Introduction
Gastric cancer (GC) is one of the most common
malignant tumors worldwide. According to GLOBOCAN estimates, GC is
the fourth most common cancer, with the third highest
cancer-associated mortality rate in males worldwide (1). In addition, GC is more prevalent in
Eastern Asian countries (2). At
present, it is accepted that surgery is the only effective cure for
GC; however, chemotherapy is the main treatment for patients with
advanced GC, which can effectively improve the quality of life and
maximize survival time of patients (3). However, chemotherapy can cause a
series of problems, including drug resistance and adverse
reactions; therefore, it is not considered ideal for the treatment
of advanced GC. Hence, the development of effective antitumor
drugs, which possess high efficiency and low toxicity, is
required.
At present, the majority of antitumor drugs are
natural products derived from plants, which have been used for the
clinical treatment of cancer, including vincristine, paclitaxel and
docetaxel (4,5). The Chinese medicinal herb
Ailanthus altissima has long been used for treatment of
colds and gastric diseases in traditional Chinese medicine
(6). Ailanthone, which is
extracted from Ailanthus altissima (7), is a type of quassinoid that has been
reported to exert obvious antitumor effects. Previous studies have
revealed that ailanthone possesses numerous biological activities,
including antimalarial, antibacterial and anti-inflammatory
activities (8,9). Previous studies have indicated that
ailanthone exerts growth-inhibitory effects against HeLa, Jurkat,
HepG2, Hep3B, R-HepG2, MCF-7, MDA-MB-231 and A549 tumor cells in
vitro (10–13). In addition, ailanthone induces
apoptosis of Huh-7 cells and significantly inhibits the growth of
Huh-7 tumors in a nude mouse xenograft model, without exerting
secondary adverse effects (14).
Apoptosis is a type of programmed cell death, which is regulated at
the gene level, and results in the elimination of damaged cells
(15). Anti-apoptotic mechanisms
serve an important role in the development and progression of
cancer, and are considered hallmarks of cancer and a potential
reason for the poor effects of cancer treatment (16,17).
Promoting apoptosis has been identified as an effective means of
cancer treatment; therefore, ailanthone may potentially be used to
treat tumors in the future.
The effects of ailanthone have yet to be reported on
GC cells. The present study aimed to investigate the inhibitory
effects of ailanthone on the SGC-7901 human GC cell line and to
elucidate its potential molecular mechanisms in vitro.
Materials and methods
Materials
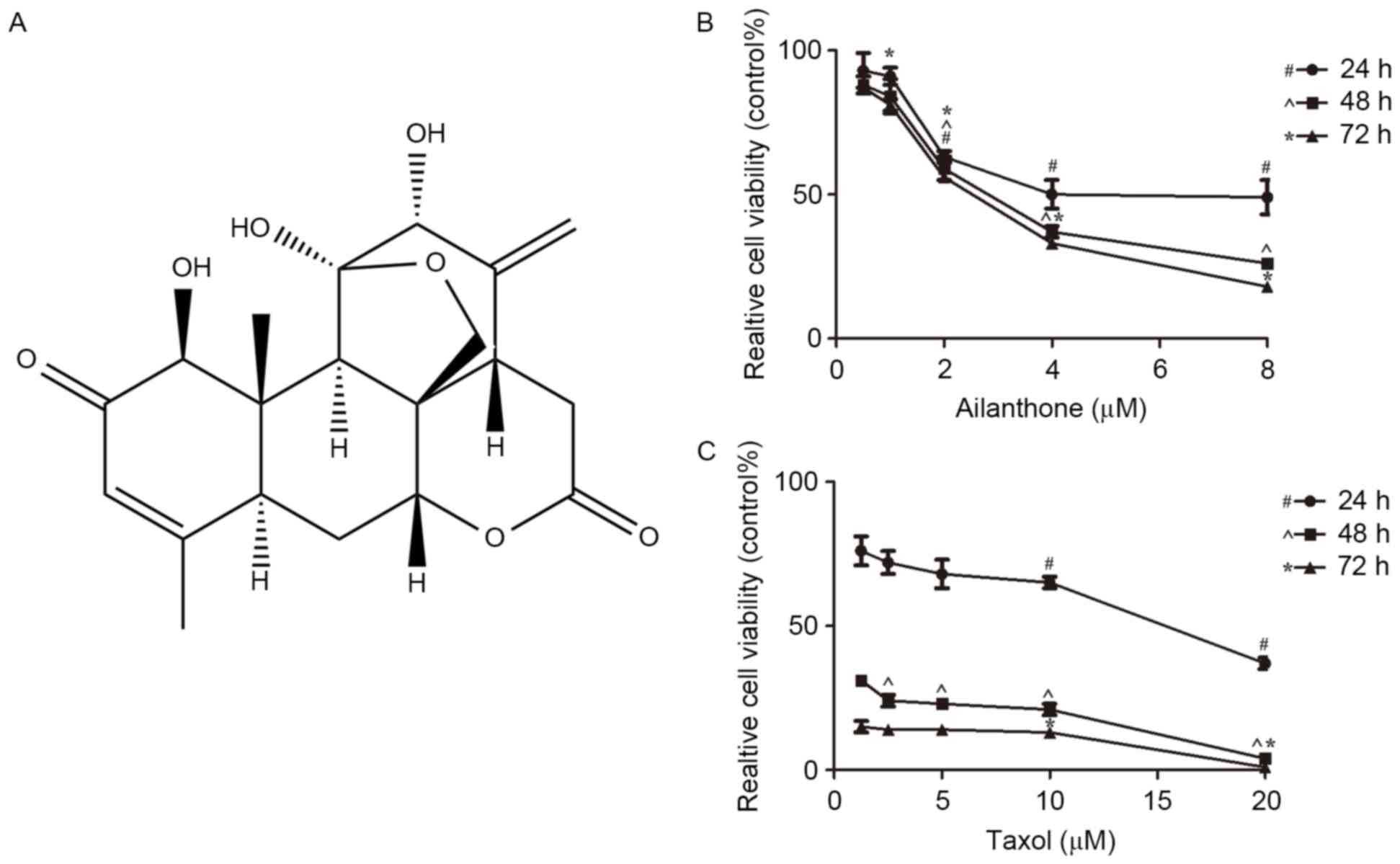
Pure ailanthone (Fig.
1A) was extracted and isolated from Ailanthus altissima.
The ailanthone sample (purity ≥98%) was provided by the Institute
of Traditional Chinese Medicine and Natural Products, Jinan
University (Guangzhou, China). Taxol was obtained from Beijing SL
Pharmaceutical Co., Ltd. (Beijing, China). Dimethyl sulfoxide
(DMSO) was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). The Cell Counting Kit-8 (CCK-8) assay (cat no. KGA317)
was obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China). RPMI-1640 (cat no. 11875-093) and penicillin-streptomycin
(PS; cat no. 15140-122) were purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Fetal bovine serum (FBS; cat
no. 100-700) was obtained from Gemini Bio Products (West
Sacramento, CA, USA). The antibodies for mouse monoclonal β-actin
(cat no. BM0626), mouse monoclonal B-cell lymphoma 2 (Bcl-2; cat
no. BM0200) and rabbit polyclonal Bcl-2-associated X protein (Bax;
cat no. BA0315-2) were purchased from Wuhan Boster Biological
Technology Ltd. (Wuhan, China). Horseradish peroxidase
(HRP)-conjugated goat anti-mouse (cat no. 62-6520) and anti-rabbit
(cat no. G-21234) immunoglobulin (Ig)G were obtained from
Invitrogen; Thermo Fisher Scientific, Inc.
Cell culture and treatment
The SGC-7901 human GC cell line (cat. no. KG026) was
obtained from Nanjing KeyGen Biotech Co., Ltd. The cells were
cultured in RPMI-1640 medium supplemented with 10% FBS and 1% PS in
a humidified incubator containing 5% CO2 and 95% air at
37°C for cell subculture and all experiments. Stock solutions of
ailanthone were prepared in DMSO, and stored at −20°C. Prior to
use, stock solutions were immediately diluted to the required
concentration with RPMI-1640 complete medium; the terminal
concentration of DMSO in the culture medium was ≤0.1%. Control
cells were treated with DMSO (0.1%), without ailanthone and
taxol.
Cell viability assay
The CCK-8 assay was used to measure cell viability.
Taxol was used in the positive control group. SGC-7901 cells in the
exponential growth phase (5×103 cells/well) were seeded
and cultured in 96-well plates for 24 h, and were then treated with
0.1% DMSO (control group), ailanthone (0.5, 1, 2, 4 and 8 µM) or
taxol (1.25, 2.5, 5, 10 and 20 µM) for 24, 48 and 72 h at 37°C,
each group was analyzed four times. Subsequently, 10 µl CCK-8
solution was added to each well. After 3 h at 37°C, the optical
density was measured at a wavelength of 450 nm using a microplate
reader (RT-6000; Rayto Life and Analytical Sciences Co., Ltd.,
Shenzhen, China). Relative cell viability was determined using the
following formula: Relative cell viability = (mean A450 of
experimental groups/mean A450 of control groups) ×100%.
Hoechst 33258 staining
Hoechst 33258 staining was used to observe the
apoptotic morphology of cells. Exponentially growing SGC-7901 cells
were cultured on glass coverslips in 6-well plates
(3×105 cells/well) for 24 h, and were then treated with
0.1% DMSO or ailanthone (1 and 2 µM) for 48 h at 37°C. The cells
were dried and were fixed in 4% paraformaldehyde for 30 min at room
temperature. Subsequently, the cells were washed three times with
phosphate-buffered saline (PBS), and were stained with 10 µg/ml
Hoechst 33258 (cat no. KGA211-10; Nanjing KeyGen Biotech Co., Ltd.)
for 10 min at room temperature. The cells were washed a further two
times with PBS and were mounted using antifade mounting medium (cat
no. P0126; Beyotime Institute of Biotechnology, Shanghai, China).
Finally, nuclear morphology was observed by fluorescence microscopy
(Olympus BX43; Olympus Corporation, Tokyo, Japan).
Annexin V-allophycocyanain
(APC)/7-amino-actinomycin D (ADD) apoptotic analysis
Exponentially growing SGC-7901 cells were seeded and
cultured in 6-well plates (3×105 cells/well) for 24 h,
and were then treated with 0.1% DMSO or ailanthone (1–4 µM) for 48
h at 37°C. The cells were washed two times with cold PBS, and were
then incubated with Annexin V APC/7-ADD (cat no. KGA1026; Nanjing
KeyGen Biotech Co., Ltd.) for 15 min at room temperature in the
dark according to the manufacturer's protocol. Subsequently, the
cells were analyzed by flow cytometry. Fluorescence was measured
using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA,
USA). The experiment was independently repeated three times, and
the proportion of apoptotic cells was calculated using the
FACSCalibur internal software system (BD Biosciences).
DNA content analysis
The SGC-7901 cells were seeded and cultured in
6-well plates (3×105 cells/well) for 24 h, and were then
treated with 0.1% DMSO or ailanthone (1–4 µM) for 48 h at 37°C.
Cells were harvested, washed with PBS and were fixed in 70% ethanol
at 4°C overnight. Subsequently, cells were incubated with 1% RNase
A at 37°C for 30 min and with propidium iodide solution (cat no.
KGA511; Nanjing KeyGen Biotech Co., Ltd.) at 4°C for 30 min in the
dark. The DNA content of cells was measured using a FACSCalibur
flow cytometer (BD Biosciences). The experiment was independently
repeated three times, and data were analyzed using the MultiCycle
DNA content and cell cycle analysis software (FlowJo, version
7.6.5; FlowJo LLC, Ashland, OR, USA).
Western blot analysis
Following treatment with 0.1% DMSO or ailanthone
(1–4 µM) for 48 h at 37°C, SGC-7901 cells were washed twice with
ice-cold PBS and suspended in radio immunoprecipitation assay lysis
buffer (cat no. KGP702; Nanjing KeyGen Biotech Co., Ltd.) on ice
for 30 min. The lysates were then cleared by centrifugation at
12,000 × g for 15 min at 4°C. Subsequently, the bicinchoninic acid
protein assay kit (cat no. KGP902; Nanjing KeyGen Biotech Co.,
Ltd.) was used to measure the total protein concentration of each
sample according to the manufacturer's protocol. Protein samples
(30 µg) from each group were separated by 15% SDS-PAGE and were
then transferred onto polyvinylidene difluoride membranes (Pall
Corporation, Port Washington, NY, USA). Membranes were blocked with
5% (w/v) non-fat dry milk dissolved in TBS containing 0.05%
Tween-20 (TBST) at room temperature for 1 h, and were then washed
three times with TBST. Subsequently, membranes were incubated with
primary antibodies against Bcl-2 (1:200), Bax (1:200) and β-actin
(1:400) overnight at 4°C. After washing three times with TBST, the
membranes were incubated with HRP-conjugated goat anti-mouse
(1:2,000) or anti-rabbit (1:2,000) IgG secondary antibodies at room
temperature for 1 h. The immunoreactive bands were visualized with
enhanced chemiluminescent substrates (cat no. 34080; Thermo Fisher
Scientific, Inc.) using an X-ray film processor (Kodak, Rochester,
NY, USA). β-actin was used as a loading control. The experiment was
independently repeated three times, and Quantity One software
(version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to analyze the densitometry of each band.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR analysis was used to analyze the mRNA
expression levels of Bcl-2 and Bax in cells treated with 0.1% DMSO
or ailanthone (1–4 µM) for 48 h at 37°C. Total RNA was extracted
from the cells by TRIzol reagent (cat no. 15596-026; Invitrogen;
Thermo Fisher Scientific, Inc.). Subsequently, first-strand cDNA
synthesis was conducted using the TransScript® One-Step
gDNA Removal and cDNA Synthesis SuperMix kit (cat no. AT311-02;
Beijing TransGen Biotech Co., Ltd., Beijing, China) according to
the manufacturer's protocol. RT-qPCR analysis was performed on the
StepOne Plus Real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in a 20-µl reaction mixture containing 10
µl AceQ® qPCR SYBR Green Master Mix (Low ROX Premixed)
(cat no. Q131-02/03; Vazyme Biotech Co., Ltd., Nanjing, China), 0.8
µl primer mixture (forward and reverse, 10 µM), 1 µl cDNA and 8.2
µl DEPC water. According to the mix kit manufacturer's protocol,
the thermocycling profile consisted of 95°C for 5 min, followed by
40 cycles at 95°C for 10 sec and 60°C for 30 sec. GAPDH was used as
an endogenous control, and data were analyzed using the
2−ΔΔCq method (18).
The experiment was performed four times. The primer sequences were
as follows: Bcl-2 forward, 5′-GGTGGGGTCATGTGTGTGG-3′ and reverse,
5′-CGGTTCAGGTACTCAGTCATCC-3′; Bax forward,
5′-CCCGAGAGGTCTTTTTCCGAG-3′ and reverse,
5′-CCAGCCCATGATGGTTCTGAT-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Data were analyzed by
one-way analysis of variance followed by Tukey's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Ailanthone induces antiproliferatice
effects on SGC-7901 cells
The CCK-8 assay was used to determine the effects of
ailanthone on the growth of SGC-7901 cells treated with 0.1% DMSO
(control group), ailanthone (0.5–8 µM) or taxol (1.25–20 µM) for
24, 48 and 72 h at 37°C. Ailanthone inhibited the viability of
SGC-7901 cells in a dose- and time-dependent manner (Fig. 1B). The half maximal inhibitory
concentration (IC50) values of ailanthone in SGC-7901
cells at 24, 48 and 72 h were 5.473, 2.906 and 2.47 µM,
respectively. Cells treated with taxol were considered the positive
control group, taxol also inhibited the growth of SGC-7901 cells in
a dose- and time-dependent manner (Fig. 1C). The IC50 value of
taxol in SGC-7901 cells was 14.47 µM at 24 h.
Ailanthone induces apoptosis of
SGC-7901 cells
A Hoechst 33258 staining assay was used to observe
the apoptotic morphology of SGC-7901 cells treated with ailanthone
(1 and 2 µM) or 0.1% DMSO for 48 h at 37°C. Characteristic
apoptotic morphology, including nuclear shrinkage and chromatin
condensation, was observed in the ailanthone groups; however,
apoptotic morphology was hardly detected in the control group
(Fig. 2A). In order to further
confirm the occurrence of apoptosis, cells were stained with
Annexin V-APC/7-ADD and were analyzed by flow cytometry. The
apoptotic rate of cells was significantly increased with
concentration of ailanthone (1–4 µM) from 12.44 to 38.71%.
Representative results are presented in Fig. 2B. As shown in Fig. 2C, following treatment with 1, 2 and
4 µM ailanthone for 48 h at 37°C, the percentage of apoptotic cells
was 12.44±0.43, 19.77±0.83 and 38.71±0.7% respectively, which was
significantly higher than in the control group (4.31±0.2%;
P<0.001). These results indicated that ailanthone induced
apoptosis of SGC-7901 cells in a dose-dependent manner.
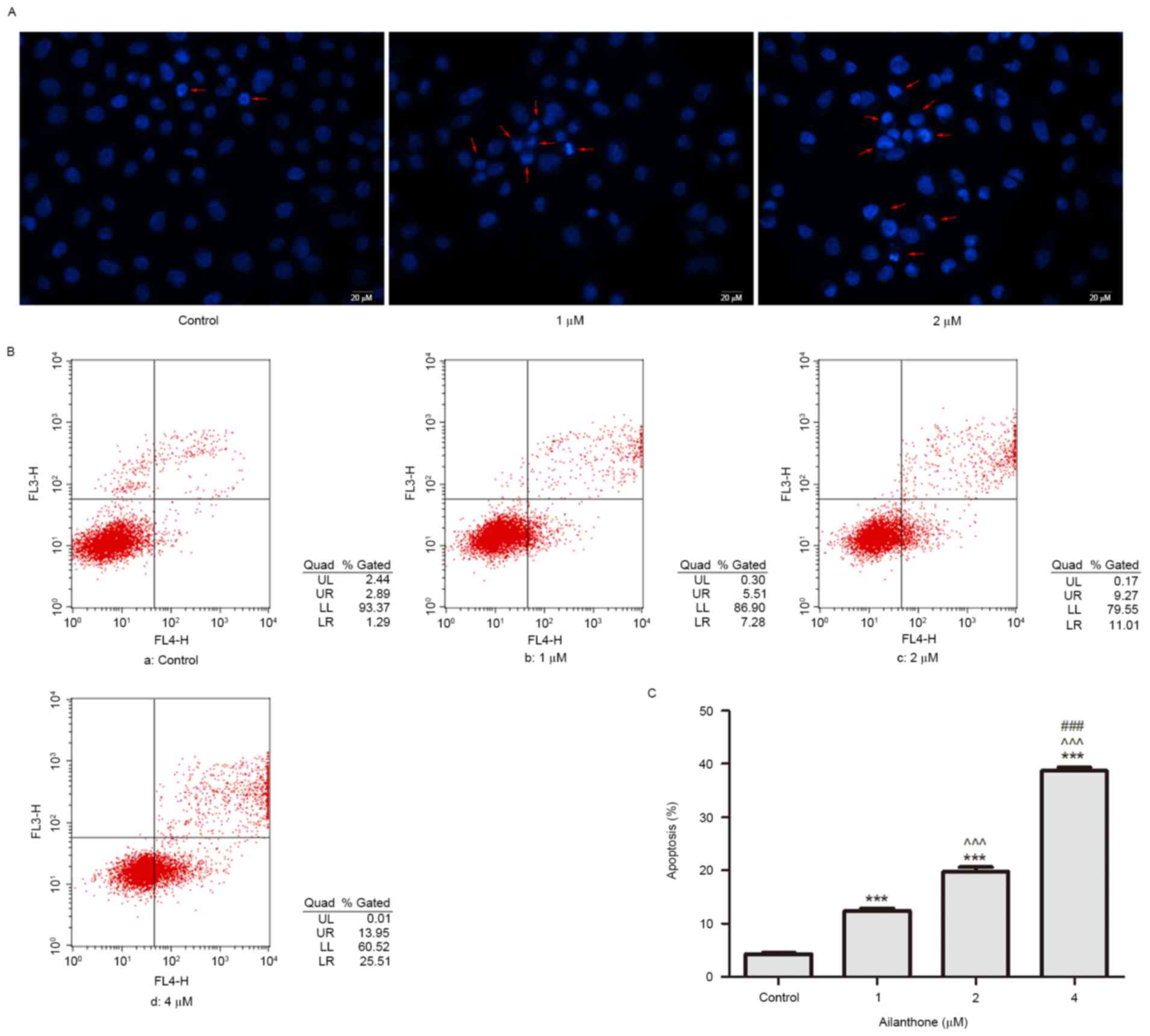 | Figure 2.Ailanthone induces apoptosis of
SGC-7901 cells. (A) Following treatment with 0.1% DMSO or
ailanthone (1 and 2 µM) for 48 h at 37°C, Hoechst 33258 staining
was used to determine the morphological alterations of cells. Red
arrows indicate characteristic apoptotic morphology. Scale bar, 20
µm. (B) Detection of apoptosis in SGC-7901 cells treated with
ailanthone for 48 h at 37°C. Early (LR quadrant) and late (UR
quadrant) apoptotic cells were detected by flow cytometry. (a)
Control cells; (b-d) cells treated with 1, 2 and 4 µM ailanthone,
respectively. The experiment was repeated three times, and
representative results are presented. (C) Percentage of apoptotic
cells. Data are presented as the mean ± standard deviation, n=3.
***P<0.001 vs. the control group; ^^^P<0.001 vs.
the 1 µM group; ###P<0.001 vs. the 2 µM group. LL,
lower left; LR, lower right; UL, upper left; UR, upper right. |
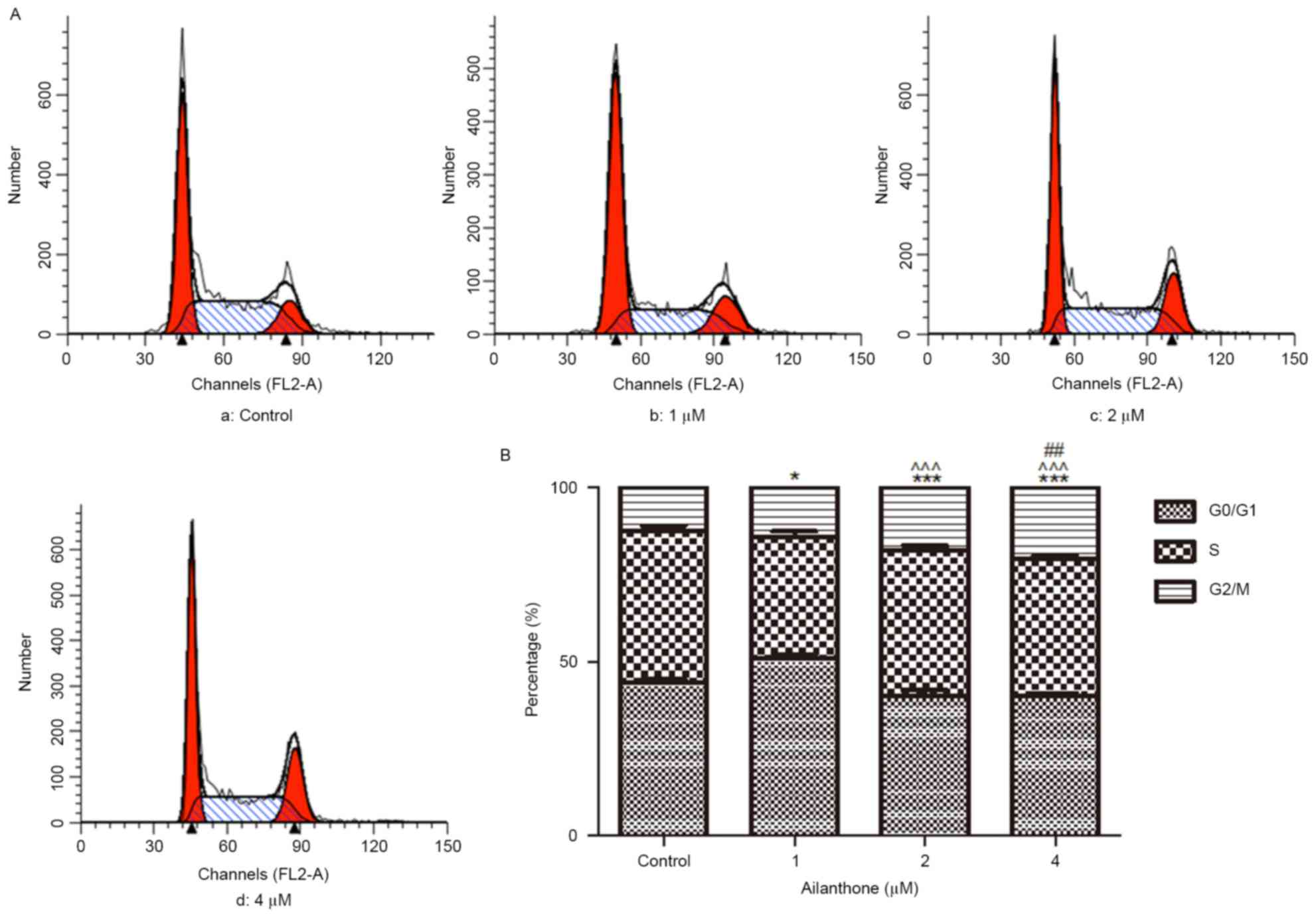
Ailanthone induces cell cycle arrest
of SGC-7901 cells
Flow cytometry was used to analyze the distribution
of SGC-7901 cells within the cell cycle following treatment with
ailanthone (1–4 µM) or 0.1% DMSO for 48 h at 37°C. G2/M
phase cell cycle arrest was observed in cells exposed to ailanthone
compared with the control group; representative results are
presented in Fig. 3A. As shown in
Fig. 3B, ailanthone induced a
G2/M phase cell cycle arrest of SGC-7901 cells in a
dose-dependent manner; the percentage of cells in G2/M
phase was 14.13±0.48, 18.10±0.56 and 20.56±0.33% in the 1, 2 and 4
µM groups, respectively. Ailanthone significantly increased the
percentage of cells in G2/M phase compared with the
control group (12.63±0.78%; P<0.05).
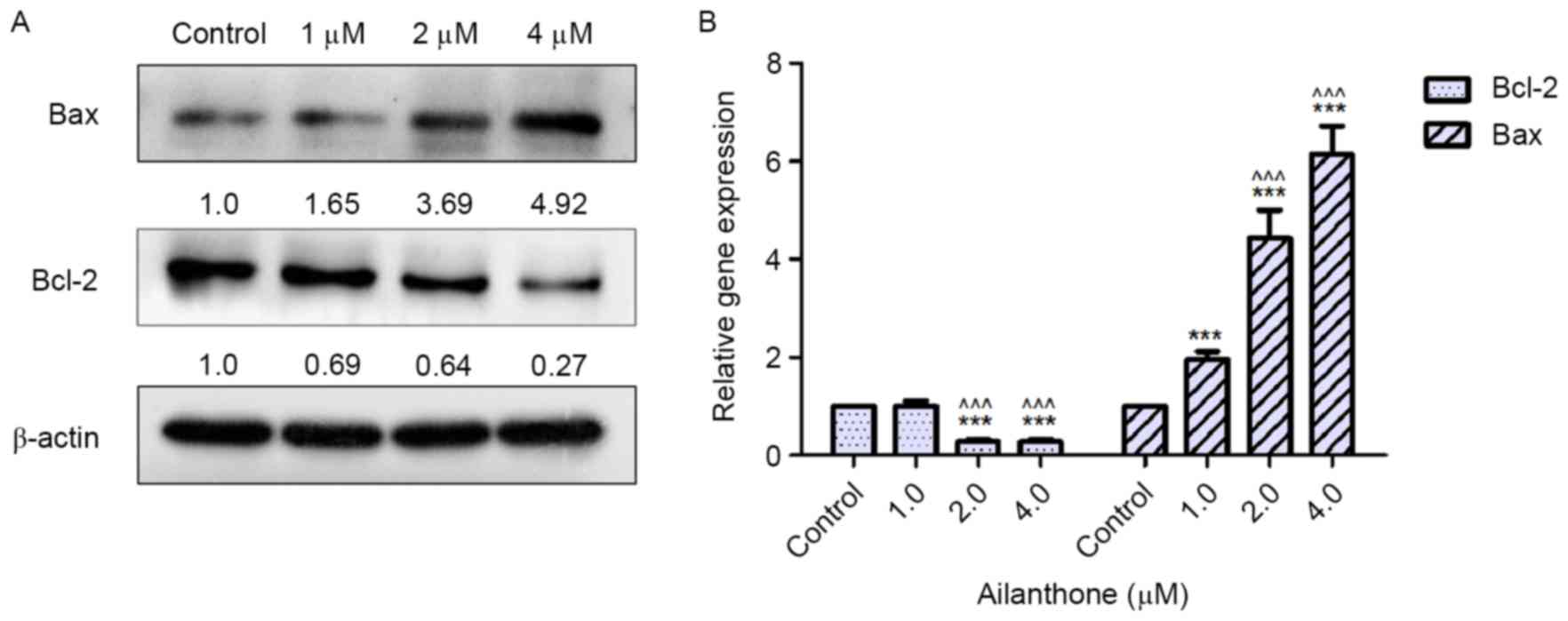
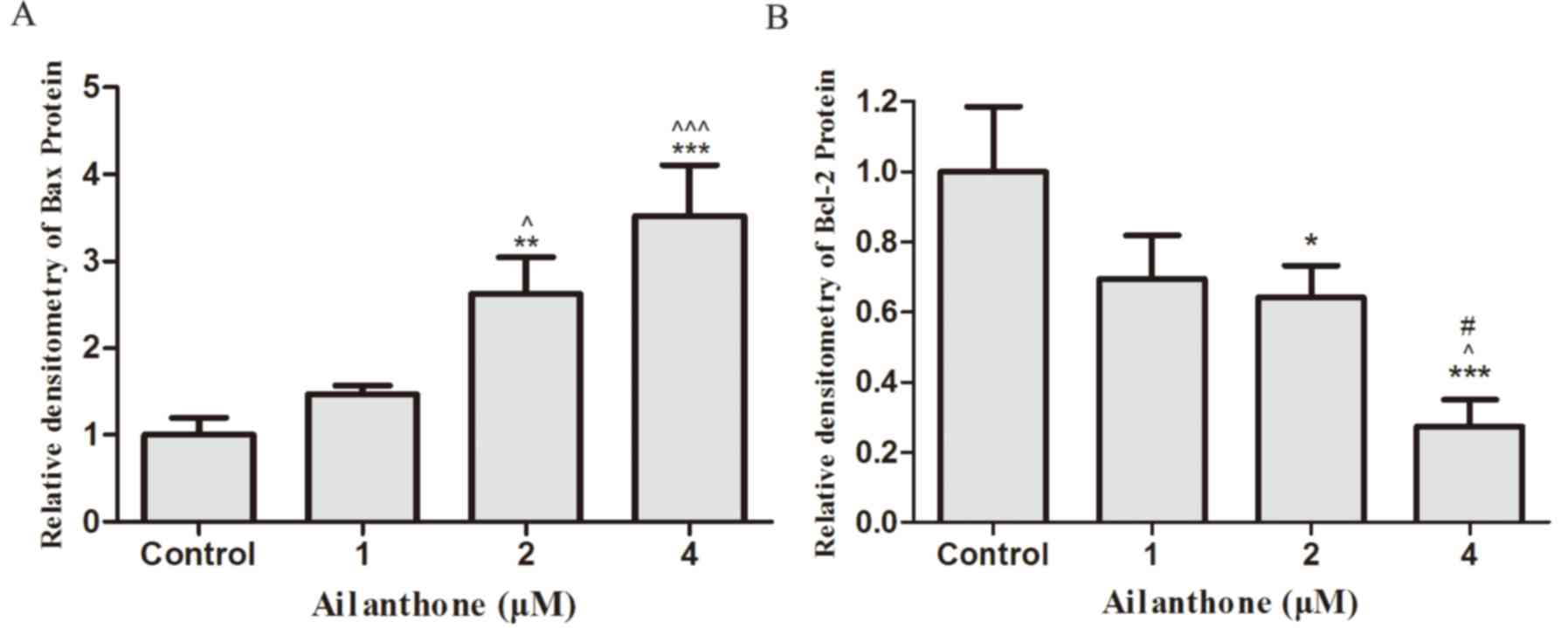
Effects of ailanthone on the protein
expression levels of Bcl-2 and Bax in SGC-7901 cells
Bcl-2 is considered an anti-apoptotic protein,
whereas Bax is considered proapoptotic. In order to detect the
protein expression levels of Bcl-2 and Bax, western blot analysis
was performed. Following treatment with ailanthone (1–4 µM) or 0.1%
DMSO for 48 h at 37°C, the protein expression levels of Bcl-2 were
decreased, whereas the protein expression levels of Bax were
increased, compared with the control group (Fig. 4A). The downregulation of Bcl-2 and
the enhanced expression of Bax were relative to ailanthone
concentration. As shown in Fig. 5,
treatment with 2 and 4 µM ailanthone significantly downregulated
the protein expression levels of Bcl-2 compared with the control
group (P<0.05, P<0.001), also significantly upregulated the
protein expression of Bax compared with the control group
(P<0.01, P<0.001).
Effects of ailanthone on the mRNA
expression levels of Bcl-2 and Bax in SGC-7901 cells
As shown in Fig.
4B, the results of RT-qPCR demonstrated that ailanthone altered
the mRNA expression levels of Bcl-2 and Bax in SGC-7901 cells
compared with in the control group. Notably, the mRNA expression
levels of Bax were significantly upregulated in the ailanthone
groups compared with the control group (P<0.001). Conversely,
treatment with 2 and 4 µM ailanthone significantly reduced the mRNA
expression levels of Bcl-2 compared with the control group
(P<0.001). However, 1 µM ailanthone had no effect on the mRNA
expression levels of Bcl-2 compared with the control group
(P>0.05).
Discussion
Compounds derived from natural products have
garnered much attention in cancer research, due to reasons of high
efficiency and low toxicity. Ailanthone, which is a potent
quassinoid, has been suggested as a potential tumor treatment in
previous studies (13,14); however, the anticancer effects and
potential mechanisms of ailanthone are unclear in SGC-7901 cells.
The present study initially investigated the anti-proliferative
activity of ailanthone against SGC-7901 cells, and demonstrated
that ailanthone significantly inhibited SGC-7901 cell viability at
low concentrations in vitro. The IC50 value for
ailanthone in SGC-7901 cells at 48 h was 2.906 µM.
The present study further analyzed the mechanisms
underlying the antiproliferative effects of ailanthone on SGC-7901
cells. Characteristic apoptotic morphology (cell nuclear shrinkage
or chromatin condensation) was observed in SGC-7901 cells treated
with ailanthone for 48 h using Hoechst 33258 staining. In addition,
the rate of apoptosis was increased in a dose-dependent manner in
ailanthone-treated SGC-7901 cells. Cancer is regarded as a disease
associated with uncontrolled cell proliferation, and anti-apoptotic
mechanisms are considered features of cancer that may result in
this uncontrolled cell proliferation (16). Apoptosis is a physiological process
that does not induce additional damage to normal cells and
surrounding tissues when cancer cells are killed in an apoptotic
manner (19). Therefore, enhanced
apoptosis is considered an effective method for cancer treatment
(20). The results of the present
study demonstrated that the anticancer effects of ailanthone on
SGC-7901 cells were partly due to the induction of apoptosis.
DNA content analysis revealed that cells exposed to
ailanthone exhibited increased cell cycle arrest at G2/M
phase compared with in the control group. In cancer cells, the
genetic control of cell division is altered, leading to unlimited
cell proliferation. Dysregulation of cell cycle progression is also
considered a common characteristic of cancer. The cell cycle
process is separated into four sequential phases: G1, S,
G2 and M phases, and is regulated at numerous positions,
known as checkpoints, by a series of proteins, including
cyclin-dependent kinases (CDKs) and cyclins (21). Cells are held at cell cycle
checkpoint for numerous reasons, including DNA damage, after which
the cell cycle process is terminated and the cells undergo
apoptosis (22). In the present
study, SGC-7901 cells were arrested at G2/M phase
following treatment with ailanthone for 48 h, as determined by flow
cytometry. In addition, the cell cycle progression of SGC-7901
cells was hindered by ailanthone, leading to inhibited cell
proliferation. Notably, in a previous study, ailanthone
significantly induced apoptosis, and arrested cells at
G1/S phase, via upregulated expression of p21 and p27,
and downregulated expression of cyclins D and E and CDKs 2, 4 and
6, in Huh-7 hepatocellular carcinoma cells (14). It should be noted that the present
study only analyzed the effects of ailanthone on cell cycle
distribution without further analyzing its underlying mechanism in
SGC-7901 cells. Combined with the previous study, it may be
hypothesized that ailanthone affects different phases of the cell
cycle in order to serve an antitumor role in various cancer cell
lines. Our future studies aim to analyze the effects and underlying
mechanisms of ailanthone on cell cycle progression in various
cancer cell lines. Similarly, the growth-inhibitory effects of
other natural products were partly associated with G2/M
phase arrest of SGC-7901 cells (23,24).
Therefore, these data indicated that ailanthone may inhibit the
proliferation of SGC-7901 cells partially via G2/M phase
arrest.
In principle, signals that induce cell death can be
blocked by upregulation of anti-apoptotic molecules and/or
downregulation of proapoptotic proteins (25). The balance between proapoptotic and
anti-apoptotic molecules is considered a key point in the
regulation of apoptosis, which decides whether cells will be killed
by apoptosis. In the majority of eukaryotic cells, there are two
major apoptotic pathways: The death receptor pathway and the
mitochondrial pathway (26,27);
the mitochondrial apoptotic pathway serves an important role in the
apoptosis of eukaryotic cells. Furthermore, by affecting the
permeability of the mitochondrial membrane, the Bcl-2 protein
family is believed to be a switch that controls the mitochondrial
apoptotic pathway (28). Bcl-2
protein family members are usually divided into three subgroups:
Anti-apoptotic proteins [Bcl-2, Bcl-extra large (xl), Bcl-2-like
protein 2, Mcl-1, Bcl-2-like protein 10 and Bcl-2-related protein
A1], proapoptotic proteins [Bax, Bcl-2 homologous antagonist/killer
(Bak) and Bcl-2 related ovarian killer] and BH3-only proteins
(Bcl-2-associated agonist of cell death, BH3 interacting-domain
death agonist and Bcl-2-interacting killer) (29). The anti-apoptotic proteins,
including Bcl-2 and Bcl-xl, suppress Bax and Bak, which serve a
critical role in the induction of mitochondrial outer membrane
permeability resulting in the release of cytochrome c, which
consequently leads to caspase activation and apoptosis (30,31).
Therefore, the ratio of pro- to anti-apoptotic Bcl-2 proteins may
regulate the sensitivity of cells to apoptosis (24). An increase in the Bax/Bcl-2 ratio
is generally believed to be a critical factor in the activation of
programmed cell death (32). To
further investigate the underlying molecular mechanisms of
ailanthone-induced apoptosis, the protein and mRNA expression
levels of Bcl-2 and Bax were detected in SGC-7901 cells treated
with ailanthone. The results revealed that ailanthone significantly
downregulated the expression of Bcl-2 and upregulated the
expression of Bax at the protein and mRNA levels. Therefore, the
present study demonstrated that ailanthone induced apoptosis of
SGC-7901 cells by altering the expression levels of Bcl-2 and
Bax.
In conclusion, the present study is the first, to
the best of our knowledge, to reveal that the anticancer effects of
ailanthone were partially due to G2/M phase cell cycle
arrest and apoptosis via modulation of Bcl-2 and Bax expression in
SGC-7901 cells. Further studies are required to explore the effects
of ailanthone on various tumor cell lines, and to evaluate the
antitumor and adverse effects of ailanthone in animal models;
however, the results of the present study indicated that ailanthone
may act as a potential antitumor agent for the treatment of GC, and
provided an experimental basis for future drug development.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81241102). The
authors would like to thank the Institute of Traditional Chinese
Medicine and Natural Products, Jinan University for providing the
pure sample of ailanthone.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cervantes A, Roselló S, Roda D and
Rodriguez-Braun E: The treatment of advanced gastric cancer:
Current strategies and future perspectives. Ann Oncol. 19 Suppl
5:v103–v107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
da Rocha AB, Lopes RM and Schwartsmann G:
Natural products in anticancer therapy. Curr Opin Pharmacol.
1:364–369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang P, Yang HL, Yang YJ, Wang L and Lee
SC: Overcome cancer cell drug resistance using natural products.
Evid Based Complement Alternat Med. 2015:7671362015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin MH, Yook J, Lee E, Lin CX, Quan Z, Son
KH, Bae KH, Kim HP, Kang SS and Chang HW: Anti-inflammatory
activity of Ailanthus altissima in ovalbumin-induced lung
inflammation. Biol Pharm Bull. 29:884–888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bray DH, Boardman P, O'Neill MJ, Chan KL,
Phillipson JD, Warhurst DC and Suffness M: Plants as a source of
antimalarial drugs 5. Activities of Ailanthus altissima stem
constituents and of some related quassinoids. Phytother Res.
1:22–24. 1987. View Article : Google Scholar
|
|
8
|
Okunade AL, Bikoff RE, Casper SJ, Oksman
A, Goldberg DE and Lewis WH: Antiplasmodial activity of extracts
and quassinoids isolated from seedlings of Ailanthus altissima
(Simaroubaceae). Phytother Res. 17:675–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kundu P and Laskar S: A brief resume on
the genus Ailanthus: Chemical and pharmacological aspects.
Phytochem Rev. 9:379–412. 2010. View Article : Google Scholar
|
|
10
|
Fukamiya N, Lee KH, Muhammad I, Murakami
C, Okano M, Harvey I and Pelletier J: Structure-activity
relationships of quassinoids for eukaryotic protein synthesis.
Cancer Lett. 220:37–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosati A, Quaranta E, Ammirante M, Turco
MC, Leone A and De Feo V: Quassinoids can induce mitochondrial
membrane depolarisation and caspase 3 activation in human cells.
Cell Death Differ. 11 Suppl 2:S216–S218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Wang WJ, Su C, Zhang DM, Xu LP, He
RR, Wang L, Zhang J, Zhang XQ and Ye WC: Cytotoxic quassinoids from
Ailanthus altissima. Bioorg Med Chem Lett. 23:654–657. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang XL, Yuan YL, Zhang DM, Li F and Ye
WC: Shinjulactone O, a new quassinoid from the root bark of
Ailanthus altissima. Nat Prod Res. 28:1432–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhuo Z, Hu J, Yang X, Chen M, Lei X, Deng
L, Yao N, Peng Q, Chen Z, Ye W and Zhang D: Ailanthone inhibits
Huh7 cancer cell growth via cell cycle arrest and apoptosis in
vitro and in vivo. Sci Rep. 5:161852015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fulda S: Evasion of apoptosis as a
cellular stress response in cancer. Int J Cell Biol.
2010:3708352010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lundberg AS and Weinberg RA: Control of
the cell cycle and apoptosis. Eur J Cancer. 35:1886–1894. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Liu X, Wu D, Zhang M, Ran G, Bi Y
and Huang H: Growth inhibition and induction of apoptosis in
SGC-7901 human gastric cancer cells by evodiamine. Mol Med Rep.
9:1147–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang C, Chen Z, Zhou X, Xu W, Wang G,
Tang X, Luo L, Tu J, Zhu Y, Hu W, et al: Cantharidin induces G2/M
phase arrest and apoptosis in human gastric cancer SGC-7901 and
BGC-823 cells. Oncol Lett. 8:2721–2726. 2014.PubMed/NCBI
|
|
25
|
Fulda S: Tumor resistance to apoptosis.
Int J Cancer. 124:511–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu G and Shi Y: Apoptosis signaling
pathways and lymphocyte homeostasis. Cell Res. 17:759–771. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bleicken S, Classen M, Padmavathi PV,
Ishikawa T, Zeth K, Steinhoff HJ and Bordignon E: Molecular details
of Bax activation, oligomerization, and membrane insertion. J Biol
Chem. 285:6636–6647. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dewson G, Kratina T, Sim HW, Puthalakath
H, Adams JM, Colman PM and Kluck RM: To trigger apoptosis, Bak
exposes its BH3 domain and homodimerizes via BH3:Groove
interactions. Mol Cell. 30:369–380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|