armc10 during zebrafish
embryonic development
To analyze the spatio-temporal expression patterns
of armc10, the present study performed RT-PCR analysis and
WISH at stages of zebrafish development from the cleavage stage
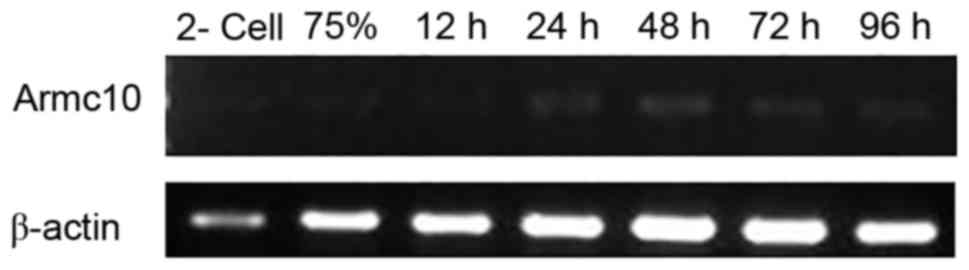
until 96 hpf. The results of the RT-PCR analysis demonstrated that
armc10 was expressed throughout early development. However,
at the cleavage (two-cell) stage, 75% epiboly stage and at 12 hpf,
the expression of armc10 was weak. The embryos showed higher
mRNA expression levels of armc10 from 24 hpf (Fig. 5). Consistent with the results of
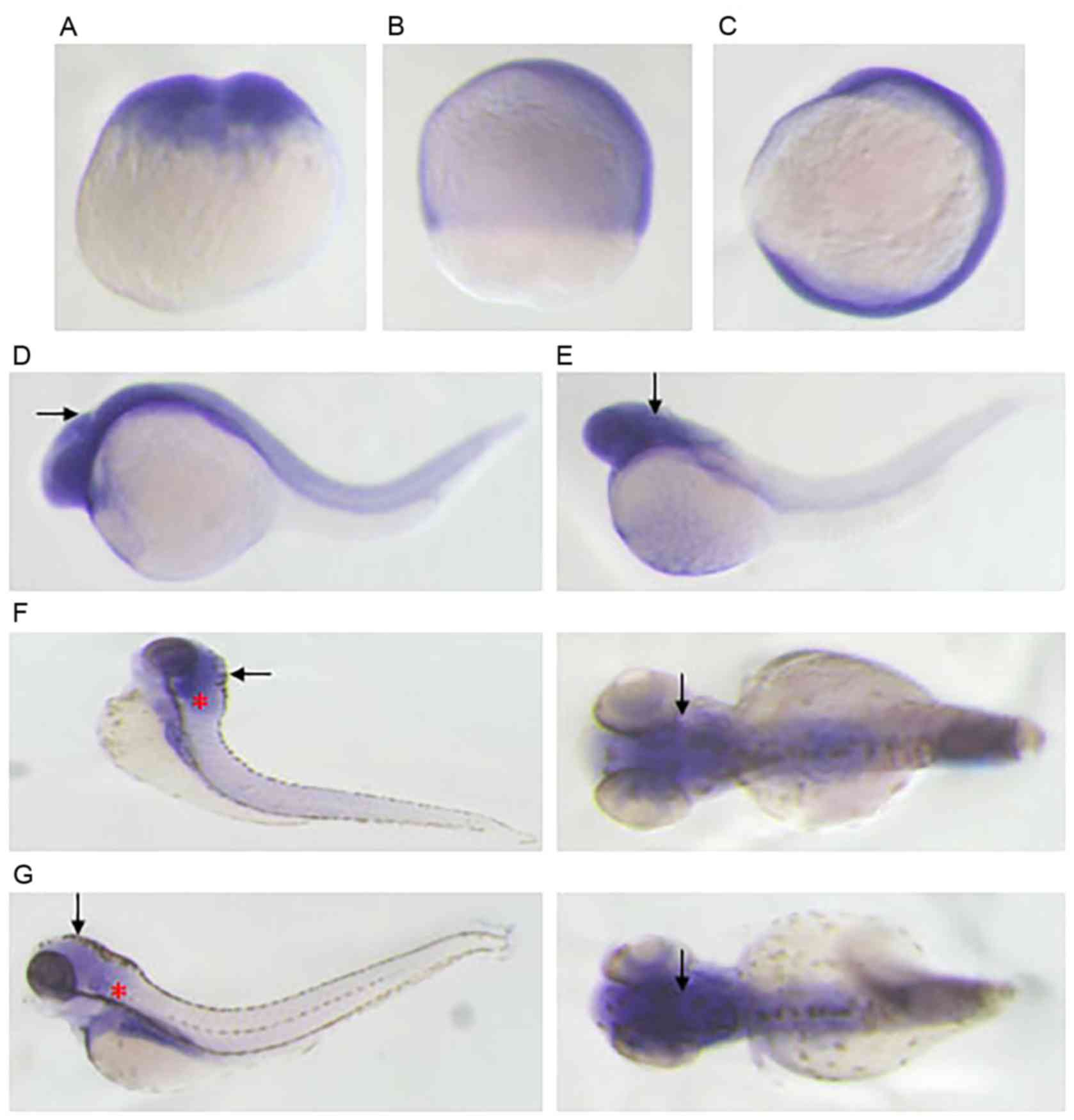
the RT-PCR analysis, WISH revealed that the hybridization signal of
armc10 was detected at the two-cell stage, indicating that
armc10 was maternally expressed (Fig. 6A). At the 75% epiboly stage and at
12 hpf, armc10 was widely expressed in the embryos (Fig. 6B and C). At 24 hpf, armc10
mRNA was expressed in the nervous system of the zebrafish head
(Fig. 6D). When the embryos were 2
days old, armc10 maintained its wide expression in the
nervous system of the zebrafish head (Fig. 6E). At 72 hpf, the armc10
mRNA was specifically expressed in otic vesicles in addition to the
nervous system of the head (Fig.
6F). At 96 hpf, the expression of armc10 remained in the
otic vesicles and the nervous system of the head (Fig. 6G).
Discussion
In the present study, to further examine the
potential molecular pathogenesis of GPRASP2, the
characterization and expression pattern of the homologous
armc10 gene in zebrafish were examined. The results of the
bioinformatics analyses showed a high degree of conservation of the
ARMC10 protein sequences among different species. The high degree
of evolutionary conservation was particularly reflected by the
presence of amino acid residues, which are important for
protein-protein interactions, including the N-terminus
transmembrane domains and armadillo domains. The high conservation
of the these domains is understandable, as it has been reported
that the armadillo repeat domain is essential for protein-protein
interactions (22–24) and is involved in diverse functions,
including embryogenesis and tumorigenesis, by interacting with
multiple binding partners (25).
The phylogenetic analysis of ARMC10 using a phylogenetic tree
demonstrated that the mammalian species formed a cluster with a
higher bootstrap value and were closely associated with zebrafish,
whereas variation was higher in lower organisms. It was concluded
that ARMC10 gradually evolved from lower organisms with more
variation, resulting in a more stable form in mammalian species.
ARMC10 is also upregulated in hepatocellular carcinoma (26). Therefore, these results suggest a
role for ARMC10 during embryogenesis and tumorigenesis.
In the present study, WISH and RT-PCR analysis were
used to detect the expression of the zebrafish armc10 gene
during early embryogenesis. The results showed that armc10
was detected at low levels prior to 12 hpf, and the expression
levels became higher at 24 hpf, distributed primarily in the
regions of the nervous system and otic vesicles. These results were
consistent to a previous finding that armc10 was widely
expressed in adult nervous tissues, particularly in the forebrain
regions of the cerebral cortex, hippocampus and thalamus (27). These sites of expression
demonstrated that the expression of zebrafish armc10 was
dynamic during embryogenesis. The spatial and temporal expression
map of armc10, together with reports that the levels of
armc10 regulate mitochondrial trafficking in neurons by
controlling the number of moving mitochondria (21), suggest a role for armc10 in
the pathophysiology of neurological diseases. Coincidentally, the
syntenic analysis performed in the present study revealed that
human GPRASP2 and zebrafish armc10 were homologous
genes. GPRASP2 has also been reported to be involved in
receptor endocytosis and postsynaptic signaling via its interaction
with the disease protein huntingtin, and that polyQ-dependent
alterations of the interaction can contribute to the pathogenesis
of Huntington's disease (28).
Therefore, the conservation of protein sequences between zebrafish
and higher vertebrates demonstrated in the present study using
syntenic and homologous analysis suggested that investigations of
zebrafish armc10 may provide important insights into these
processes in humans.
Taken together, the present study established the
gene expression map of armc10 among different stages of
zebafish embryogenesis. The expression data compiled provided
information relevant for future investigations of the role of
armc10 in the nervous system during zebrafish embryogenesis,
and provided information to assist in examining the mechanism of
GPRASP2 associated with human nervous system diseases.
Acknowledgements
This study was supported by the grants from the
National Natural Science Foundation of China (grant no. 31571302)
and the Key Research and Development Program of Jiangsu Province
(Social Development; grant no. BE2016762) to Professor Xin Cao; and
grants from the Jiangsu Health Administration of China (grant no.
LJ201120) and the Research Special Fund for Public Welfare Industry
of Health, Ministry of Health of China (grant no. 201202005) to Dr
Guangqian Xin.
References
|
1
|
Simonin F, Karcher P, Boeuf JJ, Matifas A
and Kieffer BL: Identification of a novel family of G
protein-coupled receptor associated sorting proteins. J Neurochem.
89:766–775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abu-Helo A and Simonin F: Identification
and biological significance of G protein-coupled receptor
associated sorting proteins (GASPs). Pharmacol Ther. 126:244–250.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bornert O, Møller TC, Boeuf J, Candusso
MP, Wagner R, Martinez KL and Simonin F: Identification of a novel
protein-protein interaction motif mediating interaction of
GPCR-associated sorting proteins with G protein-coupled receptors.
PLoS One. 8:e563362013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gudermann T, Kalkbrenner F, Dippel E,
Laugwitz KL and Schultz G: Specificity and complexity of
receptor-G-protein interaction. Adv Second Messenger Phosphoprotein
Res. 31:253–262. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierce KL, Premont RT and Lefkowitz RJ:
Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 3:639–650.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pierce KL and Lefkowitz RJ: Classical and
new roles of beta-arrestins in the regulation of G-protein-coupled
receptors. Nat Rev Neurosci. 2:727–733. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
West AE, Griffith EC and Greenberg ME:
Regulation of transcription factors by neuronal activity. Nat Rev
Neurosci. 3:921–931. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson D, Pusch M and Whistler JL:
Changes in G protein-coupled receptor sorting protein affinity
regulate postendocytic targeting of G protein-coupled receptors. J
Biol Chem. 282:29178–29185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuszynski GP, Rothman VL, Zheng X, Gutu M,
Zhang X and Chang F: G-protein coupled receptor-associated sorting
protein 1 (GASP-1), a potential biomarker in breast cancer. Exp Mol
Pathol. 91:608–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piton A, Gauthier J, Hamdan FF, Lafrenière
RG, Yang Y, Henrion E, Laurent S, Noreau A, Thibodeau P, Karemera
L, et al: Systematic resequencing of X-chromosome synaptic genes in
autism spectrum disorder and schizophrenia. Mol Psychiatry.
16:867–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung B, Padula D, Burtscher I, Landerer C,
Lutter D, Theis F, Messias AC, Geerlof A, Sattler M, Kremmer E, et
al: Pitchfork and gprasp2 target smoothened to the primary cilium
for hedgehog pathway activation. PLoS One. 11:e01494772016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holmfeldt P, Ganuza M, Marathe H, He B,
Hall T, Kang G, Moen J, Pardieck J, Saulsberry AC, Cico A, et al:
Functional screen identifies regulators of murine hematopoietic
stem cell repopulation. J Exp Med. 213:433–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coates JC: Armadillo repeat proteins:
Beyond the animal kingdom. Trends Cell Biol. 13:463–471. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heydorn A, Søndergaard BP, Ersbøll B,
Holst B, Nielsen FC, Haft CR, Whistler J and Schwartz TW: A library
of 7TM receptor C-terminal tails. Interactions with the proposed
post-endocytic sorting proteins ERM-binding phosphoprotein 50
(EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1
(SNX1), and G protein-coupled receptor-associated sorting protein
(GASP). J Biol Chem. 279:54291–54303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xing G, Yao J, Liu C, Wei Q, Qian X, Wu L,
Lu Y and Cao X: GPRASP2, a Novel causative gene implicated in an
X-Linked recessive syndromic hearing loss. J Med Genet. 54:426–430.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimmel CB, Ballard WW, Kimmel SR, Ullmann
B and Schilling TF: Stages of embryonic development of the
zebrafish. Dev Dyn. 203:253–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu X, Xu F, Wang X, Gao X and Zhao Q:
Molecular cloning and expression of a novel CYP26 gene (cyp26d1)
during zebrafish early development. Gene Expr Patterns. 5:733–739.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Q, Dobbs-McAuliffe B and Linney E:
Expression of cyp26b1 during zebrafish early development. Gene Expr
Patterns. 5:363–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun L, Zou Z, Collodi P, Xu F, Xu X and
Zhao Q: Identification and characterization of a second fibronectin
gene in zebrafish. Matrix Biol. 24:69–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rapaport D: Finding the right organelle.
Targeting signals in mitochondrial outer-membrane proteins. EMBO
Rep. 4:948–952. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serrat R, Mirra S, Figueiro-Silva J,
Navas-Pérez E, Quevedo M, López-Doménech G, Podlesniy P, Ulloa F,
Garcia-Fernàndez J, Trullas R and Soriano E: The Armc10/SVH gene:
Genome context, regulation of mitochondrial dynamics and protection
against Aβ-induced mitochondrial fragmentation. Cell Death Dis.
5:e11632014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCrea PD, Turck CW and Gumbiner B: A
homolog of the armadillo protein in Drosophila (plakoglobin)
associated with E-cadherin. Science. 254:1359–1361. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawasaki Y, Senda T, Ishidate T, Koyama R,
Morishita T, Iwayama Y, Higuchi O and Akiyama T: Asef, a link
between the tumor suppressor APC and G-protein signaling. Science.
289:1194–1197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spink K Eklof, Fridman SG and Weis WI:
Molecular mechanisms of beta-catenin recognition by adenomatous
polyposis coli revealed by the structure of an APC-beta-catenin
complex. EMBO J. 20:6203–6212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hatzfeld M: The armadillo family of
structural proteins. Int Rev Cytol. 186:179–224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang R, Xing Z, Luan Z, Wu T, Wu X and Hu
G: A specific splicing variant of SVH, a novel human armadillo
repeat protein, is up-regulated in hepatocellular carcinomas.
Cancer Res. 63:3775–3782. 2003.PubMed/NCBI
|
|
27
|
López-Doménech G, Serrat R, Mirra S,
D'Aniello S, Somorjai I, Abad A, Vitureira N, Garcia-Arumi E,
Alonso MT, Rodriguez-Prados M, et al: The Eutherian Armcx genes
regulate mitochondrial trafficking in neurons and interact with
Miro and Trak2. Nat Commun. 3:8142012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horn SC, Lalowski M, Goehler H, Dröge A,
Wanker EE and Stelzl U: Huntingtin interacts with the receptor
sorting family protein GASP2. J Neural Transm (Vienna).
113:1081–1090. 2006. View Article : Google Scholar : PubMed/NCBI
|