Introduction
Characterized by high incidence rate, disability
rate and cost, as well as low mortality, traumatic injury of the
spinal cord (TISC) is a common trauma in spine surgery, which
greatly influences quality of life of patients and increases the
burdens on their family members (1). With the rise of traffic and air
accidents, its morbidity is increasing year by year (1). TISC mainly occurs to young adults and
does not have good therapeutic measures (2). However, it is essential for patients
to take operative treatment, drug therapy and rehabilitative
measures to improve their functional status.
According to its mechanisms, spinal cord injury
(SCI) can be classified into primary and secondary injuries
(3). Primary injury is caused by
the initial force directly or indirectly acting on spinal cords
(4). Secondary injury occurs on
the site of primary injuries, with a series of physiochemical
mechanisms including oxidative stress and excessive release of
inflammatory response causing destructive lesions to complete
tissues surrounding the lesions and a further deepening of the
degree of injuries and the broadening of injury areas (5,6).
Oxidative stress is a series of adaptive responses triggered by the
loss of equilibrium between reactive oxygen species and the
antioxidant system (7).
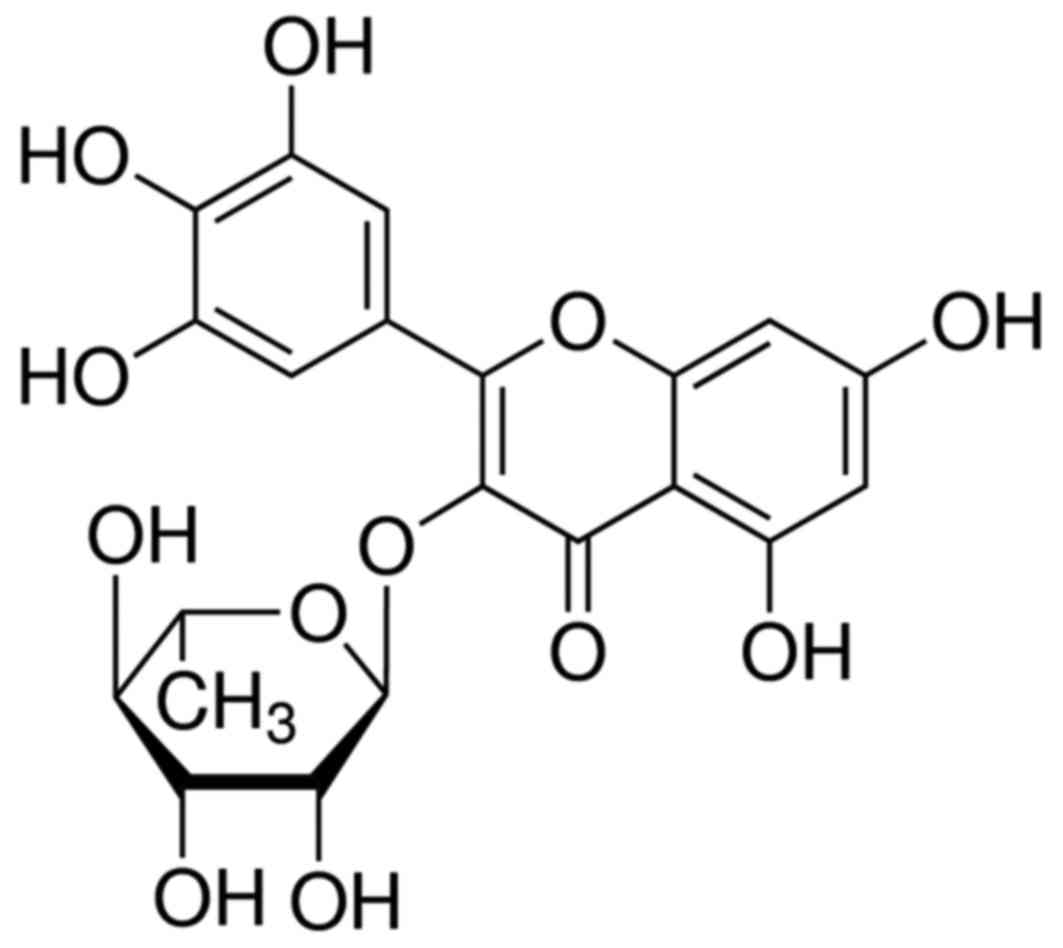
Myricitrin (Fig. 1)
is the 3-O-rhamnoside of myricetin, a flavonoid. Myricitrin and
tannin exist in waxberry extract (8). In addition to its confirmed
pharmacological functions, myricitrin is anti-inflammatory and
antineoplastic (9). Furthermore,
it can prevent tooth decay, and eliminate free radicals and
oxidative stress (10). Thus, the
present study evaluated if myricitrin ameliorated TISC, and
explored its mechanism.
Materials and methods
Animals and experimental design
All experiments were performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (National Institutes of Health, Bethesda, MD,
USA) and were approved by the Ethics Committee for Animal
Experiments, The Third Department of Orthopedics, Cangzhou Central
Hospital (Cangzhou, China). A total of 60 adult female Sprague
Dawley rats (7–8 weeks; 160–200 g) were kept in polypropylene cages
with wood shavings as bedding, 12/12 h light/dark cycle, lights on
at 8:00 a.m. at 22±2°C and had free access to water and food. The
animals were randomly divided into five groups of 12: Sham, TISC
model, myricitrin (5 mg/kg/d), myricitrin (10 mg/kg/d) and
myricitrin (30 mg/kg/d). TISC model rats were performed under
general anesthesia, using intraperitoneal ketamine (80 mg/kg) and
xylazine (10 mg/kg) injection. Rats were injured in the thoracic
level 12. A laminectomy was also performed in the thoracic level
12, and skin and muscle overlying the spinal column were cut. A
moderate-intensity weight-drop was performed using an impactor with
a diameter of 2.5 mm into the thoracic level 12. In the myricitrin
group, rats were injected intraperitoneally with 5, 10 or 30
mg/kg/d of myricitrin for 5 days.
Histopathology
Spinal cord tissue was collected and washed with
PBS. Tissue was fixed with 4% paraformaldehyde for 24 h at room
temperature, embedded in paraffin and sectioned into 4 µm sections.
Then, tissue samples were dewaxed using xylene and washed with
ethyl alcohol. Tissues were sectioned was stained with hematoxylin
and eosin.
Basso-Beattie-Bresnahan (BBB)
evaluation of locomotion and water content of spinal cord
The BBB scale evaluates the following criteria: The
rating scale ranges from 0 to 21, and scores were assigned for both
hind limbs by two independent observers blinded to the experiments.
0 on the scale refers to no observable hindlimb movement and 21 is
normal locomotion. Following myricitrin treatment, spinal cord
tissue samples were gathered and weighed as wet weight. Then, at
80°C, spinal cord tissue samples were dried for 48 h and weighed as
dry weight. The water content of the spinal cord is calculated as
dry weight/wet weight.
ELISA
Spinal cord tissue homogenate was centrifuged at
8,000 × g for 10 min at 4°C and the supernatant was harvested to
measure the protein concentration using Coomassie brilliant blue
G250 technique (Beyotime Institute of Biotechnology, Haimen,
China). A total of 50 µl protein samples were harvested, and
malondialdehyde (MDA; A003-1), superoxide dismutase (SOD; A001-3),
catalase (CAT; A007-1), glutathione peroxidase (GSH-PX; A005),
NF-κB p65 subunit (H202), tumor necrosis factor (TNF)-α (H052),
interleukin (IL)-1β (H002) and IL-6 (H007) contents were measured
using ELISA kits (Nanjing Jiancheng Biology Engineering Institute,
Nanjing, China). The absorbance value at 405 nm was determined
using a SpectraMax® M2e Multimode Microplate Reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from spinal cord tissue using
TRIzol reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A
total of 1 µg total RNA was synthesized cDNA using a one-step
RT-PCR kit (Qiagen Benelux B.V., Venlo, The Netherlands). The gene
expression levels of cyclooxygenase (COX)-2 and TGF-β1 were
analyzed by RT-qPCR, conducted using the CFX96 Real Time PCR system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The standard
amplification program included 30 cycles, 95°C with a 20 sec hold,
annealing at 60°C sec with a 20 sec hold, and extending at 72°C
with a 10 sec hold. The following primers were used for COX-2
forward, TTC CAA TCC ATG TCA AAA CCG T and reverse, AGT CCG GGT ACA
GTC ACA CTT; TGF-β1 forward, 5′-AGGGCTACCATGCCAACTTC-3′ and
reverse, 5′-CCACGTAGTAGACGATGGGC-3′; β-actin forward, GGC TGT ATT
CCC CTC CAT CG and reverse, CCA GTT GGT AAC AAT GCC ATG T.
Western blotting
Spinal cord tissue homogenate was centrifuged at
8,000 × g for 10 min at 4°C and the supernatant was harvested to
measure the protein concentration using Coomassie brilliant blue
G250 technique (Beyotime Institute of Biotechnology). Protein
samples (50 µg) were harvested using SDS-PAGE on 10–12% gel and
transferred on to a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc.). The membrane was incubated with blocking
buffer (5% skimmed milk) for 1 h at room temperature and probed
overnight at 4°C with primary antibodies against p53 (sc-1311-R;
1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Bcl-2
(sc-783; 1:400, Santa Cruz Biotechnology, Inc.), Bax (sc-6236;
1:400; Santa Cruz Biotechnology, Inc.) and β-actin (sc-7210; 1:500;
Santa Cruz Biotechnology, Inc.). The membrane was incubated with
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(sc-2004; 1:1,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature.
Statistical analysis
Experimental data are expressed as means ± standard
deviation and used SPSS software (version, 13.0; SPSS, Inc.,
Chicago, IL, USA). The Mann-Whitney U-test and Spearman's rank
correlation were used for the statistical analyses. Differences
with P<0.05 was considered to indicate a statistically
significant difference.
Results
Myricitrin inhibits histological
injury in SCI rats
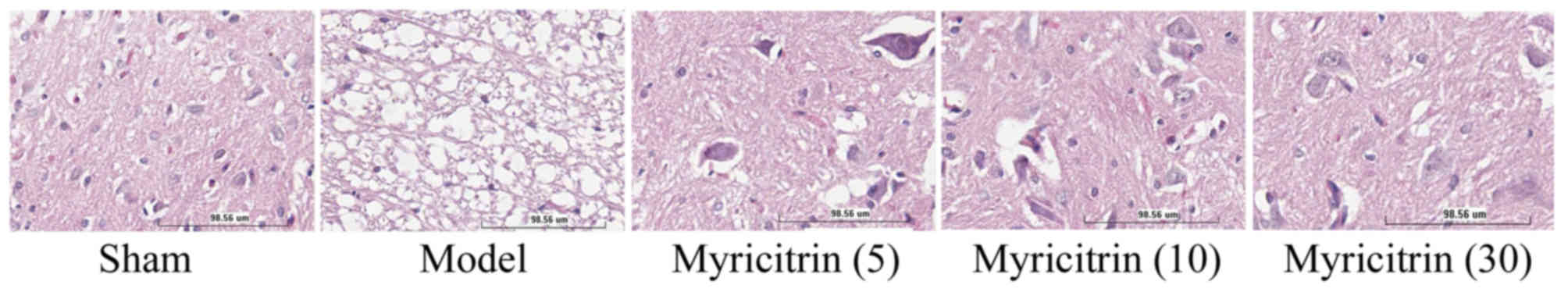
Following treatment with myricitrin, the authors
observed the histology injure of TISC model group was obviously
higher than that of the sham group (Fig. 2). However, treatment with 10 and 30
mg/kg myricitrin evidently inhibited histological injury in TISC
rats, compared with the TISC model group (Fig. 2).
Myricitrin decreases BBB score in SCI
rats
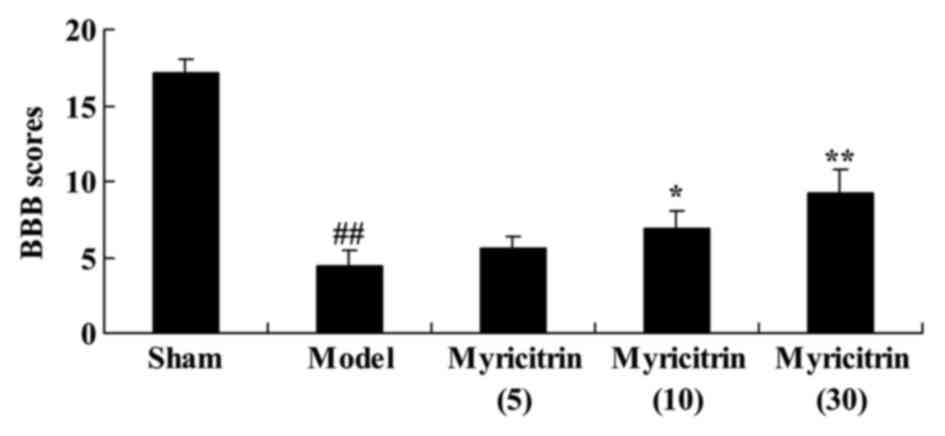
During surgery, the effect of myricitrin on BBB
score in SCI rats, the BBB locomotor rating scale, was used.
Fig. 3 indicated that the mean BBB
scores of the sham group were highest in all experiment groups.
Following treatment by myricitrin, 10 and 30 mg/kg myricitrin
significantly increased BBB score, compared with the TISC model
group (Fig. 3).
Myricitrin weakens water content of
spinal cord in SCI rats
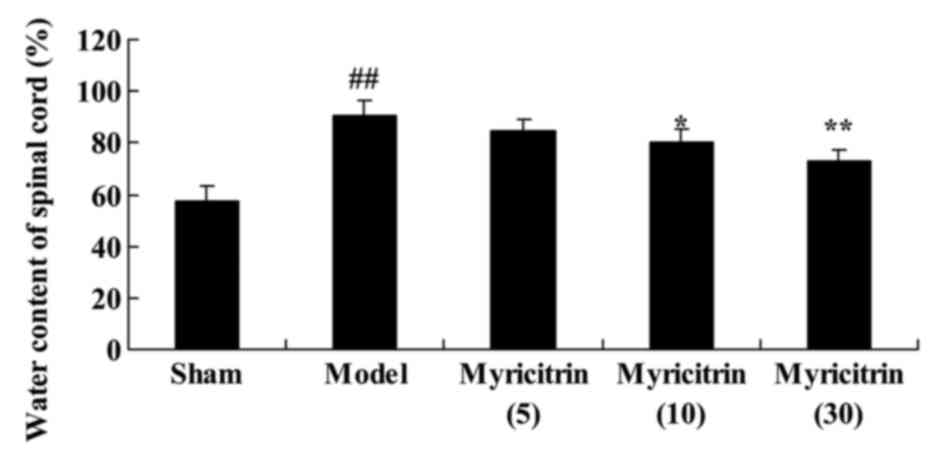
To evaluate the water content of spinal cord in SCI
rats, the water content was detected following treatment with
myricitrin. In the TISC model group, the rats presented
improvements in the water content of spinal cord compared with sham
group (Fig. 4). At 5 d, the water
content of spinal cord was significantly suppressed by treatment
with 10 and 30 mg/kg myricitrin in TISC rats, compared with the
model group (Fig. 4).
Myricitrin exhibits antioxidant
activity in SCI rats
Following the treatment with myricitrin, the authors
researched the effect that myricitrin exhibits on antioxidant
activity in SCI rats. In the TISC model group, the induction of MDA
content and inhibition of SOD, CAT and GSH-PX contents was observed
compared with sham group (Fig. 5).
In addition, treatment with 10 and 30 mg/kg myricitrin
significantly reversed the induction of MDA, SOD, CAT and GSH-PX
levels in TISC rats (Fig. 5).
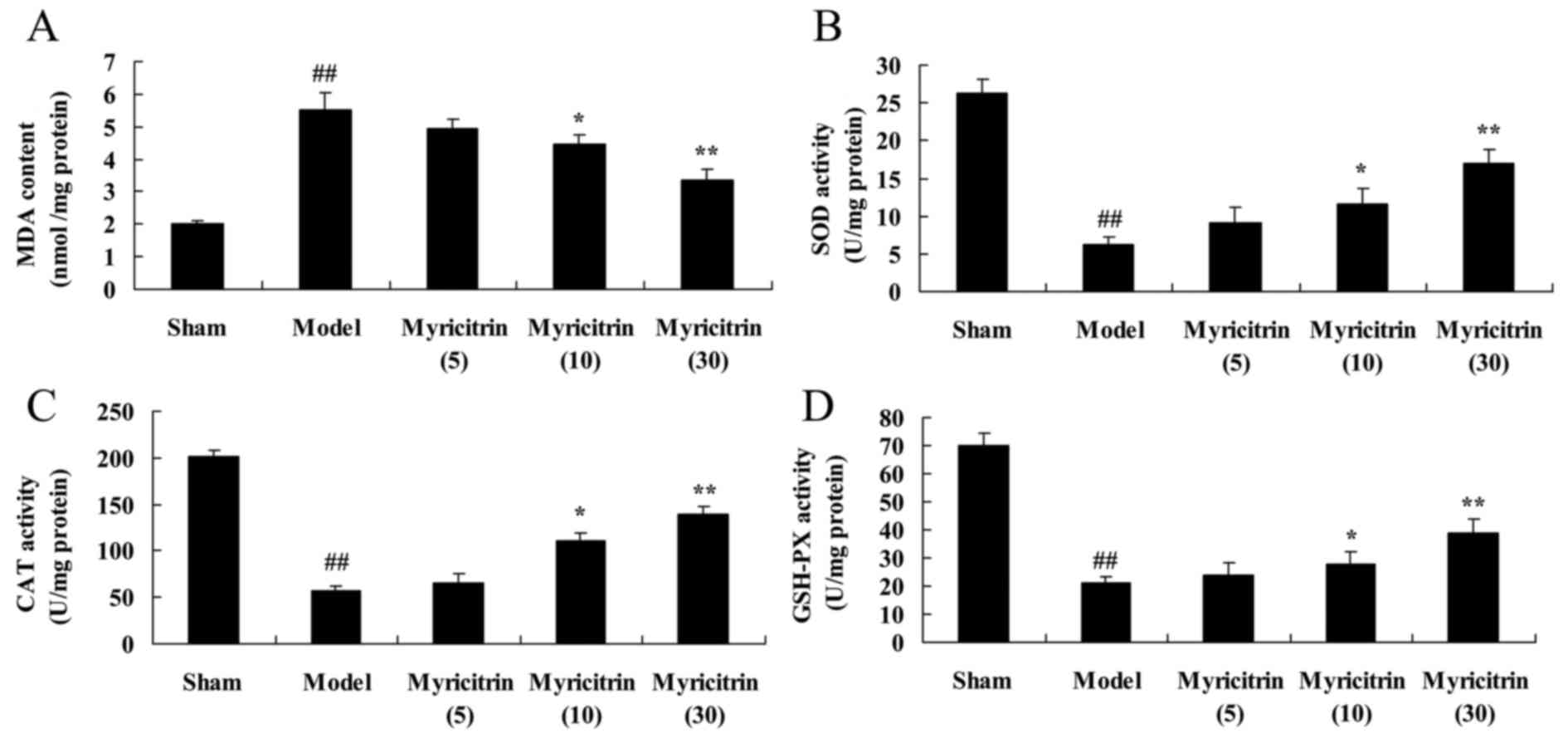 | Figure 5.Myricitrin exhibits antioxidant
activity in spinal cord injury rats. Myricitrin exhibits
antioxidant activity as reflected by (A) MDA, (B) SOD, (C) CAT and
(D) GSH-PX levels in SCI rats. Sham, sham group; Model, TISC model
group; Myricitrin (5), 5 mg/kg
myricitrin group; Myricitrin (10), 10 mg/kg myricitrin group; and
Myricitrin (30), 30 mg/kg
myricitrin group. ##P<0.01 vs. sham group,
*P<0.05, **P<0.01 vs. TISC model group. MDA, malondialdehyde;
SOD, superoxide dismutase; CAT, catalase; GSH-PX, glutathione
peroxidase; TISC, traumatic injury of the spinal cord. |
Myricitrin exhibits anti-inflammatory
activity in SCI rats
Following SCI and administration of myricitrin, to
explore the effect of myricitrin exhibits anti-inflammatory
activity in SCI rats, the NF-κB p65 subunit, TNF-α, IL-1β and IL-6
contents were measured using ELISA kits. As indicated in Fig. 6, SCI-induced NF-κB p65 subunit,
TNF-α, IL-1β and IL-6 contents were observed in the TISC model
group, compared with the sham group. Following myricitrin
administration, 10 and 30 mg/kg myricitrin significantly inhibited
the SCI-induced NF-κB p65 subunit, TNF-α, IL-1β and IL-6 contents
in TISC rats (Fig. 6).
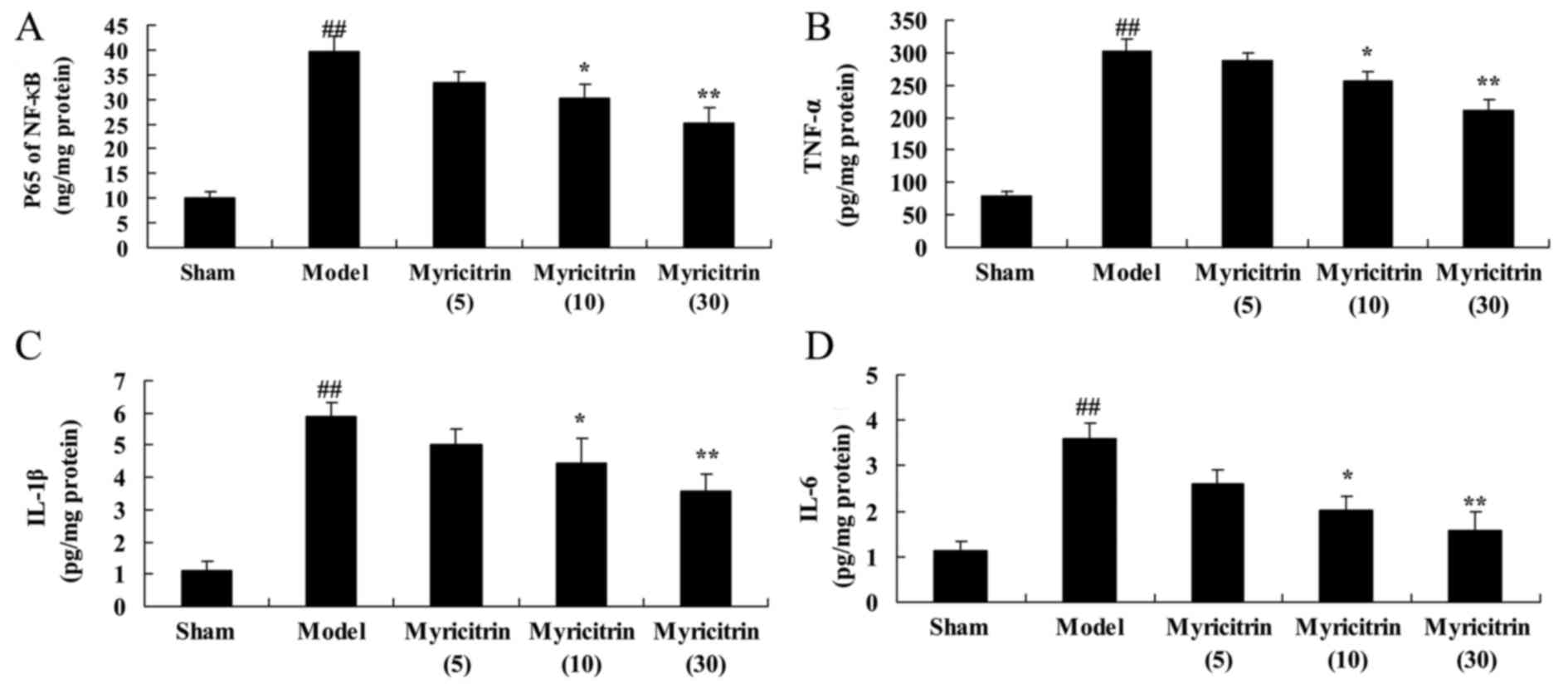 | Figure 6.Myricitrin exhibits anti-inflammatory
activity in SCI rats. Myricitrin exhibits anti-inflammatory
activity as reflected in (A) NF-κB p65 subunit, (B) TNF-α, (C)
IL-1β and (D) IL-6 levels in SCI rats. Sham, sham group; Model,
TISC model group; Myricitrin (5),
5 mg/kg myricitrin group; Myricitrin (10), 10 mg/kg myricitrin group; and
Myricitrin (30), 30 mg/kg
myricitrin group. ##P<0.01 vs. sham group,
*P<0.05, **P<0.01 vs. TISC model group. SCI spinal cord
injury; TISC, traumatic injury of the spinal cord; TNF-α, tumor
necrosis factor-α; IL, interleukin. |
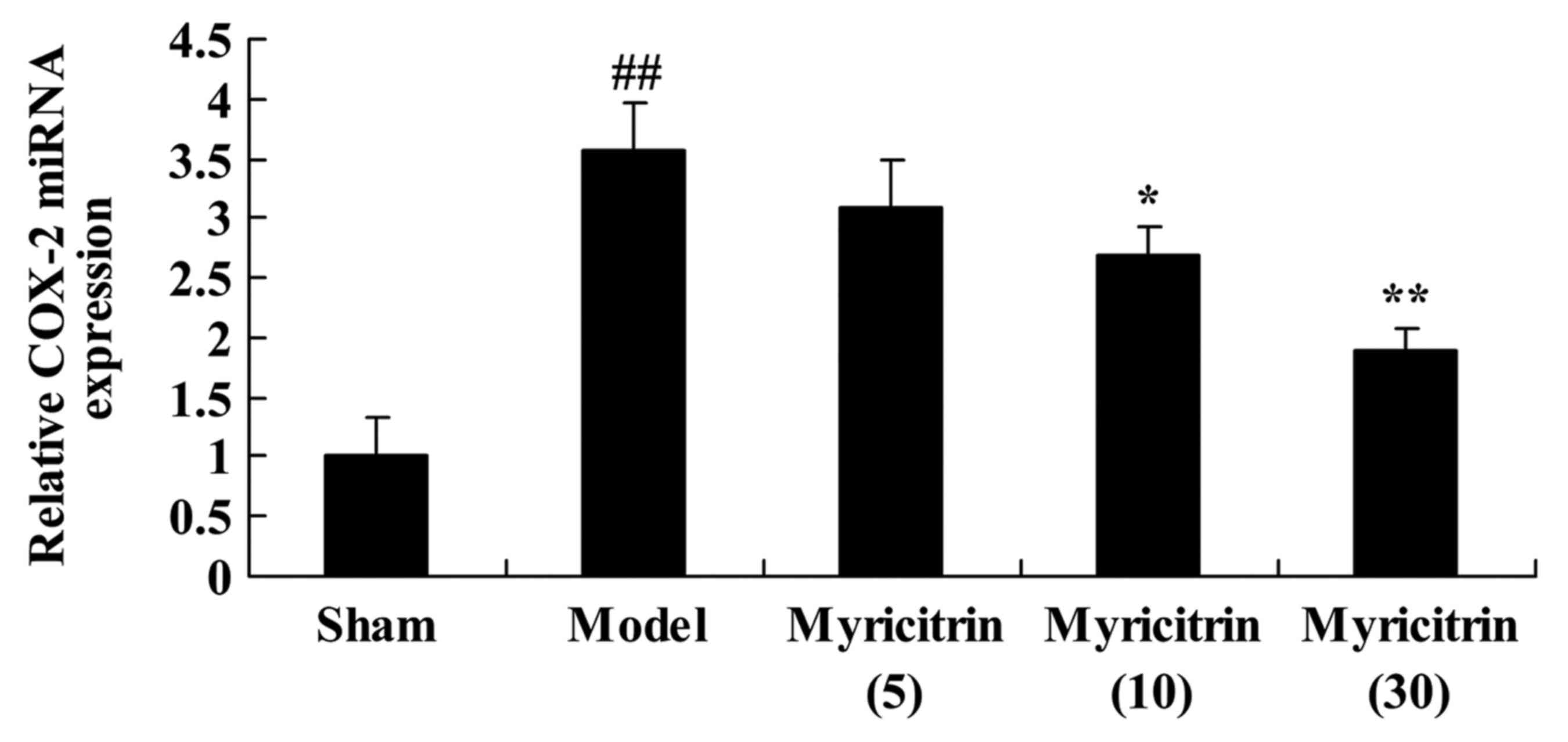
Myricitrin weakens COX-2 mRNA
expression in SCI rats
To further investigate the effect of myricitrin on
COX-2 in SCI rats, COX-2 mRNA expression was detected by Real-time
Quantitative PCR. The results revealed upregulation in COX-2 mRNA
expression of TISC model group, compared with sham group (Fig. 7). Pretreatment with 10 and 30 mg/kg
myricitrin significantly suppressed the COX-2 mRNA expression in
TISC rats (Fig. 7).
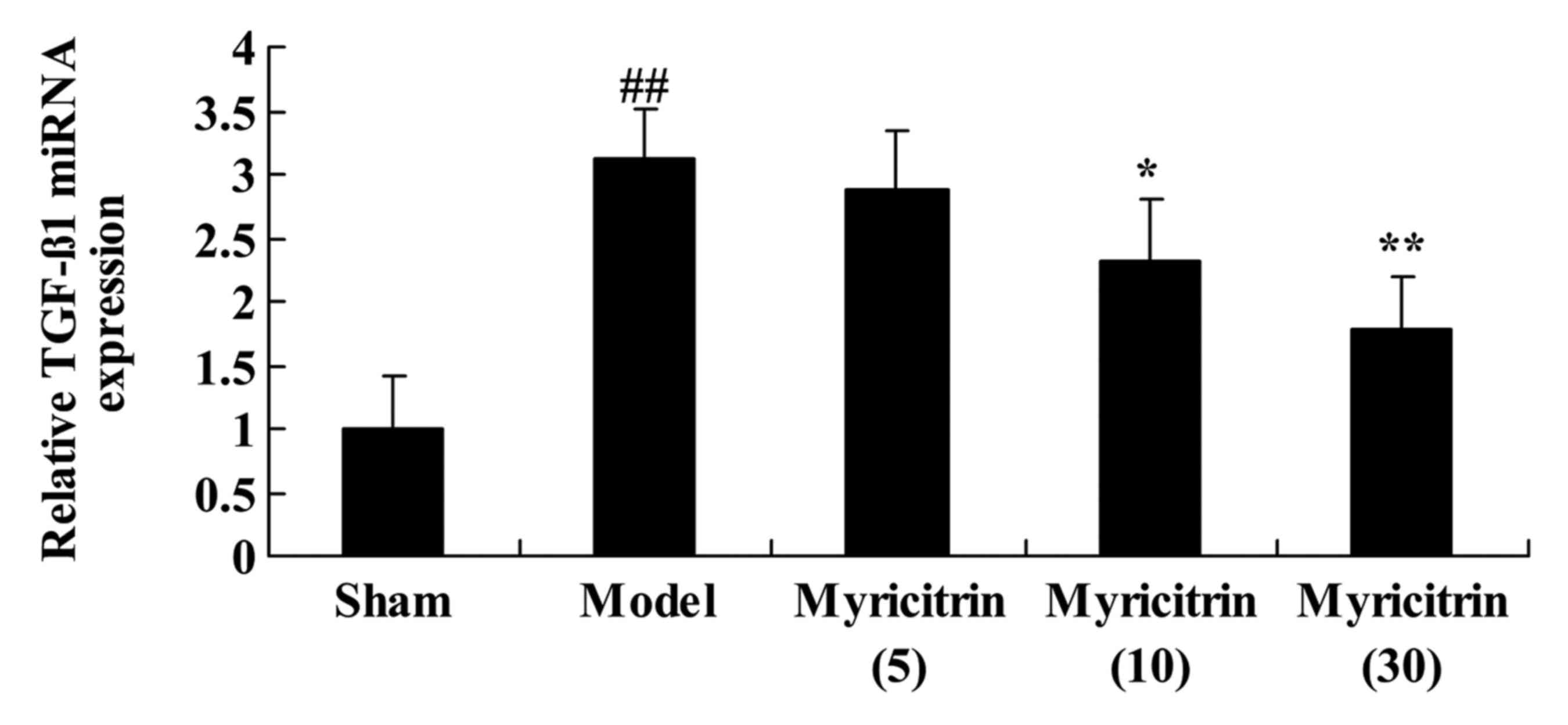
Myricitrin weakens TGF-β1 mRNA
expression in SCI rats
To examine the effect of myricitrin on TGF-β1 in SCI
rats, RT-qPCR was used to detect TGF-β1 mRNA expression. There was
a significant increase in TGF-β1 mRNA expression of the TISC model
group, compared with the sham group (Fig. 8). Following SCI and administration
of myricitrin, 10 and 30 mg/kg myricitrin significantly inhibited
TGF-β1 mRNA expression in TISC rats (Fig. 8).
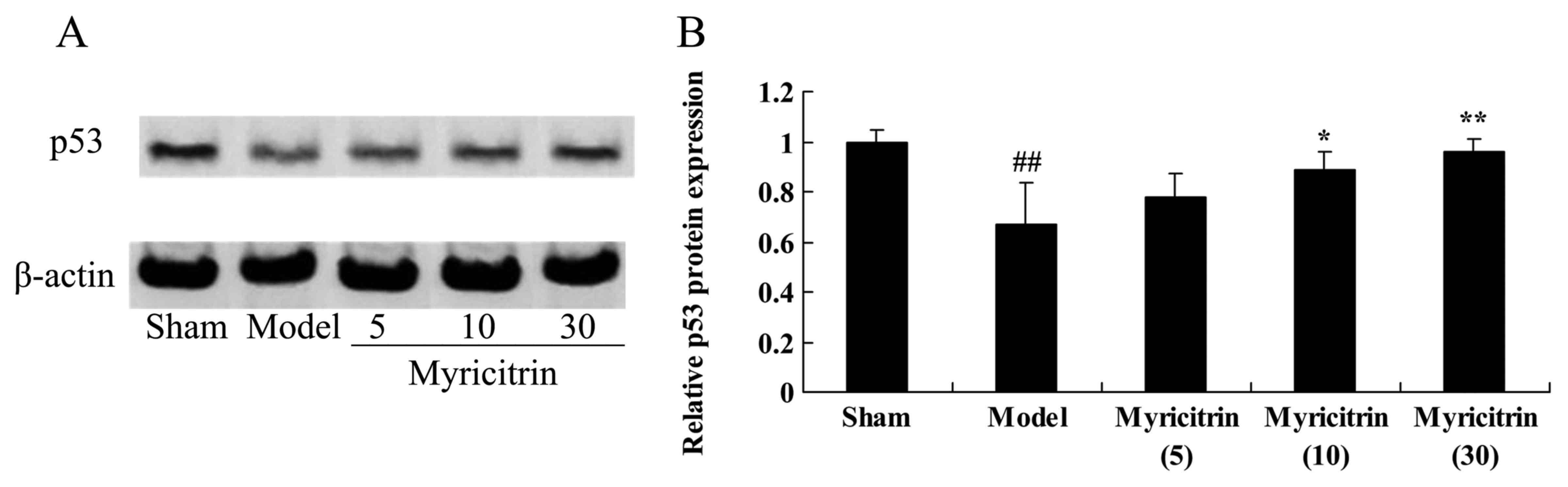
Myricitrin weakens p53 protein
expression in SCI rats
To determine the effect of myricitrin on the p53
signaling pathway in SCI rats, p53 protein expression was analyzed
using western blotting. The p53 protein expression of the TISC
model group was lower than that of the sham group (Fig. 9). Administration of 10 and 30 mg/kg
myricitrin significantly promoted the TISC-inducted inhibition of
p53 protein expression in SCI rats (Fig. 9).
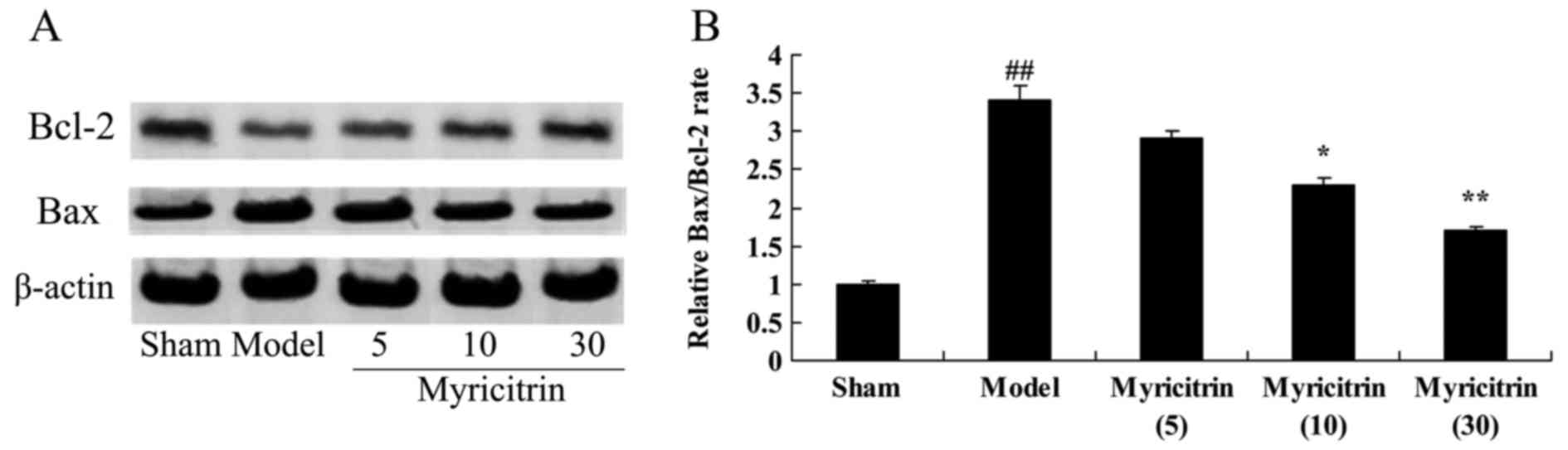
Myricitrin weakens Bcl-2/Bax rate in
SCI rats
Because Bcl-2/Bax rate mediating apoptosis, Bcl-2
and Bax protein expression was measured using western blotting. As
presented in Fig. 10, the
Bax/Bcl-2 rate of the TISC model group was higher than that of the
sham group. However, administration of 10 and 30 mg/kg myricitrin
significantly inhibited Bax/Bcl-2 rate in TISC rats (Fig. 10).
Discussion
According to pathological changes at different
phases, SCI can be divided into acute spinal cord injury and
chronic spinal cord injury (11).
At present, measures to treat SCI primarily include drug therapy,
operative treatment and functional reconstruction following SCI
(12). But the curative effects of
these therapeutic measures are not definite. Thus many patients are
still confronted with neurological dysfunction and permanent
disability. With advances of industry, agriculture and
transportation, spinal cord injury is becoming a common and
frequently occurring condition (13). Therefore, intervention and
treatment of SCI is urgent (14).
At present, 10 and 30 mg/kg myricitrin significantly increased BBB
score and suppressed the water content of spinal cord in TISC
rats.
Myricitrin treatment significantly reversed the
induction of MDA, SOD, CAT and GSH-PX contents and inhibited the
SCI-induced NF-κB p65 subunit, TNF-α, IL-1β and IL-6 contents in
TISC rats. Domitrović et al (10) suggested that myricitrin exhibited
antioxidant and anti-inflammatory actions in carbon
tetrachloride-intoxicated mice (10).
Excessive active radicals following SCI act on the
postsynaptic neurons and activate adjacent astrocytes and
microglial cells, resulting in ionic unbalance of nerve cells
(15). Oxidative stress following
SCI destabilizes the ionic homeostasis inside and outside the
membrane (16). Significant
amounts of Ca2+ enter the mitochondria and accumulate,
causing destruction of the mitochondria (17) and aerobic energy metabolism
disorders, inhibiting the synthesis of ATP (18). Excitatory toxicity triggered by
oxidative stress serves an essential role in secondary SCI; this
could extend the degeneration period of the substantia alba
medullae spinalis and accelerate the apoptosis of oligodendroglia
cells (14). It was previously
demonstrated that free radicals may increase the release of GSH,
while scavengers of free radicals have the opposite function. In
addition, oxidative stress following SCI gives rise to the
activation of gitter cells and astrocytes, and results in the
release of inflammatory cytokines and TNF-α (19). Previous studies demonstrated that
TNF-α can rapidly increase the receptor quantities of Glu in cell
membrane structures, and further enhance the sensitivity of motor
neurons to excitatory poisoning (18,20).
Following SCI, 10 and 30 mg/kg myricitrin treatment significantly
reversed the induction of MDA, SOD, CAT and GSH-PX contents and
inhibited the SCI-induced NF-κB p65, TNF-α, IL-1β and IL-6 contents
in TSCI rats. Furthermore, Domitrović et al suggested that
myricitrin may exhibit antioxidant and anti-inflammatory in carbon
tetrachloride-intoxicated mice (10).
A previous study suggested that cells in damage zone
following SCI undergo a series of changes in form, function and
metabolism (21). The causes of
these changes are that cell growth loses the internal environment
supported by nutrition. Injuries may induce a series of
immuno-inflammatory responses; a large amount of MCP-1, TNF-α, IL-6
and IL-1β released by monocyte/macrophage increases the contents of
excitatory amino acids in the damage area. In addition, the
decrease in expression of apoptosis inhibiting genes leads to cell
death and secondary injury of spinal cord occurs (21–23).
Overexpression of COX-2 is associated with the
increase of microvessel density (24). Moreover, COX-2 is closely related
with angiogenesis, which is induced by inflammatory cytokines
(25). As is well established,
bone marrow mesenchymal stem cells may secrete various inflammatory
cytokines and facilitate angiogenesis following injuries of the
central nervous system (26).
Research has discovered that obvious expression of TGF-β1 in
hematoma at damage zone occurs first (27). Then, TGF-β1 expresses in the
cytoplasm and the karyons of astrocytes and capillary endothelial
cells (inside and outside the marrow) and motor neurons would
increase (28). In the present
study, it was observed that 10 and 30 mg/kg myricitrin
significantly inhibited COX-2 and TGF-β1 mRNA expression in TISC
rats. Domitrović et al (10) suggest that myricitrin exhibits
antioxidant and anti-inflammatory effects in carbon
tetrachloride-intoxicated mice through COX-2 and TGF-β1.
The increase in p53 expression directly causes the
apoptosis or indirectly causes apoptosis by regulating other
apoptosis-related genes (29).
Transcriptional levels of p53 would rise and activate downstream
WAF/GiP1 genes to express p21, which can inhibit the activity of
cyclin dependent kinase (30).
Thus, cells can stagnant between G1 and S phases. If DNA injuries
cannot be repaired on time, apoptosis would happen (31). P53 genes decrease expression of
endogenous Bcl-2 and inhibit its functions (32). p53 can be regarded as direct
agonist of genetic transcription of Bas and increases protein
expression in Bax, it also changes the proportion of Bcl-2/Bax
proteins and facilitates apoptosis (33). In the present study, 10 and 30
mg/kg myricitrin administration significantly promoted the
TISC-inducted the inhibition of p53 protein expression and
inhibited the Bax/Bcl-2 rate in TISC rats. Sun et al
(34) suggested that the effects
of myricitrin suppressed oxidative stress damage through p53,
caspase-3, Bax/Bcl-2 and the MAPK signaling pathway in ApoE-/-
mice.
In conclusion, the current study has underlined that
myricitrin improves BBB score and suppresses the water content of
spinal cord in TISC rats through antioxidant and anti-inflammatory
effects, COX-2, TGF-β1, p53 and the Bax/Bcl-2 signaling pathway.
However, future studies are required to detail the mechanisms
underlying how myricitrin weakens TISC and the promotion of
functional recovery following SCI.
Acknowledgements
The current study was partly supported by the
Cangzhou Municipal Science and Technology Project (grant no.
131302113).
References
|
1
|
Liebscher T, Niedeggen A, Estel B and
Seidl RO: Airway complications in traumatic lower cervical spinal
cord injury: A retrospective study. J Spinal Cord Med. 38:607–614.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen-Adad J, Buchbinder B and Oaklander
AL: Cervical spinal cord injection of epidural corticosteroids:
Comprehensive longitudinal study including multiparametric magnetic
resonance imaging. Pain. 153:2292–2299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duetzmann S, Forsey LM, Senft C, Seifert
V, Ratliff J and Park J: Sacral peak pressure in healthy volunteers
and patients with spinal cord injury: With and without liquid-based
pad. Nurs Res. 64:300–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suwanna N, Thangnipon W, Kumar S and de
Vellis J: Neuroprotection by diarylpropionitrile in mice with
spinal cord injury. EXCLI J. 13:1097–1103. 2014.PubMed/NCBI
|
|
5
|
Uckermann O, Galli R, Beiermeister R,
Sitoci-Ficici KH, Later R, Leipnitz E, Neuwirth A, Chavakis T, Koch
E, Schackert G, et al: Endogenous two-photon excited fluorescence
provides label-free visualization of the inflammatory response in
the rodent spinal cord. Biomed Res Int. 2015:8590842015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Y, Fu C, Wang Z, Zhang Z, Wang H and
Liu Y: Mangiferin attenuates contusive spinal cord injury in rats
through the regulation of oxidative stress, inflammation and the
Bcl-2 and Bax pathway. Mol Med Rep. 12:7132–7138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kubota K, Saiwai H, Kumamaru H, Maeda T,
Ohkawa Y, Aratani Y, Nagano T, Iwamoto Y and Okada S:
Myeloperoxidase exacerbates secondary injury by generating highly
reactive oxygen species and mediating neutrophil recruitment in
experimental spinal cord injury. Spine (Phila Pa 1976).
37:1363–1369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glynn ER, Londono AS, Zinn SA, Hoagland TA
and Govoni KE: Culture conditions for equine bone marrow
mesenchymal stem cells and expression of key transcription factors
during their differentiation into osteoblasts. J Anim Sci
Biotechnol. 4:402013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin M, Luo Y, Meng XB, Wang M, Wang HW,
Song SY, Ye JX, Pan RL, Yao F, Wu P, et al: Myricitrin attenuates
endothelial cell apoptosis to prevent atherosclerosis: An insight
into PI3K/Akt activation and STAT3 signaling pathways. Vascul
Pharmacol. 70:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Domitrović R, Rashed K, Cvijanović O,
Vladimir-Knežević S, Škoda M and Višnić A: Myricitrin exhibits
antioxidant, anti-inflammatory and antifibrotic activity in carbon
tetrachloride-intoxicated mice. Chem Biol Interact. 230:21–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Due MR, Park J, Zheng L, Walls M, Allette
YM, White FA and Shi R: Acrolein involvement in sensory and
behavioral hypersensitivity following spinal cord injury in the
rat. J Neurochem. 128:776–786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dulin JN, Karoly ED, Wang Y, Strobel HW
and Grill RJ: Licofelone modulates neuroinflammation and attenuates
mechanical hypersensitivity in the chronic phase of spinal cord
injury. J Neurosci. 33:652–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang YG, Jiang DM, Quan ZX and Ou YS:
Insulin with chondroitinase ABC treats the rat model of acute
spinal cord injury. J Int Med Res. 37:1097–1107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conti A, Miscusi M, Cardali S, Germanò A,
Suzuki H, Cuzzocrea S and Tomasello F: Nitric oxide in the injured
spinal cord: Synthases cross-talk, oxidative stress and
inflammation. Brain Res Rev. 54:205–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown DA and Sawchenko PE: Time course and
distribution of inflammatory and neurodegenerative events suggest
structural bases for the pathogenesis of experimental autoimmune
encephalomyelitis. J Comp Neurol. 502:236–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu J, Fan HB, Guo Z, Wang Z, Li XD, Li J
and Pei GX: Salvianolic acid B attenuates spinal cord
ischemia-reperfusion-induced neuronal injury and oxidative stress
by activating the extracellular signal-regulated kinase pathway in
rats. J Surg Res. 188:222–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siddiq A, Aminova LR, Troy CM, Suh K,
Messer Z, Semenza GL and Ratan RR: Selective inhibition of
hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates
neuroprotection against normoxic oxidative death via HIF- and
CREB-independent pathways. J Neurosci. 29:8828–8838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kayali H, Ozdag MF, Kahraman S, Aydin A,
Gonul E, Sayal A, Odabasi Z and Timurkaynak E: The antioxidant
effect of beta-Glucan on oxidative stress status in experimental
spinal cord injury in rats. Neurosurg Rev. 28:298–302. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Shen H, Xie JJ, Ling J and Lu H:
Neuroprotective effect of ginseng against spinal cord injury
induced oxidative stress and inflammatory responses. Int J Clin Exp
Med. 8:3514–3521. 2015.PubMed/NCBI
|
|
20
|
Buczynski MW, Svensson CI, Dumlao DS,
Fitzsimmons BL, Shim JH, Scherbart TJ, Jacobsen FE, Hua XY, Yaksh
TL and Dennis EA: Inflammatory hyperalgesia induces essential
bioactive lipid production in the spinal cord. J Neurochem.
114:981–993. 2010.PubMed/NCBI
|
|
21
|
Bethea JR and Dietrich WD: Targeting the
host inflammatory response in traumatic spinal cord injury. Curr
Opin Neurol. 15:355–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu JH, Zheng XY, Yang JP, Wang LN and Ji
FH: Involvement of spinal monocyte chemoattractant protein-1
(MCP-1) in cancer-induced bone pain in rats. Neurosci Lett.
517:60–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bigford GE, Bracchi-Ricard VC, Keane RW,
Nash MS and Bethea JR: Neuroendocrine and cardiac metabolic
dysfunction and NLRP3 inflammasome activation in adipose tissue and
pancreas following chronic spinal cord injury in the mouse. ASN
Neuro. 5:243–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon DS, Yoo JH, Kim YH, Paik S, Han CD
and Lee JW: The effects of COX-2 inhibitor during osteogenic
differentiation of bone marrow-derived human mesenchymal stem
cells. Stem Cells Dev. 19:1523–1533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zani A, Cananzi M, Fascetti-Leon F,
Lauriti G, Smith VV, Bollini S, Ghionzoli M, D'Arrigo A, Pozzobon
M, Piccoli M, et al: Amniotic fluid stem cells improve survival and
enhance repair of damaged intestine in necrotising enterocolitis
via a COX-2 dependent mechanism. Gut. 63:300–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He T, Wang Y, Xiang J and Zhang H: In vivo
tracking of novel SPIO-Molday ION rhodamine-B™-labeled human bone
marrow-derived mesenchymal stem cells after lentivirus-mediated
COX-2 silencing: A preliminary study. Curr Gene Ther. 14:136–145.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oliveira SD, Nanini HF, Savio LE, Waghabi
MC, Silva CL and Coutinho-Silva R: Macrophage P2X7 receptor
function is reduced during schistosomiasis: Putative role of
TGF-β1. Mediators Inflamm. 2014:1349742014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiyang YB, Lu BT, Ya Z, Ya-Zhao,
Yuan-Zhang, Xia QJ, Zou Y, Zhang W, Quan XZ, Liu S, et al:
Expressional difference, distributions of TGF-b1 in TGF-b1 knock
down transgenic mouse and its possible roles in injured spinal
cord. Exp Biol Med (Maywood). 239:320–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HL, Ra H, Kim KR, Lee JM, Im H and Kim
YH: Poly(ADP-ribosyl)ation of p53 contributes to TPEN-induced
neuronal apoptosis. Mol Cells. 38:312–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Navrkalova V, Sebejova L, Zemanova J,
Jaskova Z and Trbusek M: The p53 pathway induction is not primarily
dependent on Ataxia Telangiectasia Mutated (ATM) gene activity
after fludarabine treatment in chronic lymphocytic leukemia cells.
Leuk Lymphoma. 54:1840–1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoo SH, Lim Y, Kim SJ, Yoo KD, Yoo HS,
Hong JT, Lee MY and Yun YP: Sulforaphane inhibits PDGF-induced
proliferation of rat aortic vascular smooth muscle cell by
up-regulation of p53 leading to G1/S cell cycle arrest. Vascul
Pharmacol. 59:44–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kotipatruni RR, Dasari VR, Veeravalli KK,
Dinh DH, Fassett D and Rao JS: p53- and Bax-mediated apoptosis in
injured rat spinal cord. Neurochem Res. 36:2063–2074. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee IN, Cheng WC, Chung CY, Lee MH, Lin
MH, Kuo CH, Weng HH and Yang JT: Dexamethasone reduces brain cell
apoptosis and inhibits inflammatory response in rats with
intracerebral hemorrhage. J Neurosci Res. 93:178–188. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun GB, Qin M, Ye JX, Pan RL, Meng XB,
Wang M, Luo Y, Li ZY, Wang HW and Sun XB: Inhibitory effects of
myricitrin on oxidative stress-induced endothelial damage and early
atherosclerosis in ApoE-/- mice. Toxicol Appl Pharmacol.
271:114–126. 2013. View Article : Google Scholar : PubMed/NCBI
|