Introduction
Lung cancer is a serious public health concern and
has become the leading cause of mortality among all malignant
carcinomas worldwide with the rates of morbidity and mortality
increasing each year. At present, the main treatment available to
patients with lung cancer is surgery combined with chemotherapy,
radiotherapy and targeted therapy. However, the 5-year patient
survival rate is <15%. Lung cancer mortalities in China accounts
for ~1/6 of the worldwide mortality rate (1,2).
In the majority of cases, lung cancer is detected
and diagnosed in the advanced stages. At this stage, invasion and
migration of lung cancer has occurred, and bone and brain
metastases are easily developed; however, the specific underlying
mechanisms remain unclear (3). A
number of researches have indicated that like many other
carcinomas, the development and progression of lung cancer is
closely associated with diverse gene alterations including
chromosome deficiency, gene amplification, oncogene activation,
loss of tumor suppressor genes and alterations in
apoptosis-associated genes; these abnormities can induce
tumorigenesis (3,4). In normal tissues and cells, the
levels of oncogenes are normal however, in the tumor environment,
oncogenes are mutated and/or constitutively activated, inducing
tumorigenesis.
Recent studies have revealed that, as pro-oncogenes,
cancer-testis antigens (CTAs) promote the development and
progression of tumors (5–8). As testicular tissue-specific genes,
CTAs are only expressed in normal testicular tissues and not in
other normal tissues; however, they may be abnormally overexpressed
in lung, gastric, liver, breast, lymphoma and prostate cancers
(5–8). CTAs exhibit strong immunogenicity and
thus, have been applied in clinical tumor immunotherapy (5–8).
Over 20 CTAs or CTA families, including the CT45 family, have been
reported. The CT45 family consists of six genes, CT45A1 to CT45A6.
CT45A1 is a pro-oncogene, which has been proposed as a novel
transcriptional activator (7,8).
Chen et al (9) demonstrated
that CT45A1 was positively expressed in patients with lung cancer.
Gao (10) further reported that
CT45A1 was overexpressed in lung cancer cells and that the
overexpression or gene silencing of CT45A1 markedly promoted or
inhibited the proliferation, invasion and migration of lung cancer
cells, respectively. However, the underlying molecular mechanisms
are unknown.
Therefore, in the present study, CT45A1 was targeted
by RNA interference in order to investigate the effect of CT45A1
gene silencing on the proliferation, migration and invasion of lung
cancer cells, and the mechanisms involved. The present results
revealed that CT45A1 influenced the proliferation, metastasis and
invasion of lung cancer cells, via regulating the extracellular
signal-regulated kinase (ERK)/cyclic AMP response element binding
protein (CREB) signaling pathway. These may provide theoretical a
basis for the clinical application of CT45A1.
Materials and methods
Materials and cell culture
Rabbit anti-B-cell lymphoma-2 (Bcl-2; cat. no.
ab32124), anti-Bcl-2 associated X (Bax; cat. no. ab32503),
anti-survivin (cat. no. ab76424), anti-matrix metalloproteinase 2
(MMP2; cat. no. ab37150), anti-MMP9 (cat. no. ab38898), anti-ERK1/2
(cat. no. ab126423), anti-phosphorylated ERK1/2 (p-ERK1/2) (cat.
no. ab200807), anti-CREB (cat. no. ab31387), anti-p-CREB (cat. no.
ab32096) and anti-GAPDH (cat. no. ab8245) monoclonal antibodies
were purchased from Abcam (Cambridge, MA, USA). Rabbit anti-CT45A1
(cat. no. ab170339) polyclonal antibody was purchased from Abcam.
Methyl thiazolyl tetrazolium (MTT) was purchased from Hyclone; GE
Healthcare Life Sciences (Logan, UT, USA). The horseradish
peroxidase-labeled goat anti-rabbit IgG (H+L) antibody (cat. no.
A0208), the bicinchoninic acid (BCA) kit and the Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit were obtained
from Beyotime Institute of Biotechnology (Jiangsu, China). The
Transwell insert and TRIzol® were purchased from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
CT45A1 siRNA (5′-GGAGAGAAAAGGAUCAGAUU-3′) and its negative control
(5′-UGCAGUCAGGAAGCGAUUU-3′) were prepared by Shanghai GeneChem Co.,
Ltd. (Shanghai, China).
The human lung cancer cell lines (NCI-H292, 95-D and
H1299), human non-small cell lung cancer cell lines (A549 and
NCI-H1650), SPC-A-1 human lung adenocarcinoma cell line and WI-38
human fetal lung cell line were obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in RPMI-1640 medium (Hyclone; GE Healthcare
Life Sciences) supplemented with 10% (v/v) fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences) at 37°C in a 5%
CO2 atmosphere.
CT45A1 levels
Prior to siRNA transfection, CT45A1 levels in the
various cells were evaluated by western blotting. Following siRNA
transfection, the level of CT45A1 in A549 cells was determined by
semi quantitative reverse transcription-polymerase chain reaction
(RT-PCR) and western blotting. The methodology for these procedures
is described in the following sections.
Cell transfection
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.),
CT45A1 siRNA and its negative control were each diluted with
Opti-MEM® I reduced-serum medium (Gibco; Thermo Fisher
Scientific, Inc.). Then, the CT45A1 siRNA and its negative control
were each mixed with Lipofectamine 2000 to a final concentration of
50 nmol for 30 min at room temperature. The mixture was added to
A549 cells at 50% confluence in 96-well or 6-well plates. Following
6 h, the medium was replaced by RPMI-1640 medium containing 10%
(v/v) FBS (Hyclone; GE Healthcare Life Sciences). Following
incubation for 48 h at 37°C and 5% CO2, cells were used
in the subsequent experiments.
Cell viability
A549 cells were seeded in 96-well plates and
transfected with CT45A1 siRNA and its negative control as described
above. Following 48 h, 20 µl MTT (5 mg/ml) was added to each well.
Following incubation for 4 h, the culture media was discarded and
150 µl of dimethyl sulfoxide was added to each well. Once the
crystals were fully dissolved with dimethyl sulfoxide (Beyotime
Institute of Biotechnology) under gentle agitation, absorbance was
determined at 560 nm on a microplate reader (Infinite F200/M200;
Tecan Group Ltd., Männedorf, Switzerland). Untreated cells served
as the control and the relative cell viability was calculated.
Cell apoptosis
A549 cells were seeded in 6-well plates and
transfected with CT45A1 siRNA and its negative control as
described. Following 48 h, cells were stained using the Annexin
V-FITC/PI apoptosis detection kit according to the manufacturer's
instructions. Then, the cells were assessed within 1 h on a flow
cytometer (FACSCanto; BD Biosciences, Franklin Lakes, NJ, USA) with
using the FACSCanto clinical software (BD Biosciences).
Cell migration
A549 cells were seeded in 6-well plates and
transfected with CT45A1 siRNA and its negative control. Following
48 h, ~8,000 cells were collected and transferred into the upper
chamber of the Transwell insert in serum-free RPMI-1640 medium and
RPMI-1640 medium containing 10% (v/v) FBS was added into the lower
chamber of the Transwell insert. Cells were incubated for 48 h. The
Transwell insert was taken out and the cells above the membrane
were carefully removed using cotton swabs. Then, the membrane was
fixed in 4% paraformaldehyde at room temperature for 5 min and
stained with 0.1% crystal violet at room temperature for 5 min.
Cells in the lower chamber in five visual fields were counted using
a light microscope (Eclipse TS100; Nikon Corporation, Tokyo,
Japan). The migration capacity of cells was expressed as the
average number of cells in every field of view.
Cell invasion
Matrigel was first uniformly distributed onto the
membrane (5-µm) of Transwell inserts in advance to prepare gel. For
the Transwell assay the chambers were prepared and the cells were
treated as described above. The invasion capacity of cells was also
expressed as the average number of cells in every field of
view.
RT-PCR
Total RNA was obtained using TRIzol®
according to the manufacturer's instructions; RNA purity was
determined with an ultraviolet spectrophotometer (NanoDrop 2000C;
Thermo Fisher Scientific, Inc.). RNA was reverse transcribed into
cDNA and cDNA was amplified using the SuperScript One-Step RT-PCR
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Primers (Table I) were added into the PCR system
(total volume, 25 µl) and the PCR reaction parameters were set as
follows: 35 cycles of denaturation for 45 sec at 94°C, annealing
for 45 sec at 59°C and elongation for 60 sec at 72°C. The obtained
amplification product (5 µl) was loaded for 2% agarose gel
electrophoresis (w/v) and stained with 5 µl GoldView dye (Phygene
Life Sciences, Fujian, China). Electrophoresis strips were
photographed and analyzed on a gel imaging system (ChemiDoc™ XRS;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) using ImageJ
software version 1.38e (National Institutes of Health, Bethesda,
MD, USA).
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene | Primers (5′-3′) | Base pairs |
|---|
| CT45A1 | Forward:
GCTGTCTCTCCTCCAGCAAGGAAAC | 497 |
|
| Reverse:
GACTGCAGTAGGTCCTTGCACTCCT |
|
| GAPDH | Forward:
AGCCACATCGCTCAGACA | 314 |
|
| Reverse:
TGGACTCCACGACGTACT |
|
Western blot analysis
Lung cancer cells were seeded in 6-well plates. For
evaluation following transfection, cells were transfected with
CT45A1 siRNA and its negative control as described. Following 48 h,
cells were collected and lysed in radioimmunoprecipitation buffer
(Beyotime Institute of Biotechnology) for 30 min. Cell lysates were
centrifuged at 7,000 × g and 4°C for 10 min and the supernatant was
collected to acquire total protein. Protein content was determined
using a bicinchoninic acid protein assay kit according to the
manufacturer's instructions, then protein was denatured for 10 min
and 30 ng protein samples were separated by 10% SDS-PAGE. Proteins
were transferred to a polyvinylidene difluoride membrane using the
wet transfer method. The membrane was blocked with 5% non-fat milk
for 1 h at room temperature and then incubated with the primary
antibodies (rabbit anti-Bax, anti-Bcl-2, anti-survivin, anti-MMP2,
anti-MMP9, anti-ERK1/2, anti-p-ERK1/2, anti-CREB, anti-p-CREB and
anti-GAPDH monoclonal antibodies, and rabbit anti-CT45A1 polyclonal
antibody; all 1:100) overnight at 4°C. It was then washed with TBS
containing 0.05% Tween-20 (TBST) and incubated with the secondary
antibody (1:100) for 2 h at room temperature. Following washing
again, the membrane was treated with an enhanced chemiluminescent
solution and visualized on a gel imaging system (ChemiDoc™ XRS;
Bio-Rad Laboratories, Inc.). Quantity One software version 4.62
(Bio-Rad Laboratories, Inc.) was used to determine the gray values
of the protein bands. Experiments were repeated three times.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using a Student's
t-test on SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
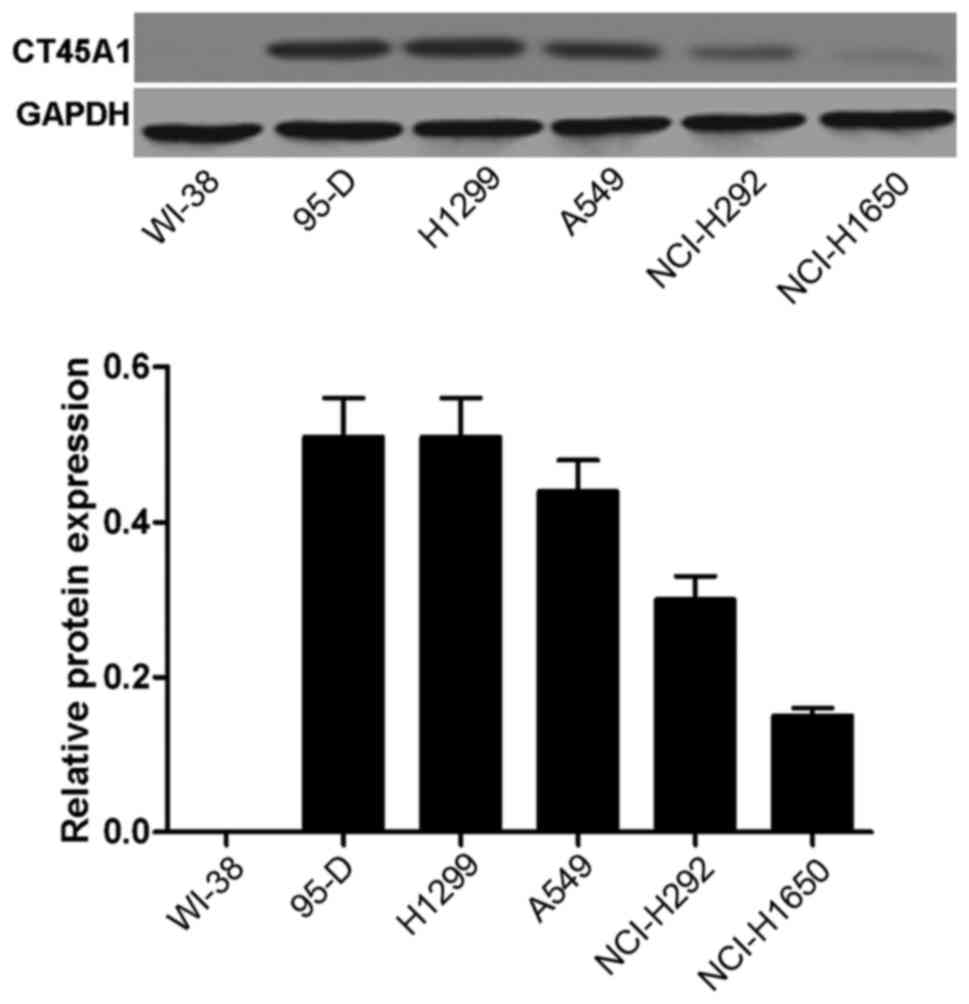
CT45A1 levels in normal lung cells and
lung cancer cells
The CT45A1 levels in normal lung cells and lung
cancer cells were evaluated by western blotting. As depicted in
Fig. 1, no band was visible and
CT45A1 was not quantitatively detected in WI-38 cells, suggesting
that CT45A1 was not expressed in normal lung cells (band A).
However, bands were observed and CT45A1 was quantitatively detected
in the 95-D, H1299, A549, NCI-H292 and NCI-H1650 cell lines. The
results indicated that CT45A1 was overexpressed in lung cancer
cells. The level of CT45A1 in A549 cells was moderate compared with
the other lung cancer cell lines, thus A549 cells were selected as
the model cells for the following investigation.
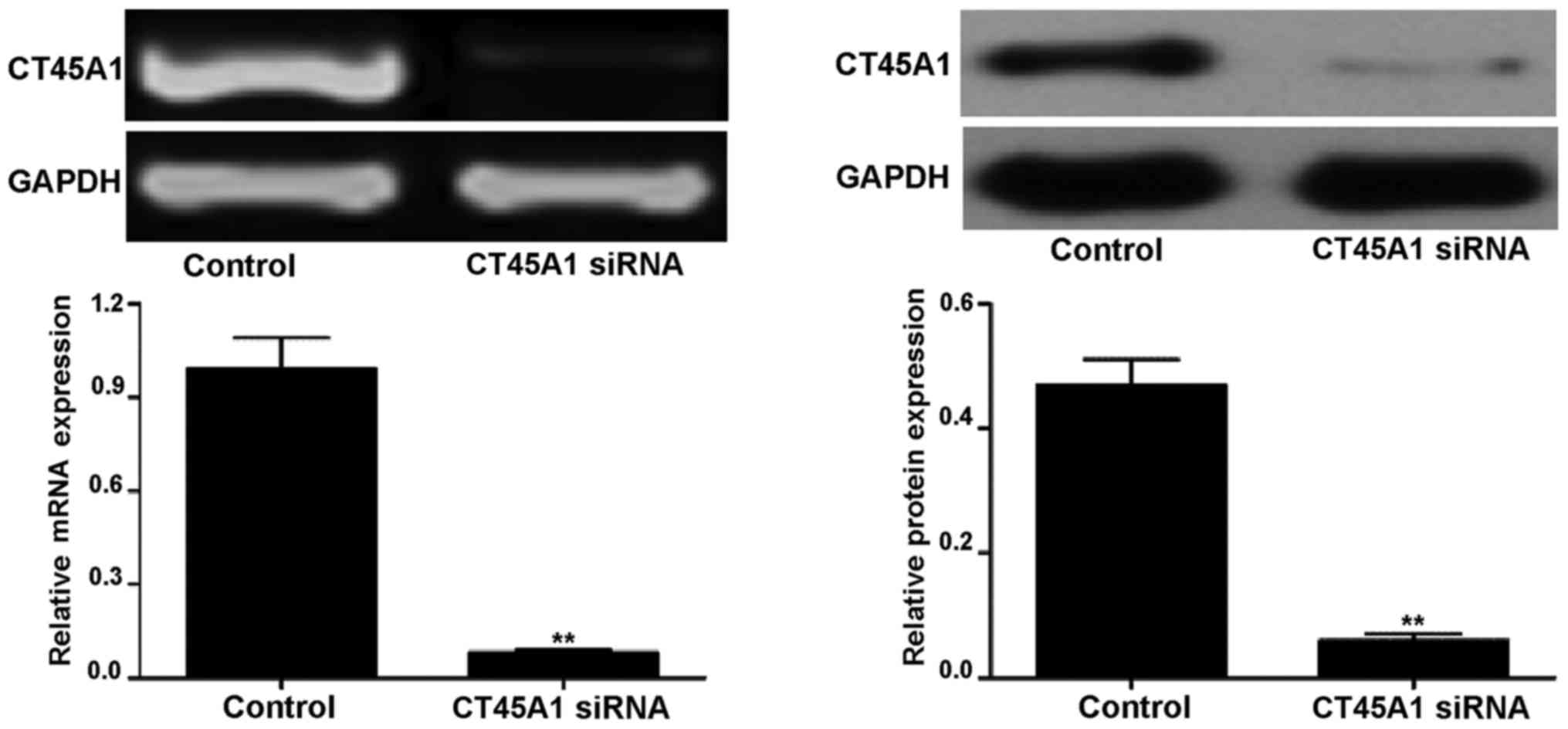
CT45A1 levels following CT45A1 siRNA
silencing
Following treatment with CT45A1 siRNA and negative
control, the level of CT45A1 in A549 cells was assessed by RT-PCR
and western blotting as demonstrated in Fig. 2. The results demonstrated that in
A549 cells, the CT45A1 mRNA and protein levels were significantly
reduced following CT45A1 siRNA silencing when compared with the
negative control (all P<0.01).
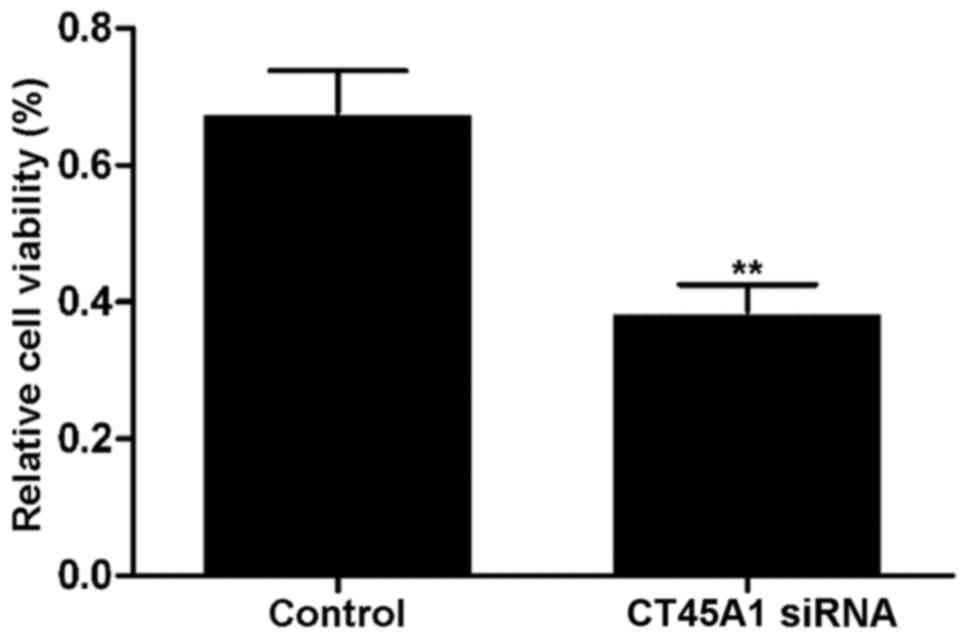
Effect of CT45A1 siRNA silencing on
cell viability
Fig. 3 demonstrates
the viability of A549 cells transfected with CT45A1 siRNA and its
negative control, which was determined by an MTT assay. The results
revealed that cell viability in the siRNA silencing group was
significantly lower than that observed in the negative control
group (P<0.01). The results indicated that CT45A1 siRNA
silencing may be capable of effectively inhibiting the
proliferation of lung cancer cells.
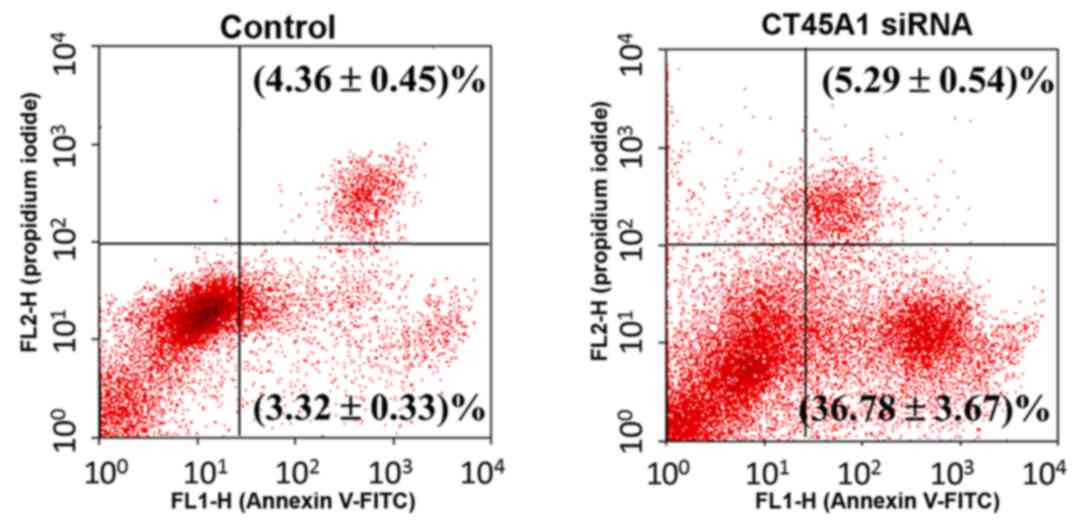
Effect of CT45A1 siRNA silencing on
cell apoptosis
Apoptosis in A549 cells following treatment with
CT45A1 siRNA and its negative control was evaluated by Annexin
V-FITC/PI staining. As presented in Fig. 4, the right lower quadrant
represents the early apoptosis cells. Following siRNA silencing,
apoptosis in A549 cells increased by >10-fold compared with the
negative control (P<0.01). This suggested that CT45A1 siRNA
silencing was able to greatly enhance apoptosis in lung cancer
cells.
Effect of CT45A1 siRNA silencing on
cell migration and invasion
Migration and invasion of A549 cells following
treatment with CT45A1 siRNA and negative control were investigated
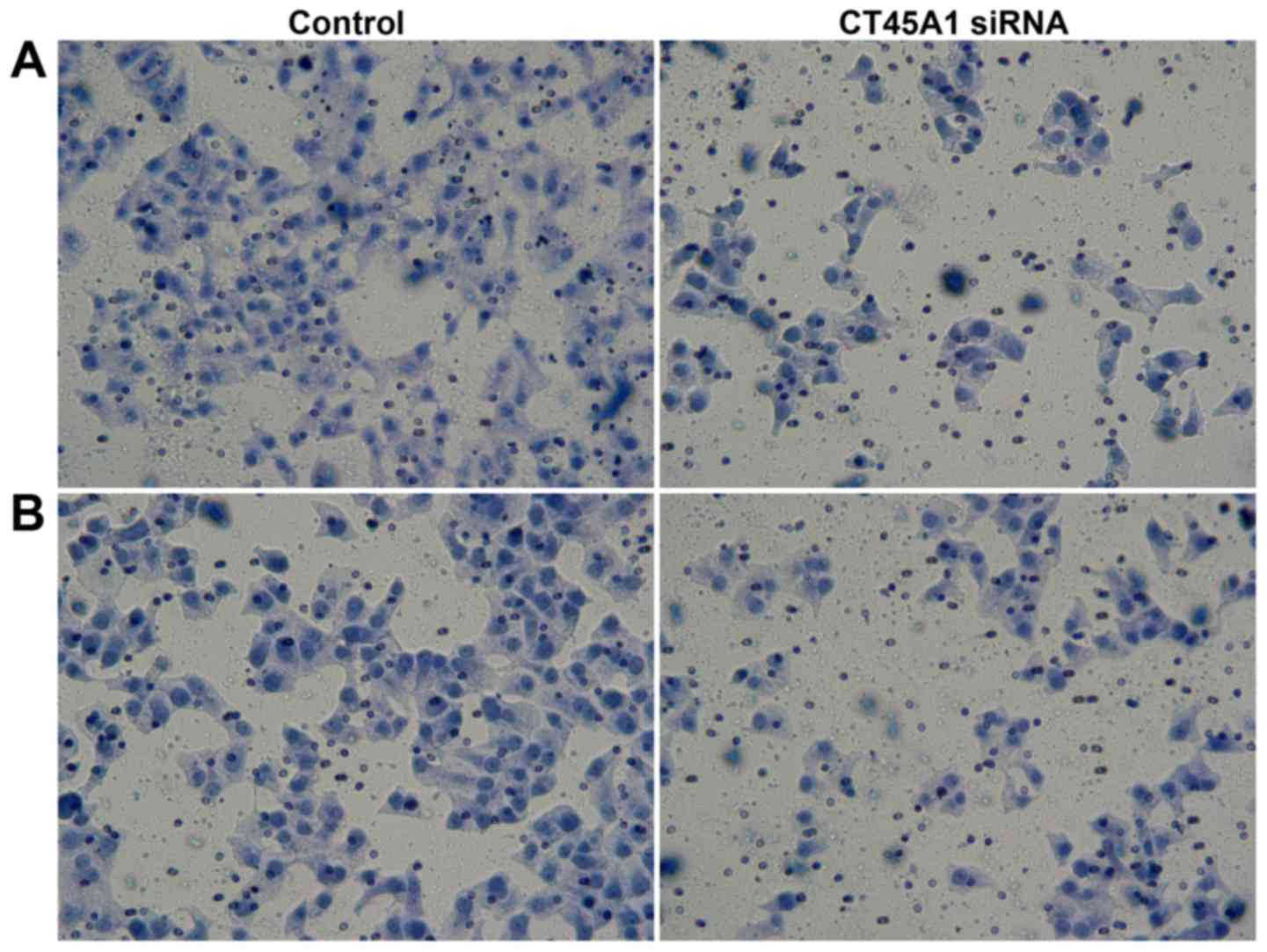
by Transwell assays. As demonstrated in Fig. 5A, in the cell migration assay, the
number of cells migrating into the lower chamber 189.36±16.94 for
CT45A1 siRNA and 59.36±5.40 in the negative control group
(P<0.01). In the cell invasion assay, the number of cells in the
lower chamber following transfection with CT45A1 siRNA was
197.35±18.28, compared to 80.67±8.67 cells in the negative control
group (P<0.01; Fig. 5B). These
results indicated that CT45A1 siRNA silencing was able to suppress
the metastasis and invasion of lung cancer cells.
Effect of CT45A1 siRNA silencing on
the expression of Bax, Bcl-2, survivin, MMP2, MMP9 and
ERK/CREB
The effect of CT45A1 siRNA transfection on the
expression of these signaling molecules in A549 cells was
investigated by western blotting as presented in Fig. 6. GAPDH was employed as the internal
control with similar blots produced among all groups, confirming
the reliable evaluation of western blot analysis.
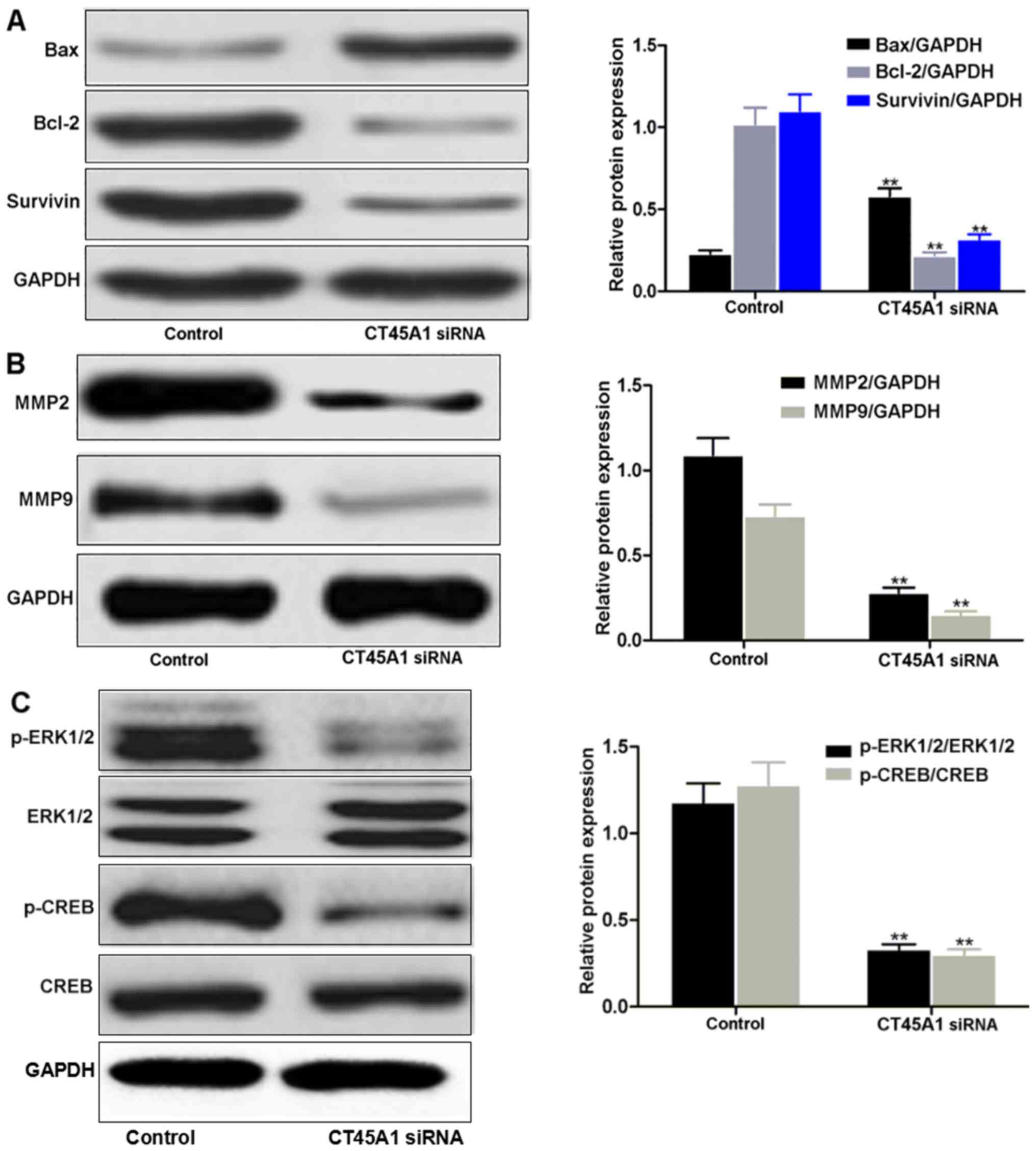 | Figure 6.Western blotting analysis of (A) Bax,
Bcl-2 and survivin, (B) MMP2 and MMP9 and (C) ERK/CREB in A549
cells transfected with CT45A1 siRNA and negative control.
**P<0.01 vs. negative control. CT45A1, cancer-testis antigen
family 45 member A1; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2
associated X; MMP, matrix metalloproteinase; ERK, extracellular
signal-regulated kinase; CREB, cyclic AMP response element binding
protein; p-, phosphorylated; siRNA, small interfering RNA. |
Much fainter bands of Bcl-2 and survivin were
observed in A549 cells transfected with CT45A1 siRNA compared with
the darker bands produced by cells transfected with the negative
control (Fig. 6A). However, the
Bax band in the CT45A1 siRNA group was much darker than that of the
negative control group. These qualitative results were in
accordance with the quantitative densitometry measurements of their
protein levels (all P<0.01), indicating that CT45A1 siRNA
silencing may downregulate the expression of Bcl-2 and surviving,
and upregulate Bax expression in lung cancer cells.
Compared with the negative control group, A549 cells
expressed less MMP2 and MMP9 when they were transfected with CT45A1
siRNA (P<0.01; Fig. 6B),
demonstrating that CT45A1 siRNA silencing decreased the expressions
of MMP2 and MMP9 in lung cancer cells. ERK1/2 and CREB bands were
present in A549 cells transfected with CT45A1 siRNA and the
negative control (Fig. 6C).
However, lower levels of p-ERK1/2 and p-CREB were detected in A549
cells transfected with CT45A1 siRNA when compared with the negative
control (both P<0.01). Thus, the results suggest that CT45A1
siRNA silencing suppressed the phosphorylation of ERK1/2 and CREB
in lung cancer cells.
Discussion
CTA was first reported by van der Bruggen et
al (11) in 1991, however, it
was revealed to only be expressed in normal testicular tissue
rather than any other normal tissues. The CT45 family genes re
located at 125 kb region of chromosome Xq26.3. The CT45 family
consists of 6 genes, CT45A1 to CT45A6, that are highly conserved
with 98% amino acid similarity. These family genes are novel ones
in biological evolution and only exist in humans and primates
rather than other animals including rats, horses and rabbits
(5–8). CT45 was demonstrated to be
overexpressed in lung, ovarian and breast cancers, and multiple
myeloma and leukemia-lymphoma, and closely associated with the
genesis, invasion and poor prognosis of tumors (5–8).
The CT45A1 gene belongs to the CT45 family, however,
there are few reports that have investigated CT45A1. Chen et
al (9) revealed that CT45A1
was positively expressed in patients with lung cancer. Gao
(10) further reported a high
level of CT45A1 in lung cancer cells and it was closely associated
with the proliferation, migration and invasion of cancer cells. It
is possible that CT45A1 may influence the genesis, metastasis and
invasion of lung cancer by regulating signaling pathways. In the
present study, expression of CT45A1 was not detected in normal lung
cells and a marked positive CT45A1 expression was observed in
various lung cancer cells, which is in accordance with previous
reports (9,10). In addition, A549 cells were used as
model cells in the following investigations for the moderate CT45A1
level among lung cancer cells. Koop et al (12) demonstrated that CT45A1 silencing in
L428 Hodgkin lymphoma cells, HT1080 fibrosarcoma cells and U266B1
myeloma cells by RNA interference produced aberrant cell
morphology, increased adhesion force and weakened the migration and
invasion abilities. The present study successfully transfected
CT45A1 siRNA into lung cancer cells with Lipofectamine 2000, and
RT-PCR and western blotting results revealed that the CT45A1
protein and mRNA levels were markedly downregulated. The results of
the MTT assay, Annexin V-FITC/PI staining and the Transwell assay
demonstrated that CT45A1 siRNA silencing markedly suppressed the
proliferation, metastasis and invasion of lung cancer cells, and
promoted apoptosis.
Cell apoptosis is programmed cell death regulated by
a number of apoptosis-associated genes (13,14).
The Bcl-2 family is the most widely investigated group of
apoptosis-associated proteins and Bcl-2 is the most common
anti-apoptotic protein (13).
Heterodimers formed by Bcl-2 and the pro-apoptotic protein Bax, and
Bax homodimers, regulate cell apoptosis by binding to the
mitochondrial outer membrane, affecting the release of caspases
from the mitochondria (13).
Another member of the anti-apoptotic protein family, survivin, also
named apoptosis inhibitory factor-5, has been observed to be the
strongest apoptosis inhibitory factor currently identified
(14). It possesses dual
functions, including apoptosis inhibition and mitosis maintenance.
Survivin inhibits cell apoptosis and promotes cell proliferation by
directly and indirectly regulating the apoptotic pathway of caspase
3 (14). Previous reports have
revealed that the expression of Bcl-2 and survivin in lung cancer
tissue was significantly higher than that observed in normal lung
tissue, while for Bax, it was markedly downregulated in lung cancer
tissues when compared with normal lung tissues (13,14).
Therefore, the downregulation of Bcl-2 and survivin, and the
upregulation of Bax may suppress the proliferation of lung cancer
cells and induce their apoptosis.
Extracellular matrix degradation is an essential
process for tumor invasion and metastasis, and MMPs are the most
important proteolytic enzymes for degrading extracellular matrix.
MMP2 prompts the invasion and metastasis of cancer cells by
degrading extracellular matrix collagen and also inducing
angiogenesis. MMP9 is the MMP with the highest molecular weight,
and promotes the migration, diffusion and invasion of cancer by
degrading the extracellular matrix and basement membrane. In
previous studies, MMP2 and MMP9 were overexpressed in lung cancer
and were closely associated with the genesis, invasion and
migration of cancer, which may make them suitable markers for lung
cancer diagnosis and prognosis (15,16).
Therefore, the downregulation of MMP2 and MMP9 may suppress the
migration and invasion of lung cancer cells to a certain
extent.
The development and progression of tumors is
regulated by multiple signaling pathways, including the
mitogen-activated protein kinase (MAPK) pathway (17). MAPK is a type of Ser/Thr protein
kinase that is widely expressed in the majority of cells. It
transduces extracellular signals to the nucleus via a cascade
reaction to regulate gene transcription (17). Thus, MAPKs have a great influence
on biological cell behaviors including proliferation, apoptosis,
differentiation and invasion. ERK is part of the classic MAPK
signalling pathway. When extracellular signals, such as ligands and
growth factors, are transmitted to the cellular membrane and bind
to growth factor receptors, ERK is activated and phosphorylated.
This activates p90 ribosomal s6 kinase and CREB, further regulating
transcription in the nucleus (18,19).
As a nuclear transcription factor in eukaryocytes, CREB regulates
transcription associated with cell growth, proliferation, apoptosis
and differentiation through autophosphorylation (18,19).
In lung cancer tissues, ERK and CREB were activated and their
phosphorylation levels were significantly elevated, suggesting
there may be a close association between them and the genesis and
development of lung cancer (18,19).
A number of reports have demonstrated that inhibiting the ERK/CREB
pathway and regulating the expression of Bax, Bcl-2 and survivin
may suppress the proliferation of cancer cells (17,20–22).
In addition, inhibition of ERK/CREB signaling pathway may suppress
the migration and invasion of human chorionic trophoblast cells and
lung cancer cells by decreasing the expression and activation of
MMP2 and MMP9 (23,24).
Shang et al (25) constructed a breast cancer model of
CT45A1 overexpression and demonstrated that the genesis, migration
and invasion of cancer were promoted by accelerating
epithelial-mesenchymal transition, enhancing the stemness of cancer
cells and activating the ERK/CREB signaling pathway. However,
silencing of CT45A1 expression in breast cancer cells reduced cell
migration and invasion (25). In
the present study, western blotting was performed to evaluate the
alterations in signaling molecules. The results demonstrated that
following the silencing of CT45A1 expression by siRNA, the
expression of Bcl-2, survivin, MMP2, and MMP9 were downregulated,
the expression of Bax was upregulated and the phosphorylation of
ERK1/2 and CREB was weakened.
In conclusion, CT45A1 may have a key role in the
genesis and development of lung cancer. In lung cancer cells,
CT45A1 was detected at a high level, which may contribute to the
aggressive biological behaviors of lung cancer. Following the
transfection of CT45A1 siRNA into lung cancer cells, CT45A1 siRNA
silencing suppressed the proliferation, metastasis and invasion of
lung cancer cells and promoted their apoptosis via a strong
targeted regulation of the ERK/CREB signaling pathway, where Bcl-2,
survivin, MMP2 and MMP9 were negatively regulated and Bax was
positively regulated. These results provide a potential therapeutic
perspective and may support the development of CT45A1 associated
drug agents and therapeutics for lung cancer.
References
|
1
|
Alvarado-Luna G and Morales-Espinosa D:
Treatment for small cell lung cancer, where are we now?-a review.
Transl Lung Cancer Res. 5:26–38. 2016.PubMed/NCBI
|
|
2
|
Awad R and Nott L: Radiation recall
pneumonitis induced by erlotinib after palliative thoracic
radiotherapy for lung cancer: Case report and literature review.
Asia Pac J Clin Oncol. 12:91–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu XZ, Zhang LT, Tong WQ, Wang W, Xu ZB
and Chen F: Research advances in molecular mechanisms of the
invasion and metastasis of lung cancer. Zhongguo Yi Xue Ke Xue Yuan
Xue Bao. 38:108–112. 2016.PubMed/NCBI
|
|
4
|
Wang H, Zhang G and Min J: Research
advances of cell apoptosis mechanisms in lung cancer. J Med Res.
40:25–27. 2011.
|
|
5
|
Chen YT, Hsu M, Lee P, Shin SJ,
Mhawech-Fauceglia P, Odunsi K, Altorki NK, Song CJ, Jin BQ, Simpson
AJ and Old LJ: Cancer/testis antigen CT45: Analysis of mRNA and
protein expression in human cancer. Int J Cancer. 124:2893–2898.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou X, Yang F, Zhang T, Zhuang R, Sun Y,
Fang L, Zhang C, Ma Y, Huang G, Ma F, et al: Heterogeneous
expression of CT10, CT45 and GAGE7 antigens and their prognostic
significance in human breast carcinoma. Jpn J Clin Oncol.
43:243–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Barger CJ, Link PA,
Mhawech-Fauceglia P, Miller A, Akers SN, Odunsi K and Karpf AR: DNA
hypomethylation-mediated activation of cancer/testis antigen 45
(CT45) genes is associated with disease progression and reduced
survival in epithelial ovarian cancer. Epigenetics. 10:736–748.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YT, Panarelli NC, Piotti KC and
Yantiss RK: Cancer-testis antigen expression in digestive tract
carcinomas: Frequent expression in esophageal squamous cell
carcinoma and its precursor lesions. Cancer Immunol Res. 2:480–486.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YT, Scanlan MJ, Venditti CA, Chua R,
Theiler G, Stevenson BJ, Iseli C, Gure AO, Vasicek T, Strausberg
RL, et al: Identification of cancer/testis-antigen genes by
massively parallel signature sequencing. Proc Natl Acad Sci USA.
102:7940–7945. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao A: The effect and mechanism of CT45A1
gene promoting the invasion and metastasis of lung cancer cells.
Suzhou University Suzhou. 2015.
|
|
11
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, van den Eynde B, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koop A, Sellami N, Adam-Klages S, Lettau
M, Kabelitz D, Janssen O and Heidebrecht HJ: Down-regulation of the
cancer/testis antigen 45 (CT45) is associated with altered tumor
cell morphology, adhesion and migration. Cell Commun Signal.
11:412013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yaren A, Oztop I, Kargi A, Ulukus C, Onen
A, Sanli A, Binicier O, Yilmaz U and Alakavuklar M: Bax, bcl-2 and
c-kit expression in non-small-cell lung cancer and their effects on
prognosis. Int J Clin Pract. 60:675–682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirano H, Maeda H, Yamaguchi T, Yokota S,
Mori M and Sakoda S: Survivin expression in lung cancer:
Association with smoking, histological types and pathological
stages. Oncol Lett. 10:1456–1462. 2015.PubMed/NCBI
|
|
15
|
Zhang DH, Zhang LY, Liu DJ, Yang F and
Zhao JZ: Expression and significance of MMP-9 and MDM2 in the
oncogenesis of lung cancer in rats. Asian Pac J Trop Med.
7:585–588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Badrawy MK, Yousef AM, Shaalan D and
Elsamanoudy AZ: Matrix metalloproteinase-9 expression in lung
cancer patients and its relation to serum mmp-9 activity,
pathologic type, and prognosis. J Bronchology Interv Pulmonol.
21:327–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng G, Wang W, Chai K, Yang S, Li F and
Jiang K: Combination treatment with triptolide and
hydroxycamptothecin synergistically enhances apoptosis in A549 lung
adenocarcinoma cells through PP2 A-regulated ERK, p38 MAPKs and Akt
signaling pathways. Int J Oncol. 46:1007–1017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv K, Shi R and Zhu J: Expression and
significance of CREB and CXCR2 in lung squamous cell carcinoma and
adenocarcinoma. Chin J Histochem Cytochem. 23:257–260. 2014.(In
Chinese).
|
|
19
|
Peng B, Lei N, Chai Y, Chan EK and Zhang
JY: CIP2A regulates cancer metabolism and CREB phosphorylation in
non-small cell lung cancer. Mol Biosyst. 11:105–114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takezawa K, Okamoto I, Nishio K, Jänne PA
and Nakagawa K: Role of ERK-BIM and STAT3-survivin signaling
pathways in ALK inhibitor-induced apoptosis in EML4-ALK-positive
lung cancer. Clin Cancer Res. 17:2140–2148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong JC, Bathina M and Fiscus RR: Cyclic
GMP/protein kinase G type-Iα (PKG-Iα) signaling pathway promotes
CREB phosphorylation and maintains higher c-IAP1, livin, survivin,
and Mcl-1 expression and the inhibition of PKG-Iα kinase activity
synergizes with cisplatin in non-small cell lung cancer cells. J
Cell Biochem. 113:3587–3598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou WJ, Wang S, Hu Z, Zhou ZY and Song
CJ: Angelica sinensis polysaccharides promotes apoptosis in human
breast cancer cells via CREB-regulated caspase-3 activation.
Biochem Biophys Res Commun. 467:562–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ko HS, Park BJ, Choi SK, Kang HK, Kim A,
Kim HS, Park IY and Shin JC: STAT3 and ERK signaling pathways are
implicated in the invasion activity by oncostatin M through
induction of matrix metalloproteinases 2 and 9. Yonsei Med J.
57:761–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryu BJ, Lee H, Kim SH, Heo JN, Choi SW,
Yeon JT, Lee J, Lee J, Cho JY, Kim SH and Lee SY: PF-3758309,
p21-activated kinase 4 inhibitor, suppresses migration and invasion
of A549 human lung cancer cells via regulation of CREB, NF-κB and
β-catenin signalings. Mol Cell Biochem. 389:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shang B, Gao A, Pan Y, Zhang G, Tu J, Zhou
Y, Yang P, Cao Z, Wei Q, Ding Y, et al: CT45A1 acts as a new
proto-oncogene to trigger tumorigenesis and cancer metastasis. Cell
Death Dis. 5:e12852014. View Article : Google Scholar : PubMed/NCBI
|