Introduction
Retinoblastoma (RB) is the most common intraocular
malignant tumour that normally affects infants and young children,
with a prevalence ranging from 1:15,000 to 1:20,000 in children
under the age of 5 years in the USA (1,2). RB
can be classified into two groups: Hereditary and nonhereditary.
Hereditary RB patients account for 30–40% cases and is usually
associated with a positive family history; those patients with no
family history are generally classified as nonhereditary (3–5).
Previous studies demonstrated that high oncogene expression level,
loss of tumour suppressors and epigenetic changes of oncogenic
methylation may be involved in RB formation and progression
(6–8). Currently, the primary treatments for
RB are enucleation, chemotherapy and focal therapy, such as laser
or cryotherapy (9). Despite
improvements in therapeutic treatments, the prognosis for patients
with RB remains poor, with a mortality rate of 50–70% among
children in underdeveloped countries (10). This poor outcome is largely due to
diagnosis and treatment delay (11). Therefore, further exploration of
the biology and molecular mechanisms of RB that cause RB procession
and metastasis and investigation of novel therapeutic methods to
improve the prognosis of patients with RB are necessary.
MicroRNAs (miRNAs or miRs) are a group of
endogenous, single-strand, non-coding 19–25 nucleotide RNAs that
modulate more than half of the genes in human cells (12). Over 1,000 miRNAs have been
identified within the human genome (13), and they may regulate gene
expression by interacting with complementary sites in the
3′-untranslated regions (3′-UTRs) of their target mRNA molecules,
thereby leading to mRNA degradation or post-transcriptional
translational repression (14).
miRNAs act as key regulators in a wide variety of physiological and
pathological processes, such as cell proliferation, cycle,
differentiation, apoptosis, metabolism and death (15–17).
An increasing number of studies reported miRNA dysregulation in
various kinds of human cancers, such as gastric cancer (18), glioma (19), lung cancer (20), bladder cancer (21) and RB (22). The abnormally expressed miRNAs are
involved in progression and development of various cancers and
serve as oncogenic and tumour suppressors by directly targeting the
oncogenes and tumour-suppressor genes, respectively (23–25).
Several miRNAs were correlated with RB tumourigenesis and
development and might serve as independent prognostic markers or
therapy targets for patients with this malignancy (26–28).
Therefore, aberrant miRNAs and their functions in RB need to be
explored to benefit the discovery of novel diagnosis biomarkers and
therapeutic strategies.
miR-382 is aberrantly expressed and play important
roles in several types of cancer (29–31).
However, the expression pattern, functional roles and underlying
molecular mechanism of miR-382 in RB remains unknown. This study
aimed to investigate the miR-382 expression levels in RB tissues
and cell lines. Furthermore, the effects of miR-382 on RB cells and
the underlying regulatory mechanism of its action were also
examined.
Materials and methods
Tissue samples
This study was approved by the Human Subjects
Committee of Beijing Tsinghua Chang Gung Hospital. Informed consent
was also obtained from all patients. A total of 26 RB tissues were
obtained from patients who underwent enucleation at Beijing
Tsinghua Chang Gung Hospital (Beijing, China). Nine normal retina
samples were collected from paediatric ruptured globe. RB tissues
were excluded from experiment if the RB patients had been treated
with chemotherapy or other treatments prior to enucleation. None of
the patients received thermotherapy, cryotherapy, chemotherapy and
radiotherapy before surgery. Tissues were immediately snap frozen
in liquid nitrogen and transferred at −80°C in the refrigerator for
storage.
Cell lines, culture conditions and
transfection
Three human RB cell lines (Y79, WERI-RB-1 and
SO-RB50) were purchased from the American Type Culture Collection
(Manassas, VA, USA) and cultured in RPMI-1640 medium (Gibco, Grand
Island, NY, USA) containing 10% heat-inactivated fetal bovine serum
(FBS; Gibco), 100 U/ml penicillin and 100 mg/ml streptomycin. All
cells were grown in a humidified atmosphere at 37°C with 5%
CO2.
miR-382 mimics and miRNA mimic negative control
(miR-NC) were obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). Brain-derived neurotrophic factor (BDNF)
overexpression vector (pCDNA3.1-BDNF) and corresponding blank
vector (pCDNA3.1) were synthesised by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). Cells were plated into 6-well plates at a
density of 60–70% confluence. After an overnight incubation, cell
transfection was performed using Lipofectamine 2000 Transfection
Reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) based on the manufacturer's instructions. Transfected cells
were incubated at 37°C in a CO2 incubator for 6 h, and
culture medium was replaced with fresh RPMI-1640 medium containing
10% FBS.
RNA isolation and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the tissues or
cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). For the
miR-382 expression analysis, reverse transcription was performed
with TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems,
Foster City, CA, USA). Quantitative PCR (qPCR) analyses were
conducted with TaqMan MicroRNA PCR kit using the Applied
Biosystems® 7900HT Fast Real-Time PCR system (both from
Applied Biosystems). To quantify the BDNF mRNA, RNA was reverse
transcribed to cDNA from 1 µg of total RNA using a PrimeScript RT
Reagent kit, followed by qPCR with SYBR Premix Ex Taq™ kit (both
from Takara Bio, Inc., Dalian, China). miR-382 and BDNF mRNA
expression levels were normalised to endogenous U6 and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), respectively.
Relative expression changes were analysed using the
2−ΔΔCq method (32).
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was used to determine RB cell proliferation. Cells
were plated in 96-well plates at a density of 3×103
cells per well. After transfection, cells were incubated at 37°C in
a 5% CO2 atmosphere for 0, 24, 48 and 72 h. CCK-8 assay
was performed at each time point. CCK-8 reagent (10 µl) was added
into each well, and cells were incubated at 37°C for an additional
4 h. Finally, the absorbance was examined at a wavelength of 450 nm
using a multifunction microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Each assay was performed in 5-well
replication and repeated three times.
Matrigel invasion assay
The invasive capacity of RB cells was examined using
24-well Transwell chambers (8 µm; Corning Costar, Cambridge, MA,
USA) pre-coated with Matrigel® (BD Biosciences, Franklin
Lakes, NJ, USA). After incubation for 48 h, the transfected cells
were collected, re-suspended with FBS-free RPMI-1640 medium as a
single-cell solution and seeded into the upper chambers at a
density of 5×104 cells/chamber. RPMI-1640 medium
supplemented with 20% FBS was added into the lower chamber as a
chemoattractant. Transwell chambers were then incubated at 37°C in
a 5% CO2 atmosphere for 24 h. Subsequently, the
non-invasive cells were carefully removed with cotton swab, whereas
the invasive cells were fixed with 100% methanol for 5 min, stained
with 0.1% crystal violet for 10 min and washed in PBS. The number
of invasive cell was counted in five randomly selected fields under
an inverted microscope (CKX41; Olympus, Tokyo, Japan).
miR-382 target prediction
The potential targets of miR-382 were analyzed with
miRNA target prediction algorithms: PicTar (http://pictar.mdc-berlin.de/) and TargetScan
(http://www.targetscan.org/).
Luciferase reporter assay
For luciferase reporter assay, reporter plasmids,
pMIRGLO-BDNF-3′-UTR wild-type (Wt) and pMIRGLO-BDNF-3′-UTR mutant
(Mut), were synthesised by Shanghai GenePharma Co., Ltd. Cells were
seeded into 24-well plates at a density of 1.5×105 per
well. After an overnight incubation, cells were transfected with
miR-382 mimics or miR-NC, along with pMIRGLO-BDNF-3′-UTR Wt or
pMIRGLO-BDNF-3′-UTR Mut, using Lipofectamine 2000 based on the
manufacturer's instructions. Cells were harvested 48 h after
transfection, and the luciferase activities were measured using
dual-luciferase reporter assay system (Promega, Madison, WI, USA)
following the manufacturer's protocol. Renilla luciferase
was used for normalisation used in this research.
Western blot analysis
The total proteins of the cell lines and tissues
were extracted using a radioimmunoprecipitation assay buffer
(Beyotime, Shanghai, China) in the presence of a proteinase
inhibitor cocktail (Sigma, St. Louis, MO, USA). The total protein
concentration was examined using the bicinchoninic acid assay
protein assay (Pierce Biotechnology, Inc., Rockford, IL, USA).
Equal amounts of protein were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). After blocking with 5% skim milk in Tris-buffered saline
containing 0.05% Tween-20 (TBST), the membranes were incubated at
4°C overnight with primary antibodies: rabbit anti-human polyclonal
BDNF (sc-20981; 1:1,000 dilution; Santa Cruz Biotechnology, Santa
Cruz, CA, USA), mouse anti-human monoclonal phosphoinositide
3-kinase (PI3K) antibody (ab86714; 1:1,000 dilution; Abcam,
Cambridge, MA, USA), rabbit anti-human polyclonal p-PI3K antibody
(cat. no. 4228; 1:1,000 dilution; Cell Signaling Technology,
Danvers, MA, USA), mouse anti-human monoclonal p-protein kinase B
(AKT) (sc-271966; 1:1,000 dilution; Santa Cruz Biotechnology),
mouse anti-human monoclonal AKT (sc-56878; 1:1,000 dilution; Santa
Cruz Biotechnology), and mouse anti-human monoclonal GAPDH
(sc-32233; 1:1,000 dilution; Santa Cruz Biotechnology). The
membranes were subsequently washed in TBST thrice and probed with
corresponding horseradish peroxidase-conjugated secondary antibody
(1:5,000 dilution; Santa Cruz Biotechnology) for 2 h at room
temperature. Finally, the protein signals were detected using
Pierce™ ECL Western Blotting Substrate (Pierce Biotechnology, Inc.)
and analysed with Quantity One® software (version 4.62;
Bio-Rad Laboratories, Inc.). GAPDH was used as a loading
control.
Statistical analysis
All data are expressed as mean ± standard deviation,
and the differences between groups were analysed with Student's
t-test or one-way analysis of variance using SPSS version 19.0
software (SPSS, Inc., Chicago, IL, USA). Spearman's correlation
analysis was utilised to analyse the correlation between miR-382
and BDNF mRNA expression levels in RB tissues. P<0.05 was
considered statistically significant.
Results
miR-382 expression is downregulated in
RB tissues and cell lines
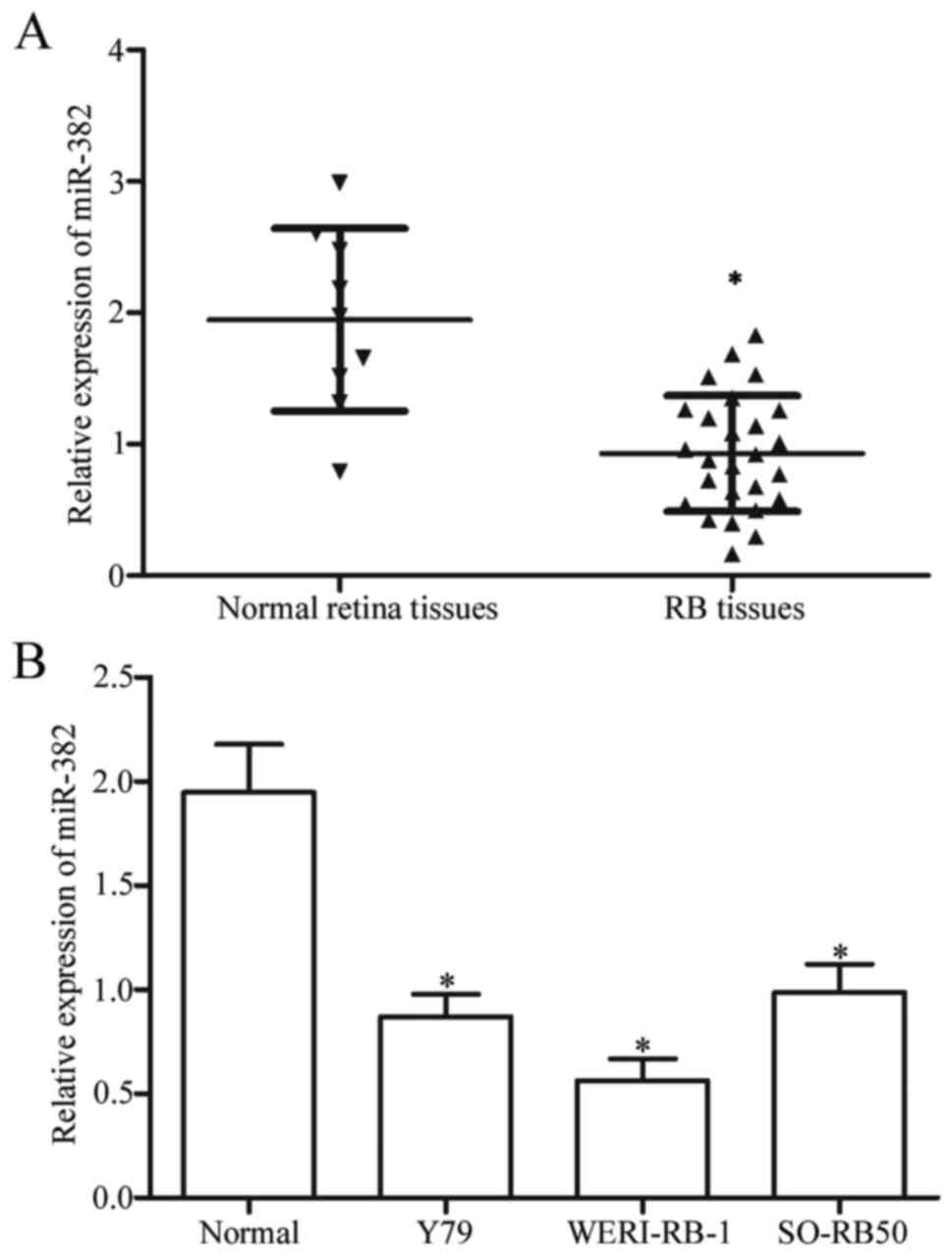
Firstly, we measured miR-382 expression in 26 RB
tissues and 9 normal retina tissues using RT-qPCR. The miR-382
expression levels were significantly downregulated in RB tissues
compared with that in normal retina tissues (Fig. 1A, P<0.05). Moreover, the miR-382
expression levels in three RB cell lines (Y79, WERI-RB-1 and
SO-RB50) were examined. As shown in Fig. 1B, miR-382 expression decreased in
RB cell lines compared with that in normal retina tissues
(P<0.05). These results suggested that miR-382 may play crucial
roles in RB occurrence and development.
miR-382 inhibits RB cell proliferation
and invasion in vitro
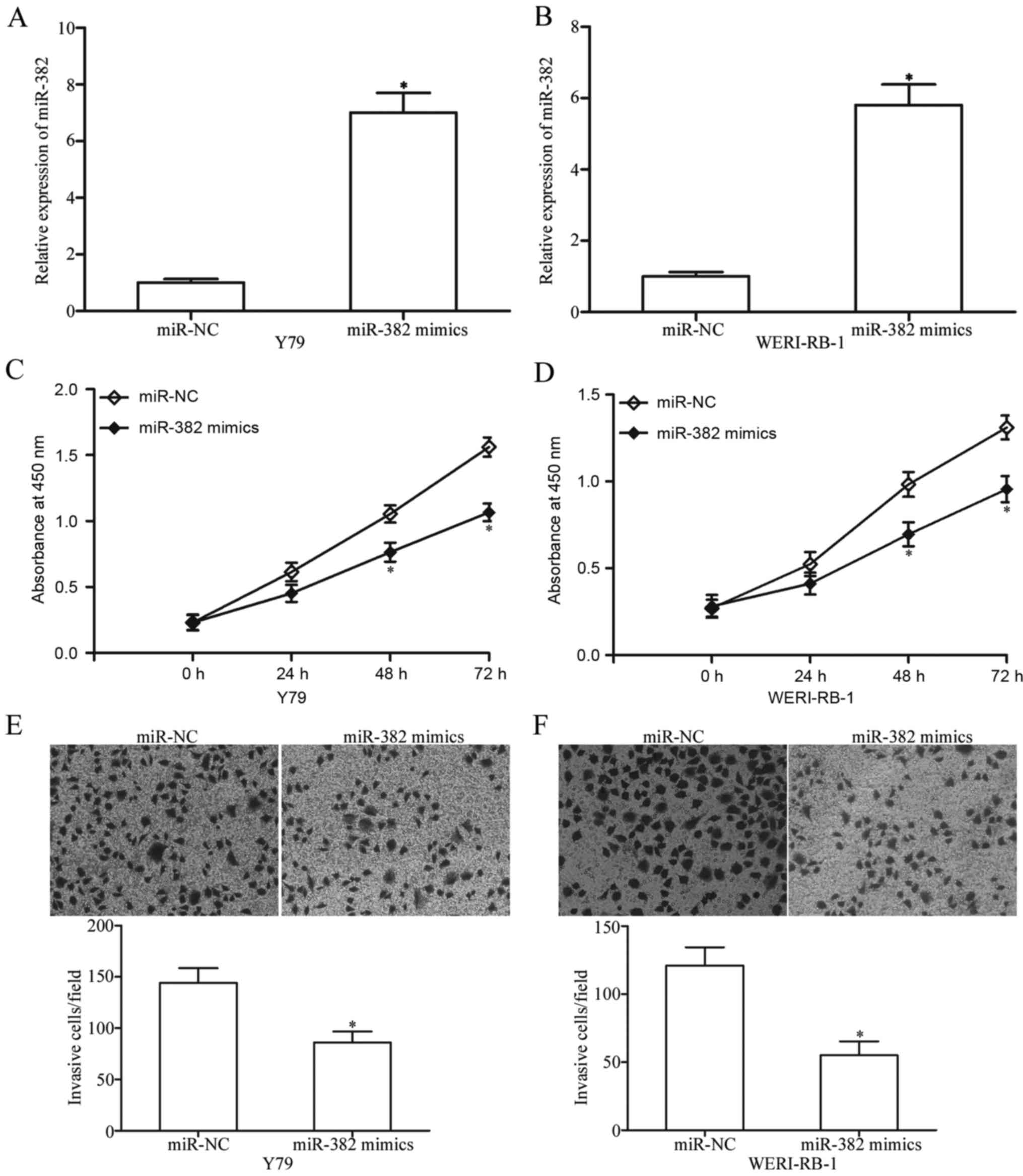
To determine the potential roles of miR-382 in RB,
Y79 and WERI-RB-1 cells were transfected with miR-382 mimics or
miR-NC. RT-qPCR confirmed that miR-382 was markedly upregulated in
Y79 and WERI-RB-1 cells after transfection with miR-382 mimics
(P<0.05; Fig. 2A and B). CCK-8
assay was performed to investigate the effect of miR-382 on RB cell
proliferation. The miR-382 upregulation significantly inhibited the
Y79 and WERI-RB-1 cell proliferation (P<0.05; Fig. 2C and D). We further performed
Matrigel invasion assay to determine the effect of miR-382 on RB
cell invasion. The invasive capacities of Y79 and WERI-RB-1 cells
were obviously suppressed in cells overexpressing miR-382 compared
with those in the miR-NC group (P<0.05; Fig. 2E and F). These results suggested
that miR-382 may play tumour-suppressing roles in RB.
BDNF is a direct target gene of
miR-382 in RB
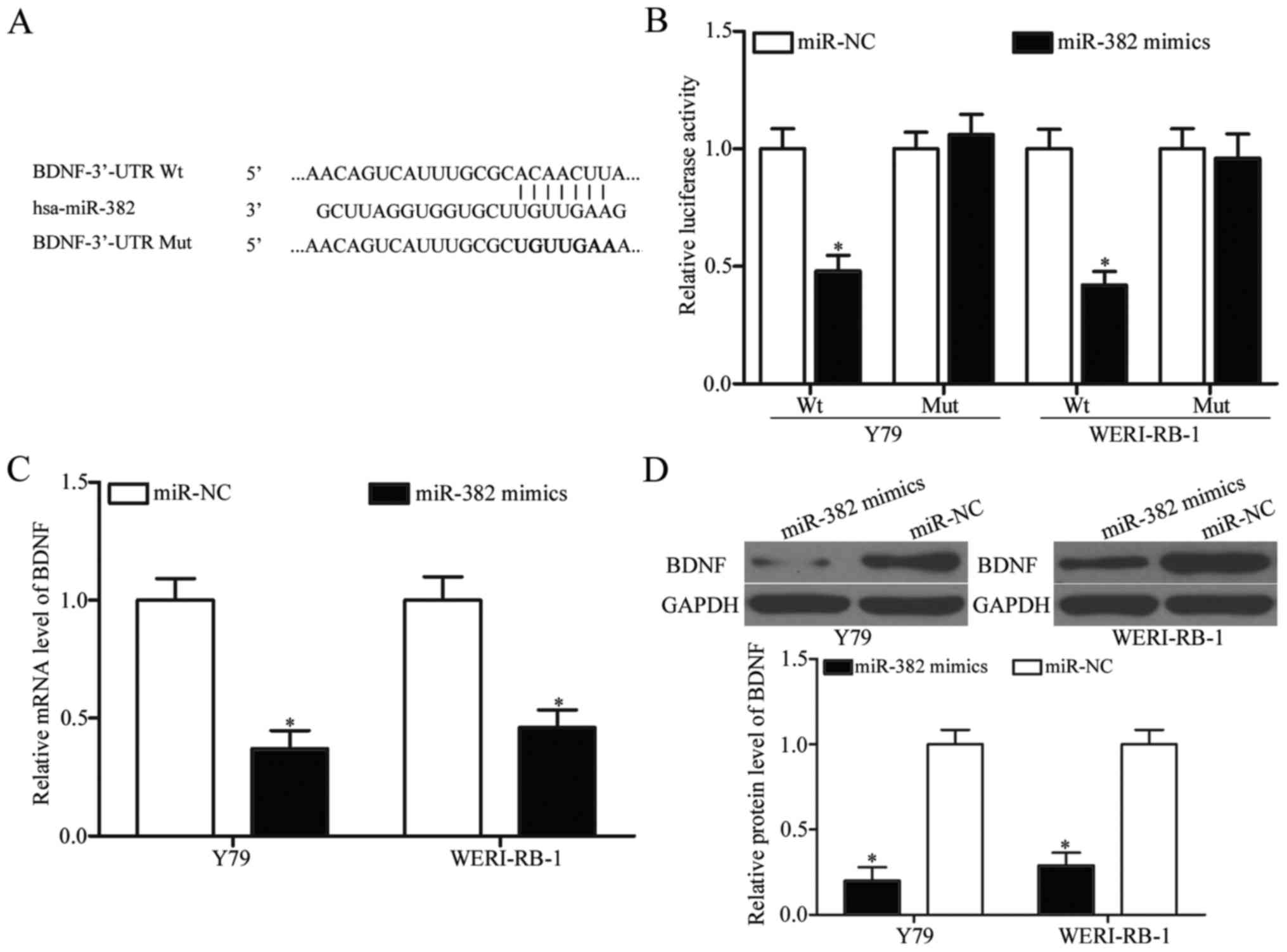
To elucidate the underlying molecular mechanisms of
miR-382 in RB, bioinformatics analysis was conducted to predict the
putative targets of miR-382. Among these potential targets, BDNF
(Fig. 3A) was selected for further
validation owing to its potential roles in RB malignancy regulation
(33,34). To confirm this prediction,
luciferase reporter assay was used to determine whether miR-382
could directly target the 3′-UTR of BDNF. The restoration
expression of miR-382 decreased the luciferase activity of reporter
plasmid that contained the wide-type BDNF 3′-UTR (P<0.05;
Fig. 3B). In contrast, no
significant inhibition was observed for the Mut reporter after
miR-382 mimic transfection. RT-qPCR and western blot analysis were
utilised to detect the BDNF expression in Y79 and WERI-RB-1 cells
transfected with miR-382 mimics or miR-NC. As shown in Fig. 3C and D, miR-382 overexpression
decreased BDNF expression at both the mRNA and protein levels in
Y79 and WERI-RB-1 cells (P<0.05). Taken together, these data
indicate that BDNF is a direct target of miR-382 in RB.
BDNF is upregulated and inversely
correlated with miR-382 expression in RB tissues
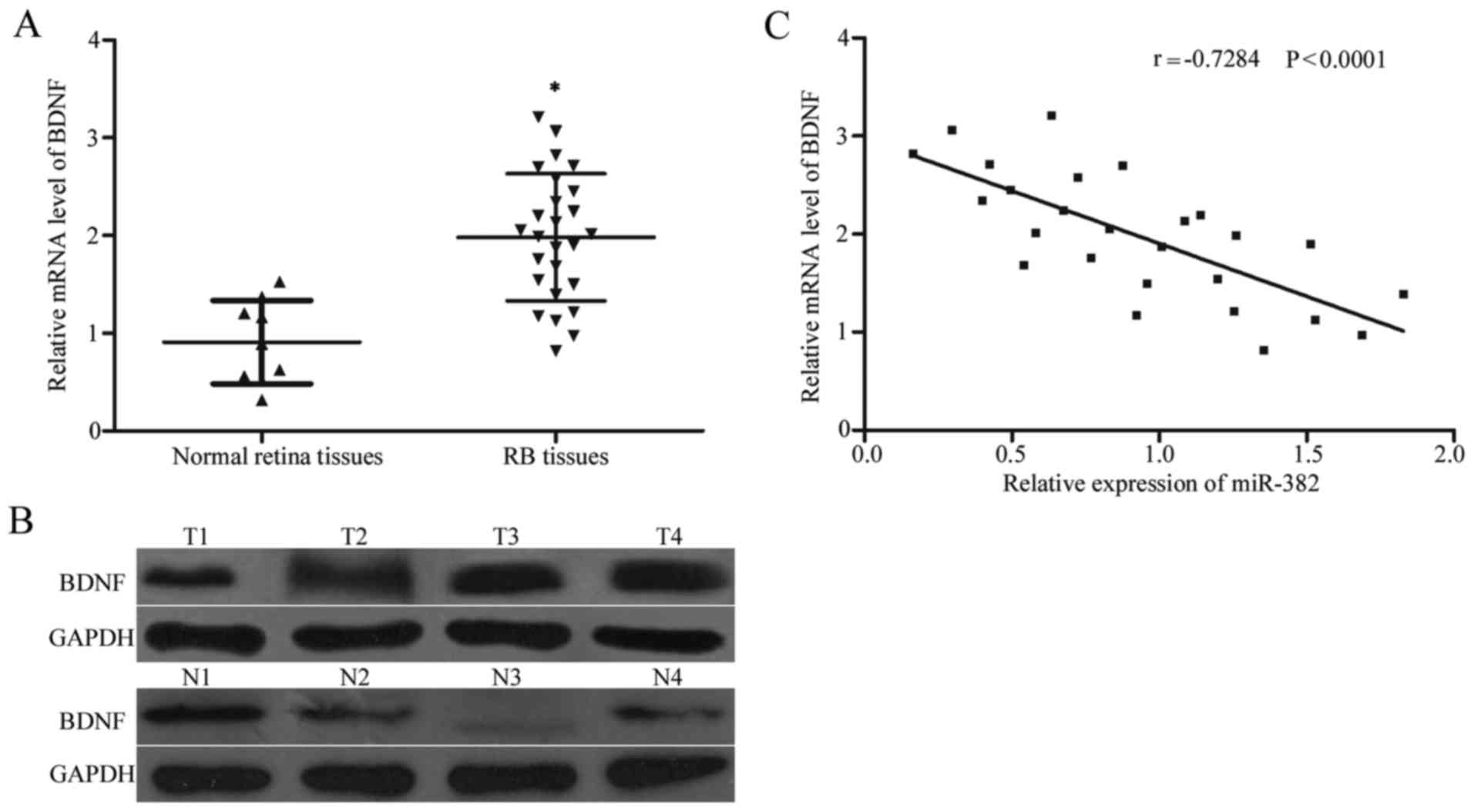
To further explore the relationship between miR-382
and BDNF, we detected the BDNF expression at the mRNA and protein
levels in 26 RB tissues and nine normal retina tissues using
RT-qPCR and western blot analysis, respectively. RB tissues had
increased BDNF expression at both the mRNA (P<0.05; Fig. 4A) and protein levels (P<0.05;
Fig. 4B) compared with the normal
retina tissues. Additionally, Spearman's correlation analysis
indicated a statistically significant inverse correlation between
miR-382 and BDNF mRNA level in RB tissues (r=−0.7284, P<0.001;
Fig. 4C).
BDNF reverses the tumour-suppressing
effects of miR-382 on RB cell proliferation and invasion
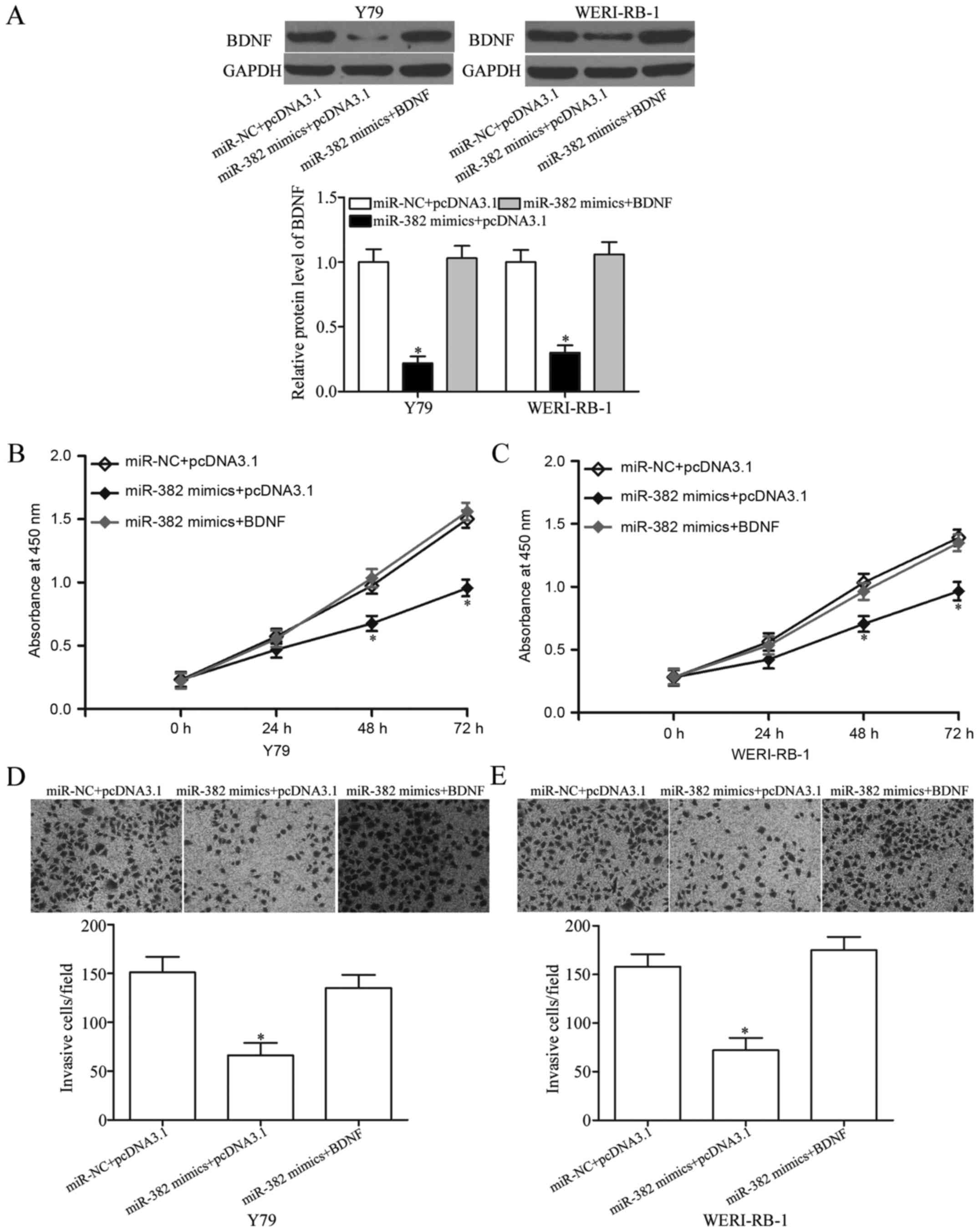
Rescue experiments were performed to evaluate
whether BDNF is responsible for the tumour-suppressing effects of
miR-382 in RB cells. Y79 and WERI-RB-1 cells were transfected with
miR-382 mimics or miR-NC and co-transfected with pcDNA3.1-BDNF or
pcDNA3.1. Western blot analysis revealed that the restoration
expression of BDNF abolished the inhibition caused by the miR-382
mimics in Y79 and WERI-RB-1 cells (P<0.05; Fig. 5A). Subsequently, CCK-8 and Matrigel
invasion assays were performed in the above mentioned cells.
Notably, the resumption expression of BDNF rescued the suppressive
effects of miR-382 on the proliferation (P<0.05; Fig. 5B and C) and invasion (P<0.05;
Fig. 5D and E) of Y79 and
WERI-RB-1 cells. These results suggested that miR-382 exerted its
tumour-suppressing roles in RB, at least in part by regulating
BDNF.
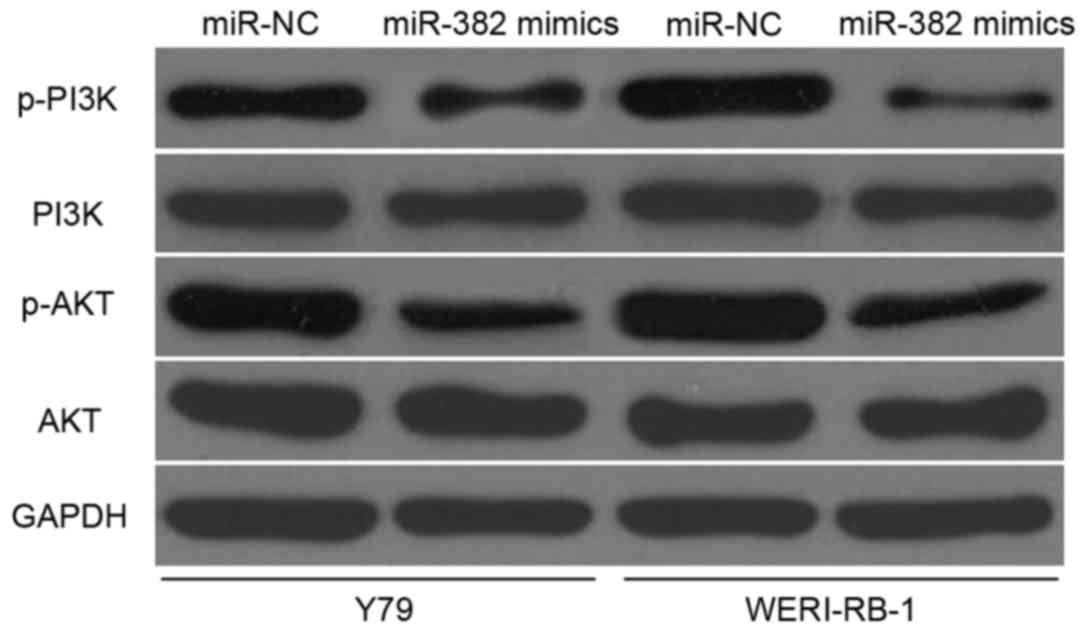
miR-382 inhibits PI3K/AKT signalling
in RB
BDNF is involved in PI3K/AKT signalling pathway
regulation (35,36). Therefore, we analysed the effects
of miR-382 on PI3K, p-PI3K, AKT and p-AKT protein expression in Y79
and WERI-RB-1 cells transfected with miR-382 mimics or miR-NC. The
miR-382 upregulation was found to reduce the p-PI3K and p-AKT
expression but did not affect the total PI3K and AKT expression in
Y79 and WERI-RB-1 cells (Fig. 6).
These results suggested that miR-382 exerts suppressive roles in RB
cells by directly regulating BDNF and indirectly regulating the
PI3K/AKT signalling pathway.
Discussion
miRNAs serve as post-transcriptional gene regulators
that potentially play critical roles in the proliferation, cycle,
differentiation, angiogenesis and metastasis of various kinds of
cancer cells (37–39). Multiple miRNAs are aberrantly
expressed in RB and contribute to RB initiation and progression
(22,40,41).
However, the expression and roles of miR-382 in RB remain unclear.
In this study, miR-382 was significantly downregulated in RB
tissues and cell lines. Furthermore, miR-382 upregulation
suppressed cell proliferation and invasion of RB in vitro.
BDNF was also demonstrated to be a direct target of miR-382 in RB.
Importantly, miR-382 inactivated PI3K/AKT signalling pathway in RB.
To the best of our knowledge, this is the first study to
investigate the expression and role of miR-382 in RB.
miR-382 was frequently found to be abnormally
expressed in multiple human cancers. For instance, miR-382 was
significantly downregulated in non-small cell lung cancer.
Additionally, low miR-382 expression level was correlated with the
tumour stage and metastasis of non-small cell lung cancer (29). The miR-382 expression level was
decreased in highly metastatic osteosarcoma cell lines and relapsed
osteosarcoma tissues. Low miR-382 expression level was inversely
associated with relapse and positively associated with
metastasis-free survival in osteosarcoma patients (42). The miR-382 expression was
relatively low in oesophageal squamous cell carcinoma for patients
with poor outcome. Kaplan-Meier analysis indicated a significant
reverse correlation between miR-382 expression and survival time of
oesophageal squamous cell carcinoma patients (43). The downregulation of miR-382 was
also observed in prostate (44),
ovarian (45) and colorectal
(30) cancers. However, miR-382
was highly expressed in tumour tissues and associated with
pathological grade and clinical stage in breast cancer (31). These findings indicated that
miR-382 expression has tissue specificity and may be a prognostic
target for cancers.
Aberrant miR-382 expression was reportedly
associated with various human cancers. Chen et al found that
ectopic miR-382 expression repressed cell proliferation, migration
and invasion in non-small cell lung cancer (29). Xu et al revealed that
miR-382 overexpression attenuated osteosarcoma cell growth,
metastasis, epithelial-mesenchymal transition and chemoresistance
(42,46). Zhang et al reported that
restoration expression of miR-382 impeded cell proliferation and
motility in prostate cancer (44).
Tan et al demonstrated that resumption expression of miR-382
decreased cell proliferation, metastasis and epithelial-mesenchymal
transition in ovarian cancer (45). Zhou et al revealed that
enforced miR-382 expression inhibited colorectal cancer cell
growth, migration and invasion in vitro (30). These studies indicate that miR-382
is a tumour suppressor in certain types of cancer. However, miR-382
serve as an oncogene in breast cancer by promoting cell viability,
clonogenicity, survival, migration and invasion in vitro and
tumourigenesis or metastasis in vivo (31). This contradiction may be explained
by the ‘imperfect complementarity’ of the interactions between
miRNAs and their target genes (47).
Several targets of miR-382 have been identified,
such as SETD8 (29) in lung
cancer, KLF12 (46), HIPK3
(46), YB-1 (42) in osteosarcoma, COUP-TFII (44) in prostate cancer, ROR1 (45) in ovarian cancer, NR2F2 in
colorectal cancer (30), and RERG
(31) in breast cancer. In this
study, BDNF was demonstrated to be a direct downstream target gene
of miR-382 in RB. To explore the mechanism underlying the
tumor-suppressing roles of miR-382 in RB, bioinformatics analysis
was performed to predict the candidate targets of miR-382. BDNF was
predicted as a potential target of miR-382. Luciferase reporter
assay was conducted to confirm this prediction, and found that
miR-382 could directly targeted the 3′-UTR of BDNF. Additionally,
RT-qPCR and western blot analysis demonstrated that miR-382
negatively regulated endogenous BDNF expression at both mRNA and
protein level in RB cells. Furthermore, BDNF was upregulated in RB
tissues and inverse correlated with miR-382 expression level.
Besides, rescue experiments indicated that BDNF overexpression
reversed the suppressive effects of miR-382 on RB cells. Taken
together, it is reasonable to suggest that alterations in miR-382
expression regulates the proliferation and invasion of RB cells via
directly targeting BDNF.
BDNF, a member of the neurotrophin family, plays an
essential role in neural maturation and differentiation (48). BDNF was upregulated in several
human malignancies, such as bladder cancer (49), glioma, gastric cancer (50), colorectal cancer (51) and breast cancer (52). Moreover, aberrant BDNF expression
was involved in tumourigenesis and tumour development. In
colorectal cancer, BDNF knockdown inhibited cell proliferation,
migration and invasion and enhanced apoptosis (51,53).
In lung cancer, BDNF downregulation suppressed cell proliferation
and invasion and increased apoptosis (54). In RB, BDNF was upregulated in
tumour tissues and cell lines (33) and contribute in RB occurrence and
progression regulation (34). All
these findings suggested that BDNF is worthy of investigation as a
potential target for the treatment of patients with RB.
In conclusion, miR-382 was significantly
downregulated in RB tissues and cells. Additionally, we
demonstrated that miR-382 acts as a tumour suppressor in RB through
direct targeting of BDNF and indirect regulation of the PI3K/AKT
signalling pathway. These results contribute evidence that miR-382
plays an important role in tumour malignant progress, and miR-382
may represent a potential gene-targeting approach for RB
treatments.
References
|
1
|
Abramson DH, Shields CL, Munier FL and
Chantada GL: Treatment of retinoblastoma in 2015: Agreement and
disagreement. JAMA Ophthalmol. 133:1341–1347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kleinerman RA, Schonfeld SJ and Tucker MA:
Sarcomas in hereditary retinoblastoma. Clin Sarcoma Res. 2:152012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marees T, Moll AC, Imhof SM, de Boer MR,
Ringens PJ and van Leeuwen FE: Risk of second malignancies in
survivors of retinoblastoma: More than 40 years of follow-up. J
Natl Cancer Inst. 100:1771–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moll AC, Imhof SM, Bouter LM and Tan KE:
Second primary tumors in patients with retinoblastoma. A review of
the literature. Ophthalmic Genet. 18:27–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benavente CA and Dyer MA: Genetics and
epigenetics of human retinoblastoma. Annu Rev Pathol. 10:547–562.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YQ, Li J and Yuan HF: Epidemiology
and risk factors of retinoblastoma in Chongqing area. Int J
Ophthalmol. 9:984–988. 2016.PubMed/NCBI
|
|
8
|
Finger PT, Harbour JW and Karcioglu ZA:
Risk factors for metastasis in retinoblastoma. Surv Ophthalmol.
47:1–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedman DL, Himelstein B, Shields CL,
Shields JA, Needle M, Miller D, Bunin GR and Meadows AT:
Chemoreduction and local ophthalmic therapy for intraocular
retinoblastoma. J Clin Oncol. 18:12–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaliki S, Shields CL, Rojanaporn D,
Al-Dahmash S, McLaughlin JP, Shields JA and Eagle RC Jr: High-risk
retinoblastoma based on international classification of
retinoblastoma: Analysis of 519 enucleated eyes. Ophthalmology.
120:997–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kozomara A and Griffiths-Jones S: miRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39(Database Issue): D152–D157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
16
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao HY, Huo FC, Wang HY and Pei DS:
MicroRNA-9 inhibits the gastric cancer cell proliferation by
targeting TNFAIP8. Cell Prolif. 50:2017. View Article : Google Scholar
|
|
19
|
Li C, Lu S and Shi Y: MicroRNA-187
promotes growth and metastasis of gastric cancer by inhibiting
FOXA2. Oncol Rep. 37:1747–1755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng Y, Zhu J, Shen D, Qin H, Lei Z, Li W,
Liu Z and Huang JA: MicroRNA-205 targets SMAD4 in non-small cell
lung cancer and promotes lung cancer cell growth in vitro and in
vivo. Oncotarget. 8:30817–30829. 2017.PubMed/NCBI
|
|
21
|
Dong F, Xu T, Shen Y, Zhong S, Chen S,
Ding Q and Shen Z: Dysregulation of miRNAs in bladder cancer:
Altered expression with aberrant biogenesis procedure. Oncotarget.
8:27547–27568. 2017.PubMed/NCBI
|
|
22
|
Wang LL, Hu HF and Feng YQ: Suppressive
effect of microRNA-143 in retinoblastoma. Int J Ophthalmol.
9:1584–1590. 2016.PubMed/NCBI
|
|
23
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer. 96
Suppl:R40–R44. 2007.PubMed/NCBI
|
|
24
|
Han RL, Wang FP, Zhang PA, Zhou XY and Li
Y: miR-383 inhibits ovarian cancer cell proliferation, invasion and
aerobic glycolysis by targeting LDHA. Neoplasma. 64:244–252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li B, Xie Z, Li Z, Chen S and Li B:
MicroRNA-613 targets FMNL2 and suppresses progression of colorectal
cancer. Am J Transl Res. 8:5475–5484. 2016.PubMed/NCBI
|
|
26
|
Sun Z, Zhang A, Jiang T, Du Z, Che C and
Wang F: MiR-145 suppressed human retinoblastoma cell proliferation
and invasion by targeting ADAM19. Int J Clin Exp Pathol.
8:14521–14527. 2015.PubMed/NCBI
|
|
27
|
Montoya V, Fan H, Bryar PJ, Weinstein JL,
Mets MB, Feng G, Martin J, Martin A, Jiang H and Laurie NA: Novel
miRNA-31 and miRNA-200a-mediated regulation of retinoblastoma
proliferation. PLoS One. 10:e01383662015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen F, Mo MH, Chen L, An S, Tan X, Fu Y,
Rezaei K, Wang Z, Zhang L and Fu SW: MicroRNA-21 down-regulates Rb1
expression by targeting PDCD4 in retinoblastoma. J Cancer.
5:804–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen T, Ren H, Thakur A, Yang T, Li Y,
Zhang S, Wang T and Chen M: miR-382 inhibits tumor progression by
targeting SETD8 in non-small cell lung cancer. Biomed Pharmacother.
86:248–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou B, Song J, Han T, Huang M, Jiang H,
Qiao H, Shi J and Wang Y: MiR-382 inhibits cell growth and invasion
by targeting NR2F2 in colorectal cancer. Mol Carcinog.
55:2260–2267. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho JY, Hsu RJ, Liu JM, Chen SC, Liao GS,
Gao HW and Yu CP: MicroRNA-382-5p aggravates breast cancer
progression by regulating the RERG/Ras/ERK signaling axis.
Oncotarget. 8:22443–22459. 2017.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stephan H, Zakrzewski JL, Bölöni R,
Grasemann C, Lohmann DR and Eggert A: Neurotrophin receptor
expression in human primary retinoblastomas and retinoblastoma cell
lines. Pediatr Blood Cancer. 50:218–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shang W, Yang Y, Zhang J and Wu Q: Long
noncoding RNA BDNF-AS is a potential biomarker and regulates cancer
development in human retinoblastoma. Biochem Biophys Res Commun.
Jan 26–2017.(Epub ahead of print). View Article : Google Scholar
|
|
35
|
Mao XY, Zhou HH, Li X and Liu ZQ:
Huperzine A alleviates oxidative glutamate toxicity in hippocampal
HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR
signaling pathway. Cell Mol Neurobiol. 36:915–925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia H, Li Y and Lv X: MicroRNA-107
inhibits tumor growth and metastasis by targeting the BDNF-mediated
PI3K/AKT pathway in human non-small lung cancer. Int J Oncol.
49:1325–1333. 2016.PubMed/NCBI
|
|
37
|
Huang Q, Liu L, Liu CH, You H, Shao F, Xie
F, Lin XS, Hu SY and Zhang CH: MicroRNA-21 regulates the invasion
and metastasis in cholangiocarcinoma and may be a potential
biomarker for cancer prognosis. Asian Pac J Cancer Prev.
14:829–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu SH, Zhang CL, Dong FS and Zhang YM:
miR-99a suppresses the metastasis of human non-small cell lung
cancer cells by targeting AKT1 signaling pathway. J Cell Biochem.
116:268–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Funamizu N, Lacy CR, Parpart ST, Takai A,
Hiyoshi Y and Yanaga K: MicroRNA-301b promotes cell invasiveness
through targeting TP63 in pancreatic carcinoma cells. Int J Oncol.
44:725–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu X, Zeng Y, Wu S, Zhong J, Wang Y and Xu
J: MiR-204, down-regulated in retinoblastoma, regulates
proliferation and invasion of human retinoblastoma cells by
targeting CyclinD2 and MMP-9. FEBS Lett. 589:645–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Wang X, Wu G, Hou D and Hu Q:
MiR-365b-3p, down-regulated in retinoblastoma, regulates cell cycle
progression and apoptosis of human retinoblastoma cells by
targeting PAX6. FEBS Lett. 587:1779–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu M, Jin H, Xu CX, Sun B, Song ZG, Bi WZ
and Wang Y: miR-382 inhibits osteosarcoma metastasis and relapse by
targeting Y box-binding protein 1. Mol Ther. 23:89–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qi B, Lu JG, Yao WJ, Chang TM, Qin XG, Ji
YH, Wang TY, Liu SG, Li HC, Liu YZ and Zhao BS: Downregulation of
microRNA-382 is associated with poor outcome of esophageal squamous
cell carcinoma. World J Gastroenterol. 21:6884–6891. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang W, Liu J, Qiu J, Fu X, Tang Q, Yang
F, Zhao Z and Wang H: MicroRNA-382 inhibits prostate cancer cell
proliferation and metastasis through targeting COUP-TFII. Oncol
Rep. 36:3707–3715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tan H, He Q, Gong G, Wang Y, Li J, Wang J,
Zhu D and Wu X: miR-382 inhibits migration and invasion by
targeting ROR1 through regulating EMT in ovarian cancer. Int J
Oncol. 48:181–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu M, Jin H, Xu CX, Sun B, Mao Z, Bi WZ
and Wang Y: miR-382 inhibits tumor growth and enhance
chemosensitivity in osteosarcoma. Oncotarget. 5:9472–9483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu Z, Ni L, Chen D, Zhang Q, Su Z, Wang Y,
Yu W, Wu X, Ye J, Yang S, et al: Identification of miR-7 as an
oncogene in renal cell carcinoma. J Mol Histol. 44:669–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McAllister AK: Neurotrophins and neuronal
differentiation in the central nervous system. Cell Mol Life Sci.
58:1054–1060. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lai PC, Chiu TH and Huang YT:
Overexpression of BDNF and TrkB in human bladder cancer specimens.
Oncol Rep. 24:1265–1270. 2010.PubMed/NCBI
|
|
50
|
Choi B, Lee EJ, Shin MK, Park YS, Ryu MH,
Kim SM, Kim EY, Lee HK and Chang EJ: Upregulation of brain-derived
neurotrophic factor in advanced gastric cancer contributes to bone
metastatic osteolysis by inducing long pentraxin 3. Oncotarget.
7:55506–55517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang X, Martin TA and Jiang WG: Biological
influence of brain-derived neurotrophic factor (BDNF) on colon
cancer cells. Exp Ther Med. 6:1475–1481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang X, Martin TA and Jiang WG: Biological
influence of brain-derived neurotrophic factor on breast cancer
cells. Int J Oncol. 41:1541–1546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang SM, Lin C, Lin HY, Chiu CM, Fang CW,
Liao KF, Chen DR and Yeh WL: Brain-derived neurotrophic factor
regulates cell motility in human colon cancer. Endocr Relat Cancer.
22:455–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang SY, Hui LP, Li CY, Gao J, Cui ZS and
Qiu XS: More expression of BDNF associates with lung squamous cell
carcinoma and is critical to the proliferation and invasion of lung
cancer cells. BMC Cancer. 16:1712016. View Article : Google Scholar : PubMed/NCBI
|