Introduction
Acute respiratory distress syndrome (ARDS), a
leading cause of morbidity and mortality in patients in critical
care (1–5), may be caused by several virulent
substances, however, the severe inflammation, which accompanies the
condition is most frequently linked to bacteria, including
lipopolysaccharide (LPS) and lipoteichoic acid (6). LPS has been previously administered
in experimental animals, causing continuous sepsis and concomitant
ARDS-like lung injuries, including polymorphonuclear neutrophil
sequestration and lung edema (7).
Although recombinant human activated protein C has been developed
for use in treating human sepsis (8), it was found not to demonstrate a
significant effect in the Prowess-Shock trial (9). Therefore, additional sepsis targets
have been established, including complement C5a (10) and its receptor (11), macrophage migration inhibitory
factor (12), high-mobility group
box 1 protein (13) and histones
(14). Pharmaceuticals are being
developed against each of these targets; however, no drugs have
achieved successful treatment of sepsis. Therefore, further
investigations to identify novel therapeutic targets are
required.
Wnt pathways, which are crucial for regulating the
mechanisms required for the proliferation, development, and
differentiation of cells and organisms, can be further
characterized as canonical and noncanonical Wnt pathway branches.
The canonical Wnt signaling pathway is key in cell proliferation
and motility, cell fate decisions, and cell polarity during
embryonic development and adult tissue homeostasis (15). Wnts and their downstream canonical
signaling pathways also have critical effects on the self-renewal
and differentiation of mesenchymal stem cells (MSCs) (16), which possess multipotency and
immunoregulatory properties, have been shown to differentiate into
alveolar epithelial cells, promote re-epithelialization, alleviate
inflammation, improve pathological impairment, and reduce mortality
rates in ARDS models (17–19). However, due to the low engraftment
of MSCs and the differentiation rates in lung tissues of these
models, they have limited therapeutic effect (20,21).
Therefore, clarification of the mechanisms underlying the Wnt
pathway in mediating MSCs or other cell functions in ARDS may lead
to improved cellular retention in injured lung tissue.
The present study focused on the use of Wnt
signaling to activate SB216763, a glycogen synthase kinase-3β
inhibitor (GSKI), to alleviate the inflammation and sepsis caused
by Wnt signaling on ARDS. An LPS-induced ARDS model was used to
investigate the condition and possible associated mechanisms. The
aim of the present study was to provide a novel perspective and
more detailed understanding of the etiology of ARDS, and provide
evidence for a potential novel therapeutic strategy.
Materials and methods
Reagents and antibodies
Murine interleukin (IL)-6, IL-8, tumor necrosis
factor (TNF)-α, IL-17, IL-18 and IL-1β enzyme-linked immunosorbent
assay (ELISA) kits were purchased from Invitrogen Life
Technologies; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Rat anti-mouse-Ly-6 G (cat. no. 551495) monoclonal antibodies
(mAbs) were purchased from BD Pharmingen (San Diego, CA, USA). Rat
anti-mouse F4/80 (clone A3-1) mAb (cat. no. MCA497GA) was acquired
from Serotec (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
FITC-conjugated sheep anti-rat IgG mAb (cat. no. PA1-28638) was
purchased from Pierce (Thermo Fisher Scientific, Inc.). Rabbit anti
mouse SP-C polyclonal Abs (cat. no. bs-10067R) was acquired from
Bioss Inc. (Woburn, MA, USA). Total protein, albumin and
keratinocyte growth factor (KGF) ELISA kits and Texas
Red-conjugated goat anti-rabbit IgG (H + L) (cat. no. ABIN287315)
were acquired from Antibodies-Online GmbH (Aachen, Germany).
Preparation of experimental
animals
A total of 144 specific pathogen-free 7–8-week-old
male C57/B6 mice weighing 20–25 g were obtained from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China), and were housed in
the animal facility of the First Affiliated Hospital of Soochow
University (Suzhou, China) under specific pathogen-free conditions.
A 12-h light/dark cycle and 19–21°C ambient temperature were
maintained during the entire course of the investigation. The
animals were housed in groups of 5, and fed regular laboratory chow
and water ad libitum. All animal experiments performed in
the present study conformed to the Guide for the Care and Use of
Laboratory Animals (22) and were
approved by the Institutional Animal Care and Use Committee of
Soochow University (Suzhou, China).
Murine model of LPS-induced ARDS
The murine model of LPS-induced ARDS was established
as previously reported (23).
Briefly, the mice were first anesthetized with an intraperitoneal
injection of 1.8% (v/v) Avertin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at a dose of 0.20 ml/10 g body weight, and
received a single dose of LPS (100 µg intratracheally) from
Escherichia coli serotype 0111:B4 (Sigma-Aldrich; Merck
KGaA) in 50 µl sterile normal saline (24). The mice were then allowed to
recover in a 100% oxygen chamber until fully awake. The control
mice received 0.9% PBS instead of LPS. The mice were then
sacrificed humanely at indicated time points of day 3 and day 14
following LPS challenge to collect tissues for analysis. The
initial experiment showed that 20 mg/kg of the GSKI (SB216763;
Selleck, Houston, TX, USA) was effective at significantly
inhibiting the effect of GSK-3β and activating WNT signaling.
Therefore, 20 mg/kg of GSKI SB216763 was used for the inhibition
experiments in the present study.
Cytokine and protein measurements in
bronchoalveolar lavage fluid (BALF)
According to a previously described method (23), BALF was collected by flushing 1 ml
ice-cold PBS back and forth three times through a tracheal cannula,
and then centrifuged at 1,000 × g at 4°C for 10 min. The protein
concentrations of IL-6 (cat. no. BMS603-2), IL-8 (cat. no.
EMCXCL15), TNF-α (cat. no. BMS607-3), IL-17 (cat. no. BMS6001),
IL-18 (cat. no. BMS618-3) and IL-1β (cat. no. EM2IL1B2) in the
supernatant were measured using murine cytokine-specific ELISA kits
(Invitrogen Life Technologies; Thermo Fisher Scientific, Inc.) in
strict accordance with the manufacturer's protocol. The quantities
of total protein (cat. no. ABIN996404), albumin (cat. no.
ABIN2756308) and KGF (cat. no. ABIN2703018) in the BALF were
measured as markers of epithelial permeability using ELISA kits
(Antibodies-Online GmbH).
Evaluation of lung edema
Lung edema was evaluated according to the ratio of
lung wet weight to body weight (LWW/BW) measured, as previously
described (25). Briefly, the
whole lung was removed and cleared of all extrapulmonary tissues,
and the LWW/BW was calculated based on the values of the respective
weights (mg/g).
Determination of neutrophils and
macrophages
According to a previously described method (22), BALF was obtained by instilling 0.9%
NaCl, containing 0.6 mmol/l ethylenediaminetetraacetic acid, in two
separate 0.5 ml aliquots. The fluid was recovered by gentle suction
and placed on ice for immediate processing. An aliquot of the BALF
was processed immediately for total and differential cell counts.
The remainder of the BALF was centrifuged at 1,200 × g at 4°C for
10 min, following which the supernatant was removed aseptically and
stored in individual aliquots at −80°C. The numbers of neutrophils
and macrophages were calculated as the percentage of neutrophils
and macrophages multiplied by the total number of cells in the BALF
sample using flow cytometry (FCM). All analyses were performed in a
blinded-manner.
Histopathology
Lung tissues were fixed in 4% paraformaldehyde at
37°C for 1 h, embedded in paraffin and cut into 5-µm thick
sections. The tissue sections were stained with hematoxylin and
eosin, and images were captured (magnification, ×200) using a
fluorescence microscope (MZ16; Leica Microsystems GmbH, Wetzlar,
Germany). An investigator, who was blinded to the identity of the
slides, evaluated the images and lung injury scores were assigned,
as previously described (23,26).
In brief, the extent of the pathological lesions was graded between
0 and 3 as shown in Table I. The
score for each animal was calculated by dividing the total score
for the number of sections observed.
 | Table I.Smith scores of the extent of
pathological lesions. |
Table I.
Smith scores of the extent of
pathological lesions.
| Score | Alveolar
hemorrhage | Extent of fibrin | Alveolar
infiltration/field |
|---|
| 0 | No hemorrhage | No fibrin in
alveolar | <5 cells |
| 1 | >5 erythrocytes
per alveolus in 1–5 alveoli | Fibrin occupation
<1/3 of field | 5–10 cells |
| 2 | >5 erythrocytes
per alveolus in 5–10 alveoli | Fibrin occupation
<2/3 but >1/3 of field | 10–20 cells |
| 3 | >5 erythrocytes
per alveolus in >10 alveoli | Fibrin occupation
>2/3 of field | >20 cells |
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical analyses were performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Comparisons among multiple
groups were performed using one-way analysis of variance followed
by Bonferroni's post hoc test if the data were normally
distributed. P<0.05 was considered to indicate a statistically
significant difference.
Results
GSKI leads to maintained body weight
and improved survival of mice with LPS-induced ARDS
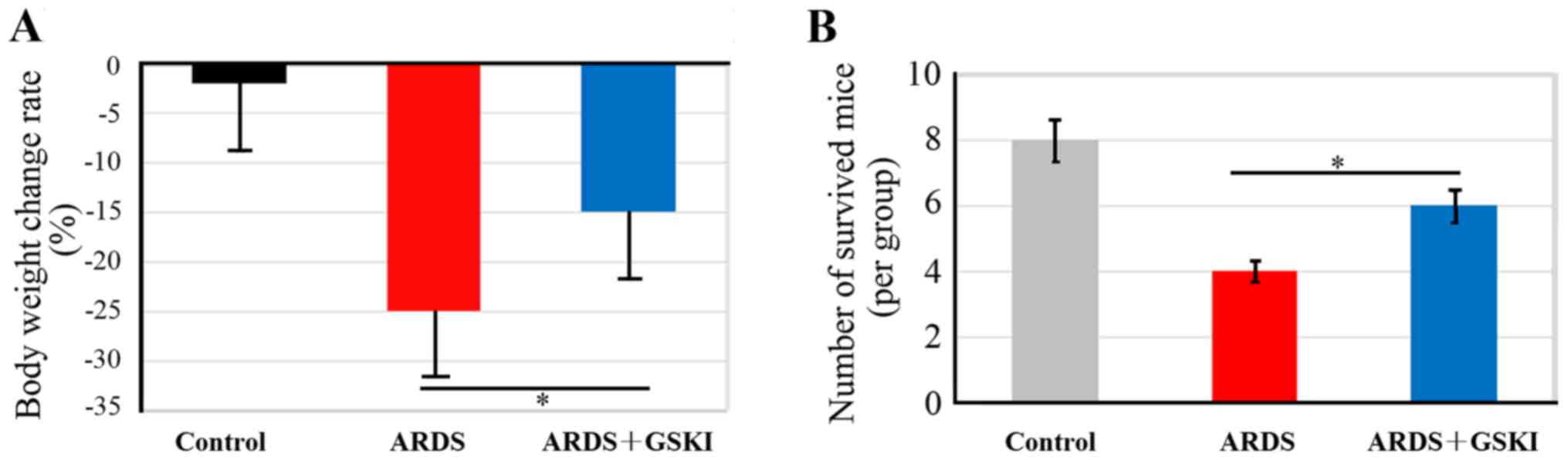
The present study evaluated the effect of GSKI
treatment on body weight maintenance and survival of mice with
LPS-induced ARDS, which had lost ~25% of their body weight
(Fig. 1A). GSKI consequently
abrogated the weight loss of the mice with LPS-induced ARDS
(P<0.05). In addition, the mortality rate was ~40% in the
LPS-induced ARDS mice, however, with GSKI treatment, the mice had
an increased survival rate, up to 60% at 40 h intervals (Fig. 1B; P<0.05), which suggested that
the Wnt signaling pathway was effective in preventing the
development of ARDS.
GSKI reduces LPS-induced lung
permeability
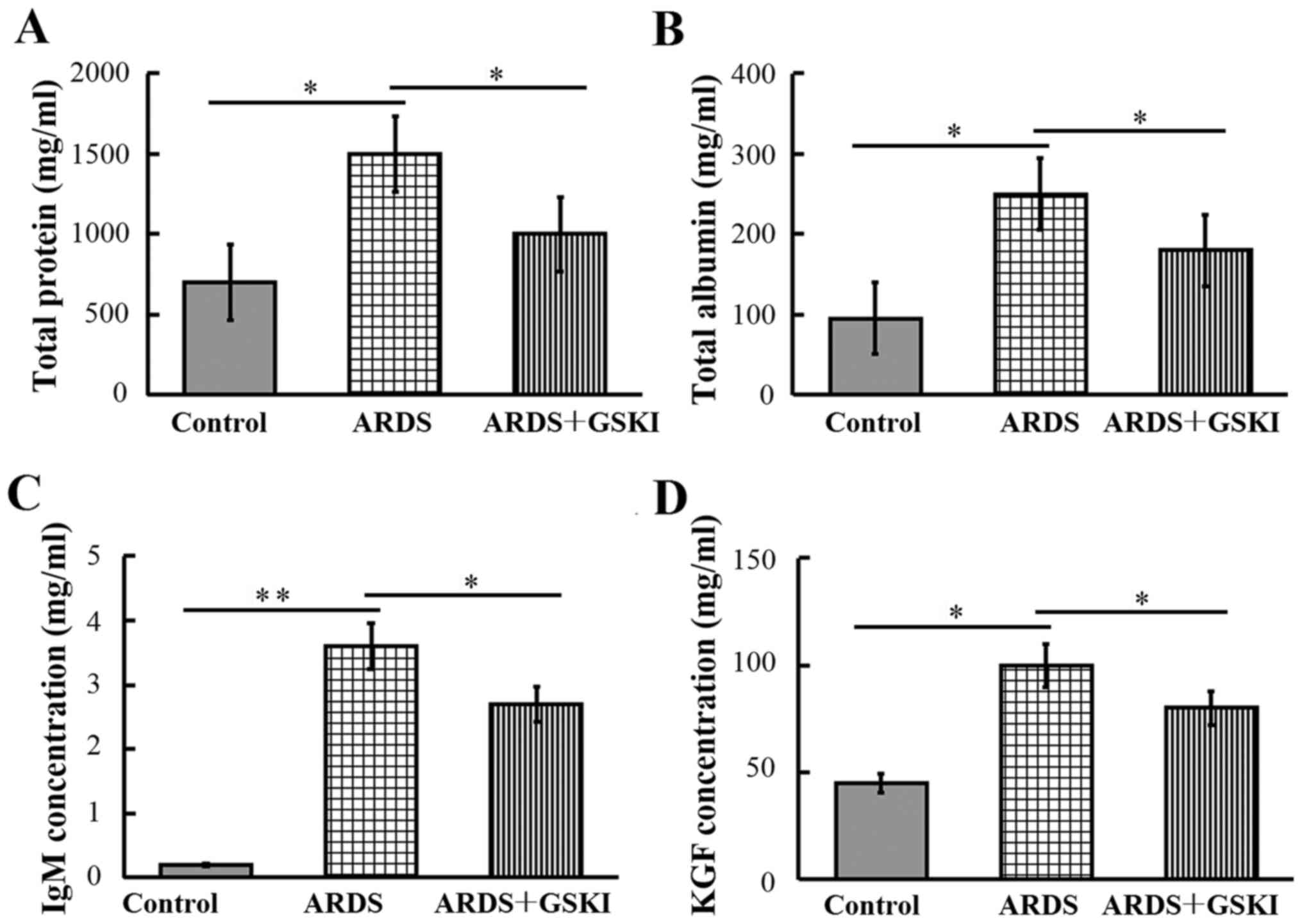
The present study subsequently examined the
concentrations of total protein, albumin, IgM and KGF in BALF, to
evaluate the integrity of the alveolar-capillary membrane barrier
and to assess pulmonary vascular leakage, the latter of which is a
marker for ARDS. The results revealed that the levels were
significantly increased in LPS-challenged mice compared with those
in the control mice (P<0.05 and P<0.01). Following GSKI
treatment, the levels of total protein, albumin, IgM and KGF were
reduced (P<0.05; Fig. 2).
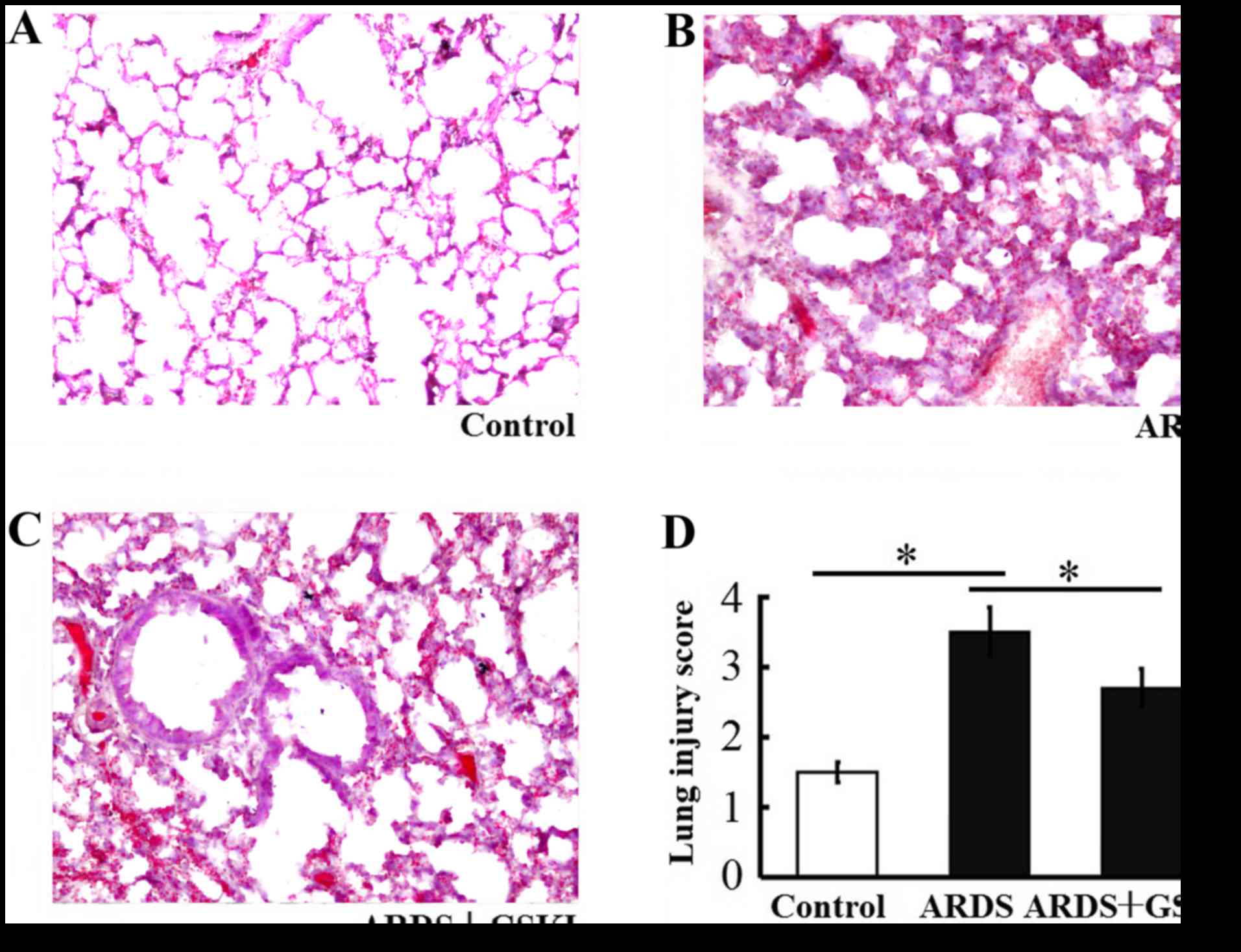
GSKI alleviates histopathological
characteristics of mice with LPS-induced ARDS
The present study found increased thickening of the
alveolar wall, alveolar and interstitial inflammatory cell
infiltration, hemorrhaging alveolar exudates, and edema in the lung
tissue of mice following LPS-induced lung injury. The Smith score
for quantifying lung injury was also increased. By contrast, the
histopathological characteristics and the Smith score were reduced
in the LPS+GSKI group, compared with those in the LPS group
(P<0.05; Fig. 3A-D).
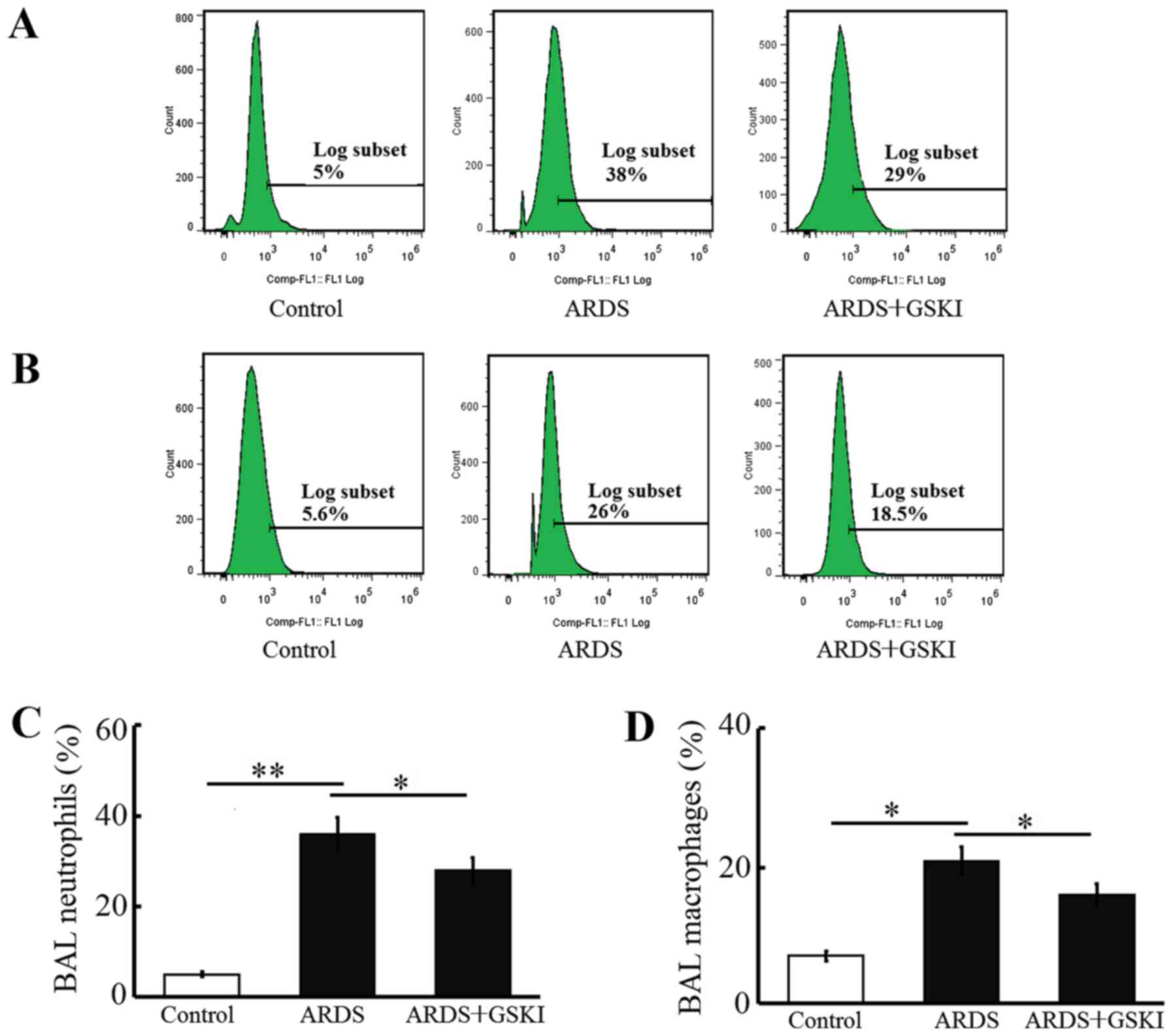
GSKI attenuates acute LPS-induced
pulmonary inflammation
The present study examined inflammatory cell
(neutrophil and macrophage) counts, pro-inflammatory cytokines and
chemokines in BALF to further assess the anti-inflammatory effect
of Wnt signaling in LPS-induced ARDS. The neutrophil and macrophage
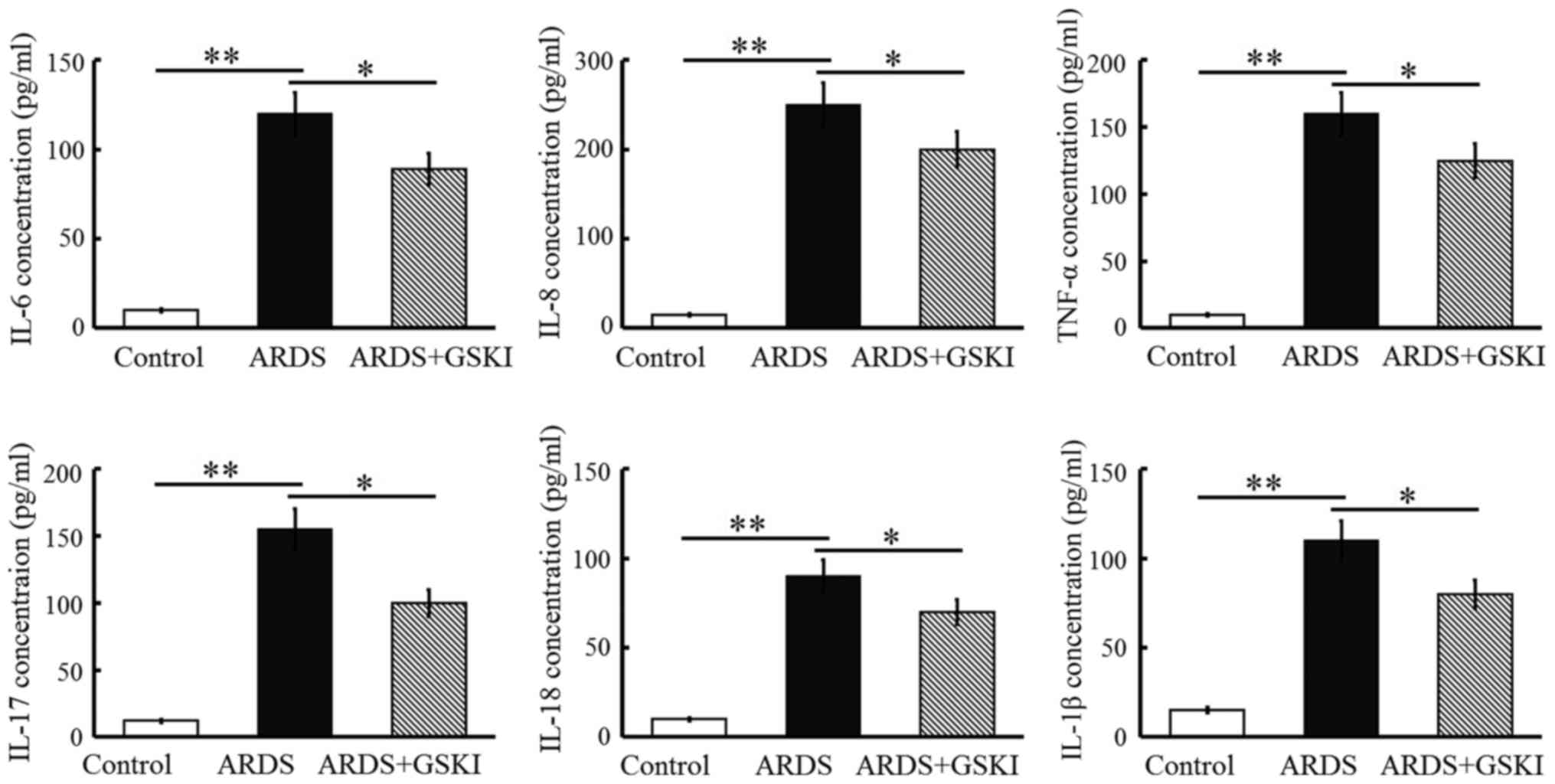
counts were increased (P<0.05 and P<0.01; Fig. 4) and the levels of pro-inflammatory
cytokines, including IL-6, IL-8, TNF-α, IL-17, IL-18, and IL-1β,
were all significantly elevated in response to the LPS challenge
(P<0.05 and P<0.01; Fig. 5).
GSKI administration was effective in decreasing the inflammatory
cell counts, and the levels of pro-inflammatory cytokines and
chemokines (P<0.05; Figs. 4 and
5).
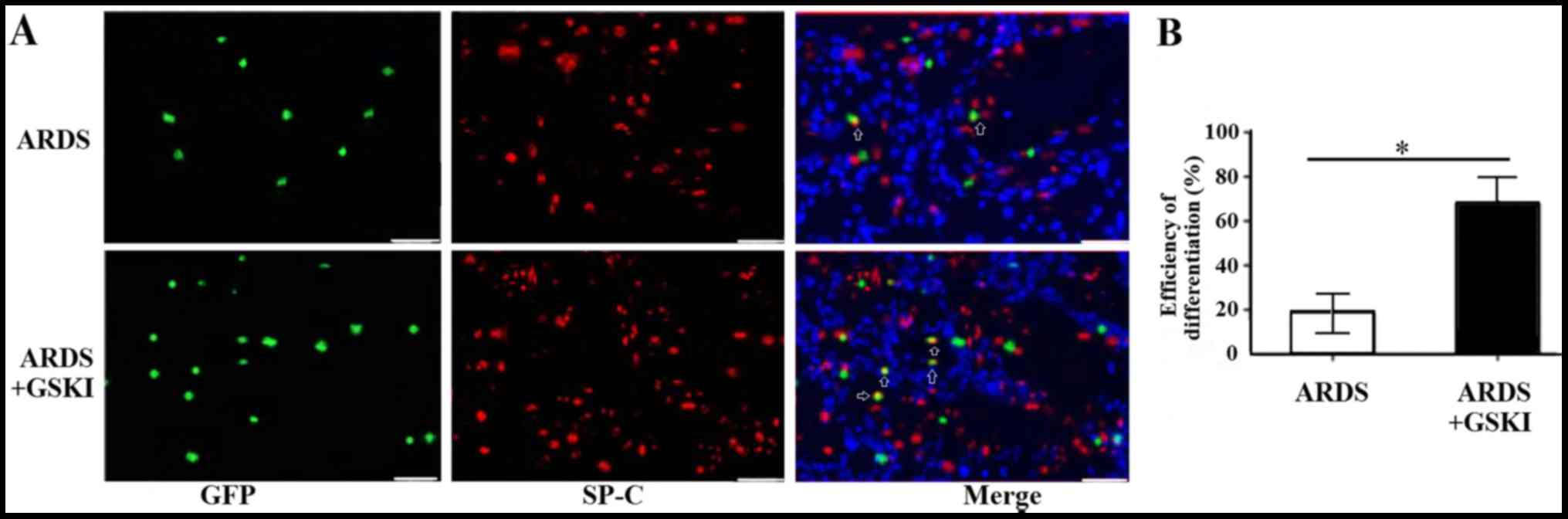
GSKI promotes the differentiation of
MSCs into ATII epithelial cells in vivo
The present study also evaluated the differentiation
of MSCs into ATII cells 14 days following treatment with GSKI by
analyzing the expression of the ATII cell marker, SP-C, through
immunofluorescence staining. The SP-C (red) and MSCs (green)
co-localized in the lung tissue (yellow) of the LPS and LPS+GSKI
groups; however, the rate of differentiation was higher following
GSKI treatment, compared with group without GSKI treatment
(P<0.05; Fig. 6A and B).
Discussion
The canonical Wnt pathway is key in the development,
differentiation, and physiological functions of cells and organisms
(27). Canonical Wnt ligands bind
to Frizzled co-receptors and low-density lipoprotein
receptor-related proteins 5 or 6, which results in GSK-3β
inhibition and the accumulation of β-catenin translocating into the
nucleus, ultimately regulating target gene expression (28). This process makes β-catenin the
pivotal signaling regulator of the canonical Wnt pathway. Due to
its importance in the pathological process of numerous diseases,
the present study examined the effect of Wnt signaling in
experimental ARDS.
GSKI has been applied as a canonical Wnt signaling
activator in previous studies. Troussard et al (29) reported that the phosphorylation of
integrin-linked kinases inhibited GSK-3I activity, but activated
AP-1 activity, resulting in regulation of the survival,
proliferation, differentiation and migration of cells. In addition,
the reduction of cAMP response element binding protein activity has
been shown to facilitate apoptosis (30,31).
Due its efficiency and stability, the present study used GSKI as
treatment reagent administered to LPS-induced ARDS mice. The
results in vivo revealed that GSKI functionally alleviated
LPS-induced acute lung injury, and abrogated inflammatory cell
infiltration into the BALF and pro-inflammatory cytokine secretion.
In addition, GSKI was shown to induce the differentiation of MSCs
into ATII epithelial cells, which is a positive outcome in ARDS
rehabilitation. Therefore, the present study revealed a novel
mechanism underlying the mediation of experimental ARDS by Wnt
signaling. Although further investigations are required, GSKI was
shown to be a prospective candidate as a chemopreventive agent in
clinical settings for therapy for ARDS.
Although it was determined that GSKI attenuated lung
injury, as shown by its effect on body weight and the survival of
the mice, and reduction of lung permeability (Figs. 1 and 2), there are several signal transduction
processes involved in the pathogenesis of ARDS, making it complex
to investigate. Investigations have focused on Wnt signaling, which
is necessary for the proliferation and migration of cells,
expression of multiple cytokines, and inflammatory responses
(32).
To the best of our knowledge, inflammatory responses
are the primary cause of LPS-induced lung injury. During the acute
pathological process, inflammatory cells, including neotrophils and
macrophages, are recruited to lung lesions, and they are induced to
secrete pro-inflammatory cytokines to further accelerate
inflammatory responses and deteriorate ARDS. Thus, effective
inhibition of inflammatory responses is fundamental treatment
strategy for ARDS. It is known that the canonical Wnt pathway is a
preventive factor in the ARDS pathological process. Consistently,
the activation of Wnt signaling by GSKI in the present study
retarded inflammatory cell infiltration and suppressed the
expression of pro-inflammatory cytokines. It was concluded that the
alleviation of experimental ARDS was associated with the effects of
Wnt signaling on the cellular and protein regulation of the
inflammatory response.
MSCs have been shown to migrate to and engraft in
injured lungs, and to differentiate into lung epithelial cells
in vivo, indicating their potential in the treatment of ARDS
(33–36). Therefore, promoting MSCs to
differentiate into lung epithelial cells may be another route to
facilitate ARDS restoration. The present study examined whether Wnt
signaling exhibits protective functions via promoting MSC
differentiation into lung epithelial cells. As expected, it was
found that activation of the canonical Wnt/β-catenin pathway by
GSKI promoted the differentiation of MSCs into ATII cells and
migration to injured lung tissues, as detected using
immunofluorescence assay, which was consistent with previous
reports (37).
In conclusion, the results of the present study
revealed that GSKI was an efficient activator for Wnt signaling in
LPS-induced ARDS, and this activating effect may facilitate MSC
differentiation into lung epithelial cells, reduce inflammatory
responses and thus alleviate lung injury. These results confirmed
GSKI as a prospective candidate chemopreventive agent in clinical
settings for ARDS therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 31270940), Jiangsu
Provincial Medical Youth Talent (grant no. QNRC2016718), the
Clinical Medical Center of Suzhou (grant no. Szzx201502) and the
Clinical Key Specialty Project of China and Suzhou Municipal
Natural Science Foundation (grant nos. SYS201448 and
SYSD2015107).
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goss CH, Brower RG, Hudson LD and
Rubenfeld GD: ARDS Network: Incidence of acute lung injury in the
United States. Crit Care Med. 31:1607–1611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendez JL and Hubmayr RD: New insights
into the pathology of acute respiratory failure. Curr Opin Crit
Care. 11:29–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quílez ME, López-Aguilar J and Blanch L:
Organ crosstalk during acute lung injury, acute respiratory
distress syndrome, and mechanical ventilation. Curr Opin Crit Care.
18:23–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Middelveld RJ and Alving K: Synergistic
septicemic action of the gram-positive bacterial cell wall
components peptidoglycan and lipoteichonic acid in the pig in vivo.
Shock. 13:297–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshinari D, Takeyoshi I, Koibuchi Y,
Matsumoto K, Kawashima Y, Koyama T, Ohwada S and Morishita Y:
Effects of a dual inhibitor of tumor necrosis factor-alpha and
interleukin-1 on lipopolysaccharide-induced lung injury in rats:
Involvement of the p38 mitogen-activated protein kinase pathway.
Crit Care Med. 29:628–634. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka S, Nishiumi S, Nishida M, Mizushina
Y, Kobayashi K, Masuda A, Fujita T, Morita Y, Mizuno S, Kutsumi H,
et al: Vitamin K3 attenuates lipopolysaccharide-induced acute lung
injury through inhibition of nuclear factor-kappaB activationce.
Clin Exp Immunol. 160:283–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranieri VM, Thompson BT, Barie PS,
Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes
A, Artigas A, et al: Drotrecogin alfa (activated) in adults with
septic shock. N Engl J Med. 366:2055–2064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huber-Lang MS, Younkin EM, Sarma JV,
McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Curnutte JT, Erickson R
and Ward PA: Complement-induced impairment of innate immunity
during sepsis. J Immunol. 169:3223–3231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riedemann NC, Guo RF, Neff TA, Laudes IJ,
Keller KA, Sarma VJ, Markiewski MM, Mastellos D, Strey CW, Pierson
CL, et al: Increased C5a receptor expression in sepsis. J Clin
Invest. 110:101–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lehmann LE, Novender U, Schroeder S,
Pietsch T, von Spiegel T, Putensen C, Hoeft A and Stüber F: Plasma
levels of macrophage migration inhibitory factor are elevated in
patients with severe sepsis. Intensive Care Med. 27:1412–1415.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ulloa L, Ochani M, Yang H, Tanovic M,
Halperin D, Yang R, Czura CJ, Fink MP and Tracey KJ: Ethyl pyruvate
prevents lethality in mice with established lethal sepsis and
systemic inflammationProc Natl Acad Sci. USA: 99. pp. 12351–12356.
2002; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Li M, Stadler S, Correll S, Li P,
Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, et al: Histone
hypercitrullination mediates chromatin decondensation and
neutrophil extracellular trap formation. J Cell Biol. 184:205–213.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouldin CM, Manning AJ, Peng YH, Farr GH
III, Hung KL, Dong A and Kimelman D: Wnt signaling and tbx16 form a
bistable switch to commit bipotential progenitors to mesoderm.
Development. 142:2499–2507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Etheridge SL, Spencer GJ, Heath DJ and
Genever PG: Expression profiling and functional analysis of wnt
signaling mechanisms in mesenchymal stem cells. Stem Cells.
22:849–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada M, Kubo H, Kobayashi S, Ishizawa K,
Numasaki M, Ueda S, Suzuki T and Sasaki H: Bone marrow-derived
progenitor cells are important for lung repair after
lipopolysaccharide-induced lung injury. J Immunol. 172:1266–1272.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotton DN, Ma BY, Cardoso WV, Sanderson
EA, Summer RS, Williams MC and Fine A: Bone marrow-derived cells as
progenitors of lung alveolar epithelium. Development.
128:5181–5188. 2001.PubMed/NCBI
|
|
20
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geburek F, Mundle K, Conrad S, Hellige M,
Walliser U, van Schie HT, van Weeren R, Skutella T and Stadler PM:
Tracking of autologous adipose tissue-derived mesenchymal stromal
cells with in vivo magnetic resonance imaging and histology after
intralesional treatment of artificial equine tendon lesions - a
pilot study. Stem Cell Res Ther. 7:212016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takaoka Y, Goto S, Nakano T, Tseng HP,
Yang SM, Kawamoto S, Ono K and Chen CL: Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced,
sepsis-related severe acute lung injury in mice. Sci Rep.
4:52042014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1. PLoS Med. 4:e2692007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang WF, Cho JH, He Q, Lin MC, Wu CC,
Voelkel NF and Douglas IS: Lipid A fraction of LPS induces a
discrete MAPK activation in acute lung injury. Am J Physiol Lung
Cell Mol Physiol. 293:L336–L344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong L, He HL, Lu XM, Yang Y and Qiu HB:
Modulation of FLT3 signaling targets conventional dendritic cells
to attenuate acute lung injury. APMIS. 120:808–818. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matute-Bello G, Winn RK, Jonas M, Chi EY,
Martin TR and Liles WC: Fas (CD95) induces alveolar epithelial cell
apoptosis in vivo: Implications for acute pulmonary inflammation.
Am J Pathol. 158:153–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Troussard AA, Tan C, Yoganathan TN and
Dedhar S: Cell-extracellular matrix interactions stimulate the AP-1
transcription factor in an integrin-linked kinase- and glycogen
synthase kinase 3-dependent manner. Mol Cell Biol. 19:7420–7427.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jean D, Harbison M, McConkey DJ, Ronai Z
and Bar-Eli M: CREB and its associated proteins act as survival
factors for human melanoma cells. J Biol Chem. 273:24884–24890.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walton M, Woodgate AM, Muravlev A, Xu R,
During MJ and Dragunow M: CREB phosphorylation promotes nerve cell
survival. J Neurochem. 73:1836–1842. 1999.PubMed/NCBI
|
|
32
|
Kurosaki T: Checks and balances on
developing B cells. Nat Immunol. 4:13–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Masterson C, Jerkic M, Curley GF and
Laffey JG: Mesenchymal stromal cell therapies: Potential and
pitfalls for ARDS. Minerva Anestesiol. 81:179–194. 2015.PubMed/NCBI
|
|
34
|
Li J, Huang S, Wu Y, Gu C, Gao D, Feng C,
Wu X and Fu X: Paracrine factors from mesenchymal stem cells: A
proposed therapeutic tool for acute lung injury and acute
respiratory distress syndrome. Int Wound J. 11:114–121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simonson OE, Mougiakakos D, Heldring N,
Bassi G, Johansson HJ, Dalén M, Jitschin R, Rodin S, Corbascio M,
El Andaloussi S, et al: In vivo effects of mesenchymal stromal
cells in two patients with severe acute respiratory distress
syndrome. Stem Cells Transl Med. 4:1199–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grove JE, Lutzko C, Priller J, Henegariu
O, Theise ND, Kohn DB and Krause DS: Marrow-derived cells as
vehicles for delivery of gene therapy to pulmonary epithelium. Am J
Respir Cell Mol Biol. 27:645–651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu AR, Liu L, Chen S, Yang Y, Zhao HJ,
Liu L, Guo FM, Lu XM and Qiu HB: Activation of canonical wnt
pathway promotes differentiation of mouse bone marrow-derived MSCs
into type II alveolar epithelial cells, confers resistance to
oxidative stress, and promotes their migration to injured lung
tissue in vitro. J Cell Physiol. 228:1270–1283. 2013. View Article : Google Scholar : PubMed/NCBI
|