Introduction
Vascular inflammation is an important process
leading to endothelium disorders and atherosclerosis. The
activation of the endothelium at inflammation sites allows
leukocytes to adhere to the vascular endothelial layer. This then
results in increased leukocyte infiltration into the vessel wall
and leukocyte differentiation into macrophages, a process that is
an important early event of endothelial dysfunction and tissue
injury (1). The production of
cytokines is another early event of atherosclerosis, due
predominantly to the large number of macrophages resident in
atherosclerotic plaques (2).
Tumor necrosis factor (TNF)-α, an endothelial
cell-derived cytokine, exists in atherosclerotic lesions and can
increase the expression of cell adhesion molecules, including
vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion
molecule-1 (ICAM-1), and endothelial-selectin (E-selectin), which
then contribute to the inflammatory process (3,4).
Interleukin (IL)-8 is found in human atheroma, and mice lacking its
receptors are less sensitive to atherosclerosis and have fewer
monocytes in vascular lesions. In addition, IL-8 serves a critical
role in the firm adhesion of monocytes to vascular endothelium,
revealing an unexpected role for this chemokine in monocyte
recruitment (5). Nuclear factor
(NF)-κB is a major transcription factor in vascular inflammation.
TNF-α is tightly regulated by transcriptional activation of NF-κB
(6) and NF-κB serves a key role in
the inflammatory process by increasing the expression of many
inflammatory mediators, mediated by degradation of its inhibitor of
κB (IκB) (7). In addition, NF-κB
regulates transcriptional activation of important pro-inflammatory
and cell adhesion molecules (8).
The mechanisms involved in NF-κB activation include the increase of
intracellular levels of reactive oxygen species (ROS), which
results from the increase in pro-inflammatory cytokines (9,10).
Therefore, regulation of the ROS/NF-κB pathway, as a critical key
mediator of inflammatory responses, may be a useful strategy
towards the treatment of vascular diseases, such as atherosclerosis
(11).
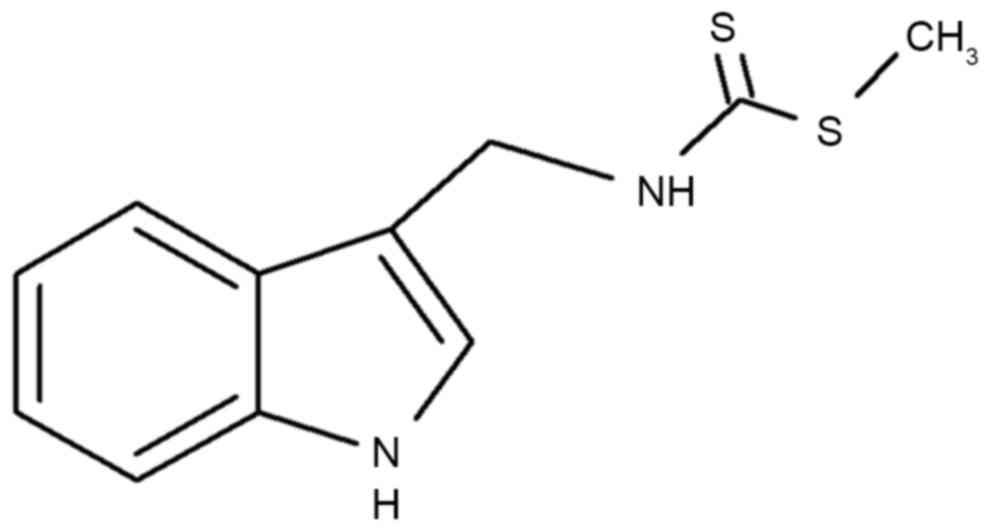
Brassinin [3-(S-methyldithiocarbamoyl) aminomethyl
indole; chemical structure illustrated in Fig. 1], is a type of indole compound
derived from cruciferous vegetables. Brassinin has demonstrated
anticancer effects in cells and animals (12). It has been reported that brassinin
can affect the effectiveness of chemotherapy against established
tumors in an autochthonous mouse model of breast cancer (13). Brassinin has also been reported to
have antiproliferative effects in cancer cells by inhibiting the
production of oxidative stress and ROS (14). However, no report exists to date
regarding the role of brassinin in vascular endothelial cells. The
present study was designed to investigate the effect of brassinin
on TNF-α-induced vascular inflammation in human umbilical vein
endothelial cells (HUVECs).
Materials and methods
Chemicals and materials
TNF-α, EDTA, antibiotic-antimycotic solutions and
fetal bovine serum (FBS) were purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Dimethyl sulfoxide
(DMSO) was purchased from Amresco, LLC (Solon, OH, USA). Brassinin,
and primary antibodies targeting VCAM-1 (sc-13160), ICAM-1
(sc-8439), E-selectin (sc-14011), IκB-α (sc-203), p-IκB-α
(sc-8404), NF-κB (sc-8008) and β-actin (sc-4778) were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and diluted
at 1/1,000 with TBS. Horseradish peroxidase (HRP)-conjugated
secondary antibodies [goat anti-mouse-IgG (A90-1169), donkey
anti-goat-IgG (A50-1019) and goat anti-rabbit-IgG (ADI-SAB-300-J)]
were obtained from Bethyl Laboratories, Inc. (Montgomery, TX, USA)
and Enzo Life Sciences, Inc. (Farmingdale, NY, USA) and diluted at
1/5,000. N-acetylcysteine (NAC) was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). All other reagents used in the
present study were of the highest purity commercially
available.
Cell culture
HUVECs and U937 monocyte cells were obtained from
the American Type Culture Collection (Manassas, VA, USA). Cells
were maintained at 5×105 cells/ml in RPMI-1640 medium D
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated FBS and 100 U/ml penicillin G and incubated at
37°C in a humidified atmosphere containing 5% CO2 and
95% air.
Monocyte-HUVEC adhesion assay
HUVECs were cultured to confluence in 6-well culture
plates, pretreated with brassinin for 30 min, and then stimulated
with TNF-α for 6 h. U937 cells were labeled with 10 µM BCECF-AM
(Sigma-Aldrich; Merck KGaA) for 1 h at 37°C and washed twice with
growth medium. Labeled U937 cells (2.5×105) were seeded
onto the HUVECs, and further incubated for 1 h. The non-adherent
U937 cells were removed from the plate by washing with PBS, and the
U937 cells bound to HUVECs were measured by fluorescence
microscopy, and then lysed with 50 mM Tris-HCI pH 8.0, containing
0.1% SDS. Fluorescence intensity was measured using a
spectrofluorometer (Infinite F200 Pro; Tecan Group Ltd., Mannedorf,
Switzerland) at excitation and emission wavelengths of 485 and 535
nm, respectively. The adhesion data were represented in terms of
percent change compared with the control group.
Western blot analysis
HUVECs were washed twice with ice-cold PBS and lysed
with buffer (radioimmunoprecipitation assay lysis buffer, WSE-7420,
ATTO Corporation, Tokyo, Japan). Protein content was determined
using the Bradford method. Cell homogenates (40 µg total protein)
were separated on 10% SDS-polyacrylamide gel electrophoresis and
transferred to nitrocellulose membranes. Blots were then washed
with TBST [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20],
blocked with 5% BSA in TBS for 1 h in room temperature and
incubated with the appropriate primary antibody in 4°C for ~8 h.
Then the membrane was washed, and primary antibodies were detected
with goat anti-rabbit-immunoglobulin (Ig) G or anti-mouse-IgG
conjugated to HRP for 2 h at room temperature, and the bands were
visualized with enhanced chemiluminescence (Atto Corporation,
Tokyo, Japan). Protein expression levels were determined by
analyzing the signals on a ChemiDoc imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). For quantitative analysis,
the average score of each band was calculated using ImageJ
(National Institutes of Health, Bethesda, MD, USA) (15).
Cell ELISA
ELISA was used to determine the levels of ICAM-1,
VCAM-1 and E-selectin expression on the cell surface, as previously
described with minor modifications. HUVECs were seeded on 96-well
plates at a concentration of 1×105 cells/well. Briefly,
HUVECs were pretreated with or without brassinin for 30 min, which
was followed by TNF-α treatment for 6 h at 37°C. Following
treatments, the cells were fixed with 1% paraformaldehyde and
exposed to mouse anti-human ICAM-1, VCAM-1 or E-selectin antibody
at 1:1,000 dilution in PBS containing 1% skim milk for 2 h at room
temperature. The cells were washed and incubated with a
HRP-conjugated secondary antibody. The expression of ICAM-1,
VCAM-1, or E-selectin was quantified by adding a peroxidase
substrate solution and measuring the absorbance of each well at 490
nm using a microplate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total mRNA was isolated from cultured HUVECs using a
commercially available kit (RNeasy Mini kit; Qiagen, Inc.,
Valencia, CA, USA). In the first step, cDNA was prepared from 500
ng mRNA by reverse transcription in a final volume of 20 µl in cDNA
synthesis kit (M-MIV cDNA Synthesis kit; EZ006S; Enzynomics, Co.,
Ltd., Daejeon, Korea). The reactions were incubated at 37°C for 60
min, 94°C for 5 min, and then cDNA was used or immediately stored
at −20°C. Template cDNA and 50 nM primers were added in PCR premix
(Rb Taq Fast qPCR 2X Premix; RT540S; Enzynomics, Co., Ltd.)
according to the manufacturer's protocol. Primer sequences were as
follows: IL-8 forward, 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ and reverse,
5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′); and GAPDH forward,
5′-ACCACAGTTCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
The thermocycling conditions were as follows: an initial step at
94°C for 15 min, followed by 45 cycles of 94°C for 20 sec, 60°C for
20 sec and 72°C for 30 sec, and a final extension step of 72°C for
5 min. The PCR products were subjected to 1% agarose gel
electrophoresis, and then detected on a ChemiDoc imaging system
(Bio-Rad Laboratories, Inc.).
Preparation of cytoplasmic and nuclear
extracts
Nuclear and cytoplasmic extracts were prepared on
ice, as previously described by Mackman et al (16). Cells were harvested and washed with
1 ml buffer A (10 mM HEPES pH 7.9, 1.5 mM MgCl2, and 19
mM KCl) for 5 min at 600 × g. The cells were resuspended in buffer
A and 0.1% NP40, left for 10 min on ice to lyse and then
centrifuged at 600 × g for 3 min. The supernatant was saved as
cytosolic extract. The nuclear pellet was then washed in 1 ml
buffer A at 4,200 × g for 3 min, re-suspended in 30 µl buffer C (20
mM HEPES pH 7.9, 25% glycerol, 0.42 M NaCl, 1.5 mM
MgCl2, and 0.2 mM EDTA), rotated for 30 min at 4°C, then
centrifuged at 14,300 × g for 20 min. The supernatant was used as
nuclear extract.
Intracellular ROS production
assay
The fluorescent probe
chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
(CM-H2DCFDA; Thermo Fisher Scientific, Inc.) was used to
determine the intracellular generation of ROS. Briefly, confluent
HUVECs in 60 mm2 dish culture plates were pretreated
with brassinin for 30 min, then stimulated with TNF-α for 6 h.
Cells were then incubated with 20 µM CM-H2DCFDA for 1 h.
The resulting fluorescence intensity was measured using a
spectrofluorometer (Infinite F200 Pro; Tecan Group Ltd.) at
excitation and emission wavelengths of 485 and 535 nm,
respectively.
Statistical analysis
Results were expressed as the mean ± standard error
of the mean of at least three independent experiments. Significance
was analyzed with one-way analysis of variance followed by a
Dunnett's test, using SigmaPlot version 10.0 (Systat Software,
Inc., San Jose, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of brassinin on TNF-α-induced
adhesion of U937 cells to HUVECs
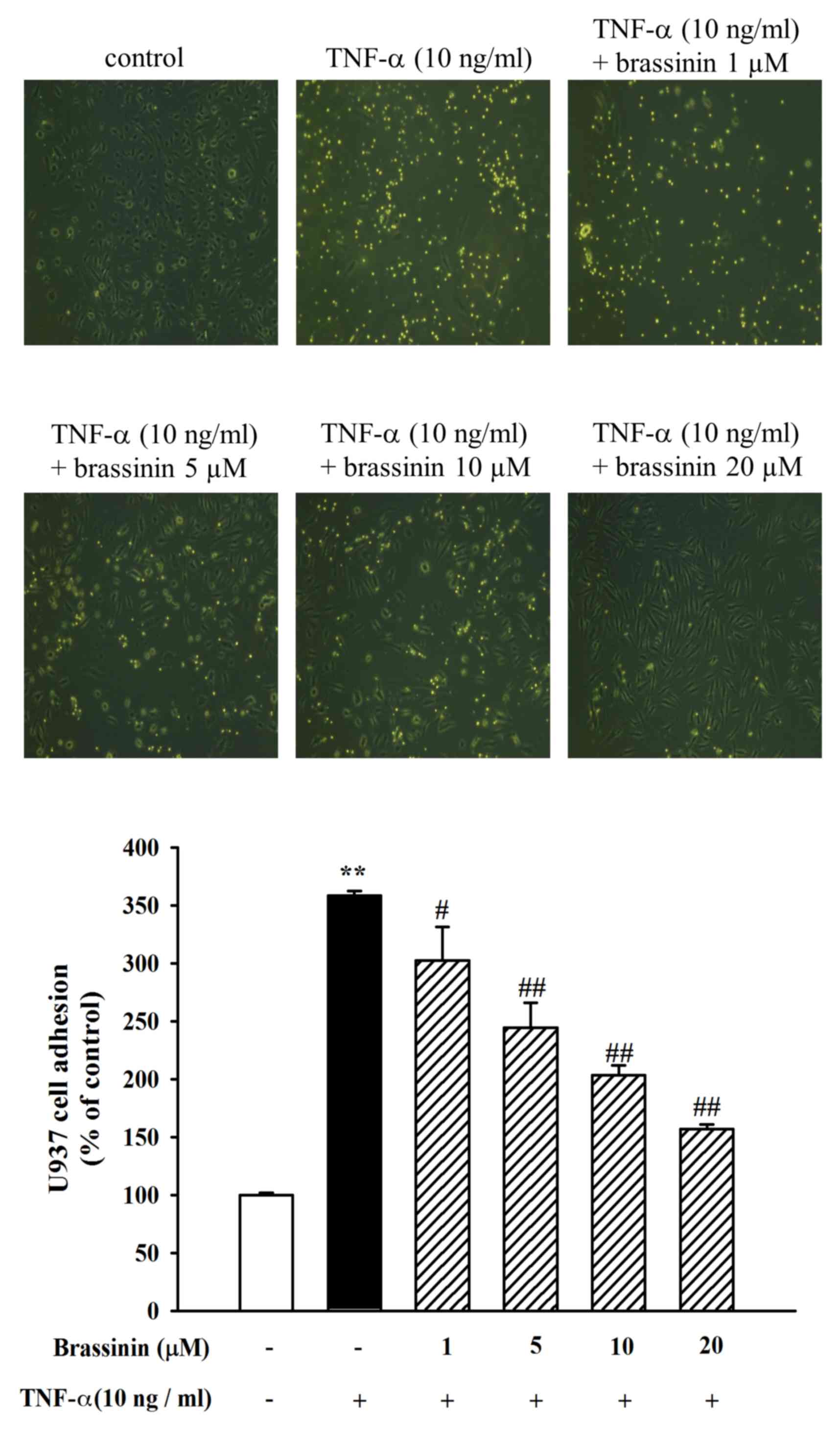
To confirm the inhibitory effect of brassinin on the
HUVEC-monocyte interaction, a cell adhesion assay was performed
between U937 cells and TNF-α-stimulated HUVECs. HUVECs were
pretreated with different concentrations of brassinin (1–20 µM) and
then stimulated with TNF-α (10 ng/ml) for 6 h. U937 cells were
allowed to adhere to the treated HUVECs and then quantified. As
illustrated in Fig. 2, control
HUVECs exhibited minimal binding to U937 cells without TNF-α
stimulation. However, adhesion of U937 cells to the HUVECs was
dramatically increased following TNF-α stimulation (Fig. 2). By contrast, pretreatment with
brassinin inhibited the adhesion of U937 cells to TNF-α-induced
HUVECs (Fig. 2). Thus, brassinin
may be effective towards preventing the early process of monocyte
adhesion to the vascular endothelium in vascular inflammation.
Effect of brassinin on
inflammation-related factors in TNF-α-induced HUVECs
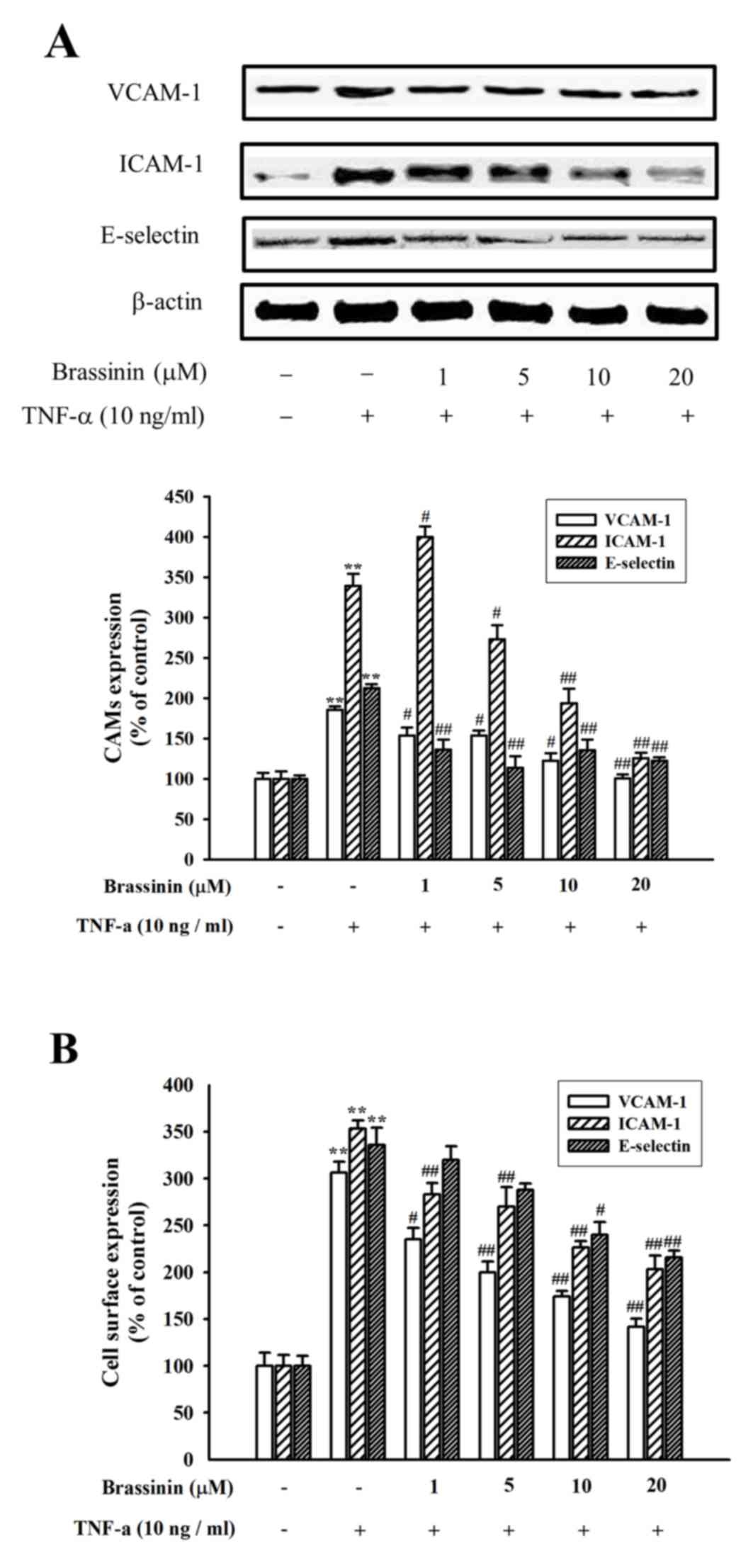
Western blot analysis was performed to confirm the
effect of brassinin on the expression of cell adhesion molecules in
HUVECs. Exposure to TNF-α for 6 h resulted in a significantly
pronounced expression of VCAM-1, ICAM-1 and E-selectin in HUVECs,
compared with control untreated cells (Fig. 3A). Pretreatment with brassinin,
however, significantly inhibited the TNF-α-induced VCAM-1, ICAM-1
and E-selectin protein expression levels (Fig. 3A). Expression of the adhesion
molecules in the cell surface of the HUVECs was also examined by
ELISA. Consistent with the results from the western blotting,
expression of VCAM-1, ICAM-1 and E-selectin on the cell surface of
HUVECs was significantly increased by TNF-α stimulation (Fig. 3B). However, TNF-α-induced cell
surface protein expression was suppressed by brassinin pretreatment
in a dose-dependent manner (Fig.
3B).
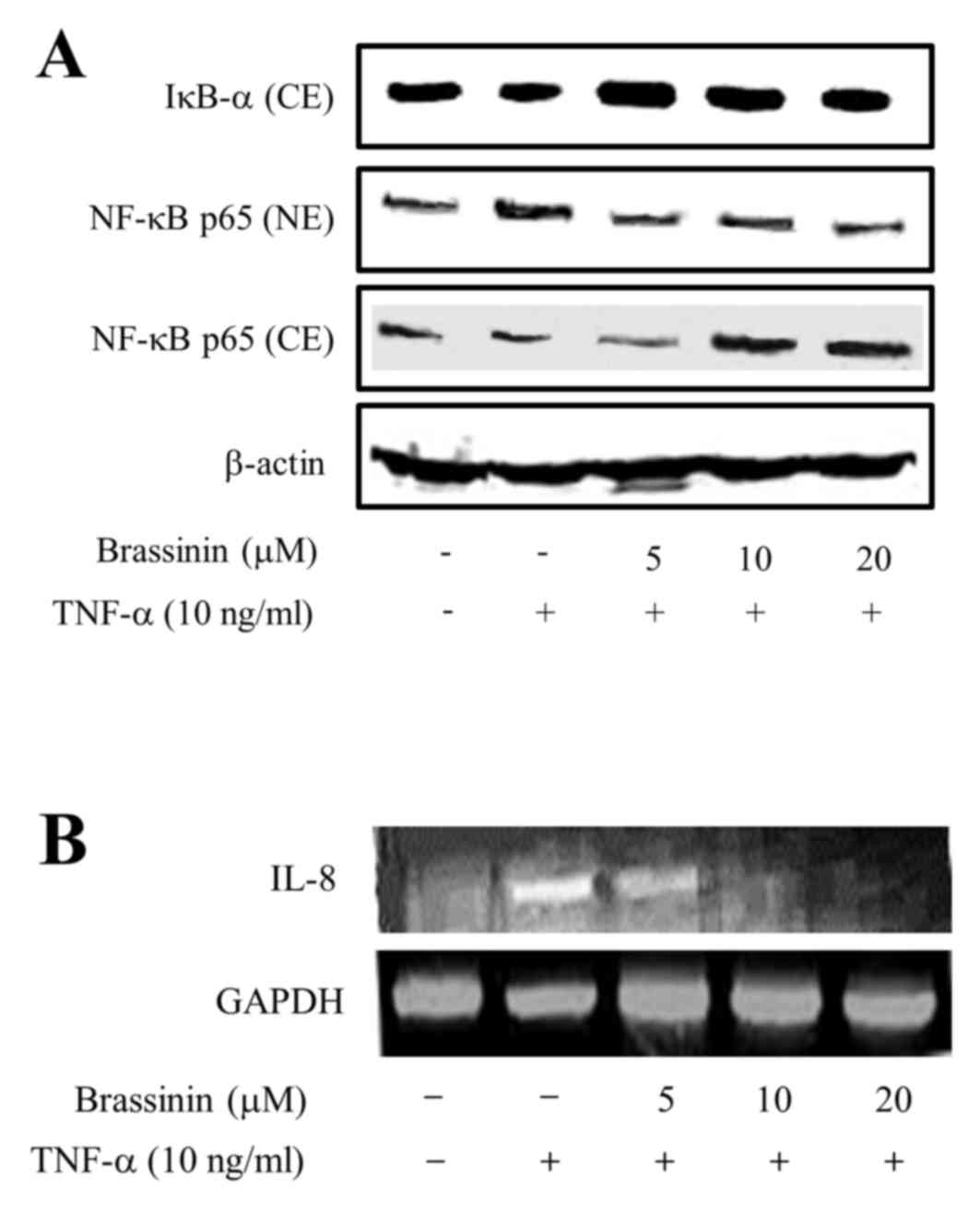
Effect of brassinin on TNF-α-induced
translocation and expression of NF-κB p65
NF-κB is activated upon cytokine stimulation via
phosphorylation and degradation of its inhibitor, IκB-α. Activation
of NF-κB requires translocation from the cytoplasm to the nucleus,
and this translocation results in transcription initiation for
genes associated with cellular growth (8). Therefore, the effect of brassinin on
NF-κB activation and translocation was examined. By western blot
analysis, IκB-α expression levels in the cytoplasm were increased
following pretreatment with brassinin of TNF-α-induced HUVECs
(Fig. 4A). In addition, western
blot analysis revealed that the levels of NF-κB p65 in the
cytoplasm decreased, while its levels in the nucleus increased
following pretreatment with brassinin, compared with cells treated
with TNF-α alone (Fig. 4A). To
confirm whether brassinin has an inhibitory effect on chemokines
such as IL-8, that is associated with human atheroma, RT-PCR
analysis was performed in the presence or absence of TNF-α and/or
brassinin. As illustrated in Fig.
4B, TNF-α stimulation obviously increased the mRNA expression
levels of IL-8 compared with untreated cells, whereas pretreatment
with brassinin markedly abrogated this TNF-α-induced IL-8 mRNA
expression in a dose-dependent manner.
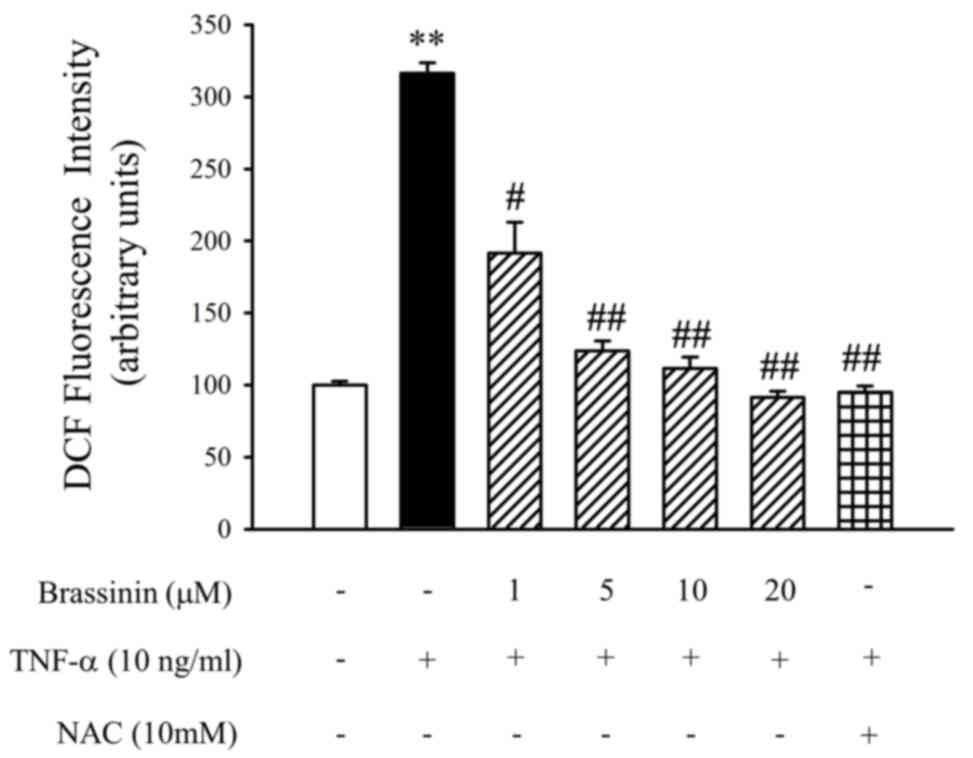
Antioxidant effect of brassinin on
TNF-α-induced ROS production
To confirm whether brassinin has an inhibitory
effect on TNF-α-induced oxidative stress, ROS levels in HUVECs were
detected using the CM-H2DCFDA probe and
spectrofluorometry. As illustrated in Fig. 5, intracellular ROS levels were
increased following TNF-α stimulation in HUVECs, compared with
untreated control cells. However, pretreatment with brassinin
significantly decreased the TNF-α-induced ROS levels (Fig. 5). In addition, NAC (10 mM) was used
as a positive control to confirm the inhibitory effect of
brassinin.
Discussion
The present study provides the first evidence
suggesting that brassinin inhibits vascular inflammation via
inhibition of TNF-α-induced adhesion molecules, NF-κB activation,
and ROS production in HUVECs.
The initiation step of vascular inflammation is
associated with the pathogenesis of atherosclerosis. Several
previous studies have focused on the importance of adhesion
molecules in atherosclerosis. This initiation step is mediated by
the interaction between monocytes and the molecules expressed on
the endothelial cells surface (17). The expression of these CAMs on the
endothelial cell membrane increases significantly when they are
stimulated with endotoxin, IL-1 and TNF-α (18). TNF-α is a very important mediator
of the inflammatory pathway, and TNF-α is closely associated with
the pathogenesis of many cardiovascular diseases, including
atherosclerosis (19). Therefore,
the present study investigated whether brassinin may attenuate the
expression of VCAM-1, ICAM-1 and E-selectin, and monocyte adhesion
to TNF-α-stimulated HUVECs. The results suggested that brassinin
exhibited an inhibitory effect to prevent the TNF-α-induced
vascular inflammatory process in endothelial cells.
NF-κB is an important transcription factor that
activates expression of many inflammatory genes (20). Expression of NF-κB is induced in
inflammatory vascular diseases. In unstimulated cells, NF-κB exists
in the cytosol as a heterodimer composed of the p50 and p65
subunits, and bound to the inhibitory protein IκB-α. When cells are
stimulated, IκB-α is phosphorylated and degraded, allowing NF-κB to
translocate to the nucleus and to start gene transcription
(21). To confirm whether
brassinin exhibits its anti-inflammatory effect on TNF-α-stimulated
endothelial cells through the NF-κB pathway, protein expression of
NF-κB and IκB-α was examined in the cytosol and the nucleus by
western blotting. The results demonstrated that brassinin
significantly suppressed TNF-α-induced accumulation of NF-κB p65
protein in the nucleus, suggesting that brassinin attenuated NF-κB
p65 nuclear translocation. Increased expression of IL-8 has been
reported in atherosclerotic lesions and circulating macrophages
from patients with atherosclerosis (22). In addition, IL-8 serves a key role
in the initiation step of inflammation by enhancing the adhesion of
monocytes to the vascular endothelium (5). In the present study, brassinin
treatment suppressed the mRNA expression levels of IL-8 in
TNF-α-induced HUVECs. These results indicated that brassinin may
have an inhibitory effect on inflammation of vascular endothelial
cells. In vascular inflammation, oxidative stress is crucial in the
initiation step of atherosclerosis, and signifies vascular
disorder. ROS is essential to the normal function of cells, but
adequate levels of antioxidant defenses are required in order to
avoid the harmful effects of excessive ROS production (23). The ROS/NF-κB pathway is recognized
as a key mediator involved in the regulation of inflammatory
responses in vascular diseases (7). Therefore, the production of ROS in
HUVECs was examined in the present study, using the
CM-H2DCFDA probe and fluorescence microscopy. The
results demonstrated that, in cells that were pretreated with
brassinin, TNF-α-induced intracellular ROS production was
significantly decreased.
In conclusion, the present study suggested that
brassinin could have a protective effect on the vascular
inflammation process, by inhibiting the expression of adhesion
molecules and the activation of the ROS/NF-κB pathway in HUVECs.
Therefore, these results provide evidence that brassinin may be
beneficial in the future for the prevention and treatment of
atherosclerosis.
Acknowledgements
The present work was supported by the National
Research Foundation of Korea funded by the Ministry of Science, ICT
and Future Planning of Korea (grant no. 2008-0062484).
References
|
1
|
Lee YJ, Moon MK, Hwang SM, Yoon JJ, Lee
SM, Seo KS, Kim JS, Kang DG and Lee HS: Anti-Inflammatory effect of
Buddleja officinalis on vascular inflammation in human umbilical
vein endothelial cells. Am J Chin Med. 38:585–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Pan L, Wang X, Gong Q and Zhu YZ:
Leonurine protects against tumor necrosis factor-α-mediated
inflammation in human umbilical vein endothelial cells.
Atherosclerosis. 222:34–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross EA, Douglas MR, Wong SH, Ross EJ,
Curnow SJ, Nash GB, Rainger E, Scheel-Toellner D, Lord JM, Salmon M
and Buckley CD: Interaction between integrin alpha9beta1 and
vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil
apoptosis. Blood. 107:1178–1183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sana TR, Janatpour MJ, Sathe M, McEvoy LM
and McClanahan TK: Microarray analysis of primary endothelial cells
challenged with different inflammatory and immune cytokines.
Cytokines. 29:256–269. 2005.
|
|
5
|
Gerszten RE, Garcia-Zepeda EA, Lim YC,
Yoshida M, Ding HA, Gimbrone MA Jr, Luster AD, Luscinskas FW and
Rosenzweig A: MCP-1 and IL-8 trigger firm adhesion of monocytes to
vascular endothelium under flow conditions. Nature. 398:718–723.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tak PP, Gerlag DM, Aupperle KR, van de
Geest DA, Overbeek M, Bennett BL, Boyle DL, Manning AM and
Firestein GS: Inhibitor of nuclear factor kappaB kinase beta is a
key regulator of synovial inflammation. Arthritis Rheum.
44:1897–1907. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen JW, Chen YH, Lin FY, Chen YL and Lin
SJ: Ginkgo biloba extract inhibits tumor necrosis
factor-alpha-induced reactive oxygen species generation,
transcription factor activation, and cell adhesion molecule
expression in human aortic endothelal cells. Arterioscler Thromb
Vasc Biol. 23:1559–1566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iademarco MF, Mcquillan JJ, Rosen GD and
Dean DC: Characterization of the promoter for vascular cell
adhesion molecule-1 (VCAM-1). J Biol Chem. 267:16323–16329.
1992.PubMed/NCBI
|
|
9
|
Gloire G, Legrand-Poels S and Piette J:
NF-kappaB activation by reactive oxygen species: Fifteen years
later. Biochem Pharmacol. 72:1493–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SM, Lee YJ, Kim YC, Kim JS, Kang DG
and Lee HS: Vascular protective role of vitexicarpin isolated from
Vitex rotundifolia in human umbilical vein endothelial cells.
Inflammation. 35:584–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar S, Sharma A, Madan B, Singhal V and
Ghosh B: Isoliquiritigenin inhibits IKappaB kinase activity and ROS
generation to block TNF-alpha induced expression of cell adhesion
molecules on human endothelial cells. Biochem Pharmacol.
73:1602–1612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SM, Oh EY, Lee JH, Nam DW, Lee SG, Lee
JH, Kim SH, Shin BS and Ahn KS: Brassinin combined with capsaicin
enhances apoptotic and anti-metastatic effects in PC-3 human
prostate cancer cells. Phytother Res. 29:1828–1836. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Banerjee T, DuHadaway JB, Gaspari P,
Sutanto-Ward E, Munn DH, Mellor AL, Malachowski WP, Prendergast GC
and Muller AJ: A key in vivo antitumor mechanism of action of
natural product-based brassinins is inhibition of indoleamine
2,3-dioxygenase. Oncogene. 27:2851–2857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kello M, Drutovic D, Chripkova M, Pilatova
M, Budovska M, Kulikova L, Urdzik P and Mojzis J: Ros-dependent
antiproliferative effect of brassinin derivative hombrassinin in
human colorectal cancer Caco2 cells. Molecules. 19:10877–10897.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mackman N, Brand K and Edgington TS:
Lipopolysaccharide mediated transcriptional activation of the human
tissue factor gene in THP-1 cells requires both activator protein-1
and nuclear factor kappa B binding sites. J Exp Med. 174:1517–1526.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourdillon MC, Poston RN, Covacho C,
Chignier E, Bricca G and McGregor JL: ICAM-1 deficiency reduces
atherosclerotic lesions in double-knockout mice
(ApoE(−/-)/ICAM-1(−/-)) fed a fat or a chow diet. Arterioscler
Thromb Vasc Biol. 20:2630–2635. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dustin ML, Rothlein R, Bhan AK, Dinarello
CA and Springer TA: Induction by IL 1 and interferion-gamma: Tissue
distribution, biochemistry, and function of a natural adherence
molecule (ICAM-1). J Immunol. 137:245–254. 1986.PubMed/NCBI
|
|
19
|
Bradley JR: TNF-mediated inflammatory
disease. J Pathol. 214:149–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hattori Y, Kasai K and Gross SS: NO
suppresses while peroxynitrite sustains NF-kappaB: A paradigm to
rationalize cytoprotective and cytotoxic actions attributed to NO.
Cardiovasc Res. 63:31–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brasier AR: The nuclear
factor-kappaB-interleukin-6 signalling pathway mediating vascular
inflammation. Cardiovasc Res. 86:211–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simonini A, Moscucci M, Muller DW, Bates
ER, Pagani FD, Burdick MD and Strieter RM: IL-8 is an angiogenic
factor in human coronary atherectomy tissue. Circulation.
101:1519–1526. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Victor VM, Rocha M, Solá E, Bañuls C,
Garcia-Malpartida K and Hernández-Mijares A: Oxidative stress,
endothelial dysfunction and atherosclerosis. Curr Pharm Des.
15:2988–3002. 2009. View Article : Google Scholar : PubMed/NCBI
|