Introduction
Myocardial infarction leads to a permanent loss of
cardiomyocytes and results in pathological remodeling (1). Despite advances in the clinical
treatment of heart failure, ischemia/reperfusion injury remains a
major cause of mortality in developed countries (2). Numerous studies have demonstrated
that autologous transplantation of bone marrow-derived mesenchymal
stem cells (BM-MSCs) has potential for repairing and regenerating
cardiomyocytes, as well as restoring heart function (3–5).
However, clinical and animal studies have reported that MSCs
derived from older donors have a reduced regenerative potential
compared with those from younger donors. As myocardial infarction
is more common in older individuals, this may limit the benefits of
treatment with MSCs (6). Long
noncoding RNAs (lncRNAs), located in the nucleus and cytosol of
cells, have been demonstrated to be integral regulatory features in
the genome (7). They have been
revealed to regulate numerous biological processes, including
transcription, differentiation, tissue development and
tumorigenesis (8–11). LincRNA-p21 is involved in
regulating tissue development and certain cellular functions,
including proliferation, apoptosis, DNA damage and endoplasmic
reticulum stress (12,13). With respect to senescence,
lincRNA-p21 is transcriptionally induced by p53, its expression is
increased in senescent cells and it represses the translation of
the proteins β-catenin and JunB, which promote cell proliferation
(14). However, the biological
function of lncRNAs in stem cell senescence remains to be fully
elucidated. LncRNAs regulate diverse biological processes by
forming lncRNA-protein and lncRNA-microRNA complexes that control
gene expression and function (7).
The Wnt/β-catenin signaling network is closely associated with stem
cell self-renewal and differentiation (15). Cytosolic β-catenin is translocated
to the nucleus in the presence of Wnt activity, where it interacts
with transcription factors to regulate genes associated with stem
cell self-renewal, proliferation and differentiation (16). Wnt/β-catenin signaling has
previously been demonstrated to be involved in the rejuvenation of
hematopoietic stem cells, by increasing their regenerative capacity
(17). β-catenin was initially
identified as a direct transcriptional target of lincRNA-p21,
thereby regulating apoptosis, DNA damage and endoplasmic reticulum
stress. In addition, a recent study demonstrated that lincRNA-p21
inhibited β-catenin signaling, thereby attenuating the viability,
self-renewal and glycolysis of cancer stem cells (CSCs) (18). Based on the role of
lincRNA-p21-regulated β-catenin signaling in stem cell senescence,
the present study aimed to determine if modulating lincRNA-p21
activated β-catenin signaling and rejuvenated aged MSCs.
Reactive oxygen species (ROS) are additionally
involved in inducing senescence under physiological and
pathological conditions (19). A
previous study demonstrated that ROS decreased the proliferative
capacity and pluripotency of aged MSCs (20), and a recent report observed that
apoptosis caused increased lincRNA-p21 expression and
ROS-associated DNA damage (21).
Endoplasmic reticulum stress induced by lincRNA-p21has been
suggested to account for its effects on the apoptosis and
proliferation of hepatocellular carcinoma cells (13). The current study investigated
whether interfering with lincRNA-p21 may attenuate oxidative stress
and promote the rejuvenation of aged MSCs.
Materials and methods
Animals
BM-MSCs were isolated from young (8-week-old) and
aged (18-month-old) male C57BL/6 mice (n=12, per age group), as
described previously (22). Mice
were purchased from the laboratory animals center of Wenzhou
Medical University (Wenzhou, China). The animals were maintained on
a 12-h light/dark cycle, at 21±2°C and with a relative humidity of
30–70%. Food and water was freely available throughout. All
procedures were approved by the Animal Care and Use Committee of
Wenzhou Medical University, and complied with the Guidelines for
the Care and Use of Laboratory Animals, published by the National
Academy Press (23).
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from HyClone (GE Healthcare Life
Sciences, Logan, UT, USA), TRIzol® reagent was from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA), and
the Transcriptor First Stand cDNA Synthesis kit, FastStart
Universal SYBR® Green Master (Rox) and X-treme GENE HP
DNA transfection reagent were purchased from Roche Diagnostics
(Basel, Switzerland). Rabbit monoclonal antibodies against
β-catenin (8480; 1/1,000) and β-actin (4970; 1,1,000) were obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA) and
horseradish peroxidase-conjugated anti-rabbit secondary antibodies
(sc-2357; 1/1,000) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Vascular endothelial growth factor (VEGF;
RAB0509), basic fibroblast growth factor (bFGF; RAB0184),
hepatocyte growth factor (HGF; RAB0214) and insulin-like growth
factor (IGF; RAB0229) ELISA kits were purchased from Sigma-Aldrich
(Merck Millipore, Darmstadt, Germany). Small interfering RNAs
(siRNAs) targeting lincRNA-p21 (sequence:
5′-UGAAAAGAGCCGUGAGCUA-3′), β-catenin (sequence:
5′-CTCACTTGCAATAATTACAAA−3′) and non-targeting siRNA-NT control
(sequence: 5′-CTCUCCGAACGUGUCACGUTT−3′) transcripts were purchased
from Thermo Fisher Scientific, Inc. Cell proliferation was assessed
using Cell Counting kit-8 (CCK-8; HaiGene Technology Co., Ltd.,
Harbin, China). Superoxide Dismutase (SOD) Activity Colorimetric
assay and Lipid Peroxidation (Malondialdehyde; MDA) assay kits were
purchased from Abcam (Cambridge, UK).
Isolation, culture and
characterization of MSCs
BM-MSCs were isolated using a standard protocol, as
described previously (22).
Briefly, mice were anaesthetized using isoflurane (5% in induction
chamber, 2% in mask; flow of oxygen, 1 litre/min; Santa Cruz
Biotechnology, Inc.), and sacrificed by cervical dislocation. Bone
marrow was isolated from the femur and tibia of mice by flushing
with PBS. Adherent MSCs were propagated and maintained at 37°C and
5% CO2 in high-glucose DMEM supplemented with 10% FBS
and 1% penicillin/streptomycin. Third-passage MSCs were used for
experiments to avoid contamination with other cell types.
Cell proliferation assay
The rate of cell proliferation was estimated using
aCCK-8 assay, according to the manufacturer's protocol. Briefly,
cells (1×105 cells/well) cultured in 96-well plates for
1, 3, 5 or 7 days were incubated with CCK-8 solution for 1 h at
37°C, following which the absorbance of each well at a wavelength
of 450 nm was recorded.
Evaluation of VEGF, bFGF, HGF and IGF
levels
The concentrations of VEGF, bFGF, HGF and IGF
secreted by MSCs were assessed by ELISA, according to the
manufacturer's protocol and as described previously (22).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression levels were analyzed by RT-qPCR.
Briefly, total cellular RNA was isolated using TRIzol and reverse
transcribed using a Transcriptor First Stand cDNA Synthesis kit,
according to the manufacturer's protocol. qPCR was performed using
the FastStart Universal SYBR Green Master (Rox). The following
primers were used: VEGF, forward, 5′-CTGTGTGCCGCTGATGCGCT-3′ and
reverse, 5′-TCGCCCTCCGGACCCAAAGT-3′; bFGF forward,
5′-AGATCATGCTTCCACCTCGT-3′ and reverse, 5′-TGGGTCCCTTTCACTTTGCC-3;
HGF forward, 5′-GGCCATGAATTTGACCTCTATGAA-3′ and reverse,
5′-TTCAACTTCTGAACACTGAGGAAT-3′; IGF, forward,
5′-TTTCAACAAGCCCACAGGGT-3′ and reverse, 5´-TTGAGGGGTGCGCAATACAT-3;
GAPDH, forward, 5′-GGAGCCAAAAGGGTCATCAT-3′ and reverse
5′-GTGATGGCATGGACTGTGGT-3′; lincRNA-p21, forward,
5′-CCTGTCCACTCGCTTTC-3′ and reverse, 5′-GGAACTGGAGACGGAATGTC-3′.
The thermocycling conditions were as follows: 40 cycles of
amplification at 95°C for 15 sec, 64°C for 20 sec and 72°C for 25
sec. The quantitation cycle (Cq) was set within the exponential
phase of the PCR reaction, and the ΔCq value for each target gene
was calculated by subtracting the Cq value for the internal control
GAPDH, from that of the target gene. Relative gene expression
levels were calculated by comparing the ΔCq values between control
and experimental conditions for each target PCR using the following
equation: Relative gene expression = 2− (ΔCq sample−ΔCq
control) (24).
Knockdown of gene expression using
siRNA
MSCs were transfected using the X-treme GENE HP DNA
Transfection reagent, according to the manufacturer's protocol.
Briefly, MSCs were cultured in6-well plates (1×106
cells/well), treated with the transfection reagent at a
reagent-to-siRNA weight ratio of 3:1 for 20 min at 37°C, followed
by the addition of a mixture containing 100 nM siRNA and incubation
in 2 ml culture medium for 48 h. Scrambled non-target-specific
siRNA (siRNA-NT) served as a control. The transfection efficiency
of siRNA-lincRNA-p21 and siRNA-β-catenin was determined by RT-qPCR
and western blotting, respectively.
Western blot analysis
Western blotting was performed as described
previously (25). Briefly, cells
were washed twice with ice-cold PBS and ruptured with lysis buffer.
The lysates were centrifuged at 4°C for 5 min at 12,000 × g and the
resulting supernatant contained total cellular protein. Proteins
were quantified using bicinchoninic acid assay, and 20 µg total
protein of each sample was resolved by 10% SDS-PAGE and transferred
onto polyvinylidene difluoride membranes. Membranes were blocked
for 1 h with 5% skim milk in TBS containing 0.1% Tween-20 and
incubated overnight at 4°C with primary antibodies. The membranes
were subsequently washed, incubated for 1 h with appropriate
horseradish peroxidase-conjugated secondary antibodies and
developed using BeyoECL Plus chemiluminescent substrate (Beyotime
Institute of Biotechnology, Jiangsu, China). The stained protein
bands were visualized using Bio-RadChemiDoc™ XRS equipment (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and quantified using
Quantity One software version 4.5.2 (Bio-Rad Laboratories,
Inc.).
ROS measurement
Levels of intracellular ROS were determined using
2,7-dichlorodihydrofluorescein diacetate, supplied in the Reactive
Oxygen Species Assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. The fluorescence
intensity of the cells was measured using a fluorescence
spectrophotometer, with excitation and emission wavelengths of 488
and 525 nm, respectively.
SOD activity
SOD activity in MSCs was determined using a
colorimetric assay kit according to the manufacturer's protocol.
Briefly, protein was isolated from MSCs using lysis buffer and SOD
activity was measured in 10 µg of total protein extract. Absorbance
was measured at a wavelength of 450 nm.
MDA lipid peroxidation assay
Lipid peroxidation was monitored using an assay kit
to measure the formation of MDA, according to the manufacturer's
protocol. Briefly, MSCs (1×106 cells) were homogenized
on ice in 300 µl MDA lysis buffer (with 3 µl 100Xbutylated
hydroxytoluene) and centrifuged (13,000 × g for 10 min at 4°C) to
remove insoluble material. The supernatant (200 µl) was added to
600 µl thiobarbituric acid and incubated at 95°C for 60 min. The
samples were cooled to room temperature in an ice bath for 10 min
and the absorbance at a wavelength of 532 nm was measured
spectrophotometrically.
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences among groups were analyzed by one-way analysis of
variance, followed by Tukey's test for multiple comparisons.
Analyses between two groups were performed using Student's t-test.
Statistical tests were performed using SPSS software version 19.0
(IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of age on MSC proliferation
and paracrine ability
As senescence is associated with reduced cellular
function, the present study compared the proliferative and
paracrine abilities of aged and young MSCs. The self-renewal
potential of young and aged MSCs was examined by CCK-8 assay, which
confirmed low rates of proliferation in aged MSCs after 1, 3, 5 and
7 days of culture (Fig. 1A). The
expression and secretion of VEGF, bFGF, HGF, and IGF was also
investigated by RT-qPCR and ELISA, respectively. mRNA expression
levels of all four factors were significantly reduced in aged
compared with younger MSCs (Fig.
1B-E) and the corresponding protein levels in the culture
medium were additionally significantly decreased in aged compared
with younger MSCs (Fig. 1F-I).
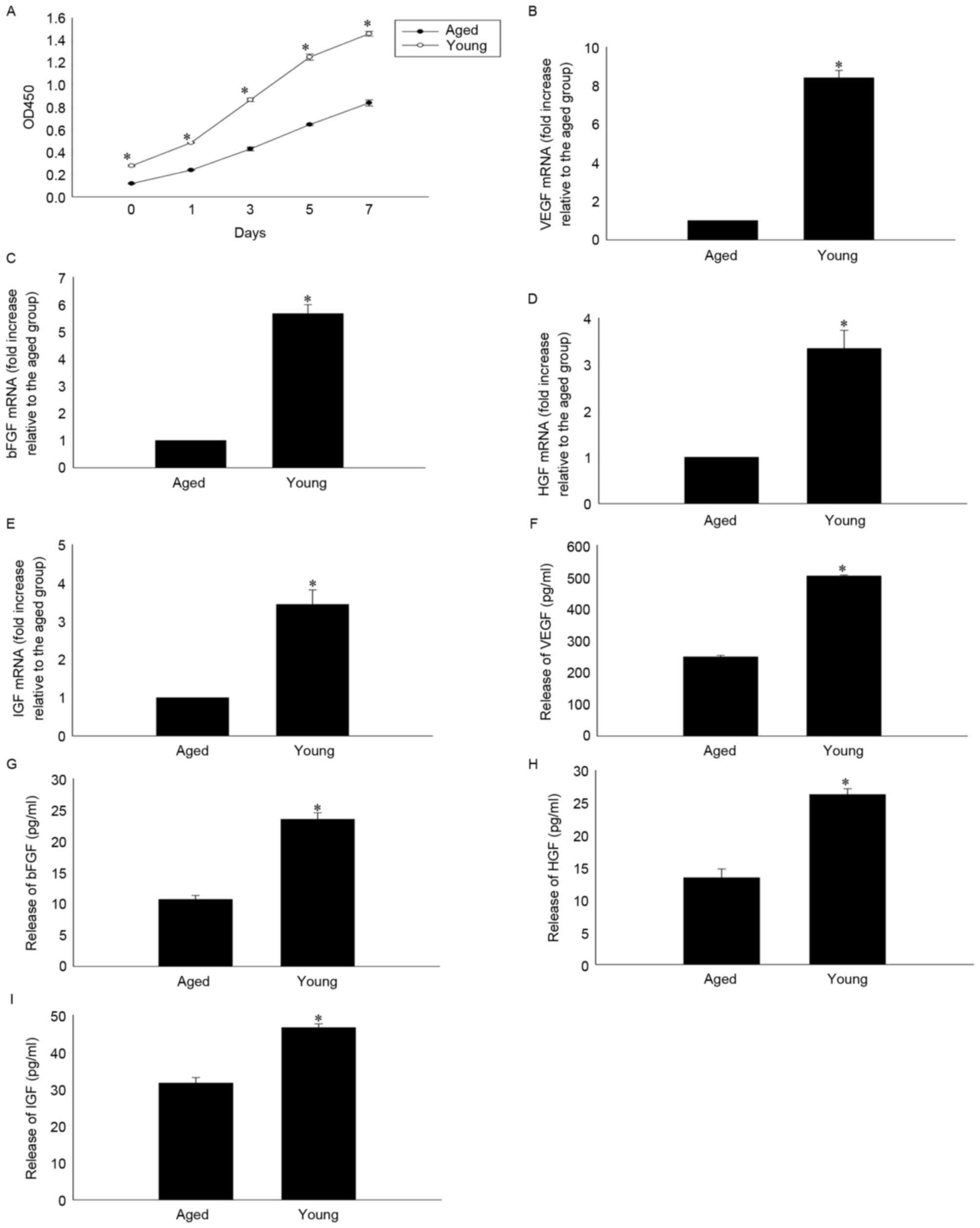 | Figure 1.Age-impaired MSC proliferation and
paracrine ability. (A) Cell proliferation curves of MSCs, as
determined by Cell Counting kit-8 assay in young and aged MSCs at
1, 3, 5 and 7 days. Relative mRNA levels of (B) VEGF, (C) bFGF, (D)
HGF and (E) IGF analyzed by reverse transcription-quantitative
polymerase chain reaction in cultures of young and aged MSCs.
Secretion of (F) VEGF, (G) bFGF, (H) HGF and (I) IGF into the
culture medium of young and aged MSCs, as analyzed by ELISA. Data
are presented as the mean ± standard deviation from three
independent experiments. *P<0.05 vs. aged MSCs. MSCs,
mesenchymal stem cells; VEGF, vascular endothelial growth factor;
bFGF, basic fibroblast growth factor; HGF, hepatocyte growth
factor; IGF, insulin-like growth factor; OD, optical density. |
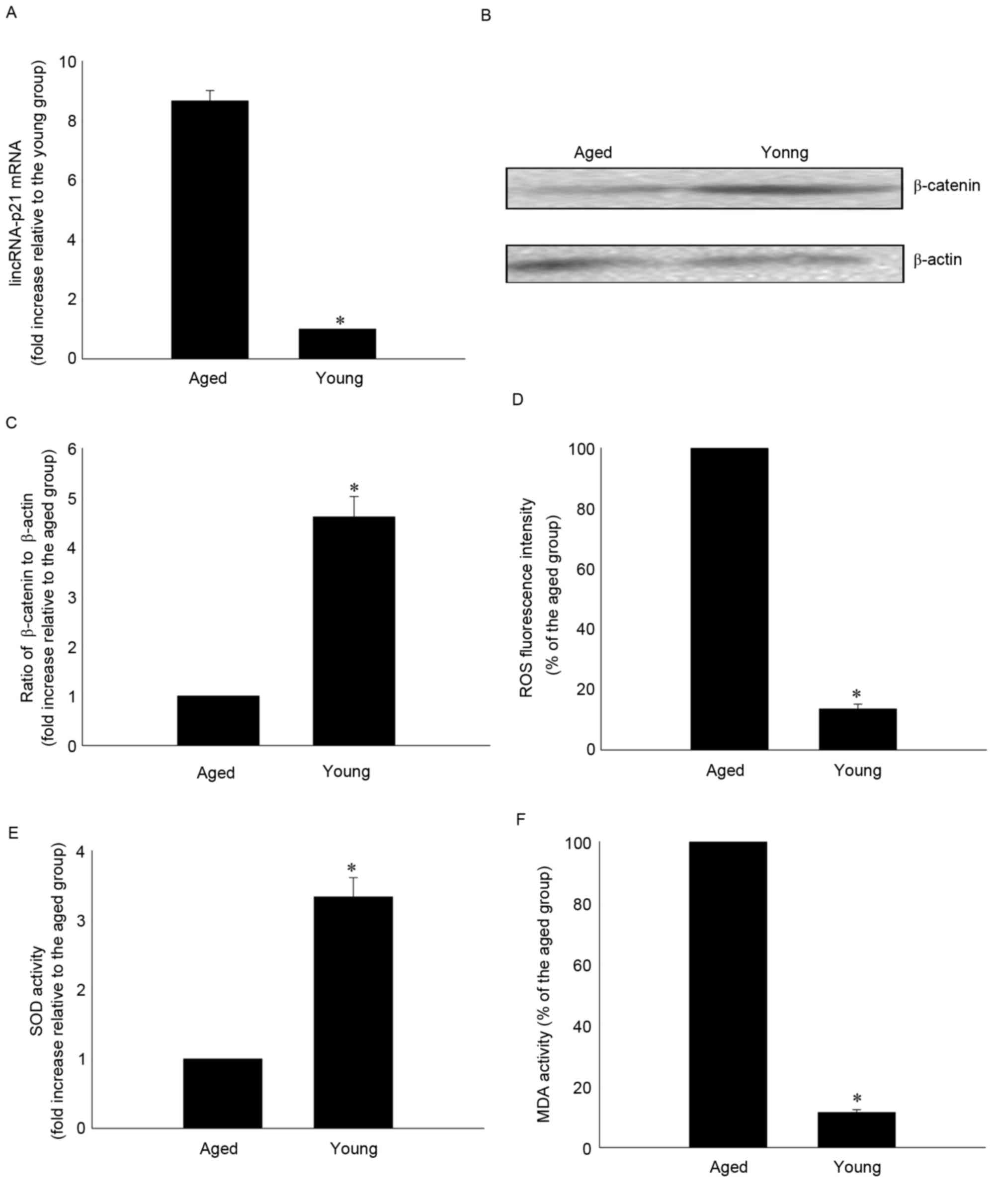
Effects of age on lincRNA-p21
expression, cellular oxidative stress and the Wnt/β-catenin
signaling pathway
lincRNA-p21 expression levels were significantly
greater in aged compared with younger MSCs (8.67±0.35 vs.
1.00±0.00; P<0.05; Fig. 2A).
lincRNA-p21 has previously been demonstrated to reduce β-catenin
protein levels in CSCs (18). In
the present study, β-catenin protein expression levels were
decreased in aged compared with younger MSCs (1.00±0.00 vs.
4.61±0.41; P<0.05; Fig. 2B and
C). To determine whether cellular senescence involved oxidative
stress, the present study investigated ROS generation, SOD
activation and lipid peroxidation by MDA assay. Senescence was
associated with a significantly increased generation of ROS
(100.00±0.00 vs. 13.58±1.60%; P<0.05; Fig. 2D), significantly reduced SOD
activity (1.00±0.00 vs. 3.33±0.28; P<0.05; Fig. 2E) and significantly increased MDA
activity (100.00±0.00 vs. 11.40±0.96%; P<0.05; Fig. 2F) compared with younger MSCs.
Effect of lincRNA-p21 knockdown on
rejuvenation of aged MSCs
The role of lincRNA-p21 in rejuvenating aged MSCs
was further investigated by knockdown of endogenous lincRNA-p21 by
siRNA. LincRNA-p21 expression levels were significantly reduced by
transfection with lincRNA-p21 siRNA compared with siRNA-NT
(10.47±1.00 vs. 100.00±0.00%; P<0.05; Fig. 3A). Knockdown of lincRNA-p21 was
associated with significantly increased expression of β-catenin
(3.92±0.29 vs. 1.00±0.00; P<0.05; Fig. 3B and C) and significantly increased
proliferation of aged MSCs (Fig.
3D) compared with the siRNA-NT control. In addition, inhibition
of lincRNA-p21 increased the paracrine ability of aged MSCs, which
was indicated by significantly increased secretion of VEGF, bFGF,
HGF and IGF (Fig. 3E-H), decreased
generation of ROS (12.22±1.07 vs. 100.00±0.00%; P<0.05; Fig. 3I), increased SOD activity
(3.25±0.17 vs. 1.00±0.00; P<0.05; Fig. 3J) and reduced MDA activity
(11.34±1.00 vs. 100.00±0.00%; P<0.05; Fig. 3K), compared with siRNA-NT. In
addition, siRNA-lincRNA-p21 transfected MSCs were compared with
young MSCs; no significant difference between the siRNA-lincRNA-p21
group and the young group in proliferation and paracrine ability
was observed (data not shown).
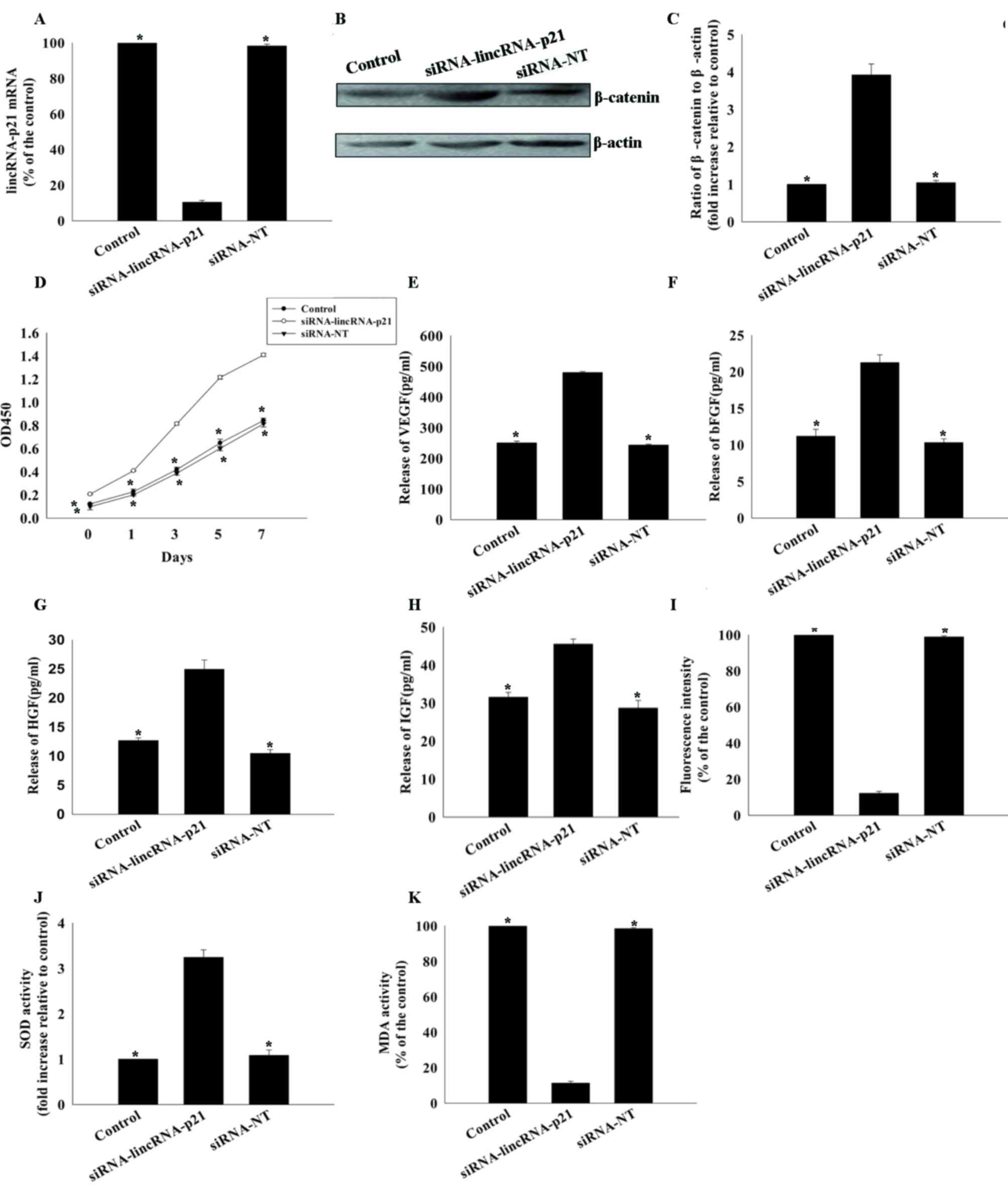 | Figure 3.Effects of lincRNA-p21 silencing on
rejuvenation and reduced oxidative stress in aged MSCs. (A) Reverse
transcription-quantitative polymerase chain reaction analysis of
lincRNA-p21expression in untransfected MSCs, and MSCs transfected
with lincRNA-p21-specific siRNA or control siRNA-NT. (B) Image and
(C) quantification of western blotting for β-catenin protein
expression levels in untransfected MSCs, and MSCs transfected with
lincRNA-p21-specific siRNA or siRNA-NT. (D) Cell proliferation
curves of untransfected MSCs, and MSCs transfected with
siRNA-lincRNA-p21 orsiRNA-NT. Concentrations of (E) VEGF, (F) bFGF,
(G) HGF and (H) IGF in the culture medium of aged untransfected
MSCs, and MSCs transfected with siRNA-lincRNA-p21 or siRNA-NT. (I)
Intracellular ROS production, (J) SOD activity and (K) MDA activity
were measured in aged untransfected MSCs, and aged MSCs transfected
with siRNA-lincRNA-p21 orsiRNA-NT. Data are presented as the mean ±
standard deviation from three independent experiments. *P<0.05
vs. siRNA-lincRNA-p21. lncRNA, long noncoding RNA; MSCs,
mesenchymal stem cells; siRNA, small interfering RNA; siRNA-NT,
non-target-specific small interfering RNA; VEGF, vascular
endothelial growth factor; bFGF, basic fibroblast growth factor;
HGF, hepatocyte growth factor; IGF, insulin-like growth factor;
ROS, reactive oxygen species; SOD, superoxide dismutase; MDA,
malondialdehyde; OD, optical density. |
Role of the Wnt/β-catenin signaling
pathway in lincRNA-p21-induced effects on MSCs
The present study further investigated the mechanism
underlying the effect of lincRNA-p21on the rejuvenation of aged
MSCs by silencing β-catenin using siRNA. β-catenin protein
expression levels were significantly reduced in cells transfected
with siRNA-β-catenin compared with siRNA-NT control (21.77±1.37 vs.
100.00±0.00%; P<0.05; Fig. 4A and
B). Knockdown of lincRNA-p21 increased the proliferation of
aged MSCs and increased their paracrine ability; these effects were
abolished by silencing β-catenin (Fig.
4C-G). The decrease in oxidative stress associated with
knockdown of lincRNA-p21 expression in aged MSCs was additionally
reversed by silencing β-catenin, with increased generation of ROS
(98.55±0.47 vs. 13.29±0.99%; P<0.05; Fig. 4H), decreased SOD activity
(1.08±0.05 vs. 3.29±0.11; P<0.05; Fig. 4I) and increased MDA activity
(98.77±0.76 vs. 11.66±0.43%; P<0.05; Fig. 4J), compared with
siRNA-lincRNA-p21-transfected cells.
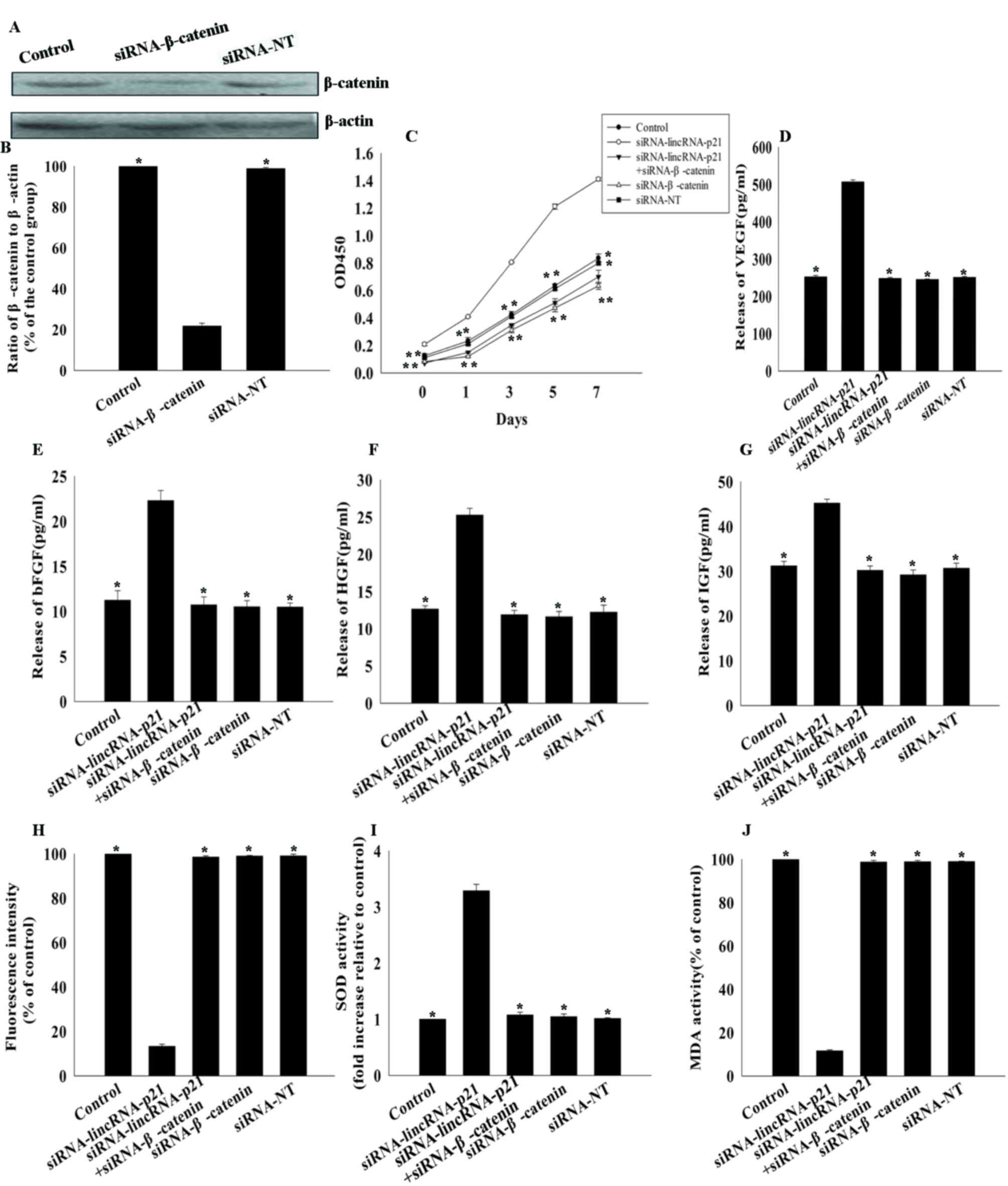 | Figure 4.Effects of lincRNA-p21 on MSC
rejuvenation via the Wnt/β-catenin pathway. (A) Image and (B)
quantification of western blotting for β-catenin protein expression
levels in untransfected MSCs, and MSCs transfected with
β-catenin-siRNA or siRNA-NT. *P<0.05 vs. siRNA-β-catenin. (C)
Cell proliferation curves, as determined by Cell Counting kit-8
assay, of untransfected MSCs, and MSCs transfected
withlincRNA-p21-siRNA, β-catenin-siRNA, lincRNA-p21-siRNA +
β-catenin-siRNA or siRNA-NT. *P<0.05 vs. siRNA-lincRNA-p21.
Concentrations of (D) VEGF, (E) bFGF, (F) HGF and (G) IGF in
culture medium of untransfected MSCs, and MSCs transfected with
lincRNA-p21-siRNA, β-catenin-siRNA, lincRNA p21-siRNA +
β-catenin-siRNA or siRNA-NT. *P<0.05 vs. siRNA-lincRNA-p21. (H)
Intracellular ROS production, (I) SOD activity and (J) MDA activity
were measured in untransfected MSCs, and MSCs transfected with
lincRNA-p21-siRNA, β-catenin-siRNA, lincRNA-p21-siRNA +
β-catenin-siRNA or siRNA-NT. *P<0.05 vs. siRNA-lincRNA-p21. Data
are presented as the mean ± standard deviation from three
independent experiments. lncRNA, long noncoding RNA; MSCs,
mesenchymal stem cells; siRNA, small interfering RNA; siRNA-NT,
non-target-specific small interfering RNA; VEGF, vascular
endothelial growth factor; bFGF, basic fibroblast growth factor;
HGF, hepatocyte growth factor; IGF, insulin-like growth factor;
ROS, reactive oxygen species; SOD, superoxide dismutase; MDA,
malondialdehyde; OD, optical density. |
Discussion
MSCs isolated from adult bone marrow are an
excellent source for therapeutic stem cell development (26). However, MSCs undergo age-associated
alterations, including reduced proliferation and paracrine
signaling (27), which decrease
their capacity for damage repair (28). Numerous strategies have been
investigated to overcome these limitations, and certain of these
have resulted in marked improvements in cardiac function,
particularly in a rodent model of acute myocardial infarction
(22). However, the mechanisms
underlying MSC senescence remain unclear. The results of the
present study identified lincRNA-p21 as an important mediator of
cellular senescence and suggested that modification of lincRNA-p21
expression rejuvenated aged MSCs via the Wnt/β-catenin signaling
pathway.
lncRNAs are non-protein-coding RNAs that are >200
nucleotides; increasing evidence has implicated them in the
regulation of various cellular functions and development processes
(29). lncRNAs differ widely in
length and function, and regulate gene transcription and the fate
of post-transcriptional mRNA (18). Collectively, lncRNAs affect a broad
range of age-associated physiological and pathological conditions
(30). The results of the current
study demonstrated that lincRNA-p21 expression was greater in MSCs
from aged mice compared with those from younger mice, and this high
level of expression was accompanied by reduced cell proliferation
and impaired paracrine ability. The role of lincRNA-p21 in MSC
senescence was confirmed by silencing with siRNA, which led to
increased cell proliferation and paracrine ability, and reduced
oxidative stress, indicating rejuvenation of aged MSCs.
Various members of the Wnt family are expressed in
stem cells (31). Wnt3a/β-catenin
are associated with canonical Wnt signaling and have been widely
investigated in relation to stem cell senescence (32), whereas β-catenin has been
previously proposed to increase hematopoietic cell self-renewal
(17). The Wnt/β-catenin network
represents a potential target for MSC rejuvenation and has been
confirmed to have an important role in restoring MSC myogenic
differentiation by increasing β-catenin bioavailability (16). In the current study, the
Wnt/β-catenin signaling pathway appeared to be inhibited in aged
MSCs, indicating that it may be involved in the rejuvenation
process. LncRNAs regulate diverse biological processes through the
formation of lncRNA-protein and lncRNA-microRNA complexes, thereby
controlling gene expression and function (7). LincRNA-p21 directly downregulates
β-catenin protein levels in various cell types, and
vector-delivered lincRNA-p21 preferentially blocked the activation
of Wnt/β-catenin signaling in CSCs. The viability, self-renewal and
tumorigenicity of CSCs were compromised by lincRNA-p21
overexpression (18). The present
study observed that the Wnt/β-catenin signaling pathway was
associated with the underlying mechanism of lincRNA-p21, and
silencing lincRNA-p21 restored β-catenin expression in aged MSCs.
In addition, the current study demonstrated that MSC rejuvenation
induced by silencing lincRNA-p21 was abolished by silencing
β-catenin, indicating that lincRNA-p21-associated aging may involve
inhibition of the Wnt/β-catenin signaling pathway.
Oxidative stress is generally associated with
induction of cell aging (33). It
is accompanied by ROS generation, increased oxidant enzyme activity
and diminished antioxidant enzyme activity (34). Cellular senescence is associated
with metabolic function disorders and recent studies have
identified roles for multiple lncRNA pathways in the control of
metabolic function (7).
LincRNA-p21 is associated with cellular DNA damage and endoplasmic
reticulum stress under oxidative stress (13). Oxidative stress diverts β-catenin
from the transcriptional pathways of the T-cell factor/lymphoid
enhancer factor family to forkhead box O, indicating that oxidative
stress-associated β-catenin downregulation may be involved in aging
in mice (35). The results of the
current study demonstrated that oxidative stress was increased in
aged MSCs, which was associated with high lincRNA-p21 and low
β-catenin expression levels, and was supported by the observation
that silencing lincRNA-p21 decreased oxidative stress, and that
this effect was reversed by silencing β-catenin.
In conclusion, the results of the present study
indicated that lincRNA-p21 is involved in MSC senescence.
Interfering with lincRNA-p21 expression may allow the rejuvenation
of aged MSCs by regulation of oxidative stress via the
Wnt/β-catenin signaling pathway. The demonstration that MSC
senescence was delayed and regenerative properties enhanced has
important therapeutic implications for vascular disorders.
Targeting lincRNA-p21 expression in aged MSCs may be a useful
strategy in cell transplantation-based repair and regeneration of
peripheral and coronary vascular lesions.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500261; to M.H.)
and the Science and Technology Planning Project of Wenzhou (grant
no. Y20160125; to M.H.).
References
|
1
|
Wollert KC, Meyer GP, Lotz J,
Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte
T, Hornig B, Messinger D, et al: Intracoronary autologous
bone-marrow cell transfer after myocardial infarction: The BOOST
randomised controlled clinical trial. Lancet. 364:141–148. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stamm C, Westphal B, Kleine HD, Petzsch M,
Kittner C, Klinge H, Schümichen C, Nienaber CA, Freund M and
Steinhoff G: Autologous bone-marrow stem-cell transplantation for
myocardial regeneration. Lancet. 361:45–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orlic D, Kajstura J, Chimenti S, Limana F,
Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A and Anversa
P: Mobilized bone marrow cells repair the infarcted heart,
improving function and survival. Proc Natl Acad Sci USA.
98:10344–10349. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stamm C, Westphal B, Kleine HD, Petzsch M,
Kittner C, Klinge H, Schümichen C, Nienaber CA, Freund M and
Steinhoff G: Autologous bone-marrow stem-cell transplantation for
myocardial regeneration. Lancet. 361:45–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimmeler S and Leri A: Aging and disease
as modifiers of efficacy of cell therapy. Circ Res. 102:1319–1330.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao XY and Lin JD: Long noncoding RNAs: A
new regulatory code in metabolic control. Trends Biochem Sci.
40:586–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kornfeld JW and Brüning JC: Regulation of
metabolism by long, non-coding RNAs. Front Genet. 5:572014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JT: Epigenetic regulation by long
noncoding RNAs. Science. 338:1435–1439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercer TR and Mattick JS: Structure and
function of long noncoding RNAs in epigenetic regulation. Nat
Struct Mol Biol. 20:300–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blume CJ, Hotz-Wagenblatt A, Hüllein J,
Sellner L, Jethwa A, Stolz T, Slabicki M, Lee K, Sharathchandra A,
Benner A, et al: p53-dependent non-coding RNA networks in chronic
lymphocytic leukemia. Leukemia. 29:2015–2023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang N, Fu Y, Zhang H, Sima H, Zhu N and
Yang G: LincRNA-p21 activates endoplasmic reticulum stress and
inhibits hepatocellular carcinoma. Oncotarget. 6:28151–28163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon JH, Abdelmohsen K, Srikantan S, Yang
X, Martindale JL, De S, Huarte M, Zhan M, Becker KG and Gorospe M:
LincRNA-p21 suppresses target mRNA translation. Mol Cell.
47:648–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paige SL, Osugi T, Afanasiev OK, Pabon L,
Reinecke H and Murry CE: Endogenous Wnt/beta-catenin signaling is
required for cardiac differentiation in human embryonic stem cells.
PLoS One. 5:e111342010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brunt KR, Zhang Y, Mihic A, Li M, Li SH,
Xue P, Zhang W, Basmaji S, Tsang K, Weisel RD, et al: Role of
WNT/β-catenin signaling in rejuvenating myogenic differentiation of
aged mesenchymal stem cells from cardiac patients. Am J Pathol.
181:2067–2078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Florian MC, Nattamai KJ, Dörr K, Marka G,
Uberle B, Vas V, Eckl C, Andrä I, Schiemann M, Oostendorp RA, et
al: A canonical to non-canonical Wnt signalling switch in
haematopoietic stem-cell ageing. Nature. 503:392–396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Lei ZJ, Guo Y, Wang T, Qin ZY,
Xiao HL, Fan LL, Chen DF, Bian XW, Liu J and Wang B:
miRNA-regulated delivery of lincRNA-p21 suppresses β-catenin
signaling and tumorigenicity of colorectal cancer stem cells.
Oncotarget. 6:37852–37870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Y, Nikulenkov F, Zawacka-Pankau J, Li
H, Gabdoulline R, Xu J, Eriksson S, Hedström E, Issaeva N, Kel A,
et al: ROS-dependent activation of JNK converts p53 into an
efficient inhibitor of oncogenes leading to robust apoptosis. Cell
Death Differ. 21:612–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gharibi B, Farzadi S, Ghuman M and Hughes
FJ: Inhibition of Akt/mTOR attenuates age-related changes in
mesenchymal stem cells. Stem Cells. 32:2256–2266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hall JR, Messenger ZJ, Tam HW, Phillips
SL, Recio L and Smart RC: Long noncoding RNA lincRNA-p21 is the
major mediator of UVB-induced and p53-dependent apoptosis in
keratinocytes. Cell Death Dis. 6:e17002015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madonna R, Taylor DA, Geng YJ, De Caterina
R, Shelat H, Perin EC and Willerson JT: Transplantation of
mesenchymal cells rejuvenated by the overexpression of telomerase
and myocardin promotes revascularization and tissue repair in a
murine model of hindlimb ischemia. Circ Res. 113:902–914. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin
L, Zhang MM and Yu B: Novel mechanism of inhibition of dendritic
cells maturation by mesenchymal stem cells via interleukin-10 and
the JAK1/STAT3 signaling pathway. PLoS One. 8:e554872013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou M, Cui J, Liu J, Liu F, Jiang R, Liu
K, Wang Y, Yin L, Liu W and Yu B: Angiopoietin-like 4 confers
resistance to hypoxia/serum deprivation-induced apoptosis through
PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells.
PLoS One. 9:e858082014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang H, Hou H, Yi W, Yang G, Gu C, Lau
WB, Gao E, Ma X, Lu Z, Wei X, et al: Increased expression of
pigment epithelium-derived factor in aged mesenchymal stem cells
impairs their therapeutic efficacy for attenuating myocardial
infarction injury. Eur Heart J. 34:1681–1690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stenderup K, Justesen J, Clausen C and
Kassem M: Aging is associated with decreased maximal life span and
accelerated senescence of bone marrow stromal cells. Bone.
33:919–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang JH, Sampogna S, Morales FR and Chase
MH: Age-related ultrastructural changes in hypocretinergic
terminals in the brainstem and spinal cord of cats. Neurosci Lett.
373:171–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greco S, Gorospe M and Martelli F:
Noncoding RNA in age-related cardiovascular diseases. J Mol Cell
Cardiol. 83:142–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malhotra S and Kincade PW: Wnt-related
molecules and signaling pathway equilibrium in hematopoiesis. Cell
Stem Cell. 4:27–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nemeth MJ, Topol L, Anderson SM, Yang Y
and Bodine DM: Wnt5a inhibits canonical Wnt signaling in
hematopoietic stem cells and enhances repopulation. Proc Natl Acad
Sci USA. 104:15436–15441. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henson SM, Lanna A, Riddell NE, Franzese
O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK,
Tooze SA and Akbar AN: p38 signaling inhibits mTORC1-independent
autophagy in senescent human CD8+ T cells. J Clin
Invest. 124:4004–4016. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Almeida M, Han L, Martin-Millan M, O'Brien
CA and Manolagas SC: Oxidative stress antagonizes Wnt signaling in
osteoblast precursors by diverting beta-catenin from T cell factor-
to forkhead box O-mediated transcription. J Biol Chem.
282:27298–27305. 2007. View Article : Google Scholar : PubMed/NCBI
|