Introduction
Hearing impairment (HI) is a genetically
heterogeneous disorder in humans, with an incidence of
approximately 1:1,000 children worldwide; over half of these cases
may be attributed to a genetic cause (1). Nonsyndromic HI (NSHI) accounts for
60–70% of inherited hearing impairment and involves more than 100
genes with autosomal dominant (20–25%), autosomal recessive
(75–80%), X-linked (1–2%) and maternal inheritance (≥1%) patterns
(2). Previous reports conducted in
China have suggested that mutations in the gap junction protein β2
(GJB2), solute carrier family 26 member 4 (SLC26A4) and
mitochondrially encoded 12S RNA (mtDNA12SrRNA) genes are the
predominant genetic causes of HI; mutations in these three genes
may explain 26.7–35.7% of the etiology of NSHI (3–5).
Mutations in deafness-related genes are currently detected using
several different methods, including direct sequencing, microarray
analysis, polymerase chain reaction (PCR)-restriction fragment
length polymorphism analysis and denatured high-performance liquid
chromatography. Although direct sequencing is the gold standard
approach for the detection of gene mutations, this strategy is
expensive, time-consuming and inefficient for the sequencing of
large fragments. The SNPscan technique is high-throughput and
cost-effective, and several studies have demonstrated the accuracy,
sensitivity and specificity of this technique (6–8).
Therefore, SNPscan is considered to be a valid tool for the genetic
diagnosis of inherited HI.
Northwest China is home to numerous ethnic groups,
including >40 ethnic minority groups, some of which are found
only in the northwest region, such as the Tu ethnicity. This region
is populated by individuals with diverse ethnicities, however, the
majority of the residents are of Hui or Tibetan ethnicity. These
ethnic minorities reside in separate ethnic neighborhoods and have
distinct genetic backgrounds, which are relatively conserved.
Therefore, individuals of the Hui, Tibetan and Tu ethnicities
residing in this region may present unique mutation spectra of
deafness-related genes. The objective of the present study was to
investigate the molecular etiology of NSHI using the SNPscan
technique, in patients with HI from the Hui, Tibetan and Tu
ethnicities residing in northwest China. The information obtained
within the present study may provide a scientific basis for the
diagnosis, intervention and genetic counseling of patients with HI
and their families.
Materials and methods
Patient selection
A total of 283 individuals with NSHI from unrelated
families were enrolled; 194 were from Lanzhou Special Education
School (Lanzhou, China) and 89 were from Xining Special Education
School (Xining, China). An additional 150 region-, age- and
ethnicity-matched control individuals with normal hearing were
recruited for participation in this study. The study protocol was
approved by the Ethics Committee of the Second Hospital of Lanzhou
University (Lanzhou, China). Informed consent was obtained from all
subjects prior to blood sampling. The medical history of each
patient was acquired, and this included age of onset, family
history, mother's health during pregnancy, previous history of
infection, head trauma and use of aminoglycoside antibiotics. The
patients with NSHI received routine physical and
otorhinolaryngology examinations. Age-appropriate audiological
examinations, including pure-tone audiometry or auditory brainstem
response testing, immittance testing, and distortion product
otoacoustic emissions testing (9)
were performed. All HI subjects demonstrated moderate to profound
bilateral sensorineural hearing impairment on audiograms. Patients
with middle ear disorders or syndromic HI were excluded from the
study. Genomic DNA was extracted from the peripheral blood
leukocytes of 283 patients with nonsyndromic hearing impairment and
150 controls with normal hearing using a DNA extraction kit
(Axygen; Corning Incorporated, Corning, NY, USA), according to the
manufacturer's protocol.
SNPscan for mutation detection
A total of 115 mutations in GJB2, SLC26A4 and
mtDNA12SrRNA that were previously identified in ≥2 individuals were
selected from a mutation database generated by direct
exon-sequencing of the GJB2, mtDNA12SrRNA, and SLC26A4 genes in
>7,000 patients with HI (10).
SNPscan genotyping was performed using a custom-by-design 2×48-plex
SNPscan kit (Shanghai Genesky Biotech, Co., Ltd., Shanghai, China).
This kit was developed according to a SNP genotyping technology
patented by Genesky Biotech, Co., Ltd., based on double ligation
and multiplex fluorescence PCR. A ligation mixture was prepared at
a volume of 20 µl containing 1X Ligase buffer, 1X probe mix, 0.5 µl
ligase and 100–200 ng DNA sample. The ligation reaction was
performed using an ABI 2720 thermal cycler (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) under the
following cycling conditions: 2 min at 98°C, followed by 5 cycles
of 1 min at 94°C, 3 h at 60°C, 2 min at 94°C, hold at 58°C and
immediately stopped with the addition of 20 µl 20 mM EDTA.
Multiplex fluorescence PCR reactions were performed for each
ligation product. Each PCR mixture was prepared at a volume of 20
µl containing, 1X PCR buffer, 1 µl primer mix, 0.3 µl heat
activated Taq DNA polymerase and 1 µl ligation product. The PCR
cycling conditions were as follows: 2 min at 95°C; 9 cycles of 20
sec at 94°C, 40 sec cycle at 63–0.5°C and 1.5 min at 72°C; 25
cycles of 20 sec at 94°C, 40 sec at 60°C, and 1.5 min at 72°C; 1 h
at 68°C; and hold at 4°C. PCR amplification with blue and red
fluorescent dye modified universal primers were performed. The red
fluorescence dye was acridine orange and the blue fluorescence dye
was 4′,6-diamidino-2-phenylindole (both from Shanghai Genesky
Biotech, Co., Ltd.). PCR products were separated and detected by
capillary electrophoresis on an ABI 3130XL sequencer (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Raw data were analyzed
using GeneMapper v4.0 (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The genotypes at each locus were determined
based on the color of the dye label and the fragment sizes of the
allele-specific ligation PCR products. For quality control, the
assay was randomly repeated for 4% of the samples and concordant
results were obtained. All SNPs were genotyped successfully with a
call rate of >98%.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Intergroup differences in
rate or frequency were compared using the two-tailed χ2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Results overview
The patient cohort consisted of 94 Hui [age,
12.0±3.1 (range 3–35) years; female/male ratio, 54/40], 96 Tibetan
[age, 12.4±2.8 (range 8–30) years; female/male ratio, 45/51] and 93
Tu [age, 13.8±3.2 (range 4–25) years; female/male ratio, 44/49]
individuals. A total of 32/283 patients had a family history of HI
(16 Hui, 12 Tibetan and 4 Tu individuals). The remaining 251
patients were diagnosed with sporadic HI. For the control group,
150 individuals with normal hearing from the same region as the
case group were recruited, including Hui (n=50), Tibetan (n=50) and
Tu (n=50) individuals. The mutation detection rates of the three
deafness-related genes in the three ethnicities are presented in
Table I. A representative section
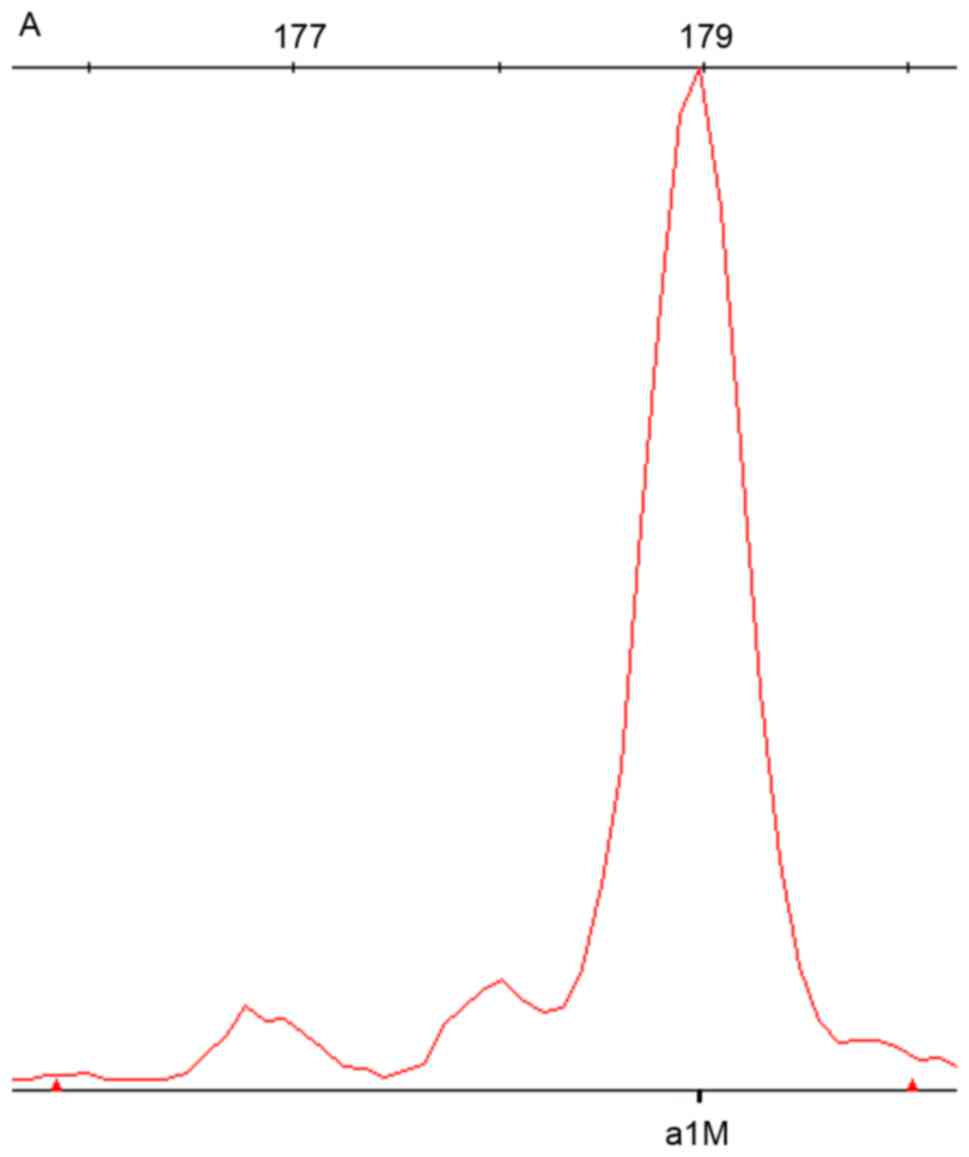
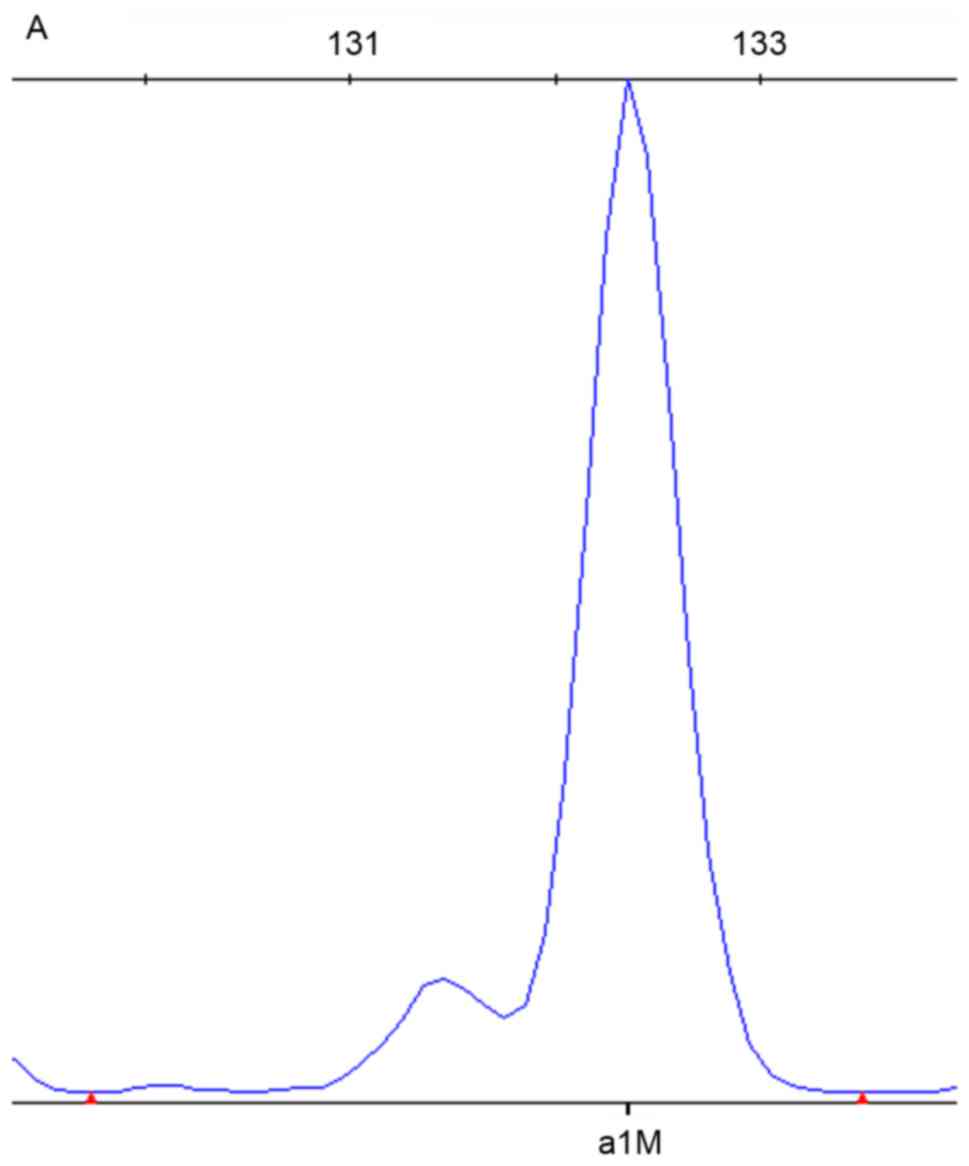
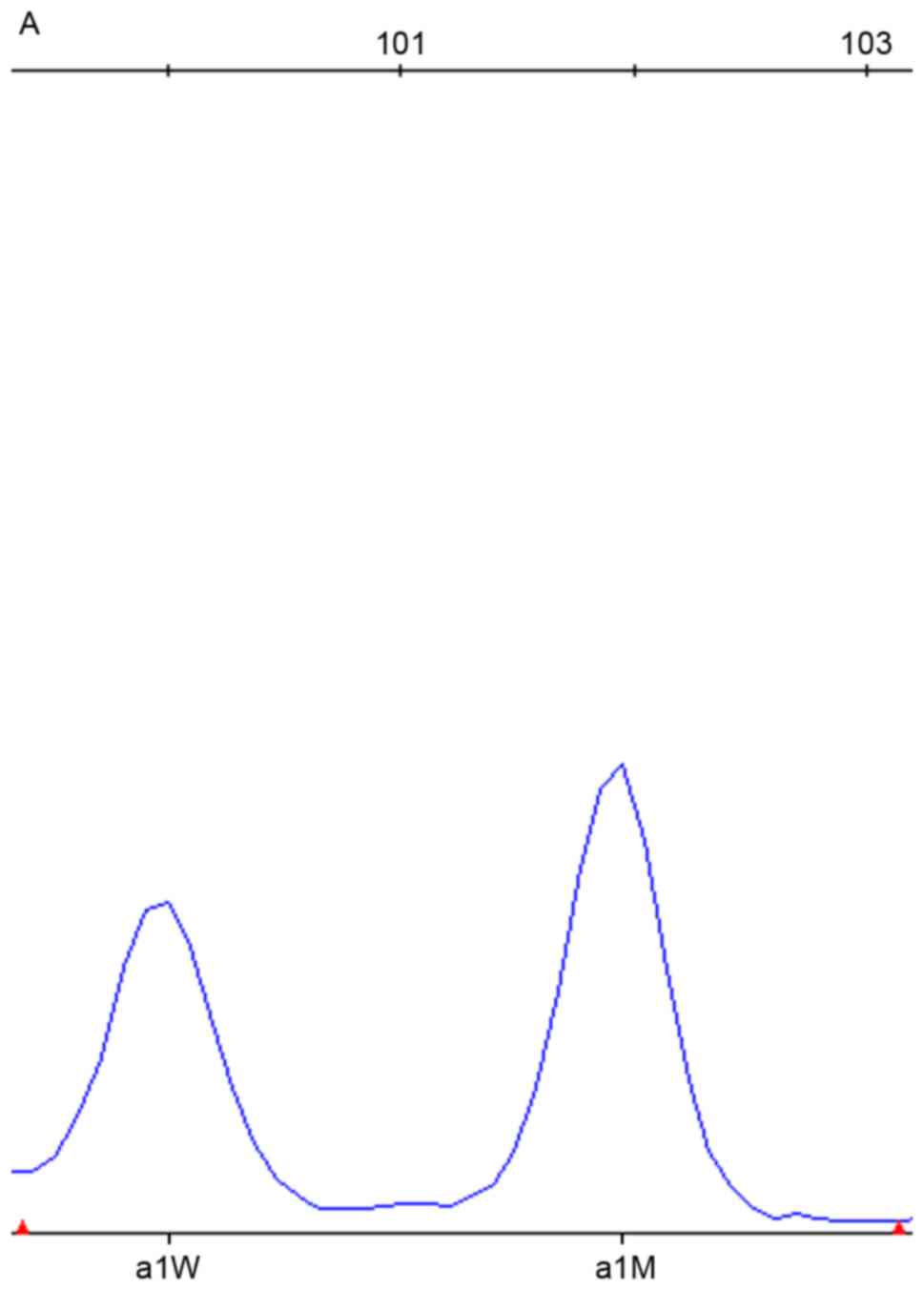
of the SNPscan results are depicted in Figs. 1–3. DNA samples with the mtDNA12SrRNA
m.1494C>T mutation were detected by the red dye labeled probe
set in the assay; the wild-type is presented in Fig. 1. DNA samples heterozygous for the
235delC mutation in GJB2 were detected by the blue dye labeled
probe set in the assay; the wild-type is presented in Fig. 2. DNA samples homozygous for the
919-2A>G mutation in SLC26A4 were detected by the blue dye
labeled probe set in the assay; the wild-type is presented in
Fig. 3.
 | Table I.The detection rates of GJB2, SLC26A4
and mtDNA12SrRNA mutations in 283 Hui, Tibetan and Tu patients with
nonsyndromic hearing impairment. |
Table I.
The detection rates of GJB2, SLC26A4
and mtDNA12SrRNA mutations in 283 Hui, Tibetan and Tu patients with
nonsyndromic hearing impairment.
| Gene | GJB2 | SLC26A4 | mtDNA12SrRNA |
|---|
|
|
|
|
|---|
| Ethnicity | No. of mutations | Rate (%) | No. of mutations | Rate (%) | No. of mutations | Rate (%) |
|---|
| Hui | 14 | 14.89 | 10 | 10.64 | 1 | 1.06 |
| Tibetan | 9 | 9.37 | 6 | 6.25 | 5 | 5.21 |
| Tu | 11 | 11.83 | 8 | 8.6 | 5 | 5.38 |
| Total | 34 | 12.01 | 24 | 8.48 | 11 | 3.89 |
GJB2
A total of 6GJB2 variants were identified in the
patient cohort (Table II), of
which5 were pathogenic, including c.299_300delAT, c.235delC,
c.35delG, c.176_191del16 and c.257C>G. The c.109G>A SNP was a
nucleotide change of unknown significance. The detection rate of
GJB2 mutations in the Hui patients was 14.89% (14/94), and five
variants were observed, these were: c.235delC, c.299_300delAT,
c.109G>A, c.35delG and c.176_191del16, with allele frequencies
of 6.91% (13/188), 2.66% (5/188), 2.13% (4/188), 0.53% (1/188) and
0.53% (1/188), respectively. The c.235delC SNP was the most
prevalent mutation, accounting for 54.17% of all mutant alleles.
The detection rate of GJB2 mutations was 9.37% (9/96) in the
Tibetan patients, and four variants were detected, these were:
c.109G>A, c.235delC, c.299_300delAT and c.257C>G, with allele
frequencies of 2.6% (5/192), 2.08% (4/192), 0.52% (1/192) and 0.52%
(1/192), respectively. The c.109G>A SNP exhibited the highest
allele frequency, accounting for 45.45% of all mutant alleles. The
detection rate of GJB2 mutations was 11.83% (11/93) in the Tu
patients, and three variants were observed, these were: c.235delC,
c.109G>A and c.176_191del16, with allele frequencies of 8.06%
(15/186), 1.07% (2/186) and 0.54% (1/186), respectively. The
c.235delC SNP was the most prevalent mutation, accounting for
83.33% of all mutant alleles.
 | Table II.Gap junction protein β2 genotypes of
283 minority patients with nonsyndromic hearing impairment. |
Table II.
Gap junction protein β2 genotypes of
283 minority patients with nonsyndromic hearing impairment.
| Genotype | Hui patients (n) | Tibetan patients
(n) | Tu patients (n) |
|---|
| 235delC/235delC | 5 | 1 | 6 |
|
299_300delAT/299_300delAT | 2 | 0 | 0 |
|
235delC/299_300delAT | 1 | 0 | 0 |
| 35delG/235delC | 1 | 0 | 0 |
|
176_191del16/235delC | 1 | 0 | 1 |
|
257C>G/299_300delAT | 0 | 1 | 0 |
| 235delC/wt | 0 | 2 | 2 |
| 109G>A/wt | 4 | 5 | 2 |
| Total | 14 | 9 | 11 |
No significant differences in the detection rate of
GJB2 mutations were found amongst the three ethnic groups
(P>0.05). A significant difference in the allele frequency of
the c.235delC mutation was observed between the Hui and Tibetan
patients (χ2=5.189, P<0.05), and between the Tu and
Tibetan patients (χ2=7.08, P<0.05), however, no
significant difference in this frequency was detected between the
Hui and Tu patients (χ2=0.178, P>0.05). In the
control group, four individuals (2.67%, 4/150) were revealed to be
heterozygous carriers of GJB2 mutations, including one with
c.235delC, one with c.299_300delAT, and two with c.109G>A.
SLC26A4
A total of 7 mutations were identified in this
cohort, including c.919-2A>G, c.2168A>G, c.1226G>A,
c.754T>C, c.1520delT, c.1517T>G and c.249G>A, (Table III), The detection rate of
SLC26A4 mutations in the Hui patients was 10.64% (10/94), the
identified SNPs included c.919-2A>G, c.2168A>G, c.1520delT,
c.1517T>G and c.249G>A. The allele frequency of c.919-2A>G
was the highest at 4.79% (9/188), followed by c.1517T>G at 2.13%
(4/188), c.249G>A at 1.06% (2/188), c.2168A>G at 0.53%
(1/188) and c.1520delT at 0.53% (1/188). Thec.919-2A>G and
c.1517T>G mutations were the most prevalent in the Hui patients,
accounting for 52.94% and 23.53% of all mutant alleles,
respectively. The detection rate of SLC26A4 mutations was 6.25%
(6/96) in the Tibetan patients. The identified SNPs included
c.919-2A>G, c.2168A>G and c.1226G>A. The allele frequency
of c.919-2A>G was the highest at 3.12% (6/192), followed by
c.1226G>A at 1.04% (2/192) and c.2168A>Gat 0.52% (1/192). The
c.919-2A>G and c.1226G>A mutations were the most prevalent in
the Tibetan patients, accounting for 66.67% and 22.22% of all
mutant alleles, respectively. The detection rate of SLC26A4
mutations was 8.6% (8/93) in the Tu patients. The identified SNPs
included c.919-2A>G, c.2168A>G and c.754T>C. The allele
frequency of c.919-2A>G was the highest at 6.45% (12/186),
followed by c.2168A>G at 1.07% (2/186) and c.754T>C at 0.54%
(1/186). The c.919-2A>G and c.2168A>G mutations were the most
prevalent in the Tu patients, accounting for 80% and 13.33% of all
mutant alleles, respectively.
 | Table III.Solute carrier family 26 member 4
genotypes of 283 minority patients with nonsyndromic hearing
impairment. |
Table III.
Solute carrier family 26 member 4
genotypes of 283 minority patients with nonsyndromic hearing
impairment.
| Genotype | Hui patients
(n) | Tibetan patients
(n) | Tu patients
(n) |
|---|
|
919-2A>G/919-2A>G | 3 | 1 | 5 |
|
919-2A>G/2168A>G | 0 | 1 | 1 |
|
754T>C/919-2A>G | 0 | 0 | 1 |
|
1226G>A/1226G>A | 0 | 1 | 0 |
| 1520
delT/2168A>G | 1 | 0 | 0 |
|
1517T>G/1517T>G | 2 | 0 | 0 |
| 249
G>A/249G>A | 1 | 0 | 0 |
| 919-2A>G/wt | 3 | 3 | 0 |
| 2168A>G/wt | 0 | 0 | 1 |
| Total | 10 | 6 | 8 |
No significant differences in the detection rate of
SLC26A4 mutations were found amongst the three ethnic groups
(P>0.05). Furthermore, no significant differences in the allele
frequency of the c.919-2A>G mutation were found amongst the
three ethnic groups (P>0.05). In the control group, two
individuals (1.33%, 2/150) were heterozygous for the c.919-2A>G
mutation.
mtDNA12SrRNA
Three Tibetan patients (3.12%, 3/96) carried the
m.1555A>G mutation, and two (2.08%, 2/96) possessed the
m.1494C>T mutation. Five Tu patients (5.38%, 5/93) and one Hui
patient (1.06%, 1/94) carried the m.1555A>G mutation. A total of
10/11 patients with the m.1555A>G or the m.1494C>T mutations
had a history of aminoglycoside use. All of the mutations detected
in mtDNA12SrRNA were in a homoplasmic state. No significant
differences in the mutation rates of mtDNA12SrRNA were observed
amongst the three ethnic groups (P>0.05). None of the 150
control individuals were found to carry the m.1555A>G mutation
or the m.1494C>T mutation.
Discussion
The present study performed mutation analysis using
the SNPscan technique to evaluate 283 patients with moderate to
profound sensorineural NSHI. The detection rates of GJB2 mutations
were 14.89, 9.37 and 11.83% in the Hui, Tibetan and Tu patients,
respectively. The detection rates of SLC26A4 mutations were 10.64,
6.25 and 8.6% in the Hui, Tibetan and Tu patients, respectively.
The mutations rates of mtDNA12SrRNA in the Hui, Tibetan and Tu
patients were 1.06, 5.21 and 5.38%, respectively. No significant
differences in the detection rates of GJB2, SLC26A4 and
mtDNA12SrRNA mutations were found amongst the three ethnic groups.
Notably, the mutation spectra of the common deafness-related genes
differed amongst the three ethnic groups.
Previous reports have suggested that mutations in
GJB2 are the most common causes of NSHI in several populations
(11,12). The prevalence of GJB2 mutations
appears to vary amongst different ethnic groups. The results of
this study indicated that the detection rates of GJB2 mutations in
the Hui, Tibetan and Tu patients were 14.89, 9.37, and 11.83%,
respectively. However, Dai et al (13) reported that the detection rate of
GJB2 mutations was 19.1% (313/1640) in Han Chinese NSHI patients
from different regions of China. A significant difference in this
rate was observed between the Tibetan patients and the
aforementioned Han Chinese patients (χ2=5.66,
P<0.05), consistent with the report of Dai et al
(13). No significant differences
were discovered between the Han Chinese group and Hui and Tu ethnic
groups in the present study (P>0.05). The mutation spectra of
GJB2 are distinct amongst HI populations of different ethnicities.
Numerous studies have demonstrated that c.35delG, c.167delT and
c.235delC are the most prevalent mutations in Caucasians, Ashkenazi
Jews and a pan-Asian cohort, respectively (14). In the present study, c.235delC was
the most prevalent mutation in Hui and Tu patients. This finding is
consistent with the observation that the c.235delC mutation is the
most common mutation in Eastern Asians (15). Furthermore, the allele frequency of
c.235delC was lower in the Tibetan patients compared with the Hui
and Tu patients, in agreement with the report of Dai et al
(15). The c.109G>A mutation
exhibited the highest allele frequency in the Tibetan patients.
This mutation is common in East Asians, with an allele frequency of
6.7% (185/2744) in Han Chinese patients with NSHI (13). No statistical difference in this
frequency was observed between the Tibetan patients and the
aforementioned Han Chinese patients (χ2=2.275,
P>0.05). The pathogenicity of the c.109G>A variant is
controversial; recent research has revealed that it is associated
with mild to moderate hearing impairment (16–17).
In the present study, the allele frequency of c.109G>A was 2.6%
(5/192) in the Tibetan patients and 2% (1/50) in the Tibetan
control subjects; no significant difference in this frequency was
found between the Tibetan patient and control groups
(χ2=0.06, P>0.05).
Mutations in SLC26A4 are responsible for both
syndromic and nonsyndromic hearing impairment; they are the second
most common genetic cause of NSHI in China (5). The prevalence of SLC26A4 mutations
varies amongst different ethnic groups. In the present study, the
detection rates of SLC26A4 mutations were 10.64, 6.25 and 8.6% in
the Hui, Tibetan and Tu patients, respectively. Yuan et al
(18) reported that the detection
rate of SLC26A mutations was 16.55% (315/1903) in Han Chinese NSHI
patients from different regions of China. A significant difference
in this rate was found between the Han Chinese patients and Tibetan
patients (χ2=7.197, P<0.05), this finding is
consistent with a report by Yuan et al (18) that the detection rate of SLC26A4
mutations in the Tibetan HI population was significantly lower
compared with the Han Chinese population. No significant
differences were observed between the aforementioned Han Chinese
group and Hui and Tu ethnic groupsin the present study (P>0.05).
The mutation spectra of SLC26A4 varied amongst different ethnic
groups. Previous studies have indicated that the most common
mutations in the SLC26A4 gene are p.T416P and IVS8+1G>A in
northern European populations and c.2168A>G in Japanese or
Korean populations (19–21), whereas the most common SLC26A4
mutations are c.919-2A>G and c.2168A>G in Han Chinese
populations (22,23). In the present study, it was
observed that different ethnicities demonstrated distinct SLC26A4
mutation spectra. The c.919-2A>G was the most common mutation in
the Hui, Tibetan and Tu patients, however, c.1517T>G,
c.1226G>A and c.2168A>G were the second most common mutations
in these patients, respectively. These results suggest that ethnic
background is a significant factor contributing to differences in
the SLC26A4 mutation spectra amongst these ethnicities.
The A1555 G and C1494T mutations in mtDNA12SrRNA
have been associated with aminoglycoside-induced deafness and
nonsyndromic hearing impairment in several families of different
ethnicities (24–27). In particular, the A1555 G mutation
is the most commonly detected, and its prevalence fluctuates with
ethnic variation (28). Compared
with the A1555 G mutation, the C1494T mutation exhibits a lower
carrier frequency in Chinese patients with NSHI (29). Published reports have indicated
that the incidence of the m.1555A>G mutation is higher in Asian
HI populations compared with Caucasian populations (30). In the present study, the mutation
rates of m.1555A>G were 3.12, 5.38 and 1.06% in the Hui, Tibetan
and Tu patients, respectively. Guo et al (31) reported that the mutation rate of
m.1555A>G was 6.09% (113/1856) in Han Chinese NSHI patients in
northwest China. No significant differences in this rate were
observed between the aforementioned Han Chinese group and Tibetan
and Tu ethnic groups in the present study (P>0.05). However,
significant differences were observed between the Han Chinese
patients and Hui patients (χ2=4.103, P<0.05). The
differences in the m.1555A>G mutation rate may be due to
geographical and environmental differences. Given that ethnic
background influences the spectrum of gene mutations present, the
western Eurasian-specific haplogroup frequency of mtDNAs in the Hui
population was 6.7%; however, no western Eurasian type was found in
Han samples from the same region (32). Furthermore, the A1555 G and C1494T
mutations in the highly conserved coding site of mtDNA12SrRNA are
the predominant ototoxic targets of aminoglycoside antibiotics,
especially the A1555 G mutation. These mutations increase
sensitivity to aminoglycoside ototoxicity, thus leading to these
ototoxic effects (33). In the
present study, 10/11 patients with A1555 G or C1494T mutations had
a history of aminoglycoside use. One Tibetan patient without a
history of aminoglycoside exposure carried the C1494T mutation and
exhibited late-onset deafness. Therefore, it may be hypothesized
that active genetic counseling and intervention combined with
avoidance of aminoglycoside use in patients with HI, and their
matrilineal relatives, may limit the occurrence of HI.
The present study demonstrated that mutation
screening using the SNPscan assay is a powerful and effective
method for the evaluation of a large deaf cohort. However, the
sample size of the present study was too small to allow for final
conclusions to be made, and more patients with nonsyndromic hearing
impairment will be recruited for future studies. A total of 24.38%
of the patients with HI demonstrated evidence of genetic
involvement; 12.01, 8.48 and 3.89% of the patients demonstrated
inherited hearing impairment caused by GJB2, SLC26A4 and
mtDNA12SrRNA mutations, respectively. Furthermore, the mutation
spectra of the common deafness-related genes differed amongst HI
populations of the Hui, Tibetan and Tu ethnicities. The information
reported in this study will be helpful in designing a protocol for
genetic testing for HI, and for achieving accurate molecular
diagnoses in northwest China.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172765).
References
|
1
|
Morton NE: Genetic epidemiology of hearing
impairment. Ann N Y Acad Sci. 630:16–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bitner-Glindzicz M: Hereditary deafness
and phenotyping inhumans. Br Med Bull. 63:73–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo YF, Liu XW, Guan J, Han MK, Wang DY,
Zhao YL, Rao SQ and Wang QJ: GJB2, SLC26A4 and mitochondrial DNA
A1555G mutations in prelingual deafness in Northern Chinese
subjects. Acta Otolaryngol. 128:297–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan Y, You Y, Huang D, Cui J, Wang Y,
Wang Q, Yu F, Kang D, Yuan H, Han D and Dai P: Comprehensive
molecular etiology analysis of nonsyndromic hearing impairment from
typical areas in China. J Transl Med. 7:792009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xin F, Yuan Y, Deng X, Han M, Wang G, Zhao
J, Gao X, Liu J, Yu F, Han D and Dai P: Genetic mutations in
nonsyndromic deafness patients of Chinese minority and Han
ethnicities in Yunnan, China. J Transl Med. 11:3122013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Li S, Yang Y, Yang X, Liu Y, Liu
Y, Hu W, Jin L and Wang X: Genome-wide association study validation
identifies novel loci for atherosclerotic cardiovascular disease. J
Thromb Haemost. 10:1508–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang LL, Chen FY, Wang H, Hu XL, Dai X,
Mao J, Shen ZT, Wu YH, Wang SM, Hai J, et al: Haplotype analysis of
eight genes of the monoubiquitinated FANCD2-DNA damage-repair
pathway in breast cancer patients. Cancer Epidemiol. 37:311–317.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin J, Wang L, Shi Y, Shao A, Tang W, Wang
X, Ding G, Liu C, Chen S and Gu H: Interleukin 17A rs4711998 A>G
polymorphism was associated with a decreasedrisk of esophageal
cancer in a Chinese population. Dis Esophagus. 27:87–92. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erenberg A, Lemons J, Sia C, Trunkel D and
Ziring P: Newborn and infant hearing loss: Detection and
intervention. American Academy of Pediatrics. Task Force on Newborn
and Infant Hearing, 1998–1999. Pediatrics. 103:527–530.
1999.PubMed/NCBI
|
|
10
|
Du W, Cheng J, Ding H, Jiang Z, Guo Y and
Yuan H: A rapid method for simultaneous multi-gene mutation
screening in children with nonsyndromic hearing loss. Genomics.
104:264–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilcox SA, Saunders K, Osborn AH, Arnold
A, Wunderlich J, Kelly T, Collins V, Wilcox LJ, Gardner RJ
McKinlay, Kamarinos M, et al: High frequency hearing loss
correlated with mutations in the GJB2 gene. Hum Genet. 106:399–405.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Estivill X, Fortina P, Surrey S, Rabionet
R, Melchionda S, D'Agruma L, Mansfield E, Rappaport E, Govea N,
Milà M, et al: Connexin-26 mutations in sporadic and inherited
sensorineural deafness. Lancet. 351:394–398. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai P, Yu F, Han B, Liu X, Wang G, Li Q,
Yuan Y, Liu X, Huang D, Kang D, et al: GJB2 mutation spectrum in
2,063 Chinese patients with nonsyndromic hearing impairment. J
Transl Med. 7:262009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kenneson A, Van Naarden Braun K and Boyle
C: GJB2 (connexin 26) variants and nonsyndromic sensorineural
hearing loss: A HuGE review. Genet Med. 4:258–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai P, Yu F, Han B, Yuan Y, Li Q, Wang G,
Liu X, He J, Huang D, Kang D, et al: The prevalence of the 235delC
GJB2 mutation in a Chinese deaf population. Genet Med. 9:283–289.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Lu J, Tao Z, Huang Q, Chai Y, Li X,
Huang Z, Li Y, Xiang M, Yang J, et al: The p.V37I exclusive
genotype of GJB2: A genetic risk indicator of postnatal permanent
childhood hearing impairment. PLoS One. 7:e366212012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gallant E, Francey L, Tsai EA, Berman M,
Zhao Y, Fetting H, Kaur M, Deardorff MA, Wilkens A, Clark D, et al:
Homozygosity for the V37I GJB2 mutation in fifteen probands with
Mild to Moderate sensorineural hearing impairment: Further
confirmation of pathogenicity and haplotype analysis inAsian
populations. Am J Med Genet. 161A:1–2157. 2013.PubMed/NCBI
|
|
18
|
Yuan Y, Guo W, Tang J, Zhang G, Wang G,
Han M, Zhang X, Yang S, He DZ and Dai P: Molecular epidemiology and
functional assessment of novel allelic variants of SLC26A4 in
nonsyndromic hearing loss patients with enlarged vestibular
aqueduct in China. PLoS One. 7:e499842012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campbell C, Cucci RA, Prasad S, Green GE,
Edeal JB, Galer CE, Karniski LP, Sheffield VC and Smith RJ: Pendred
syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel
mutations and possible genotype-phenotype correlations. Hum Mutat.
17:403–411. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsukamoto K, Suzuki H, Harada D, Namba A,
Abe S and Usami S: Distribution and frequencies of PDS (SLC26A4)
mutations in Pendred syndrome and nonsyndromic hearing loss
associated with enlarged vestibular aqueduct: A unique spectrum of
mutations in Japanese. Eur J Hum Genet. 11:916–922. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz
S, Ghosh M, Kim HN, Moon SK, Abe S, Tukamoto K, et al: Origins and
frequencies of SLC26A4 (PDS) mutations in east and south Asians:
Global implications for the epidemiology of deafness. J Med Genet.
40:242–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang QJ, Zhao YL, Rao SQ, Guo YF, Yuan H,
Zong L, Guan J, Xu BC, Wang DY, Han MK, et al: A distinct spectrum
of SLC26A4 mutations in patients with enlarged vestibular aqueduct
in China. Clin Genet. 72:245–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai P, Li Q, Huang D, Yuan Y, Kang D,
Miller DT, Shao H, Zhu Q, He J, Yu F, et al: SLC26A4 c.919-2A>G
varies among Chinese ethnic groups as a cause of hearing loss.
Genet Med. 10:586–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jacobs HT, Hutchin TP, Käppi T, Gillies G,
Minkkinen K, Walker J, Thompson K, Rovio AT, Carella M, Melchionda
S, et al: Mitochondrial DNA mutations in patients with postlingual,
nonsyndromic hearing impairment. Eur J Hum Genet. 13:26–33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li R, Xing G, Yan M, Cao X, Liu XZ, Bu X
and Guan MX: Cosegregation of C-insertion at position 961 with
A1555G mutation of mitochondrial 12S rRNA gene in a large Chinese
family with maternally inherited hearing loss. Am J Med Genet A.
124A:1–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Young WY, Yan Q, Li R, Cao J, Wang
Q, Li X, Peters JL, Han D and Guan MX: Functional characterization
of the mitochondrial 12S rRNA C1494T mutation associated with
aminoglycoside-induced and non-syndromic hearing loss. Nucleic
Acids Res. 33:1132–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan HJ, Chen J, Liu X, Cheng J, Wang X,
Yang L, Yang S, Cao J, Kang D, Dai P, et al: Coexistence of
mitochondrial 12S rRNA C1494T and CO1/tRNA(Ser(UCN)) G7444A
mutations in two Han Chinese pedigrees with aminoglycoside-induced
and non-syndromic hearing loss. Biochem Biophys Res Commun.
362:94–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prezant TR, Agapian JV, Bohlman MC, Bu X,
Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, et
al: Mitochondrial ribosomal RNA mutation associated with both
antibiotic-induced and non-syndromic deafness. Nat Genet.
4:289–294. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Yuan YY, Huang DL, Han DY and Dai P:
Rapid screening for the mitochondrial DNA C1-494T mutation in a
deaf population in China using real-time quantitative PCR. Acta
Otolaryngol. 132:814–818. 2012.PubMed/NCBI
|
|
30
|
Li R, Greinwald JH Jr, Yang L, Choo DI,
Wenstrup RJ and Guan MX: Molecular analysis of the mitochondrial
12S rRNA and tRNASer(UCN) genes in paediatric subjects with
non-syndromic hearing loss. J Med Genet. 41:615–620. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo YF, Liu XW, Xu BC, Zhu YM, Wang YL,
Zhao FF, Wang DY, Zhao YL, Ji YB and Wang QJ: Analysis of a
large-scale screening of mitochondrial DNA m.1555A>G mutation in
2417 deaf-mute students in northwest of China. Genet Test Mol
Biomarkers. 14:527–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao YG, Kong QP, Wang CY, Zhu CL and Zhang
YP: Different matrilineal contributions to genetic structure of
ethnic groupsin the silk road region in China. Mol Biol Evol.
21:2265–2280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao H, Li R, Wang Q, Yan Q, Deng JH, Han
D, Bai Y, Young WY and Guan MX: Maternally inherited
aminoglycoside-induced and nonsyndromic deafnessis associated with
the novel C1494T mutation in the mitochondrial12S rRNA gene in a
large Chinese family. Am J Hum Genet. 74:139–152. 2004. View Article : Google Scholar : PubMed/NCBI
|