Introduction
In recent years, gene therapy has become a
widely-used approach to treat a variety of inherited and malignant
diseases. It requires safe, efficient and specific gene delivery
systems. Despite the safety advantages, the efficiency of non-viral
vectors is usually poor (1,2).
Therefore, the development of efficient non-viral transfection
systems to improve transfection efficiency is an important area of
research.
In 1962, Feldherr (3) described the process by which
nucleocytoplasmic transport of colloidal gold particles occurs
through specialized pores in the nuclear envelope known as nuclear
pore complexes (NPCs). Transport through NPCs is selective and
energy dependent (4). Ions and
small molecules with a diameter of <9 nm enter the nucleus
passively, whereas larger molecules require nuclear localization
sequences (NLSs) that are recognized by the cytoplasmic transport
receptors responsible for mediating nuclear uptake (5). The most studied, and therefore best
known, NLSs are virus-derived peptides, such as the Tat
(transactivating) protein or Antennapedia homeodomain protein.
However, arginine/lysine-rich NLSs, such as the Simian virus 40
(SV40) large T antigen (PKKKRKV), appear to be far more efficient
(6–8). The immunogenicity of this peptide has
been reported to be markedly lower when compared with additional
NLS-peptides (9,10). In addition, this peptide binds
proteins and DNA via electrostatic interactions. Therefore,
conjugation of this peptide to DNA may improve nuclear import and
thus increase the transfection efficiency of non-viral gene
delivery systems (8,11).
Cationic lipids and polymers have been employed as
non-viral gene transfer agents. These cationic substances form
complexes with anionic DNA via electrostatic interactions. The
subsequent cationic DNA complexes are taken up by cells via
electrostatic interactions due to the negative charge of the cell
surface (12). Among these
complexes, cationic liposomes are widely used for almost all animal
cells, as they demonstrate nonspecific ionic interactions and a low
level of toxicity (13).
Therefore, a number of polymeric cationic systems including
polyethylenimine (PEI), cationic peptides (poly L-lysine), cationic
dendrimers and chitosan have been studied. In a previous study, PEI
has been proposed as a safer alternative to other non-viral vectors
(14).
Among the novel strategies under investigation,
ultrasound-targeted microbubble destruction (UTMD) has been
demonstrated to be particularly promising for the enhancement of
gene and drug delivery (15–17).
A number of studies have demonstrated that UTMD significantly
increases the permeability of a cell membrane, thereby improving
the efficiency of gene transfection (15–17).
In addition, the combination of ultrasound (US) irradiation with
PEI markedly improves transfection efficiency (18,19)
and specific targeting (20). Yoo
and Jeong (21) demonstrated the
plasmid DNA/PEI complexes containing NLS attached to psoralen, a
nucleic acid-intercalating agent, increased transfection
efficiencies in COS-1 cells, indicating that this complex may be
used as a potential DNA carrier for therapeutic applications.
Current research is primarily focused on whether the combined
effect of UTMD and PEI/DNA/NLS complexes may be more efficient than
previously studied methods without increasing cell damage.
The present study investigated the use of SV40 large
T-antigen as a nuclear localization signal non-covalently bound to
a PEI/DNA complex, in an attempt to increase transfection
efficiency using the unique physical non-viral method of US
irradiation.
Although US is a simple, convenient and targeted
method, the transfection efficiency achieved by US remains low
(22). Therefore, the aim of the
present study was to combine US with PEI/DNA/NLS complexes to
produce a novel gene transfer system, and to investigate the safety
of the new gene delivery system, as well as its effect on
transfection efficiency.
Materials and methods
Ultrasound irradiation equipment
Ultrasound irradiation was generated by a UGT 2007
ultrasonic gene transfection apparatus (Ultrasonic Research
Institute, Chongqing Medical School, Chongqing, China) with a
varied pulse time of 1 to 10 sec and a probe area of 0.8
mm2. The irradiation frequency ranged from 0.5 to 2 MHz
with a continuous wave. The intensity of ultrasound exposure was
set at 1.5 W/cm2 and the duration was 30 sec, as previously
described (23).
Plasmid
The 4.7-kb pEGFP-N3 plasmid, an expression vector
for the enhanced green fluorescent protein (EGFP) gene, was
obtained from Clontech Laboratories, Inc. (Mountainview, CA, USA).
The plasmid was used to transform chemically-competent DH5α
Escherichia coli (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), which is performed according to the
manufacturer's protocol. Cultures of transformed bacteria were
grown in the presence of 20 µg/ml kanamycin (Shine Star (Hubei) Bio
Engineering Co., Ltd., Jingzhou, China). The plasmid DNA was
extracted and purified using a QIAquick PCR Purification kit (Shine
star (Hubei) Bio Engineering Co., Ltd.). The concentration of
isolated plasmid DNA was determined by measuring the absorbance at
260 nm (A260) using UV spectrophotometry (Beckman DU-640; Beckman
Coulter, Inc., Brea, CA, USA). Following determination of the
concentration, the plasmid DNA was resuspended to a final
concentration of 1 µg/µl in elution buffer [2.5 mM Tris-HCl, (pH
8.5)]. In addition, the A260/A280 ratio was between 1.8 and 2.0,
indicating that the purified plasmid DNA was free of
contaminants.
NLS peptide
The NLS peptide, PKKKRKV, originates from the
large-T antigen in the SV40 virus (molecular weight: 1386;
high-performance liquid chromatography analysis: 98.612%), and was
obtained from Shanghai Science Peptide Biological Technology Co.,
Ltd. (Shanghai, China). The peptide powder was stored at −20°C,
away from light sources at room temperature for 30 min, before it
was centrifuged at 37°C and 400 × g for 5 min. A total of 1 mg
peptide was dissolved in 1 ml phosphate-buffered saline (PBS) in a
sterile environment, and was stored at −20°C away from light
sources.
Preparation and characterization of
PEI/DNA complexes with and without NLS
Branched PEI with an average molecular weight of 25
kDa was obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). An aqueous stock solution of PEI was prepared by diluting
1 mg of the commercial solution in 1,000 ml ddH2O, neutralizing
with HCl and filtering through a 0.2-µm filter (EMD Millipore,
Billerica, MA, USA). The PEI/DNA complexes were prepared at various
PEI nitrogen: DNA phosphate ratios (N:P) by adding the pEGFP-N3
plasmid solution to the PEI solution, the N:P ratios are 0:1,
0.25:1, 0.5:1, 1:1, 2:1, 4:1, 8:1 and 16:1. The mixture was gently
mixed using a pipette for 3 to 5 sec to initiate complex formation,
before the NLS solution was added and incubated for 30 min at room
temperature.
Preparation of SonoVue/DNA,
SonoVue/PEI/DNA and SonoVue/PEI/DNA/NLS complexes
The SonoVue™ microbubble suspension
(Bracco Suisse SA, Manno, TI, Switzerland) was reconstituted
immediately prior to use by injecting 5 ml 0.9% saline solution.
The mean diameter of the microbubbles was 2.5 µm, and the
concentration was 2×108 to 5×108
microbubbles/ml. Prior to the experiments, 400 µl SonoVue was mixed
with the pEGFP-N3 plasmid, PEI/DNA complexes or
PEI/DNA/NLS complexes. The complex formation was confirmed by gel
electrophoresis. All complexes were prepared by incubation for 30
min at room temperature.
Gel electrophoresis assays
In the first set of experiments, the aim was to
evaluate whether PEI can be combined with the pEGFP-N3
plasmid using static electricity adsorption. Therefore, the
pEGFP-N3 plasmid (1/1 µl) was incubated with PEI at N:P
ratios ranging from 0:1 to 16:1. In the second set of experiments,
NLS (120 µg) was added to each well (5). Agarose gels were prepared with a 0.7%
agarose solution in Tris-acetate buffer containing ethidium bromide
(0.5 µg/ml). Electrophoresis was performed for 60 min at 90 V.
Effect of NLS peptide and PEI on DNA
integrity using a deoxyribonuclease (DNase) protection assay
To determine whether the PEI/NLS complexes protect
the pEGFP-N3 plasmid from DNase degradation reference
earlier works (24) in the present
study, the pEGFP-N3 plasmid was incubated with the PEI at N:P
ratios of 6:1 (19) with or
without 120 µg NLS (24) for 1 h
at room temperature. DNase endonuclease (0.25 U; Thermo Fisher
Scientific, Inc.) was then added to pEGFP-N3 plasmid, PEI/DNA and
PEI/DNA/NLS solutions for 5, 10 or 15 min at 37°C. Following
incubation at 80°C for 10 min using a thermo cycler to terminate
enzyme digestion, the samples were transferred to wells at the top
of a 1.0% agarose gel. Electrophoresis was performed at 100 V for 1
h.
Cell culture and transfection
groups
A total of 2×106 293T cells (China Center
for Type Culture Collection, Wuhan, China) were placed into 6-well
culture plates in 90% Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc.). The cell cultures were
maintained at 37°C and 5% CO2 for 24 h until the cell
adherence rate was ~95%. The different transfection conditions were
grouped as follows: Group 1 (control group), 10 µg pEGFP-N3 plasmid
was added to a single well, and the plate was incubated in 5%
CO2 at 37°C for 2 h; group 2, pEGFP-N3 plasmid+PEI
(PEI/DNA), whereby 10 µg pEGFP-N3 plasmid and 7.8 µg PEI (N:P, 6:1)
were added to a single well, and the plate was incubated in 5%
CO2 at 37°C for 24 h; group 3, pEGFP-N3 plasmid+PEI+NLS
peptide (PEI/DNA/NLS), whereby 10 µg pEGFP-N3 plasmid, 7.8 µg PEI
(N:P, 6:1) and 120 µg NLS peptide were added to a single well, and
the plate was incubated in 5% CO2 at 37°C for 24 h;
group 4, UTMD+pEGFP-N3 plasmid (UTMD+DNA), whereby 400
µl SonoVue (20% of total volume) and 10 µg pEGFP-N3
plasmid were added into one well before the plate was exposed to US
generated by the UGT 2007 ultrasonic gene transfection apparatus.
The probe was placed under the 6-well plate at a distance of 3 to 5
mm, and the intensity of the ultrasound exposure was set at 1.5
W/cm2 for 30 sec. The plate was then incubated in 5%
CO2 at 37°C for 24 h; group 5: UTMD+pEGFP-N3
plasmid+PEI (UTMD+PEI/DNA), whereby 400 µl SonoVue (20% of total
volume), 10 µg pEGFP-N3 plasmid and 7.8 µg PEI (N:P,
6:1) were added to a single well and the plate was exposed to US
generated by a UGT 2007 ultrasonic gene transfection apparatus
using the same conditions described for the UTMD+DNA group. The
plate was then incubated in 5% CO2 at 37°C for 24 h;
group 6, UTMD+pEGFP-N3 plasmid+PEI+NLS peptide
(UTMD+PEI/DNA/NLS), whereby 400 µl SonoVue (20% of total volume),
10 µg pEGFP-N3 plasmid, 7.8 µg PEI (N:P, 6:1) and 120 µg
NLS peptide were placed into a single well and the plate was
exposed to the US generated by a UGT 2007 ultrasonic gene
transfection apparatus using the aforementioned conditions. The
plate was then incubated in 5% CO2 at 37°C for 24 h.
Following 24 h of incubation, the expression of GFP were observed
using an inverted microscope (Olympus IX51; Olympus Corporation,
Tokyo, Japan).
Flow cytometry analysis
Gene transfection efficiency was examined by flow
cytometry analysis (FACS Calibur™; BD Biosciences,
Franklin Lakes, NJ, USA). Both the control and treated 293T cells
were collected to measure the expression of the plasmid-encoded
EGFP gene. Flow cytometry with the excitation setting at 488 nm was
used to analyze the transfection efficiency. The 293T cells were
digested with 0.25% trypsin and washed three times in 1 ml
pre-cooled PBS. At least 10,000 cells were acquired for each test
measurement. All experiments were performed in triplicate. The data
was analyzed by FlowJo software (version 10; FlowJo, LLC., Ashland,
OR, USA).
Evaluation of cytotoxicity using the
cell counting kit (CCK)-8 assay
293T cells were seeded in a 96-well plate at a
density of 5×103 cells/cm2 in 100 µl of
growth medium, and incubated for 24 h in 5% CO2 at 37°C
to ensure that the cell adherence rate was ~90%. Prior to
transfection, the medium was removed, and the cells were washed
with PBS. The cells were then divided into the aforementioned
groups and treated accordingly in 100 µl of mixed medium.
Experiments performed on each sample were repeated three times. The
cells were then incubated with 10 µl CCK-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) and 100 µl growth medium for 4
h. Relative cell viability was calculated by measuring the A450
values using a microplate reader (Victor3; PerkinElmer, Inc.,
Waltham, MA, USA). The viability of untreated control cells was
arbitrarily defined as 100%.
Statistical analysis
Variables were normally distributed and presented as
the mean ± standard deviation. Differences between the data prior
to and following gene transfection were analyzed using the
Student's t-test. Comparisons among multiple stages were made using
one-way analysis of variance with post hoc analysis by
Student-Newman-Keuls test. Statistical analyses were performed
using SPSS software (version, 19.0; IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of DNA/PEI complexes
and DNA/PEI/NLS complex interactions
Gel retardation experiments were used to measure the
DNA binding ability of PEI (Fig.
1). The polymers could neutralize the negative charge of DNA
and prevent the DNA from moving to the anode (12). However, plasmid (p)-DNA with
complexes were retained in the gel loading well at an N:P ratio
>1, indicating that PEI/DNA complexes were formed (Fig. 1). In contrast to the preparation of
the PEI/DNA complexes containing 120 µg NLS peptides, even though
the PEI/DNA complexes possessed a N:P ratio <2, the PEI/DNA
complexes were retained in the gel loading wells. This indicated
that the PEI/DNA/NLS complexes were formed, and the NLS peptide
exhibited a positive charge.
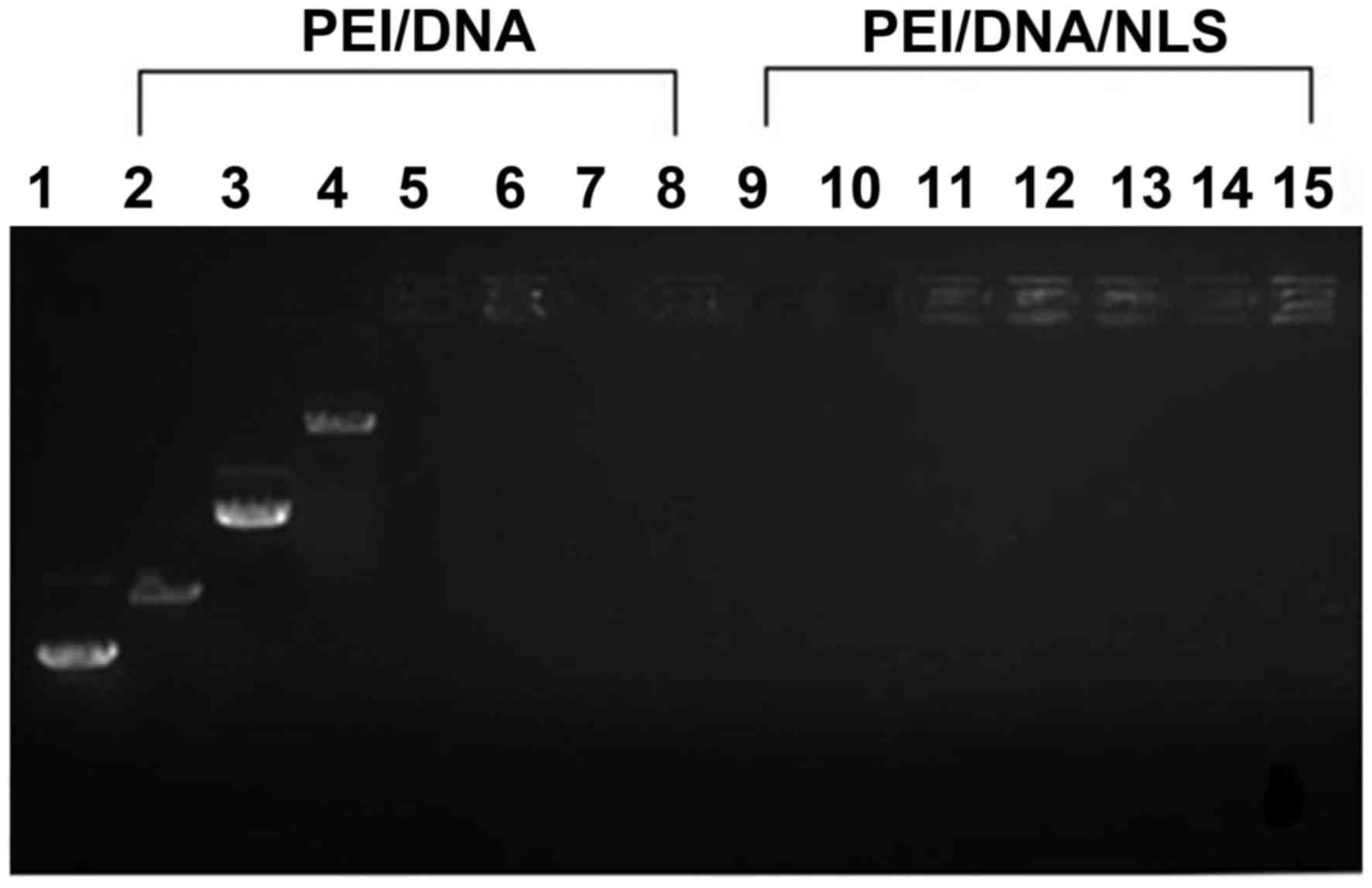 | Figure 1.Gel retardation analysis of PEI/DNA
complexes generated in the presence or absence of NLS. Lane 1,
pEGFP-N3 plasmid; lanes 2 to 8, PEI/DNA complexes at N:P
ratios 0.25:1, 0.5:1, 1:1, 2:1, 4:1, 8:1 and 16:1, respectively;
lanes 9 to 15, PEI/DNA complexes with 120 µg of NLS at weight
ratios of at N:P ratios 0.25:1, 0.5:1, 1:1, 2:1, 4:1, 8:1 and 16:1,
respectively. PEI, polyethylenimine; NLS, nuclear localization
sequence; PEI/DNA, pEGFP-N3 plasmid plus PEI. |
Effect of PEI and NLS on protection of
pDNA from DNase I
To evaluate the ability of the PEI and NLS to
protect pDNA from cytosolic nucleases, DNase I was added to the
pEGFP-N3 plasmid, DNA/PEI and PEI/DNA/NLS solutions for different
durations. The pEGFP-N3 plasmid was digested into small fragments
following 5 min of incubation with DNase I, as demonstrated by the
smeared bands at the bottom of the gel (lane 2; Fig. 2). By contrast, the PEI/DNA complex
was not degraded until 10 min (lane 6), and the PEI/DNA/NLS complex
was not degraded until 15 min (lane 10; Fig. 2). This indicated PEI and NLS may
have a protective effect on pDNA against degradation by lysosomes
and degeneration enzymes in the cytoplasm; however, this function
appeared to be time-dependent.
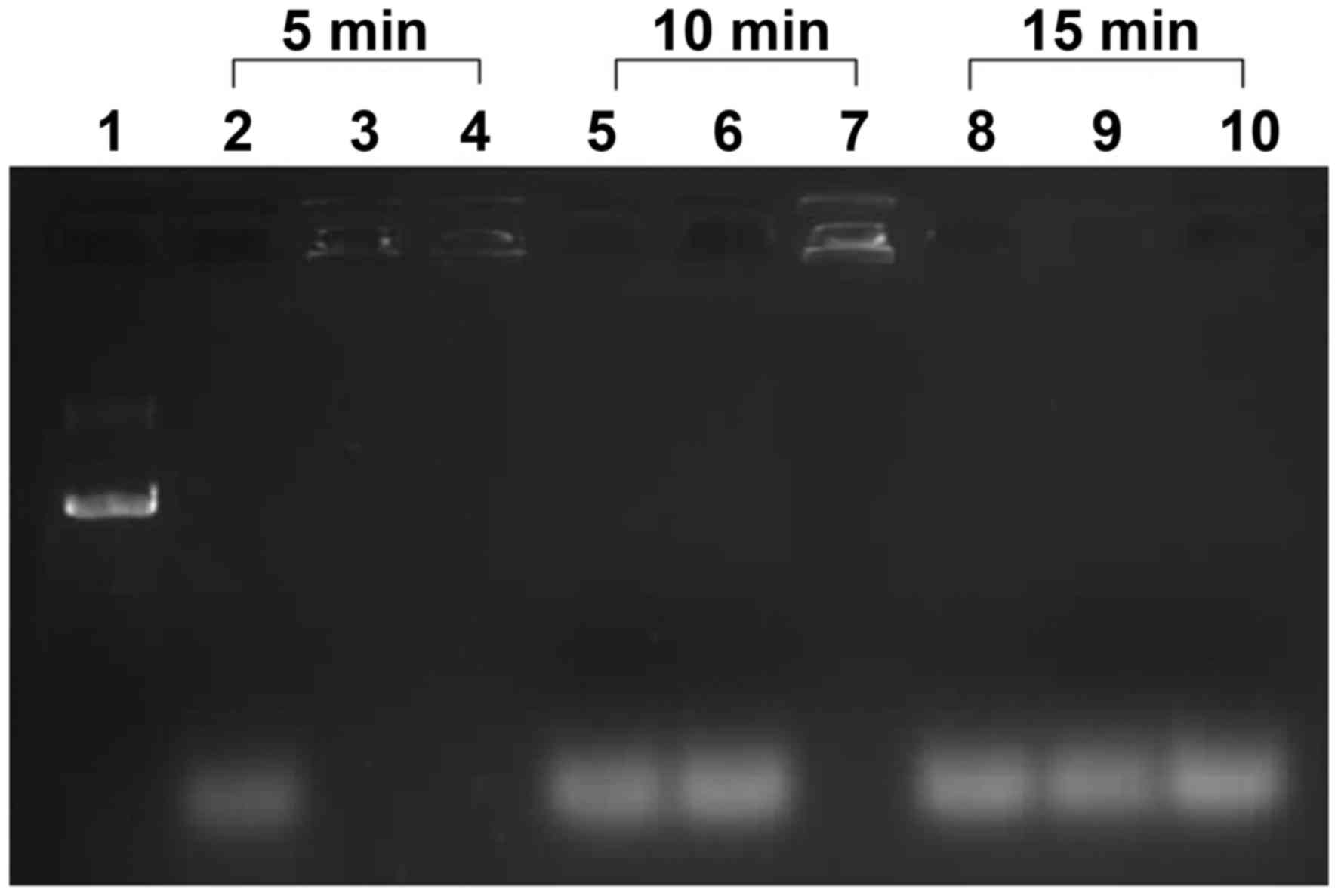 | Figure 2.The effect of PEI and NLS on the
protection of pDNA from DNase digestion as determined by gel
electrophoresis. The pEGFP-N3 plasmid was incubated with
the PEI at N:P ratios of 6:1 and in the presence or absence of 120
µg NLS. Lane 1, pEGFP-N3 plasmid; lanes 2 to 4, the
pEGFP-N3 plasmid, PEI/DNA and PEI/DNA/NLS, respectively,
following exposure to DNase for 5 min; lanes 5 to 7, the
pEGFP-N3 plasmid, PEI/DNA and PEI/DNA/NLS, respectively,
following exposure to DNase for 10 min; lanes 8 to 10, the
pEGFP-N3 plasmid, PEI/DNA and PEI/DNA/NLS, respectively
following exposure to DNase for 15 min. PEI, polyethylenimine; NLS,
nuclear localization sequence; pDNA, plasmid DNA; DNase,
deoxyribonuclease; N:P ratio, PEI nitrogen: DNA phosphate ratio;
PEI/DNA, pEGFP-N3 plasmid plus PEI; PEI/DNA/NLS,
pEGFP-N3 plasmid plus PEI and NLS. |
Expression of GFP under an inverted
fluorescence microscope
pEGFP-N3 is an expression vector for the
EGFP gene; the presence of which can be observed by detecting GFP
expression in 293T cells following transfection under an inverted
fluorescence microscope. Fig. 3
reveals almost no expression of GFP in the pEGFP-N3
plasmid-only group (Fig. 3A). By
contrast, group 3 exhibited a higher level of green fluorescence
when compared with group 2 (Fig. 3B
and C), demonstrating that the non-covalent binding of pDNA to
NLS may improve the efficiency of transfection. In addition, the
highest level of GFP expression was observed in group 6 (Fig. 3). This indicated that UTMD combined
with PEI/DNA/NLS complexes may improve the transfection efficiency
when compared with PEI/DNA/NLS and UTMD+PEI/DNA.
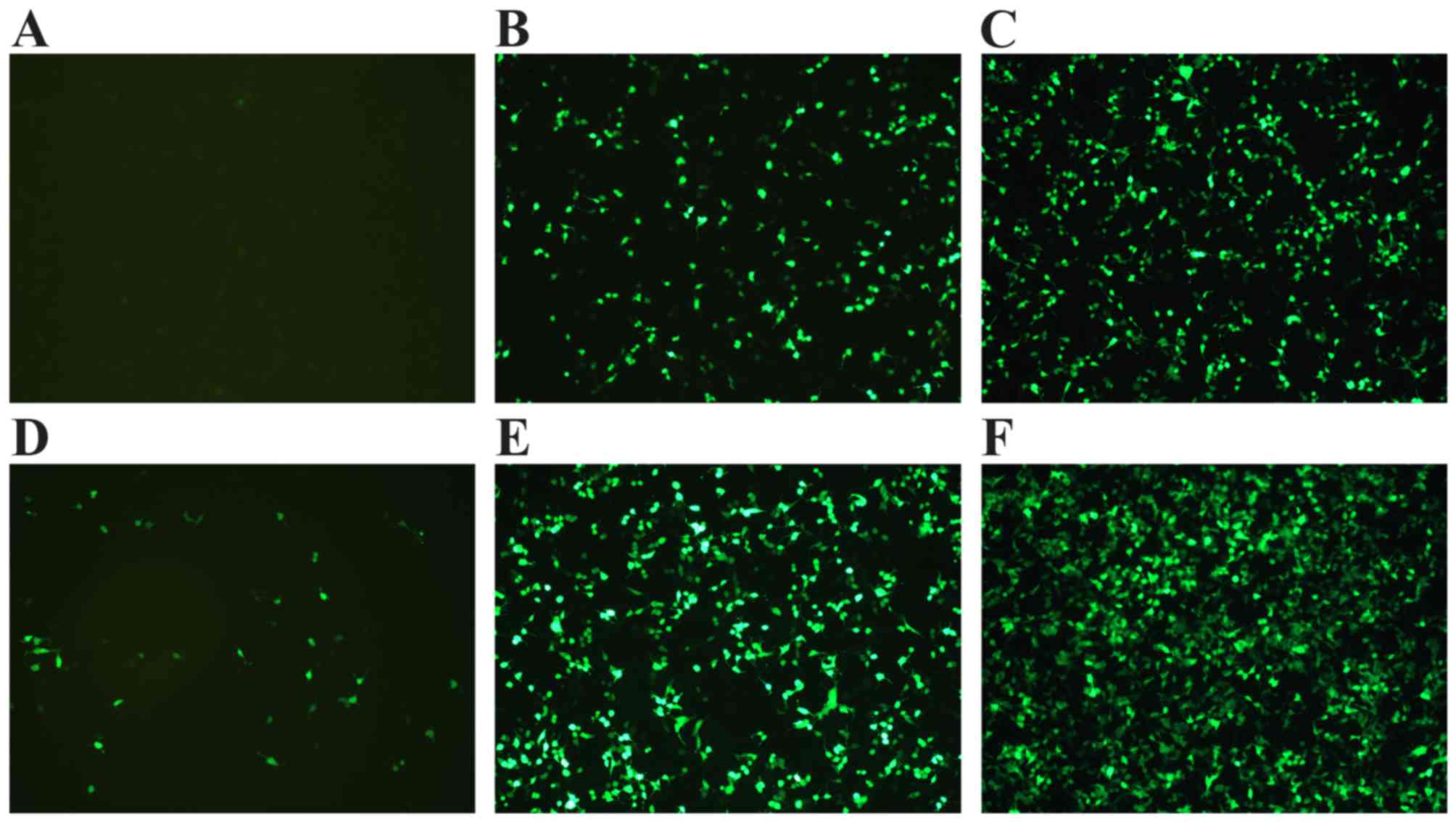 | Figure 3.Expression of green fluorescent
protein was observed under an inverted fluorescence microscope
(magnification, ×400). (A) pEGFP-N3 plasmid, (B)
pEGFP-N3 plasmid with PEI, (C) pEGFP-N3
plasmid with PEI and NLS, (D) pEGFP-N3 plasmid treated
by UTMD, (E) pEGFP-N3 plasmid with PEI treated by UTMD,
(F) pEGFP-N3 plasmid with PEI and NLS treated by UTMD.
PEI, polyethylenimine; NLS, nuclear localization sequence, UTMD,
ultrasound-targeted microbubble destruction. |
Effect of UTMD combined with
PEI/DNA/NLS complexes on transfection efficiency
Fig. 4 depicts the
transfection efficiency in each group by flow cytometry analysis.
All groups displayed a significantly higher level of transfection
efficiency when compared with the DNA group (P<0.05). In
addition, the transfection efficiency in group 6 was significantly
higher when compared with groups 3 and 5, respectively indicating
that UTMD combined with PEI/DNA/NLS complexes may express an
increased quantity of GFP compared with PEI/DNA/NLS or
UTMD+PEI/DNA. These results implied that UTMD combined with
PEI/DNA/NLS may be an effective method to increase the expression
of the target gene (both P<0.05).
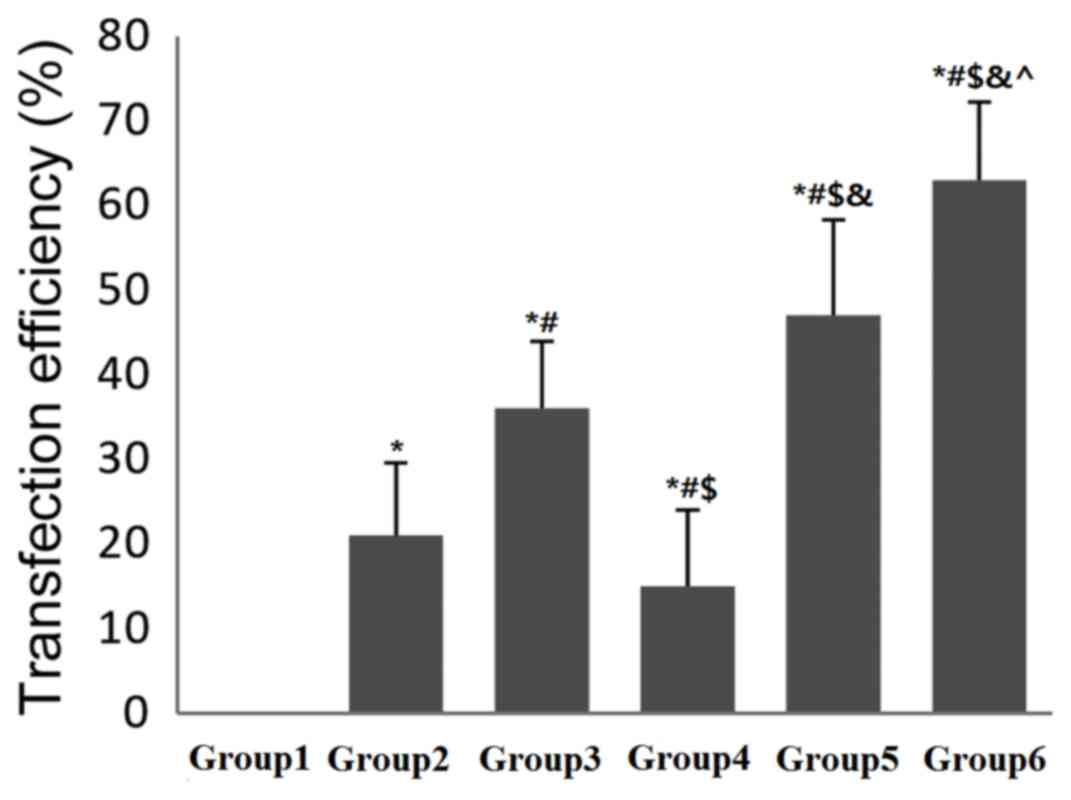 | Figure 4.Effect of NLS on PEI/DNA complexes
following US-mediated transfection of 293T cells. US was applied at
1.5 W/cm2 for 30 sec. Transfection efficiency was
detected by flow cytometry following 24 h. *P<0.05 vs. DNA;
#P<0.05 vs. PEI/DNA; $P<0.05 vs.
PEI/DNA/NLS; &P<0.05 vs. UTMD+DNA;
^P<0.05 vs. UTMD+PEI/DNA. NLS, nuclear localization
sequence; PEI, polyethylenimine; US, ultrasound; UTMD,
ultrasound-targeted microbubble destruction; group 1, DNA,
pEGFP-N3 plasmid only; group 2, PEI/DNA,
pEGFP-N3 plasmid with PEI; group 3, PEI/DNA/NLS,
pEGFP-N3 plasmid with PEI and NLS; group 4, UTMD+DNA,
pEGFP-N3 plasmid treated by UTMD; group 5, UTMD+PEI/DNA,
pEGFP-N3 plasmid with PEI treated by UTMD; group 6,
UTMD+PEI/DNA/NLS, pEGFP-N3 plasmid with PEI and NLS
treated by UTMD. |
Effect of combining UTMD with
PEI/DNA/NLS complexes on cell viability
One of the major requirements for cationic polymer
vectors in gene delivery is low cytotoxicity. In the present study,
cells in the different groups were incubated for 4 h following
treatment, and cell viability was assessed using a CCK-8 assay and
a microplate reader. As shown in Fig.
5, cell viability decreased as a result of US irradiation and
PEI. However, as the differences were not statistically significant
and cell viability was >80% among all groups, the level of cell
toxicity was considered to be acceptable (Fig. 5).
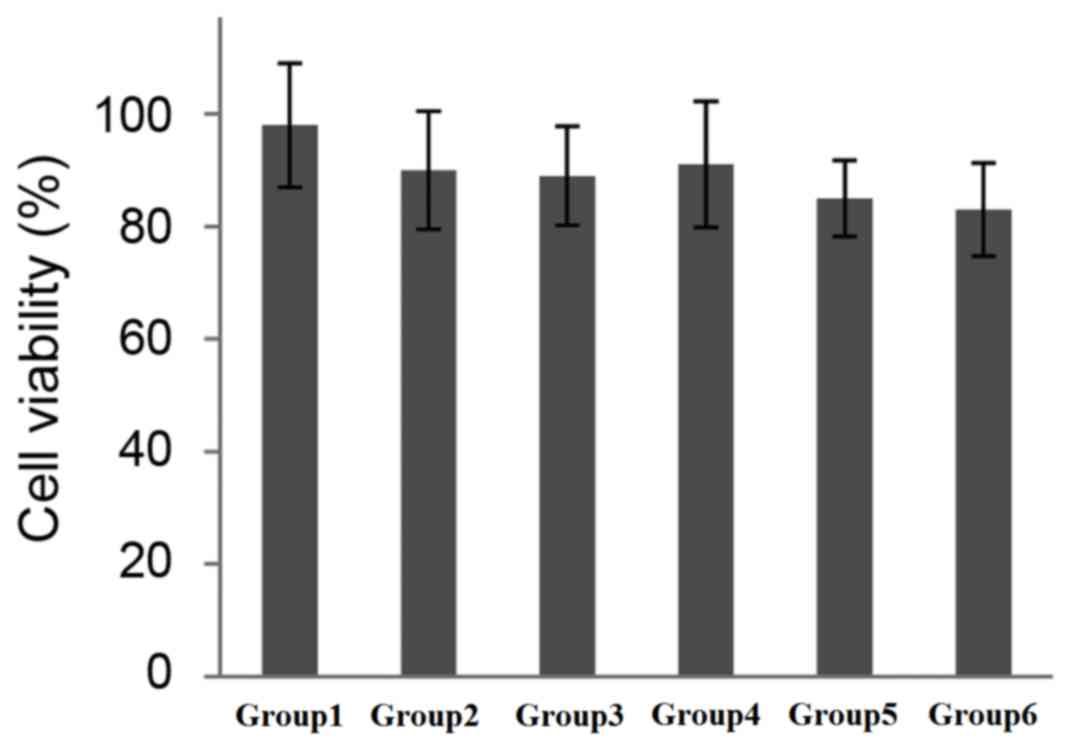 | Figure 5.Cytotoxic effect of NLS peptide
interaction with PEI/DNA complexes following US mediated
transfection of 293T cells. Cell viability was measured using the
Cell Counting Kit-8 assay. The viability of the treated cells were
expressed as a percentage of untreated controls (100%). NLS,
nuclear localization sequence; PEI, polyethylenimine; US,
ultrasound; UTMD, ultrasound-targeted microbubble destruction;
group 1, DNA, pEGFP-N3 plasmid only; group 2, PEI/DNA,
pEGFP-N3 plasmid with PEI; group 3, PEI/DNA/NLS,
pEGFP-N3 plasmid with PEI and NLS; group 4, UTMD+DNA,
pEGFP-N3 plasmid treated by UTMD; group 5, UTMD+PEI/DNA,
pEGFP-N3 plasmid with PEI treated by UTMD; group 6,
UTMD+PEI/DNA/NLS, pEGFP-N3 plasmid with PEI and NLS
treated by UTMD. |
Discussion
Successful gene therapy requires the development of
technologies capable of transferring exogenous genes into a wide
variety of cells and tissues safely and effectively. To enhance
exogenous DNA delivery, the therapy must demonstrate the ability to
affect membrane permeability, to bind and condense DNA in a
reversible manner and to promote nuclear delivery of DNA through
non-viral gene delivery methods (25). When considering the above
requirements, UTMD presents a number of advantages for gene
therapy, as it is capable of reversibly opening the cell membrane
in a safe manner, and demonstrates a high targeting ability and
visibility (26). Therefore, the
present study employed US as the chosen method of transfection.
Previous studies have demonstrated that UTMD improves cell membrane
permeability (26,27); however, additional studies have
suggested that UTMD only facilitates gene entry to the cytoplasm,
and not to the nucleus (28,29).
These discrepancies may be due to differences in US irradiation
frequency, the radiation pattern and experiment selections.
However, a previous study confirmed that US irradiation facilitates
access of DNA to the nucleus (5).
Duvshani-Eshet et al (15,16)
demonstrated that US induces exogenous genes to enter the nucleus
of three different cell types, including baby hamster kidney (BHK)
cells, human prostate carcinoma PC-3 cells and basal cell
epithelioma (BCE) cells; this is achieved only when the frequency
of US irradiation and total radiation energy is sufficiently high.
When compared with the control group, this increased target gene
expression to 1,200-fold higher levels, increased the transfection
efficiency to 28% and reduced the cell mortality rate to <20%.
In addition, several studies have demonstrated that, regardless of
whether it is combined with microbubble technology, long-pulse
ultrasonic irradiation leads to entry of DNA to the cytoplasm and
the nucleus, while maintaining a cell survival rate of >80%
(15,16). In the present study, ultrasonic
irradiation conditions were set according to the previously
described methods (30). PEI was
used as a non-viral gene transfer agent, as PEI is cationic and has
demonstrated the capacity to produce strong DNA compaction, thus
providing effective DNA protection (31). The negatively-charged plasmid DNA
may be protected from degradation by PEI during mechanical
processes (32). PEI serves an
important role in this process of transfection.
To promote nuclear delivery of target DNA in the
present study, the SV40 large T antigen (PKKKRKV) was applied as a
type of NLS. NLS sequences bind to receptors on the nuclear
membrane, thus mediating the delivery of DNA into the nucleus.
Previous studies have demonstrated that NLS effectively promote DNA
delivery into the nucleus, thus improving exogenous gene expression
(33,34); however, a number of studies have
provided evidence to suggest that NLS-coupled DNA does not promote
the transport of DNA to the nucleus (8). It is possible that the non-covalent
coupling method does not effectively combine NLS with DNA, or that
the covalent coupling method does not expose the NLS epitope.
Although previous studies have confirmed that NLS promotes DNA
entry to the nucleus, it may impede gene expression following
covalent coupling (8,35). Fixed-point coupling technologies,
including peptide nuclear acids (36), triple helix formation
oligonucleotides (37) and
restriction enzyme recognition domain structures (38), combine the NLS to the outside of
the target gene expression cassette and promote DNA delivery to the
nucleus without affecting gene expression. However, due to a number
of limitations with these techniques, they are unable to be widely
used for gene therapy. These limitations include the tedious
preparation of the complex, the difficult generation of a
connection with exogenous genes and the challenging detection of
successful complex construction. Taking the cost into
consideration, the present study used NLS peptides combined with
PEI/DNA produced by electrostatic adsorption to form the complex,
as electrostatic adsorption does not affect the properties of
microbubbles, NLS, PEI and DNA. The results indicated that PEI and
NLS may demonstrate protective effects on DNA against degradation
by lysosomes and degenerative enzyme in the cytoplasm.
The present study applied UTMD as the method of
transfection due to the cavitation effect, which reversibly
increases the permeability of the cell membrane (15–17).
In addition, the surface of the SonoVue microbubbles, cell
membranes and DNA are all negatively charged, whereas the PEI and
NLS peptide were positively charged. The present study therefore
combined the NLS peptide with the PEI/DNA complex by electrostatic
interactions, which reduced the cost of peptide synthesis and
simplified the procedure. US irradiation is considered to be an
alternative approach for non-viral gene delivery, as it increases
the opportunity for interactions between non-viral vectors and
target cells, thereby facilitating the targeted release of
PEI/DNA/NLS complexes. The destruction of microbubbles stimulates
binding of PEI/DNA/NLS complexes to the cell membrane and promotes
endocytosis, thus enhancing gene transfection.
The results of the current study indicated that
PEI/DNA complexes and PEI/DNA/NLS complexes were formed
successfully. In addition, PEI and NLS may demonstrate protective
effects on DNA integrity. Although the protective effect was
time-dependent, it induced the delivery of more exogenous genes
into the nucleus, and this result is consistent with the study by
Collins et al (39). The
results indicated that combining UTMD with the PEI/DNA/NLS
complexes may effectively increase the rate of exogenous gene
transfection and expression. Even though cell viability was
decreased through the mechanical damage of the ultrasonic
irradiation and the low level of cytotoxicity of PEI, no
significant difference in cell viability in all groups was observed
when compared with group 1. In addition, the cell survival rate was
>80%. Therefore, the combination of UTMD and PEI/DNA/NLS
complexes as a method of gene therapy may be worthy of further
investigation in future studies.
The results of the present study indicated that
combining UTMD with PEI/DNA/NLS complexes promotes the delivery of
target genes and improves the rate of transfection and gene
expression effectively. However, further studies are required to
clarify the mechanism by which the complex facilitated gene
delivery. In addition, as the PEI/DNA/NLS complexes are coated in
microbubbles instead of connected to the surface of the
microbubbles, further studies are required to determine whether
additional genes could be delivered to the cells. In addition, as
the present study was conducted in vitro, the effect of UTMD
combined with PEI/DNA/NLS complexes requires further verification
through in vivo studies. Finally, the use of liposomes with
low or no toxicity requires further investigation.
In conclusion, the results of the current study
suggest that UTMD is a simple, effective, non-invasive targeting
technology, which has been demonstrated to be a feasible approach
to enhance the efficiency of non-viral transfection. The
combination of UTMD and PEI/DNA/NLS complexes was developed as a
gene delivery method; however, it requires improvement prior to its
use in clinical applications. It is a promising technique for gene
therapy and may serve an important role in future treatments. The
present study successfully produced a UTMD-PEI/DNA/NLS complex gene
delivery system, and the results demonstrated that the transfection
efficiency of the system was superior to that of UTMD+PEI or
PEI/DNA/NLS complexes alone, without subsequently increasing the
rate of cell injury. Therefore, it may be an effective method for
the clinical application of gene transfection.
Acknowledgements
The present study was supported by the Chinese
National Science Foundation Committee (grant nos. 81471674 and
81501495).
Glossary
Abbreviations
Abbreviations:
|
UTMD
|
ultrasound-targeted microbubble
destruction
|
|
PEI/DNA/NLS
|
polyethylenimine/pEGFP-N3
plasmid/nuclear localization sequence
|
References
|
1
|
Bonci D, Cittadini A, Latronico MV,
Borello U, Aycock JK, Drusco A, Innocenzi A, Follenzi A, Lavitrano
M, Mont MG, et al: Advanced generation lentiviruses as efficient
vectors for cardiomyocyte gene transduction in vitro and in vivo.
Gene Ther. 10:630–636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oberle V, de Jong G, Drayer JI and
Hoekstra D: Efficient transfer of chromosome-based DNA constructs
into mammalian cells. Biochim Biophys Acta. 1676:223–230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldherr CM: The nuclear annuli as
pathways for nucleocytoplasmic exchanges. J Cell Biol. 14:65–72.
1962. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richardson WD, Mills AD, Dilworth SM,
Laskey RA and Dingwall C: Nuclear protein migration involves two
steps: Rapid binding at the nuclear envelope followed by slower
translocation through nuclear pores. Cell. 52:655–664. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duvshani-Eshet M, Keren H, Oz S,
Radzishevsky IS, Mor A and Machluf M: Effect of peptides bearing
nuclear localization signals on therapeutic ultrasound mediated
gene delivery. J Gene Med. 10:1150–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bremner KH, Seymour LW, Logan A and Read
ML: Factors influencing the ability of nuclear localization
sequence peptides to enhance nonviral gene delivery. Bioconjug
Chem. 15:152–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ciolina C, Byk G, Blanche F, Thuillier V,
Scherman D and Wils P: Coupling of nuclear localization signals to
plasmid DNA and specific interaction of the conjugates with
importin alpha. Bioconjug Chem. 10:49–55. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hébert E: Improvement of exogenous DNA
nuclear importation by nuclear localization signal-bearing vectors:
A promising way for non-viral gene therapy? Biol Cell. 95:59–68.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schirmbeck R, König-Merediz SA, Riedl P,
Kwissa M, Sack F, Schroff M, Junghans C, Reimann J and Wittig B:
Priming of immune responses to hepatitis B surface antigen with
minimal DNA expression constructs modified with a nuclear
localization signal peptide. J Mol Med (Berl). 79:343–350. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng C, Juhls C, Oswald D, Sack F,
Westfehling I, Wittig B, Babiuk LA and van Drunen Littel-van den
Hurk S: Effect of different nuclear localization sequences on the
immune responses induced by a MIDGE vector encoding bovine
herpesvirus-1 glycoprotein D. Vaccine. 24:4625–4629. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escriou V, Carrière M, Scherman D and Wils
P: NLS bioconjugates for targeting therapeutic genes to the
nucleus. Adv Drug Deliv Rev. 55:295–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Opanasopit P, Apirakaramwong A,
Ngawhirunpat T, Rojanarata T and Ruktanonchai U: Development and
characterization of pectinate micro/nanoparticles for gene
delivery. AAPS Pharm Sci Tech. 9:67–74. 2008. View Article : Google Scholar
|
|
13
|
Ruozi B, Forni F, Battini R and Vandelli
MA: Cationic liposomes for gene transfection. J Drug Target ١١:
٤٠٧-٤١٤, ٢٠٠٣.
|
|
14
|
Mao S, Sun W and Kissel T: Chitosan-based
formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev.
62:12–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duvshani-Eshet M, Baruch L, Kesselman E,
Shimoni E and Machluf M: Therapeutic ultrasound-mediated DNA to
cell and nucleus: Bioeffects revealed by confocal and atomic force
microscopy. Gene Ther. 13:163–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duvshani-Eshet M and Machluf M:
Therapeutic ultrasound optimization for gene delivery: A key factor
achieving nuclear DNA localization. J Control Release. 108:513–528.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noble ML, Kuhr CS, Graves SS, Loeb KR, Sun
SS, Keilman GW, Morrison KP, Paun M, Storb RF and Miao CH:
Ultrasound-targeted microbubble destruction-mediated gene delivery
into canine livers. Mol Ther. 21:1687–1694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deshpande MC and Prausnitz MR: Synergistic
effect of ultrasound and PEI on DNA transfection in vitro. J
Control Release ١١٨: ١٢٦-١٣٥, ٢٠٠٧.
|
|
19
|
Lee YH and Peng CA: Nonviral transfection
of suspension cells in ultrasound standing wave fields. Ultrasound
Med Biol ٣٣: ٧٣٤-٧٤٢, ٢٠٠٧.
|
|
20
|
Chumakova OV, Liopo AV, Andreev VG,
Cicenaite I, Evers BM, Chakrabarty S, Pappas TC and Esenaliev RO:
Composition of PLGA and PEI/DNA nanoparticles improves
ultrasound-mediated gene delivery in solid tumors in vivo. Cancer
Lett ٢٦١: ٢١٥-٢٢٥, ٢٠٠٨.
|
|
21
|
Yoo HS and Jeong SY: Nuclear targeting of
non-viral gene carriers using psoralen-nuclear localization signal
(NLS) conjugates. Eur J Pharm Biopharm. 66:28–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen ZY, Yang F, Lin Y, Zhang JS, Qiu RX,
Jiang L, Zhou XX and Yu JX: New development and application of
ultrasound targeted microbubble destruction in gene therapy and
drug delivery. Curr Gene Ther. 13:250–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahim A, Taylor SL, Bush NL, ter Haar GR,
Bamber JC and Porter CD: Physical parameters affecting
ultrasound/microbubble-mediated gene delivery efficiency in vitro.
Ultrasound Med Biol ٣٢: ١٢٦٩-١٢٧٩, ٢٠٠٦.
|
|
24
|
Zhao W, Liu K, Chen S, Zhu MM, Lv H, Hu J
and Mao Y: Polyethylenimine derivate conjugated with RGD-TAT-NLS as
a novel gene vector. Biomed Mater Eng. 24:1933–1939.
2014.PubMed/NCBI
|
|
25
|
Sadeghian F, Hosseinkhani S, Alizadeh A
and Hatefi A: Design, engineering and preparation of a multi-domain
fusion vector for gene delivery. Int J Pharm. 427:393–399. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan Z, Kumon RE, Park J and Deng CX:
Intracellular delivery and calcium transients generated in
sonoporation facilitated by microbubbles. J Control Release.
142:31–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang D, Tan KB, Gao YH, Liu H and Yang WX:
Effects of diagnostic ultrasound-targeted microbubble destruction
on permeability of normal liver in rats. Ultrasonics. 52:1065–1071.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin LF, Li F, Wang HP, Wei F, Qin P and Du
LF: Ultrasound targeted microbubble destruction stimulates cellular
endocytosis in facilitation of adeno-associated virus delivery. Int
J Mol Sci. 14:9737–9750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schlicher RK, Hutcheson JD, Radhakrishna
H, Apkarian RP and Prausnitz MR: Changes in cell morphology due to
plasma membrane wounding by acoustic cavitation. Ultrasound Med
Biol. 36:677–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Q, Chen JL, Chen Q, Wang X, Deng Q,
Hu B and Guo RQ: Optimization of transfection parameters for
ultrasound/SonoVue microbubble-mediated hAng-1 gene delivery in
vitro. Mol Med Rep. 6:1460–1464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fukumoto Y, Obata Y, Ishibashi K, Tamura
N, Kikuchi I, Aoyama K, Hattori Y, Tsuda K, Nakayama Y and
Yamaguchi N: Cost-effective gene transfection by DNA compaction at
pH 4.0 using acidified, long shelf-life polyethylenimine.
Cytotechnology. 62:73–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lentz YK, Anchordoquy TJ and Lengsfeld CS:
DNA acts as a nucleation site for transient cavitation in the
ultrasonic nebulizer. J Pharm Sci. 95:607–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Opanasopit P, Rojanarata T, Apirakaramwong
A, Ngawhirunpat T and Ruktanonchai U: Nuclear localization signal
peptides enhance transfection efficiency of chitosan/DNA complexes.
Int J Pharm. 382:291–295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawazu T, Kanzaki H, Uno A, Azuma H and
Nagasaki T: HVJ-E/importin-β hybrid vector for overcoming
cytoplasmic and nuclear membranes as double barrier for non-viral
gene delivery. Biomed Pharmacother. 66:519–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sebestyén MG, Ludtke JJ, Bassik MC, Zhang
G, Budker V, Lukhtanov EA, Hagstrom JE and Wolff JA: DNA vector
chemistry: The covalent attachment of signal peptides to plasmid
DNA. Nat Biotechnol. 16:80–85. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braun K, von Brasch L, Pipkorn R, Ehemann
V, Jenne J, Spring H, Debus J, Didinger B, Rittgen W and Waldeck W:
BioShuttle-mediated plasmid transfer. Int J Med Sci. 4:267–277.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neves C, Byk G, Scherman D and Wils P:
Coupling of a targeting peptide to plasmid DNA by covalent triple
helix formation. FEBS Lett. 453:41–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lavigne MD, Yates L, Coxhead P and Górecki
DC: Nuclear-targeted chimeric vector enhancing nonviral gene
transfer into skeletal muscle of Fabry mice in vivo. FASEB J.
2097–2107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Collins E, Birchall JC, Williams JL and
Gumbleton M: Nuclear localisation and pDNA condensation in
non-viral gene delivery. J Gene Med. 9:265–274. 2007. View Article : Google Scholar : PubMed/NCBI
|



















