Introduction
Alzheimer's disease (AD) is the most common
neurodegenerative disorder with progressive deterioration of memory
in the elderly. There are nearly 40 million AD patients worldwide
and prevalence is increasing because of increased longevity and the
aging population. Therefore, it is critical to understand the
underlying pathology of AD and to find promising therapies.
Beta amyloid (Aβ) cascades, the posttranslational
modification of tau proteins and cholinergic dysfunctions, are
considered the most important hypotheses of AD pathology, but no
theory alone is sufficient to explain the diversity of biochemical
pathways and abnormalities leading to AD. Currently, the majority
of FDA-approved therapies for AD are designed to restore
cholinergic function. Inhibitors of acetylcholinesterase (AChE),
for example, are an effective method to correct cholinergic
deficiency in some AD cases (1).
However, their effectiveness diminishes over time because of the
progressive loss of cholinergic neurons. In contrast, it has been
reported that levels of M1 muscarinic acetylcholine receptors
(mAchRs) are well preserved in the brains of AD patients relative
to other mAchRs and nicotinic receptors (2). Therefore, M1 receptor agonists might
be an alternative method of restoring cholinergic function for AD
treatment. In support of this notion, it has been demonstrated that
activation of M1 receptors can attenuate AD-related pathology
including memory impairment, amyloid deposition, and tau
phosphorylation (3–6). One agonist of the M1 receptor,
xanomeline, has been a promising candidate for such treatment.
Xanomeline has indeed been shown to induce dose-dependent
improvement of cognitive function and behavioral performance in AD
patients, but its clinical utility is limited owing to intolerable
muscarinic adverse effects (3). In
order to reduce the unwanted adverse effects of this agent, a
derivative of xanomeline named EUK1001 has been developed, which
showed higher affinity for M1 mAChRs and significantly less
toxicity than xanomeline (7).
Moreover, EUK1001 has been shown to improve cognitive function,
enhance hippocampal synaptic plasticity in aged mice (7,8), and
to induce hippocampal neurogenesis in adult mice (9). Most importantly, EUK1001 can
ameliorate recognition memory, attenuate brain degeneration, and
suppress brain tau hyperphosphorylation in the presenilin-deficient
mouse model (6), suggesting that
EUK1001 might be a promising therapeutic agent for AD treatment.
Although presenilin1/presenilin2 conditional double knockout mice
(PS cDKO mice) show AD-like neurodegenerative phenotypes, these
mice have decreased Aβ levels in the brain (10–13),
inconsistent with AD patients. Thus, these mice might not be a
suitable animal model for testing anti-AD agents. Therefore, the
effects of EUK1001 on neurodegeneration need to be further assessed
in other animal models of AD.
It is well known that the triple transgenic mice
expressing human Swedish double-mutated APP (APPsw), human mutated
four-repeat tau (tauP301L), and human mutated presenilin1
(PS1M146V) (3×Tg-AD mice) progressively develop Aβ plaques and
neurofibrillary tangles and are the best presently available AD
animal model (14). In the present
study, we explored the effects of EUK1001 on the cognitive
phenotype of this 3×Tg-AD mouse model and analyzed possible
underlying mechanisms.
Materials and methods
Animals
Triple transgenic mice (3×Tg-AD) harboring hAPPswe,
htauP301L, and hPS1M146V transgenes were originally generated by
Oddo et al (14). The
transgenic mice used in this study were purchased from the colony
maintained by the Jackson Laboratory and bred in our lab. Wild-type
mice on the same strand background (B6129SF2/J) served as controls
according to the instructions of the Jackson Laboratory. All mice
were maintained in a specific-pathogen-free (SPF) facility on a
12-h light/dark cycle (20–26°C and 40–70% humidity). All animal
procedures were approved by the Committee for the Care and Use of
Laboratory Animals at ECNU, China.
Compounds and treatment
Both xanomeline and EUK1001 (7) were synthesized in the laboratory of
Dr. Xiaoping Lei at Peking University. Male and female 3×Tg-AD mice
were divided into different groups because previous studies have
demonstrated that behavior performance of 3×Tg-AD mice varies by
sex with females performing worse than males (15). Six-month-old male 3×Tg-AD mice were
randomly divided into two groups (EUK1001 and control) and female
mice into three groups (EUK1001, xanomeline, and control; n=10–12
per group). For each group, mice were injected intraperitoneally
with xanomeline (1 mg/kg), EUK1001 (1 mg/kg), or saline (as the
vehicle control) daily for three months. Male and female wild-type
mice were also divided into two groups (EUK1001 and control;
n=10–12 per group) and were administered EUK1001 or saline
identically to the 3×Tg-AD mice. These compounds were also
administered during the two weeks of behavioral testing.
Water maze
We used a circular pool (1.2 m in diameter) with a
platform placed in the center of any quadrant, submerged 1 cm
beneath the water surface. The maze was located in a room
containing several simple extra-maze visual cues. The training
protocol consisted of four sessions, with one session (four trials)
per day. The starting quadrant was randomly assigned for each
animal but each session (four trials) included all four starting
quadrants. To reduce stress, in both the hidden and cued versions
of the task, mice were placed on the platform for 10 sec prior to
the first training trial. Mice were allowed to find and escape onto
the submerged platform. If a mouse failed to find the platform
within 60 sec, we manually guided it to the platform and allowed to
remain there for 30 sec. After this, each mouse was placed into a
holding cage under a warming lamp for 25 sec until the next trial.
During the transfer test, the platform was removed and the mice
were allowed to swim in the pool for 60 sec. We recorded the time
spent in each quadrant by each mouse and the time until each mouse
crossed the platform after searching.
Novel object recognition
This task was conducted as previously described
(16). Briefly, mice were
extensively handled and then individually habituated to an open
field box (50×50×25 cm) for 15 min every day for 3 days. During
each training session, each mouse was allowed to explore freely for
15 min in the open field box with two novel objects placed in the
center. In the 1-h and 24-h retention tests, mice were placed back
into the same box for 15 min, and one of the objects during the
training session was replaced with a novel object. The
discrimination index is calculated as the ratio of the time spent
exploring the novel object divided by the total exploration time
for both objects, and is used to evaluate recognition memory.
Aβ ELISA
Aβ measurements were performed by the Invitrogen
Human Aβ40 and Aβ42 ELISA kit (Life Technologies, New York, NY,
USA) following the manufacturer's protocols. Cortex or hippocampus
samples were sonicated (5 sec) in four volume protein lysis buffer
containing protease inhibitors. The lysate was centrifuged (18,000
× g, for 10 min at 4°C). The supernatant was considered soluble and
pellets were further extracted by sonicating with formic acid and
further centrifugation. Prior to the ELISA, insoluble samples were
diluted 3 times with Tris 1 M, pH 10, and soluble samples were
diluted 5 times in ELISA buffer. Results are expressed as pg/mg of
protein measured by the Bradford method, using BSA as standard.
Plasmid construction
The whole coding sequence (CDS) of the human
muscarinic M1 receptor (about 1.4 kb, accession no. NM_000738) was
obtained by PCR and inserted into the HindIII and EcoRI sites of
pcDNA3.1(+). The whole CDS of human APP Swedish mutant (APPsw)
(about 2.1 kb, accession no. NM_201414) was obtained by PCR using
brain cDNA of 3×Tg-AD mice. The PCR product was digested by
restricted enzymes HindIII and Xba I and subcloned into
pcDNA3.1(+). The positive clones of pcDNA3.1-hM1 (hM1) and
pcDNA3.1-hAPPsw (hAPPsw) were validated by sequencing and their
expression was tested in N2a cells after transfection by western
blotting.
Cell culture and treatment
N2a cells were cultured in Dulbecco's Modified
Eagle's Medium (high glucose with L-glutamine; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% Fetal
Bovine Serum (FBS; PAA Laboratories Inc., Dartmouth, MA, USA), and
1% penicillin/streptomycin (PAA Laboratories Inc.), pH 7.2, in a
humidified 5% CO2 water-jacketed incubator (Thermo Forma
Inc., Waltham, MA, USA). To analyze the effects of M1 receptor
activation on APP processing in N2a cells, N2a cells were
cotransfected with the following combined plasmids: (1) hAPPsw/hM1, (2) hAPPsw/pc (pcDNA3.1), (3) pc/hM1, (4) pc/pc, using Lipofectiome 2000
according to the manufacturer's protocol. Forty-eight h after
transfection, N2a cells were rinsed twice with FBS-free Opti-MEM,
and treated for 6 h with or without xanomeline or EUK1001 in
Opti-MEM.
Western blot analysis
After xanomeline or EUK1001 treatment, the
cotransfected cells and their medium were collected. The medium was
centrifuged for 5 min at 14,000 × g at 4°C and the supernatant was
subjected to western blotting after the addition of BSA (final
concentration: 10 µg/ml), which served as a loading control. The
treated cotransfected cells were lysed on ice using a lysis buffer
(Beyotime, Shanghai, China) containing 1 mM PMSF, followed by
centrifugation at 14,000 × g at 4°C for 5 min. Protein
concentrations were determined using a BCA kit (Beyotime). Equal
amounts of protein were resolved by SDS-PAGE and electrically
transferred onto nitrocellulose membranes (Millipore, Billerica,
MA, USA). After blocking for 1 h with 5% non-fat milk at room
temperature, the blots were incubated with the primary antibodies
for 1 h, and then with the secondary antibodies for 1 h at room
temperature. Proteins were visualized with an ECL kit (CWBio,
Beijing, China). A Quantity One GelDoc XR gel imaging system
(Bio-Rad Laboratories, Hercules, CA, USA) was used for detection
and analysis of band intensity. Relative protein levels were
normalized to BSA or β-actin. Antibodies used were: anti-β-Amyloid
(DE2B4) (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-Amyloid β-Precursor Protein (LN27) (1:1,000; Invitrogen),
anti-BSA (1:500; Bioss, Beijing, China), β-actin (1:1,000; Cell
Signaling Technology, Beverly, MA, USA) and proper HRP-conjugated
secondary antibodies (1:5,000; KangChen Bio-tech, Shanghai,
China).
Statistical analysis
Results, presented as mean ± SEM, were analyzed by
two way ANOVA and post-hoc Holm-Sidak method using Graphpad Prism.
Student's t-test or Mann-Whitney U test was used for direct
comparison of two groups using Sigmaplot 10.0 or SPSS 10.0
software.
Results
Effect of EUK1001 on cognitive
function
To evaluate the therapeutic effects of the
xanomeline derivative, EUK1001, on the cognitive phenotype of AD,
six-month-old homozygous 3×Tg-AD and wild-type mice were
administered EUK1001, xanomeline, or saline daily for three months.
Based on previous results of in vivo pharmacological and
behavioral tests, we tested the doses of 1 mg/kg xanomeline and
EUK1001 (7). We evaluated the
effects of these drugs on learning and memory using two different
behavioral paradigms: Morris water maze and novel object
recognition. Both tasks are dependent on the the hippocampus, a
site where the Aβ and tangle pathology is most severe in 3×Tg-AD
mice (14). We discussed the
results of these tests in turn.
In the Morris water maze task, both male and female
saline-treated AD mice (ct-ad-m and ct-ad-f) exhibited greater
escape latencies than saline-treated wild-type mice (Fig. 1A and B; P<0.01). Both male and
female EUK1001-treated mice performed as well as control mice
(Fig. 1A and B; P>0.05).
Xanomeline-treated female AD mice spent less time to find the
platform compared to saline-treated AD mice (Fig. 1A; P<0.05). Similarly, in the
probe trial, time spent in the target quadrant was significantly
shorter for ct-ad-m and ct-ad-f mice compared to wild type
(Fig. 1C and D; P<0.05), while
this time was not significantly different between wild-type and
EUK1001-treated mice (Fig. 1C and
D; P>0.05). Xanomeline treatment also ameliorated the
performance of x-ad-f mice in the probe trial (Fig. 1D; P<0.05). We found no
significant difference between xanomeline-treated female mice and
EUK1001-treated female mice (Fig.
1D; P>0.05). These results suggest that EUK1001 treatment
can rescue impairment of spatial memory in AD mice although it has
no evident effect on the performance of wild-type mice.
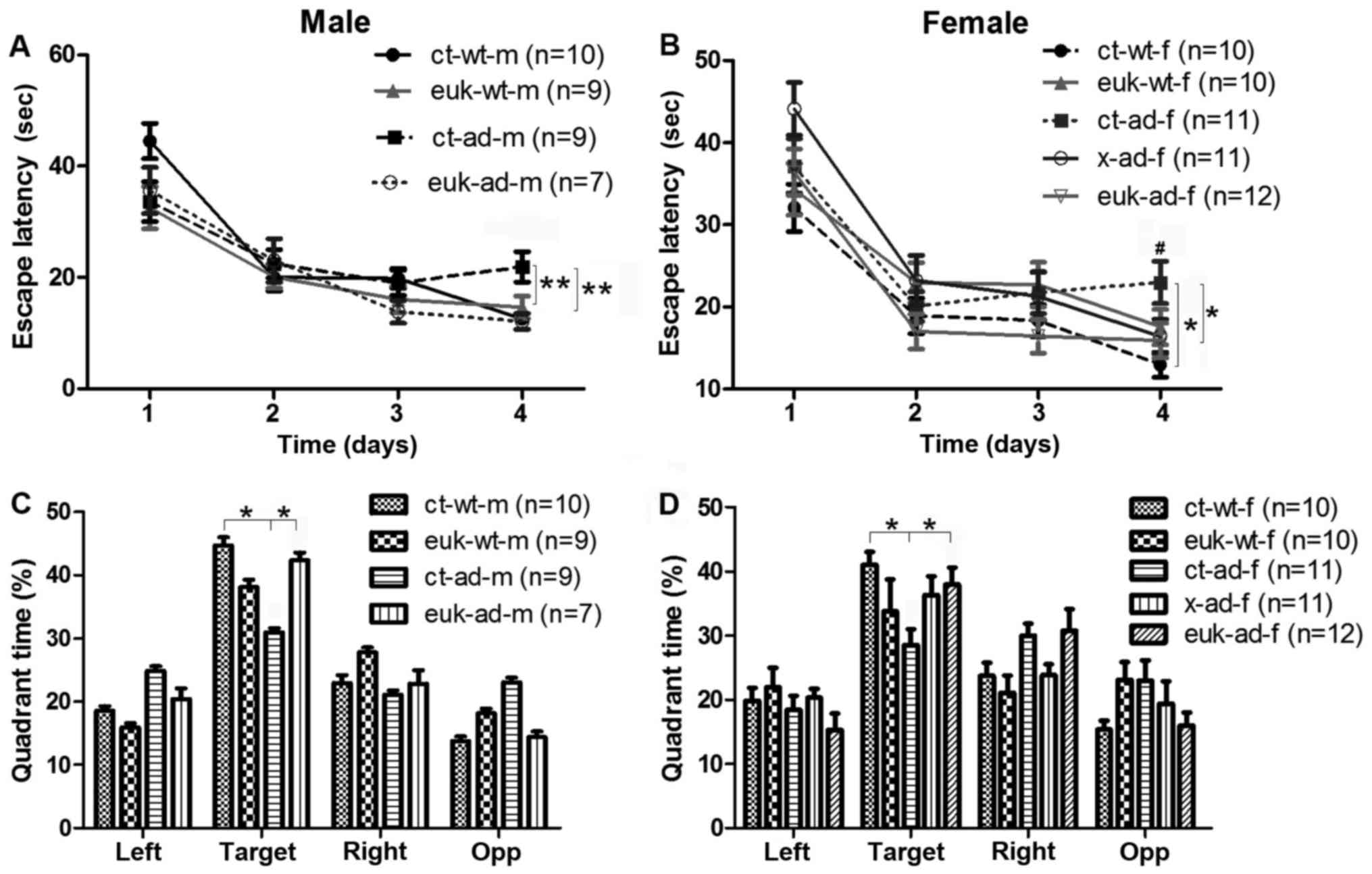 | Figure 1.EUK1001 treatment rescued the spatial
memory deficits of 3×Tg-AD mice. (A and B) During training, both
male (A) and female (B) AD mice exhibited a greater latency than
wild-type mice. In contrast, EUK1001-treated AD mice had a similar
latency to wild-type. (C and D) During the probe session, both male
(C) and female (D) AD mice spent less time in the target quadrant
than wild-type mice, while EUK1001-treated AD mice spent a similar
duration of time in the target quadrant as wild-type mice.
**P<0.01, *P<0.05: ct-ad vs. ct-wt;
euk-ad vs. ct-ad. #P<0.05: X-ad
vs. ct-ad. x, xanomeline; euk, EUK1001; ct, saline; ad,
Alzheimer's disease; m, male; f, female; wt, wild-type; |
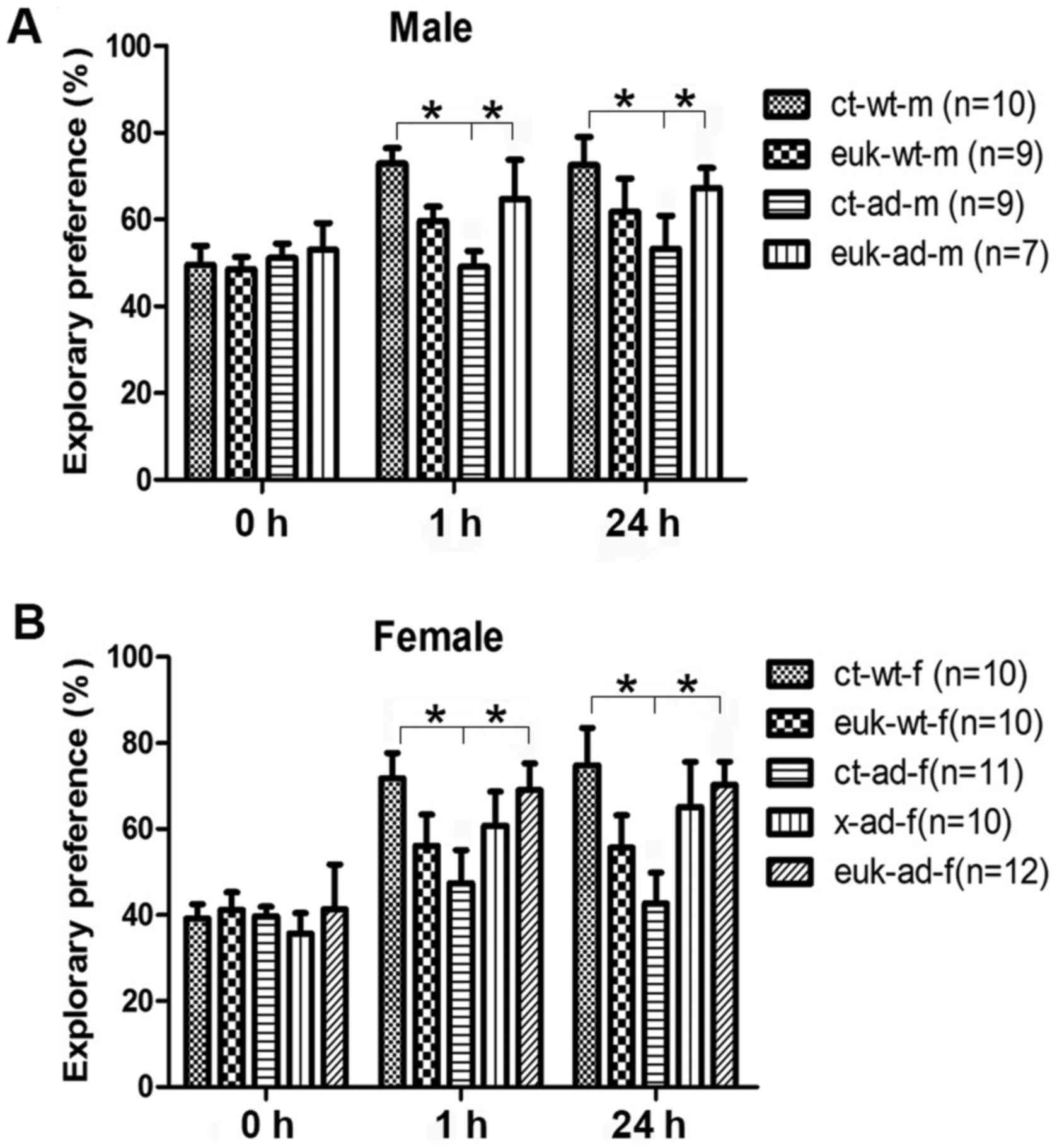
In the novel object recognition task, we found no
preference between the two objects in any groups during the
training sessions. However, both the ct-ad-m and ct-ad-f mice spent
less time exploring the novel object during both 1 and 24-h
retention tests than wild-type controls (Fig. 2A and B; P<0.05). The
EUK1001-treated AD mice showed significantly more exploring of the
novel object than control AD mice (Fig. 2A and B; P<0.05).
Xanomeline-treated female mice performed similarly to control AD
mice (Fig. 2A and B; P>0.05).
EUK1001 treatment did not induced any significant changes to
exploring preference in wild type mice (Fig. 2A and B; P>0.05). These results
suggest that EUK1001 treatment also enhanced recognition memory of
AD mice.
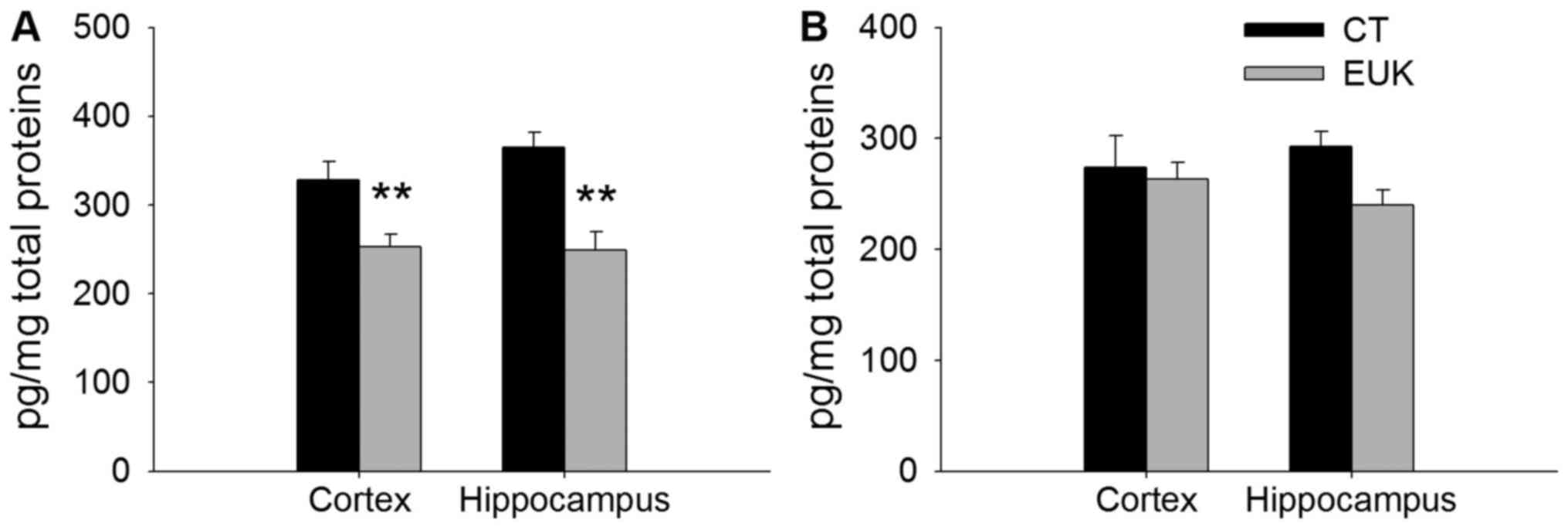
Effect of EUK1001 on Aβ level in the
brain
All mice were sacrificed 24 h after the final novel
object recognition trial, and their brains were isolated and
processed for biochemical evaluation. To determine the effects of
EUK1001 administration on the AD neuropathology present in the
3×Tg-AD mice, we compared the Aβ40 and Aβ42 levels in the cortices
and hippocampi of saline- and EUK1001-treated AD mice using
sandwich ELISA. The results showed that EUK1001 treatment led to a
significant decrease of Aβ42 level in both the hippocampi and
cortices of AD mice (Fig. 3A;
P<0.01), but had no significant effect on Aβ40 level (Fig. 3B; P>0.05).
Effect of EUK1001 on APP processing in
vitro
It is well known that APP undergo sequential
proteolytic processing by two pathways: The α pathway and the β
pathway. If APP is cleaved by α-secretase, which produces sAPPα,
the pathway is non-anyloidogenic. So we wondered whether the
EUK1001 treatment had an effect on Aβ42 levels via altered APP
processing to α pathway. In this regard, APPsw and M1
receptor-overexpressing N2a cell models were established for the
analysis of sAPPα (alpha-secretase-cleaved APP) secretion (The
overexpression was validated by real-time PCR experiments, Figs. 4A and B). Cell media was collected
for detection of sAPPα by western blotting using DE2B4 antibody,
which recognizes sAPPα and amino acids 1–17 of Aβ. Cell lysate was
used to detect the expression of APPsw using the LN27 antibody
which recognizes the N-terminus of APP. Results indicated that
xanomeline and EUK1001 both increased sAPPα levels in the media of
N2a cell cotransfected with APPsw and M1 without affecting total
expression levels of APPsw (Fig.
4C-E; P<0.01). Conversely, xanomeline and EUK1001 had no
effect on the secretion of sAPPα in APPsw/pcDNA3.1 cotransfected
N2a cells (Fig. 4F-H; P>0.05),
which suggests that the effect of xanomeline and EUK1001 was
dependent on the M1 receptor.
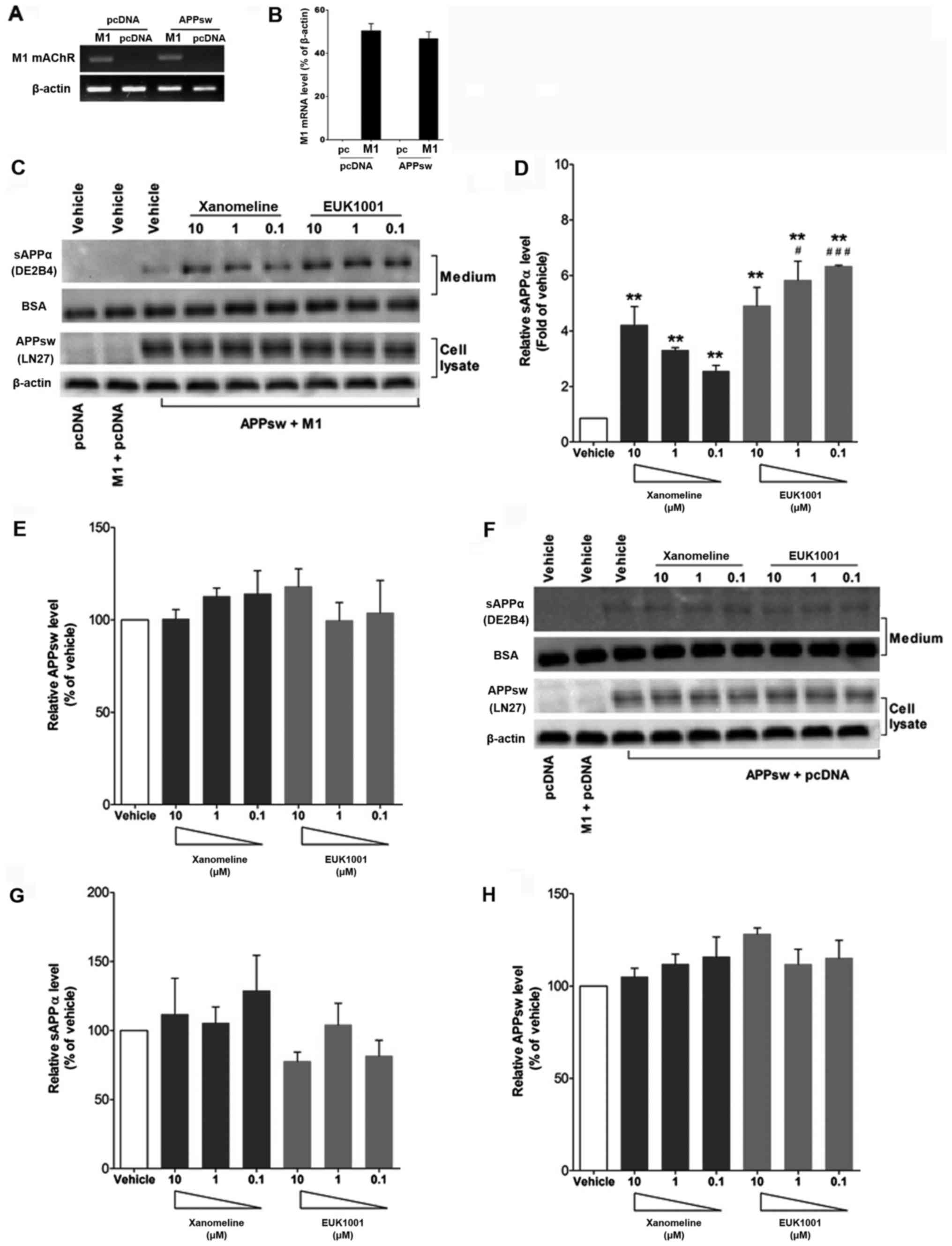 | Figure 4.EUK1001 treatment increased sAPPα by
activating M1 receptors in transfected N2a cells. N2a cells were
transiently cotransfected with hAPPsw and M1 receptors or hAPPsw
and pcDNA3.1. Potent M1 receptor expression was observed in
APPsw/M1-N2a and pc/M1-N2a cells, but not in APPsw/pc-N2a and
pc/N2a cells, as revealed by (A) PCR and (B) reverse
transcription-qPCR. Then the cells were treated with or without
xanomeline or EUK1001 in Opti-MEM. Proteins in cell medium and
lysate were collected for western blotting. For the detection of
sAPPα in the medium, BSA was added and served as a loading control.
Relative protein levels were normalized to β-actin (BSA for sAPPα)
and are shown +SEM. (C and D) Xanomeline and EUK1001 effectively
increased sAPPα levels in the medium, (C and E) without affecting
the total expression levels of APPsw. (F-H) Xanomeline and EUK1001
had no effect on secreted sAPPα levels or total APPsw level in
hAPPsw/pcDNA3.1 cotransfected N2a cells. **P<0.01, vs. vehicle;
#P<0.05, ###P<0.001, vs. Xanomeline at
the same concentration. hAPPsw, human Swedish double-mutated
amyloid precursor protein; sAPP, soluble amyloid precursor protein;
mAchR, muscarinic acetylcholine receptor; BSA, bovine serum
albumin; PCR, polymerase chain reaction. |
Discussion
Activation of muscarinic receptors by M1 agonists
including xanomeline has been demonstrated to be an effective
therapeutic strategy for AD. However, their clinic utility has so
far been limited owing to severe adverse effects (3). The derivative of xanomeline, EUK1001,
has lower toxicity and in previous research has been shown to
attenuate AD-like neurodegeneration in presenilin-deficient mice
(6). The mice, however, do not
mimic all of the hallmarks of AD patients without Aβ production
(12). The present study provides
evidence for the beneficial effects of EUK1001 for AD pathology
using the well-known 3×Tg-AD mouse model. Our results indicate that
three-month treatment with EUK1001 can ameliorate the impairment of
spatial memory and recognition memory in both male and female
3×Tg-AD mice. This is consistent with previous studies showing that
selective M1 receptor agonist, AF267B, rescues cognitive deficits
in 3×Tg-AD mice (4).
Additionally, we have found that EUK1001 treatment
led to a decrease of Aβ42 level in AD mice and induced an increase
of sAPPα production in N2a cells, suggesting that activation of M1
receptors by EUK1001 might alter APP processing to an alternative
nonamyloidgenic pathway, which will be confirmed in our ongoing
work. A similar effect was found in previous experiments (4). These results also demonstrated that
the effect of M1 receptor activation on APP processing is mediated
by the activation of PKC and ERK1/2 (4,17).
Therefore, EUK1001 administration might enhance cognitive function
of 3×Tg-AD mice by activating M1 mAChRs, which leads to the
generation of α-secretase-generated products through activation of
PKC and ERK1/2. Further study will be done to elucidate whether the
effect of M1 receptor activation on APP processing is mediated by
the activation of PKC and ERK1/2 pathway M1 mAchRs represent a
viable target for AD treatment, and highly selective allosteric
agonists of M1 receptors are promising compounds for AD therapies
(18). The principal challenge has
been to find selective compounds that activate the M1 receptor
subtype without also activating M2 or M3 receptors, which are
associated with undesirable adverse effects. All previous compounds
have been discontinued owing to inadequate selectivity. Therefore,
the suitability of EUK1001 as a therapy for AD lies in its
specificity. Studies in our lab have indicated that the effects of
EUK1001 on hippocampal long-term potentiation are blocked by
pirenzepine, a selective M1 antagonist, but not by the selective
M2/M4 antagonist methoctramine (7). This suggests that EUK1001 was able to
affect hippocampal long-term potentiation via M1 receptor
stimulation. Moreover, EUK1001 has been shown to have low toxicity
in mice (7). Thus, EUK1001 remains
a promising therapeutic agent for the treatment of AD. However,
these effects and possible adverse effects still require further
investigation before and during clinical application.
Currently, a fully selective muscarinic M1 receptor
agonist, HTL9936, is undergoing Phase 1 clinical trials in order to
develop a new medicine for improving cognitive function in patients
with AD (19). The present paper
has identified another agent, EUK1001, which could alleviate memory
impairment by decreasing Aβ pathology. This drug also attenuated
the neurodegenerative phenotypes in another AD-like rodent model
(6). Thus, future work should be
performed to investigate the potential role of EUK1001 for AD
therapy.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shanghai, no. 16ZR1410500. We thank the
English native speaker in Editage for professional language editing
of the manuscript.
References
|
1
|
Knapp MJ, Knopman DS, Solomon PR,
Pendlebury WW, Davis CS and Gracon SI: A 30-week randomized
controlled trial of high-dose tacrine in patients with Alzheimer's
disease. The Tacrine Study Group. JAMA. 271:985–991. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nordberg A, Alafuzoff I and Winblad B:
Nicotinic and muscarinic subtypes in the human brain: Changes with
aging and dementia. J Neurosci Res. 31:103–111. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bodick NC, Offen WW, Levey AI, Cutler NR,
Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K,
Bymaster FP, et al: Effects of xanomeline, a selective muscarinic
receptor agonist, on cognitive function and behavioral symptoms in
Alzheimer disease. Arch Neurol. 54:465–473. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caccamo A, Oddo S, Billings LM, Green KN,
Martinez-Coria H, Fisher A and LaFerla FM: M1 receptors play a
central role in modulating AD-like pathology in transgenic mice.
Neuron. 49:671–682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher A: M1 muscarinic agonists target
major hallmarks of Alzheimer's disease-an update. Curr Alzheimer
Res. 4:577–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang D, Yang L, Su J, Niu Y, Lei X, Xiong
J, Cao X, Hu Y, Mei B and Hu JF: Attenuation of neurodegenerative
phenotypes in Alzheimer-like presenilin 1/presenilin 2 conditional
double knockout mice by EUK1001, a promising derivative of
xanomeline. Biochem Biophys Res Commun. 410:229–234. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui Y, Wang D, Si W, Lv W, Niu Y, Lei X,
Hu Y and Cao X: Enhancement of memory function in aged mice by a
novel derivative of xanomeline. Cell Res. 18:1151–1153. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui YH, Si W, Yin L, An SM, Jin J, Deng SN
and Cao XH: A novel derivative of xanomeline improved memory
function in aged mice. Neurosci Bull. 24:251–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang XL, Gong Q, Zhang S, Wang L, Hu Y,
Shen H and Dong S: 3-[3-(3-florophenyl-2-propyn-1-ylthio)-1,2,
5-thiadiazol-4-yl]-1,2,5,6-tetrahydro-1-methylpyridine oxalate, a
novel xanomeline derivative, improves neural cells proliferation
and survival in adult mice. Neural Regen Res. 7:24–30.
2012.PubMed/NCBI
|
|
10
|
Beglopoulos V, Sun X, Saura CA, Lemere CA,
Kim RD and Shen J: Reduced beta-amyloid production and increased
inflammatory responses in presenilin conditional knock-out mice. J
Biol Chem. 279:46907–46914. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng R, Wang H, Wang J, Shrom D, Zeng X
and Tsien JZ: Forebrain degeneration and ventricle enlargement
caused by double knockout of Alzheimer's presenilin-1 and
presenilin-2. Proc Natl Acad Sci USA. 101:8162–8167. 2004;
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saura CA, Choi SY, Beglopoulos V, Malkani
S, Zhang D, Rao BS Shankaranarayana, Chattarji S, Kelleher RJ III,
Kandel ER, Duff K, et al: Loss of presenilin function causes
impairments of memory and synaptic plasticity followed by
age-dependent neurodegeneration. Neuron. 42:23–36. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang X, Zhang D, Shi J, Chen Y, Zhang P
and Mei B: Increased inflammatory response both in brain and in
periphery in presenilin 1 and presenilin 2 conditional double
knock-out mice. J Alzheimers Dis. 18:515–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oddo S, Caccamo A, Shepherd JD, Murphy MP,
Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y and LaFerla
FM: Triple-transgenic model of Alzheimer's disease with plaques and
tangles: Intracellular Abeta and synaptic dysfunction. Neuron.
39:409–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clinton LK, Billings LM, Green KN, Caccamo
A, Ngo J, Oddo S, McGaugh JL and LaFerla FM: Age-dependent sexual
dimorphism in cognition and stress response in the 3×Tg-AD mice.
Neurobiol Dis. 28:76–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong S, Li C, Wu P, Tsien JZ and Hu Y:
Environment enrichment rescues the neurodegenerative phenotypes in
presenilins-deficient mice. Eur J Neurosci. 26:101–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haring R, Fisher A, Marciano D, Pittel Z,
Kloog Y, Zuckerman A, Eshhar N and Heldman E: Mitogen-activated
protein kinase-dependent and protein kinase C-dependent pathways
link the m1 muscarinic receptor to beta-amyloid precursor protein
secretion. J Neurochem. 71:2094–2103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caccamo A, Fisher A and LaFerla FM: M1
agonists as a potential disease-modifying therapy for Alzheimer's
disease. Curr Alzheimer Res. 6:112–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
HTL-9936 is a selective muscarinic M1
agonist designed to improve cognitive function in patients with AD
and other diseases. http://newdrugapprovals.org/2014/05/05/htl-9936-is-a-selective-muscarinic-m1-agonist-designed-to-improve-cognitive-function-in-patients-with-ad-and-other-diseases/
|