Introduction
Rheumatoid arthritis (RA) is a chronic systemic
inflammatory disease with etiology that remains to be fully
elucidated. At present, it is widely recognized that RA is an
autoimmune disease. The disease is often presented in facet joints,
including hands, wrists and feet with symmetric distribution
(1). Patients at an early stage of
the disease, often present with pain in red, swelled and heated
joints and functional barriers, whereas patients at an advanced
stage of the disease present ankylosis or malformation at varying
degrees, accompanied with atrophy of bone muscle, bone necrosis and
numerous disabling symptoms (2).
The specific pathogenesis of the disease is not clear and may be
associated with multiple factors, including social differences,
geographical conditions, internal secretion alterations, vocational
status, nutrition and metabolic disturbance and bacterial and viral
infections (3). RA as of yet has
no effective treatment and its therapeutic regimen primarily
involves relieving pain and inflammation (4).
Arthrocele at early stage results from synovium
congestion, edema and exudation of articular cavity, in addition to
periarticular tissue edema (5). In
rheumatic synovitis, fenestra expansion of synovial endothelial
cells and stimulation of inflammatory mediators, including
histamine, bradykinin, prostaglandin E2, free radicals and
neurosecretion of substance P result in hemangiectasis, increase in
capillary permeability and exudation, which lead to synovial
membrane and periarticular tissue edema (6). Collagen and protein polysaccharide
degradation are mediated by cytokines interleukin (IL)-1, IL-6,
IL-8 and tumor necrosis factor (TNF)-α, chondrocyte swelling and
degeneration are mediated by nitric oxide (NO) and NO-induced
oxygenase expression in cartilage (5,7).
Adhesion of hemameba, T cells, cell endothelial cells and
fibrinogen mediated by adhesion molecules promote formation of a
fibrin clot, resulting in disturbed blood vessel function and a
subsequent synovium inflammatory response (8). Growth hormone inhibiting factor,
TNF-α and IL increase in synovium results in increasing T cell
expression with autoantigen reactivity, resulting in arthritic
symptoms (9).
The phosphoinositide 3-kinase (PI3K), Akt
serine/threonine kinase (Akt) signaling pathway is an important
intracellular transduction pathway and has previously been
demonstrated to be associated with the development of RA (10). The active state of the signaling
pathway is tightly regulated by the negative regulatory factor
phosphatase and tensin homolog (PTEN), and the regulatory factors
TNF-α, transforming growth factor (TGF)-β and TNF-related
apoptosis-inducing ligand (11).
In the synovial cells of RA patients, the signaling pathway is
maintained in the abnormal activated state, resulting in high
expression of downstream anti-apoptosis genes and a subsequent
impact on multiple downstream effector molecules (12). Furthermore, it is important in the
synovial cell proliferation and apoptotic imbalance of RA patient
joints. Excessively-grown synovial cells infiltrate and grow in the
cartilage articularis and bone tissues, resulting in joint
deformity and dysfunction. Therefore, the inhibition of the
PI3K-AKT signal pathway may negatively regulate proteins, reverse
excessive proliferation of RA synoviocytes, and may provide a novel
target for curing the disease (13).
The primary constituents of gamboge are composed of
70–80% resin, 15–25% gum and include gambogic acid, neogambogic
acid, allogambogic acid, morellin, isomorellin, morellic acid and
isomorellic constituents. Gambogic acid is the primary effective
constituent (14). The
pharmacological and pharmacokinetic properties of preparation of
gambogic acid have previously been studied (15). The anti-tumor effects of gambogic
acid involve induction of tumor cell apoptosis, restraining the
cell cycle and impacting oncogenes, cancer suppressor genes and
expression of associated proteins (16). The present study therefore aimed to
investigate the anti-inflammatory effects of gambogic acid in RA
rats and to reveal the potential signaling pathways involved.
Materials and methods
Experimental rat model
All study protocols employed were in accordance with
the guidelines of the Animal Care and Use Committee of Baodi
District People's Hospital of Tianjin City (Tianjin, China). The
study was approved by the Ethics Committee of Sichuan Provincial
People Hospital (Chengtu, China). A total of 30 male Sprague-Dawley
rats (age, 8–10 weeks; weight, 250–300 g) were obtained from
Laboratory Animal Center of Tianjin Medical University (Tianjin,
China) and maintained in individual cages under standard conditions
(temperature, 22–23°C; humidity, 55–60%; 12-h light/dark cycle),
and provided with free access to food and water.
Grouping and model establishment
The experimental rats were randomly divided into 5
groups (6 rats/group): Sham, RA model, gambogic acid (1 mg/kg/day),
gambogic acid (5 mg/kg/day) and gambogic acid (10 mg/kg/day) groups
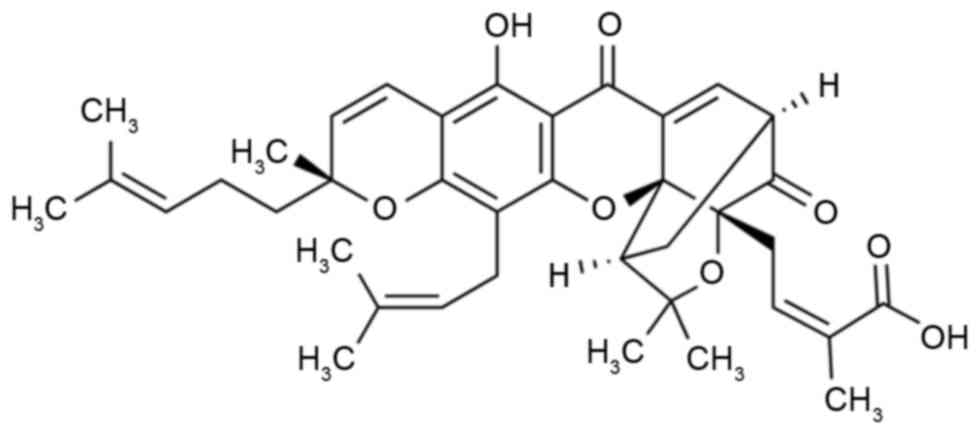
for 3 weeks. The chemical structure of gambogic acid (≥95%, high
performance liquid chromatography) is indicated in Fig. 1 and was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). In the RA model,
gambogic acid (1 mg/kg/day), gambogic acid (5 mg/kg/day) and
gambogic acid (10 mg/kg/day) groups, rats were anesthetized with an
intraperitoneal injection of 40 mg/kg sodium pentobarbital
(Sigma-Aldrich; Merck KGaA). A total of 10 mg/ml Freund's complete
adjuvant (Sigma-Aldrich; Merck KGaA) was subcutaneously injected
into the 2nd and 3rd toes of the right foot. The right ankle then
demonstrated acute inflammatory swelling.
Swelling degree measurement
The posterior right limb was inserted in 20 ml
water, and the volume of displaced water was measured as
representative of foot volume. The swelling degree was measured as
follows: (Foot volume 7 days following RA induction-foot volume
prior to RA induction)/(foot volume prior to RA
induction)x100%.
Clinical arthritic scoring
measurement
The rats were observed according to a macroscopic
scoring system, as follows: 0, no sign of arthritis; 1–5, 2 joints
involved; 6–10, >2 joints involved; 11–15; severe arthritis of
the entire paw and digits. Clinical arthritic scoring was performed
three times in each session.
Pain threshold measurement
Pain threshold measurement was conducted as
previously described (17). The
pressure pain threshold (g) was detected using an electronic
pressure pain detector (Somedic AB, Sösdala, Sweden). Pain
threshold measurement was conducted three times in each
session.
Inflammatory effects measurement
Following sacrifice of rats using 100 mg/kg sodium
pentobarbital (Sigma-Aldrich; Merck KGaA), blood was extracted from
the inferior vena cava (3 ml) and centrifuged at 3,000 × g for 10
min at 4°C. The supernatant was used to analyze IL-1β (cat. no.
ER008-96) and IL-6 (cat. no. ER003-96) activities with ELISA kits,
according to the manufacturer's protocol (Genetimes Technology,
Inc., Shanghai, China).
Western blotting
The synovium was digested with
radioimmunoprecipitation assay lysis buffer (Roche Diagnostics,
Basel, Switzerland) at 4°C for 30 min and centrifuged at 12,000 × g
for 10 min at 4°C. The supernatant was used to analyze the protein
content using a bicinchoninic acid kit (Fermentas; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Equal amounts of extracted
protein samples (50 µg) were separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked using 5% skim milk
powder in TBS containing 0.1% Tween-20 at 37°C for 1 h and
incubated with the following primary antibodies at 4°C overnight:
Anti-Akt (1:300; cat. no. sc-8312), anti-phosphorylated (p)-Akt
(1:500; cat. no. sc-7985-R), anti-mammalian target protein of
rapamycin (mTOR; 1:300; cat. no. sc-8319), anti-p-mTOR (1:500; cat.
no. sc-101738), anti-vascular endothelial growth factor (VEGF;
1:300; cat. no. sc-13083), anti-hypoxia-inducible factor-1α
(HIF-1α; 1:300; cat. no. sc-10790) and anti-GAPDH (1:300; cat. no.
sc-367714), all obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Membranes were then incubated with goat
anti-rabbit immunoglobulin G (1:2,000; cat. no. 14708; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 37°C for 1 h.
Protein bands were visualized using enhanced chemiluminescence
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Blots were
semi-quantified using ImageJ software version 1.41 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
of 3 independent experiments. One-way analysis of variance followed
by the Tukey's post hoc test was used to determine the differences
among groups using SPSS software, version 11.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
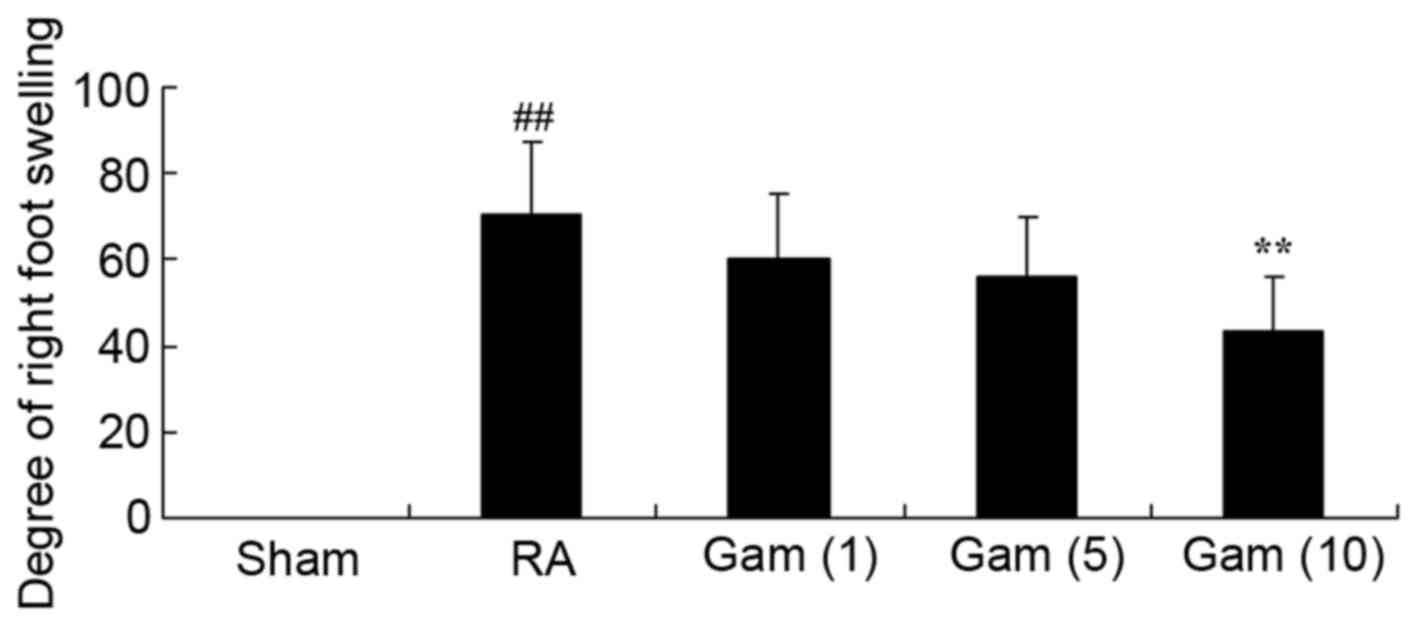
Gambogic acid decreases degree of
right foot swelling
The constitutional formula of gambogic acid is
presented in Fig. 1. The present
study firstly examined the potential effects of gambogic acid on
the degree of right foot swelling in RA rats. The degree of right
foot swelling was increased in the RA model group compared with
sham group (Fig. 2). The results
demonstrated that treatment with gambogic acid at 10 mg/kg/day
significantly inhibited the degree of right foot swelling in RA
rats (Fig. 2).
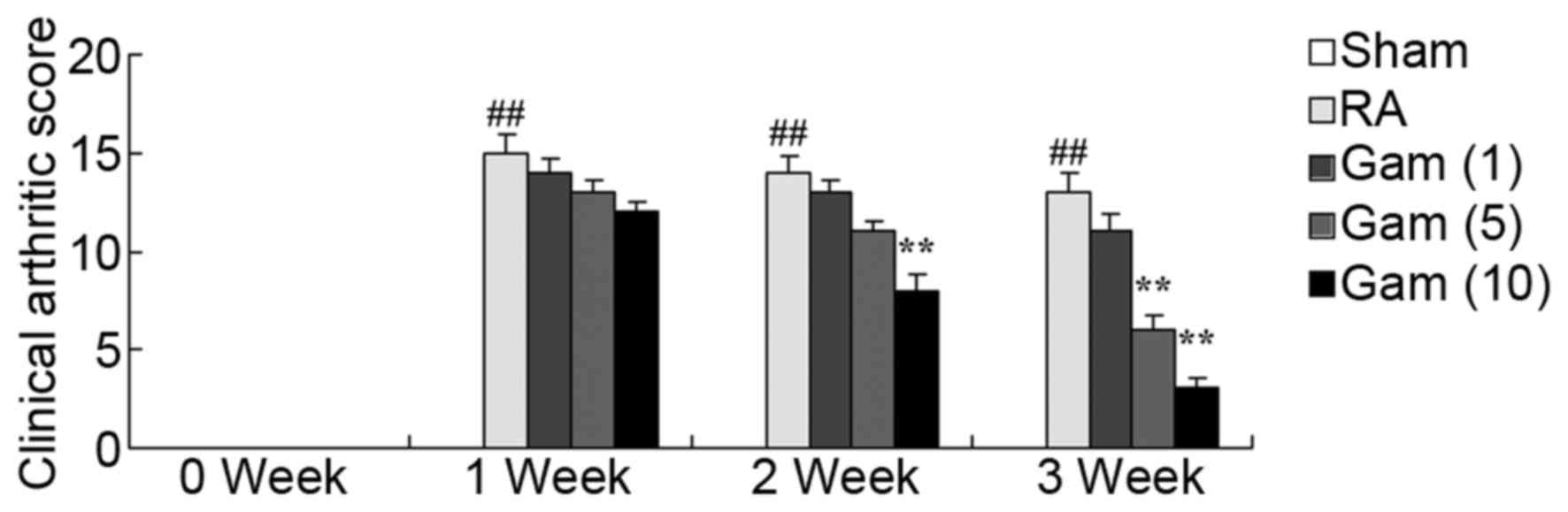
Gambogic acid decreases clinical
arthritic score of RA rats
The potential effects of gambogic acid on the
clinical arthritic score of RA rats was next investigated. As
presented in Fig. 3, the clinical
arthritic score of the RA rat model group significantly increased
over the period of 3 weeks, compared with sham group. However,
administration of 10 mg/kg/day gambogic acid significantly reduced
the clinical arthritic score of RA rats, over this time period
(Fig. 3).
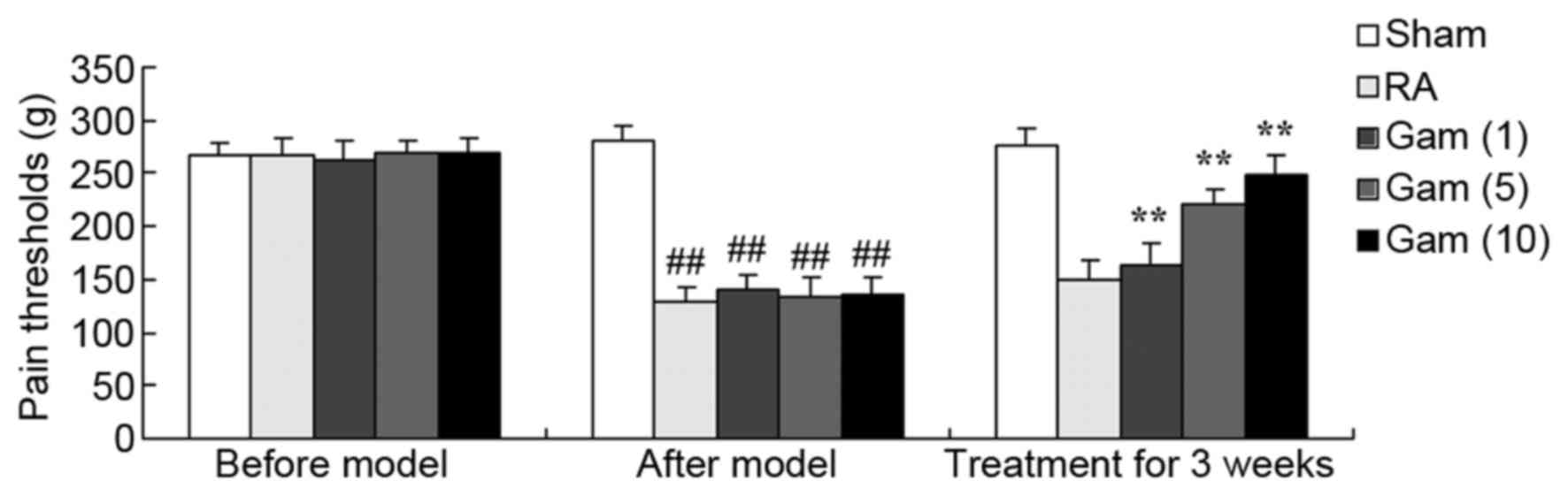
Gambogic acid increases pain threshold
of the RA rat model
To elucidate the potential effects of gambogic acid
on the RA rat model, the pain threshold of the RA rat model was
measured. As presented in Fig. 4,
compared with sham group, the pain threshold of the RA rat model
was decreased. Treatment with gambogic acid significantly increased
the pain threshold of the RA rats (Fig. 4).
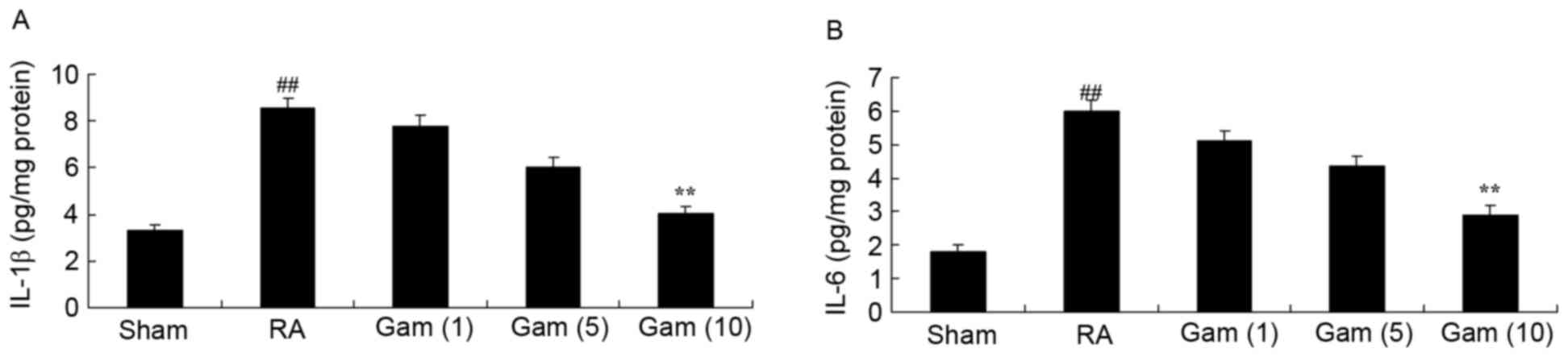
Gambogic acid decreases inflammation
of the RA rat model
To elucidate the anti-inflammatory effect of
gambogic acid in the RA rat model, IL-1β and IL-6 activities were
analyzed using ELISA kits. Notably, RA significantly promoted IL-1β
and IL-6 activities in the RA rat model, compared with sham group
(Fig. 5). Following treatment with
gambogic acid, the promotion of IL-1β and IL-6 activities in RA
rats was significantly suppressed (Fig. 5).
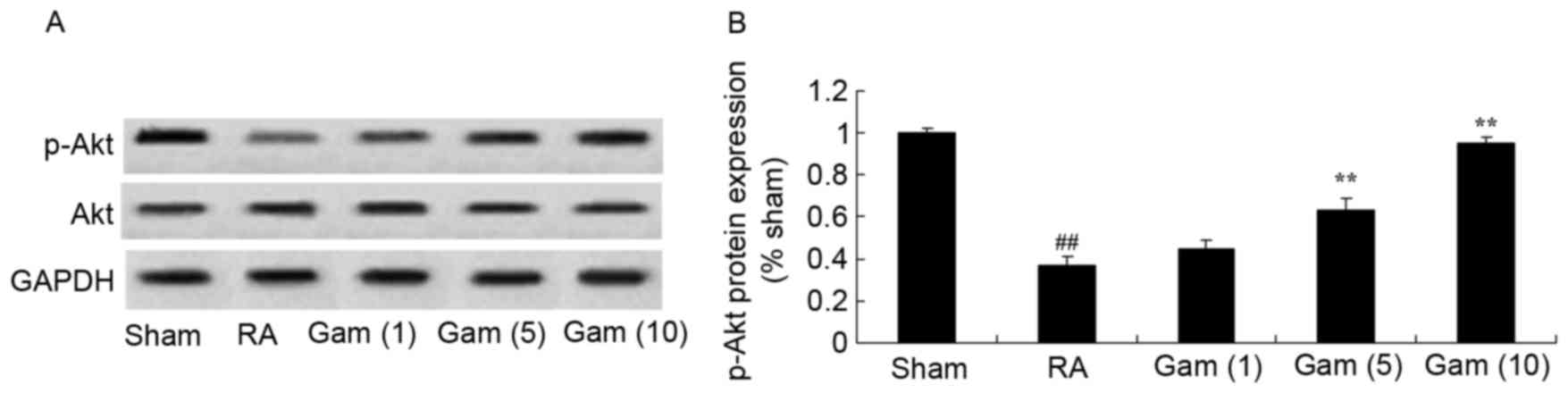
Gambogic acid suppresses inhibition of
the Akt signaling pathway in the RA rat model
p-Akt expression was examined from tissue via
western blotting, in order to analyze the potential effects of
gambogic acid on RA. The p-Akt protein expression was significantly
inhibited in the RA rat model, compared with the sham group
(Fig. 6). Notably, gambogic acid
significantly suppressed the inhibition of p-Akt protein expression
in RA rats (Fig. 6).
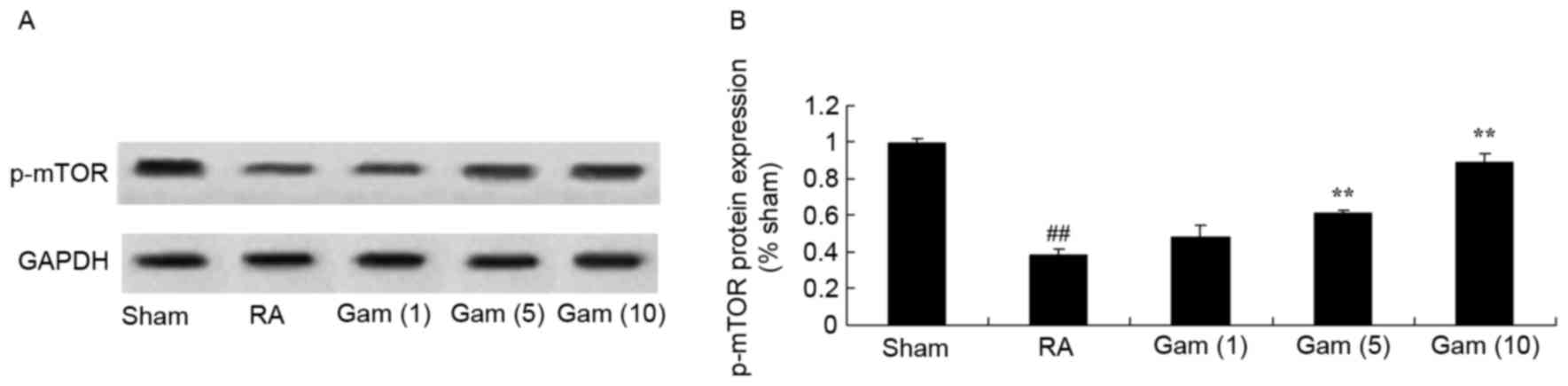
Gambogic acid activates mTOR signaling
pathway in the RA rat model
To explore if gambogic acid activates the mTOR
signaling pathway in RA rats, the models were treated with
differing doses. The p-mTOR protein expression levels in the RA rat
model group were decreased compared with sham group (Fig. 7). However, treatment with gambogic
acid significantly activated the mTOR signaling pathway via
increased p-mTOR protein expression in RA rats (Fig. 7).
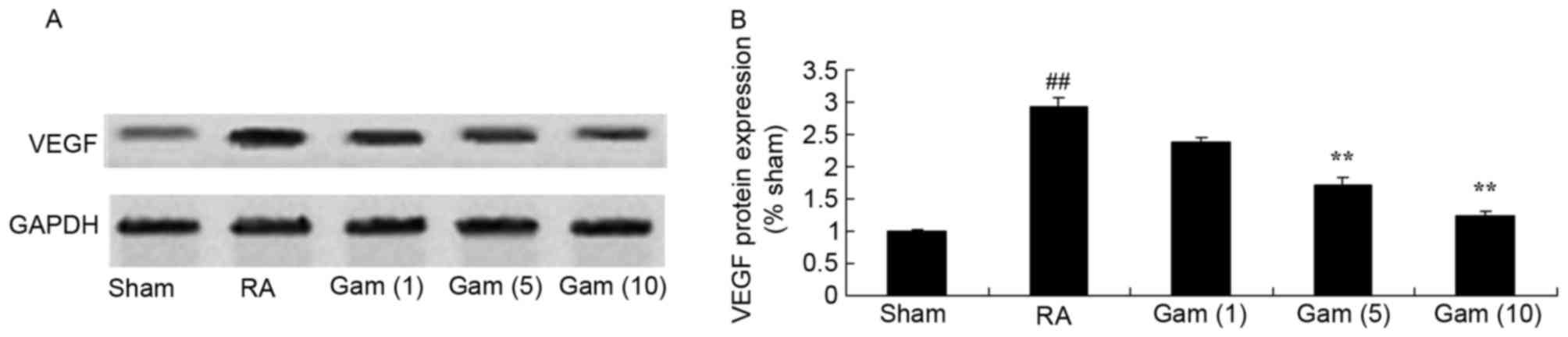
Gambogic acid inhibits VEGF signaling
pathway in the RA rat model
To examine the role of the VEGF signaling pathway in
the potential effects of gambogic acid on RA, VEGF protein
expression was measured using western blotting. As presented in
Fig. 8, the activation of VEGF
protein expression was significantly increased in the RA model
group, compared with sham group. Gambogic acid significantly
suppressed the activation of VEGF protein expression in RA rats
(Fig. 8).
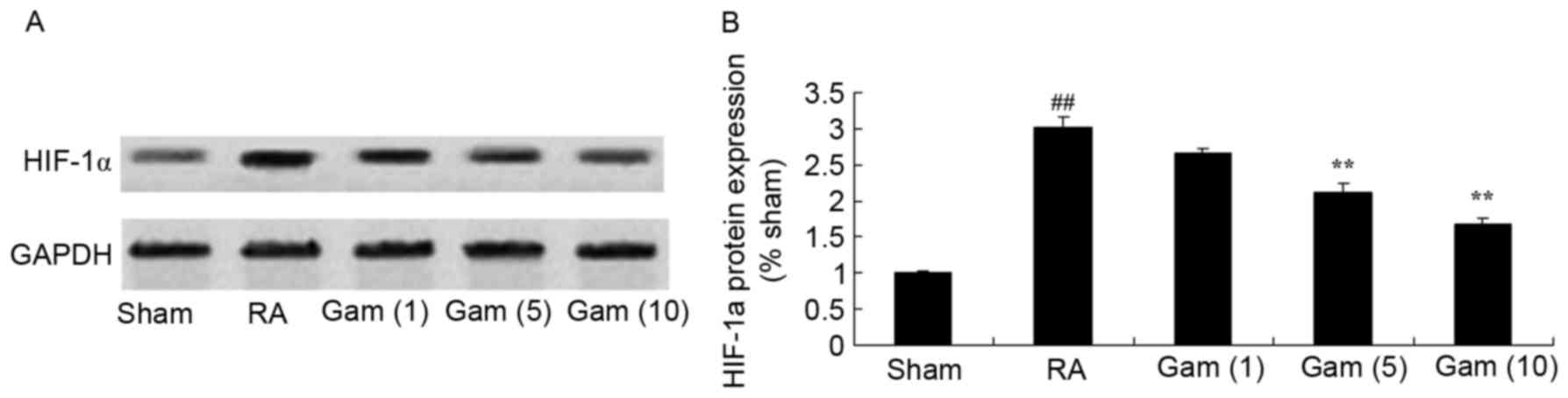
Gambogic acid inhibits HIF-1α
signaling pathway in the RA rat model
To investigate the role of the HIF-1α signaling
pathway in mediating the potential effects of gambogic acid on RA,
HIF-1α protein expression was measured via western blotting. There
was a significant increase in HIF-1α protein expression in the RA
rat model group, compared with sham group (Fig. 9). Correspondingly, treatment with
gambogic acid significantly suppressed the activation of HIF-1α
protein expression in RA rats (Fig.
9).
Discussion
RA is a systemic autoimmune disease with a primary
manifestation of corrosive arthritis. The predominant pathological
alterations are chronic inflammation of synovial tissues (18). Lesions violate multiple joints of
body, generally beginning with facet joints, including hands and
feet, present symmetry, and may additionally result in systematic
diseases, including rheumatoid, vasculitis and pericarditis
(19). Mortality rates are
positively correlated with age. The disability rate of the disease
is high, having a strong impact on physical and psychological
health and daily quality of life (20). At present, anti-inflammatory drugs,
slow acting anti-rheumatic drugs, glucocorticoids and biological
agents are used as therapeutic agents in clinics, however long-term
application of these drugs may result in serious gastrointestinal
symptoms and damage of the liver and kidney (21,22).
In the present study, gambogic acid significantly inhibited the
degree of right foot swelling, reduced the clinical arthritic score
of RA and increased the pain threshold of the RA rats. These
results suggest that gambogic acid may act as a potential future
therapeutic reagent in the treatment of RA.
At the later stages of RA, cartilago articularis and
bone tissues of patients are seriously corroded and ultimately
result in damage and functional loss of the entire joint structure
(9). RA affects ~1% of the total
population worldwide (23). It is
reported that inflammatory cells and chemotactic factors are
important in the development and progression of RA and autoimmune
diseases. TNF-α, IL-1β and IL-6 are important in mediating
RA-associated mortality, the synovial inflammatory response and the
process of osteoclasia (21). At
present, inhibitors of IL-1β and IL-6 have already been used for
clinical research and therapy of RA and exhibit beneficial effects
(24). The findings of the present
study revealed that gambogic acid significantly suppressed the
promotion of IL-1β and IL-6 activities in RA rats.
PI3K/Akt signaling regulates proliferation and
differentiation of B lymphocytes by activating mTOR (12). mTOR is the target molecule of
sirolimus and is vital in the regulation of cell growth and
proliferation. mTOR forms two types of complexes (C) with different
functions, including mTORCl and mTORC2 (11). mTORC1 is sensitive to sirolimus,
whereas mTORC2 is not sensitive to sirolimus. mTORC1 reinforces
protein synthesis via phosphorylation of 4E-binding protein 1 and
ribosomal protein S6 kinase B1. mTOR signaling impacts the cell
cycle, cell growth and cell proliferation (25). In numerous human cancers, various
important tumor-inhibiting factors (PTEN, tuberous sclerosis 1/2,
liver kinase B1) in the mTOR signaling pathway are deficient. Cell
mutation and gene amplification in PI3CA (P110α subtype of PI3K),
in addition to an Akt activated mutation, result in increases in
cell proliferation (13). The
present study demonstrated that gambogic acid significantly
prevented the decrease in p-Akt and p-mTOR protein expression
induced by RA in the rat models. Activation of the PI3K/Akt/mTOR
signaling pathway was important in the effect of gambogic acid on
RA. Liu et al (14)
suggested that gambogic acid induces G0/G1 cell cycle arrest via
the PI3K/Akt/mTOR signaling pathway.
Vascular endothelial growth factor is additionally
termed vascular permeability enhancement factor (26). It participates in human embryonic
development, wound healing, hair growth and diabetic retinopathy,
with pathological and physiological roles. The molecular weight of
VEGF is ~46 kDa. It is a dimer glycoprotein molecule comprised of
two monomers combined, and has two important physiological
functions (27). Pro-blood vessel
endothelium proliferation is the leading function of VEGF. It is an
endothelial cell mitogen and promotes fission of endothelial cells
in order to promote neovascularization. The VEGF receptor is
located in vascular endothelial cells (28). Therefore, the function of VEGF is
concentrated and has stronger specificity (27). VEGF may additionally increase
permeability of blood capillaries and is the blood vessel
anti-reflection molecule that exhibits the strongest biological
effect, >5 million times more potent compared with histamine
substances (28). The results of
the present study suggested that gambogic acid significantly
suppressed the activation of VEGF protein expression, blocking
activation of the VEGF signaling pathway in RA rats. Lu et
al (16) suggested that
gambogic acid may be a structurally novel angiogenesis inhibitor
via suppressing VEGF production.
HIF-1α is an important regulatory factor of
histiocyte anoxia. RA intra-articular anoxia results in an
upregulation of HIF-1α expression in synovial tissues and regulates
multiple signaling pathways, including inflammation, angiogenesis,
cell invasion and proliferation, and intensifies the occurrence and
development of RA (29). In the
pathological state, HIF-1α expression is abnormal. The RA
intra-articular environment is an anaerobic environment which leads
to high expression of HIF-1α in synovial tissues (30). High expression of HIF-1α may
promote RA in synovial tissues, secrete chemotactic factors,
recruit monocytes, T and B lymphocytes, reinforce differentiation
of T helper 17 cells directly and participate in the overall
inflammatory response of RA (31).
Furthermore, HIF-1α may additionally promote VEGF expression and
the subsequent generation of blood vessels (31). In the present study, gambogic acid
significantly suppressed the activation of HIF-1α protein
expression in RA rats. Lu et al (16) demonstrated that gambogic acid
inhibited angiogenesis via suppression of the HIF-1α pathway. These
findings suggested that gambogic acid downregulates HIF-1α, in
treatment of RA.
In conclusion, the present study demonstrated that
gambogic acid significantly inhibited the degree of right foot
swelling, increased pain threshold, reduced clinical arthritic
score and suppressed inflammation in RA rats, via regulation of the
Akt, mTOR, VEGF and HIF-1α signaling pathways. Therefore, gambogic
acid may act as a potential future therapeutic reagent in the
treatment for RA.
References
|
1
|
Meyfroidt S, Stevens J, De Lepeleire J,
Westhovens R, De Cock D, Van Der Elst K, Vanhaecht K and
Verschueren P: A general practice perspective on early rheumatoid
arthritis management: A qualitative study from Flanders. Eur J Gen
Pract. 21:231–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abram SG, Nicol F, Hullin MG and Spencer
SJ: The long-term outcome of uncemented low contact stress total
knee replacement in patients with rheumatoid arthritis: Results at
a mean of 22 years. Bone Joint J. 95-B:1–1499. 2013. View Article : Google Scholar
|
|
3
|
Avina-Zubieta JA, Abrahamowicz M, De Vera
MA, Choi HK, Sayre EC, Rahman MM, Sylvestre MP, Wynant W, Esdaile
JM and Lacaille D: Immediate and past cumulative effects of oral
glucocorticoids on the risk of acute myocardial infarction in
rheumatoid arthritis: A population-based study. Rheumatology
(Oxford). 52:68–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halabi H, Alarfaj A, Alawneh K, Alballa S,
Alsaeid K, Badsha H, Benitha R, Bouajina E, Al Emadi S, El Garf A,
et al: Challenges and opportunities in the early diagnosis and
optimal management of rheumatoid arthritis in Africa and the Middle
East. Int J Rheum Dis. 18:268–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersson U and Tracey KJ: HMGB1 as a
mediator of necrosis-induced inflammation and a therapeutic target
in arthritis. Rheum Dis Clin North Am. 30:627–637, xi. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szabo-Taylor KE, Eggleton P, Turner CA,
Faro ML, Tarr JM, Tóth S, Whiteman M, Haigh RC, Littlechild JA and
Winyard PG: Lymphocytes from rheumatoid arthritis patients have
elevated levels of intracellular peroxiredoxin 2 and a greater
frequency of cells with exofacial peroxiredoxin 2, compared with
healthy human lymphocytes. Int J Biochem Cell Biol. 44:1223–1231.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calmon-Hamaty F, Combe B, Hahne M and
Morel J: Lymphotoxin alpha stimulates proliferation and
pro-inflammatory cytokine secretion of rheumatoid arthritis
synovial fibroblasts. Cytokine. 53:207–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsiao HB, Hsieh CC, Wu JB, Lin H and Lin
WC: Kinsenoside inhibits the inflammatory mediator release in a
type-II collagen induced arthritis mouse model by regulating the T
cells responses. BMC Complement Altern Med. 16:802016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavelka K, Kavanaugh AF, Rubbert-Roth A
and Ferraccioli G: Optimizing outcomes in rheumatoid arthritis
patients with inadequate responses to disease-modifying
anti-rheumatic drugs. Rheumatology (Oxford). 51 Suppl 5:v12–v21.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan H, Yang P, Zhou D, Gao W, Qiu Z, Fang
F, Ding S and Xiao W: Knockdown of sphingosine kinase 1 inhibits
the migration and invasion of human rheumatoid arthritis
fibroblast-like synoviocytes by down-regulating the PI3K/AKT
activation and MMP-2/9 production in vitro. Mol Biol Rep.
41:5157–5165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitra A, Raychaudhuri SK and Raychaudhuri
SP: IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR
signaling cascade. Cytokine. 60:38–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiu YC, Lin CY, Chen CP, Huang KC, Tong
KM, Tzeng CY, Lee TS, Hsu HC and Tang CH: Peptidoglycan enhances
IL-6 production in human synovial fibroblasts via TLR2 receptor,
focal adhesion kinase, Akt, and AP-1-dependent pathway. J Immunol.
183:2785–2792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li GQ, Zhang Y, Liu D, Qian YY, Zhang H,
Guo SY, Sunagawa M, Hisamitsu T and Liu YQ: PI3 Kinase/Akt/HIF-1α
pathway is associated with hypoxia-induced epithelial-mesenchymal
transition in fibroblast-like synoviocytes of rheumatoid arthritis.
Mol Cell Biochem. 372:221–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Li W, Ye C, Lin Y, Cheang TY, Wang
M, Zhang H and Wang S, Zhang L and Wang S: Gambogic acid induces
G0/G1 cell cycle arrest and cell migration inhibition via
suppressing PDGF receptor β tyrosine phosphorylation and Rac1
activity in rat aortic smooth muscle cells. J Atheroscler Thromb.
17:901–913. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang SW, Okada M, Sayeed I, Xiao G, Stein
D, Jin P and Ye K: Gambogic amide, a selective agonist for TrkA
receptor that possesses robust neurotrophic activity, prevents
neuronal cell death. Proc Natl Acad Sci USA. 104:16329–16334. 2007;
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu N, Yang Y, You QD, Ling Y, Gao Y, Gu
HY, Zhao L, Wang XT and Guo QL: Gambogic acid inhibits angiogenesis
through suppressing vascular endothelial growth factor-induced
tyrosine phosphorylation of KDR/Flk-1. Cancer Lett. 258:80–89.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia Z and He J: Paeoniflorin ameliorates
rheumatoid arthritis in rat models through oxidative stress,
inflammation and cyclooxygenase 2. Exp Ther Med. 11:655–659. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de la Torre I, Leandro MJ, Edwards JC and
Cambridge G: Baseline serum immunoglobulin levels in patients with
rheumatoid arthritis: Relationships with clinical parameters and
with B-cell dynamics following rituximab. Clin Exp Rheumatol.
30:554–560. 2012.PubMed/NCBI
|
|
19
|
Roberts CA, Dickinson AK and Taams LS: The
interplay between monocytes/macrophages and CD4(+) T cell subsets
in rheumatoid arthritis. Front Immunol. 6:5712015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Kady IM and El-Masry SA:
Pro-inflammatory and anti-inflammatory cytokines profile in
rheumatoid arthritis patients. Egypt J Immunol. 15:109–114.
2008.PubMed/NCBI
|
|
21
|
Ferraccioli G and Gremese E:
Thrombogenicity of TNF alpha in rheumatoid arthritis defined
through biological probes: TNF alpha blockers. Autoimmun Rev.
3:261–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu D, Guo M, Hu Y, Liu T, Yan J, Luo Y,
Yun M, Yang M, Zhang J and Guo L: Effect of sanhuangwuji powder,
anti-rheumatic drugs, and ginger-partitioned acupoint stimulation
on the treatment of rheumatoid arthritis with peptic ulcer: A
randomized controlled study. J Tradit Chin Med. 35:273–280. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cobo-Ibáñez T, Descalzo MÁ,
Loza-Santamaría E, Carmona L and Munoz-Fernandez S: Serious
infections in patients with rheumatoid arthritis and other
immune-mediated connective tissue diseases exposed to anti-TNF or
rituximab: Data from the Spanish registry BIOBADASER 2.0. Rheumatol
Int. 34:953–961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Zhu Q, Song J, Liu H, Miao Y, Yang
F, Wang F, Cheng W, Xi Y, Niu X, et al: Regulatory effect of
iguratimod on the balance of Th subsets and inhibition of
inflammatory cytokines in patients with rheumatoid arthritis.
Mediators Inflamm. 2015:3560402015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu T and Mohan C: The AKT axis as a
therapeutic target in autoimmune diseases. Endocr Metab Immune
Disord Drug Targets. 9:145–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oranskiy SP, Yeliseyeva LN, Tsanaeva AV
and Zaytseva NV: Body composition and serum levels of adiponectin,
vascular endothelial growth factor, and interleukin-6 in patients
with rheumatoid arthritis. Croat Med J. 53:350–356. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hetland ML, Christensen IJ, Lottenburger
T, Johansen JS, Svendsen MN, Hørslev-Petersen K, Nielsen L and
Nielsen HJ: Circulating VEGF as a biological marker in patients
with rheumatoid arthritis? Preanalytical and biological variability
in healthy persons and in patients. Dis Markers. 24:1–10. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hah YS, Koh YJ, Lim HS, Kim HO, Cheon YH,
Noh HS, Jang KY, Lee SY, Lee GM, Koh GY and Lee SI:
Double-antiangiogenic protein DAAP targeting vascular endothelial
growth factor A and angiopoietins attenuates collagen-induced
arthritis. Arthritis Res Ther. 15:R852013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li GF, Qin YH and Du PQ: Andrographolide
inhibits the migration, invasion and matrix metalloproteinase
expression of rheumatoid arthritis fibroblast-like synoviocytes via
inhibition of HIF-1α signaling. Life Sci. 136:67–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brouwer E, Gouw AS, Posthumus MD, van
Leeuwen MA, Boerboom AL, Bijzet J, Bos R, Limburg PC, Kallenberg CG
and Westra J: Hypoxia inducible factor-1-alpha (HIF-1alpha) is
related to both angiogenesis and inflammation in rheumatoid
arthritis. Clin Exp Rheumatol. 27:945–951. 2009.PubMed/NCBI
|
|
31
|
Seo Y, Ji YW, Lee SM, Shim J, Noh H, Yeo
A, Park C, Park MS, Chang EJ and Lee HK: Activation of HIF-1α
(hypoxia inducible factor-1α) prevents dry eye-induced acinar cell
death in the lacrimal gland. Cell Death Dis. 5:e13092014.
View Article : Google Scholar : PubMed/NCBI
|