Introduction
Adhesive Capsulitis, also known as Frozen Shoulder
(1), pericapsulitis and
periarthritis (2) is a common
disease of unclear cause and significant morbidity (3). It is characterized by pain and a
progressive loss of both active and passive range of motion. These
symptoms can last up to 2 years or longer (4,5).
Patients who underwent the arthroscopic capsular release procedure
experienced significant reductions in pain, improvements in range
of motion (6).
It has long been recognized that glucose and lipid
metabolism disorders have a close association with idiopathic
adhesive capsulitis. Although the incidence in the general
population is <2% (7,8), the incidence is about 10% in Type I
diabetics and up to 29% in Type II diabetics (9–11).
Sung et al (12)
demonstrated that hypercholesterolemia and inflammatory
lipoproteinemias, especially hyper-low-density lipoproteinemia and
hyper-non-high-density lipoprotein cholesterolemia, may contribute
to the development of primary idiopathic adhesive capsulitis. Won
et al (13) demonstrates
that the anterior-inferior capsular portion is the main pathologic
site of idiopathic adhesive capsulitis and reveals significant
correlations with metabolic parameters on 18F-FDG PET/CT.
The diagnostic criteria, which still holds true
today, was initially described by Codman (14) in 1934 based upon the recognition of
selective restriction of passive external rotation with pain. The
macroscopic and histological features of idiopathic adhesive
capsulitis indicate that it is mediated by an inflammatory process
(2,15), fibrotic process (16–21),
or inflammatory process with subsequent reactive capsular fibrosis
(22). However, the underlying
pathological processes and molecular pathogenesis remain poorly
understood (23).
Due to the lack of understanding of the causes of
this disease, current treatment involves mainly relieving symptoms.
We conducted a transcriptional analysis of samples from patients
with idiopathic adhesive capsulitis and compared them with control
healthy samples, in order to gain insight into the molecular
mechanisms that contribute to the pathogenesis of this disease.
Thus, to test this hypothesis and further understand this disease,
we conducted a transcriptional analysis.
Materials and methods
To acquire broader and deeper insights into the
mechanisms of idiopathic adhesive capsulitis development, we
performed RNA-seq on five idiopathic adhesive capsulitis samples
(part of shoulder capsule, subacromial bursa and synovial) and two
matched adjacent normal tissues (some part of the shoulder capsule,
subacromial bursa and synovial from the acromioclavicular
dislocation patients).
RNA-seq and quality analysis of raw
data
Each subject signed the informed consent form before
participating in our study. This study was approved by the Ethics
Committee of The First Affiliated Hospital of Shenzhen University
and was conducted in conformity with the guidelines outlined in the
Declaration of Helsinki statement. After obtaining the written
informed consent, tissue samples for genetic analysis were obtained
from the idiopathic adhesive capsulitis patients and control
subjects.
The RNA extraction method was followed by the
article (24), then the mRNA is
enriched using oligo (dT) magnetic beads after incubating total
issue samples with DNase I. Then the mRNA was fragmented into short
fragments which were then used to synthesize the cDNA by using
random hexamer-primer, Buffer, dNTPs, RNase H, and DNA polymerase
I. Following cDNA purification, end repair, 3′-end single
nucleotide A (adenine) addition, and sequencing adaptors ligation,
we performed PCR amplification. RNA sequencing was performed via
Illumina HiSeq™ 2000 after the QC step by using Agilent 2100
Bioanalyzer and ABI Step One Plus Real-Time PCR System. Primary
sequencing data produced by Illumina HiSeq™ 2000 were subjected to
quality control (QC) methods. Before data analysis, we removed the
dirty raw reads, which contain adapter sequences, high content of
unknown bases, and low quality reads.
Calculation of expression values and
identification of differentially expressed genes (DEGs)
First, we used Burrows-Wheeler Aligner (BWA)
(25) and Bowtie software (v2.3.0)
(26) to map clean reads to genome
reference. Secondly, we used RSEM (27) to quantify gene expression level
followed by FPKM (28) method to
calculate expression level. We calculated FPKM value for
normalization. The Hg19 version of the human genome reference was
used in the present study.
Thirdly, we used Noiseq package method (29) to find differentially expressed
genes using the following criteria: Fold change ≥2 and diverge
probability ≥0.8. Noiseq is available at http://bioinfo.cipf.es/noiseq or Bioconductor.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis
Gene Ontology analysis was performed with QuickGO,
which is a web-based tool for Gene Ontology annotations. Moreover,
we performed the Biological Process (BP), Cell Component (CC) and
Molecular Function (MF) enrichment analysis.
QuickGO (30) was
used to conduct GO functional annotation and enrichment analysis of
DEGs. The enrichment analysis by the hypergeometric test was done
to test whether a GO term is statistically enriched for the given
set of genes. KEGG (31), the
major public pathway-related database, was used to perform pathway
enrichment analysis of DEGs.
Protein-protein interaction (PPI)
analysis
STRING (Search Tool for the Retrieval of Interacting
Genes/Proteins) (32) was used to
predict protein-protein interactions.
Cytoscape analysis
Cytoscape is a software for visualizing complex
networks and integrating these with any type of attribute data.
STRING (Search Tool for the Retrieval of Interacting
Genes/Proteins) provides protein-protein interactions data and
cytoscape visualization.
Results
Identification of DEGs
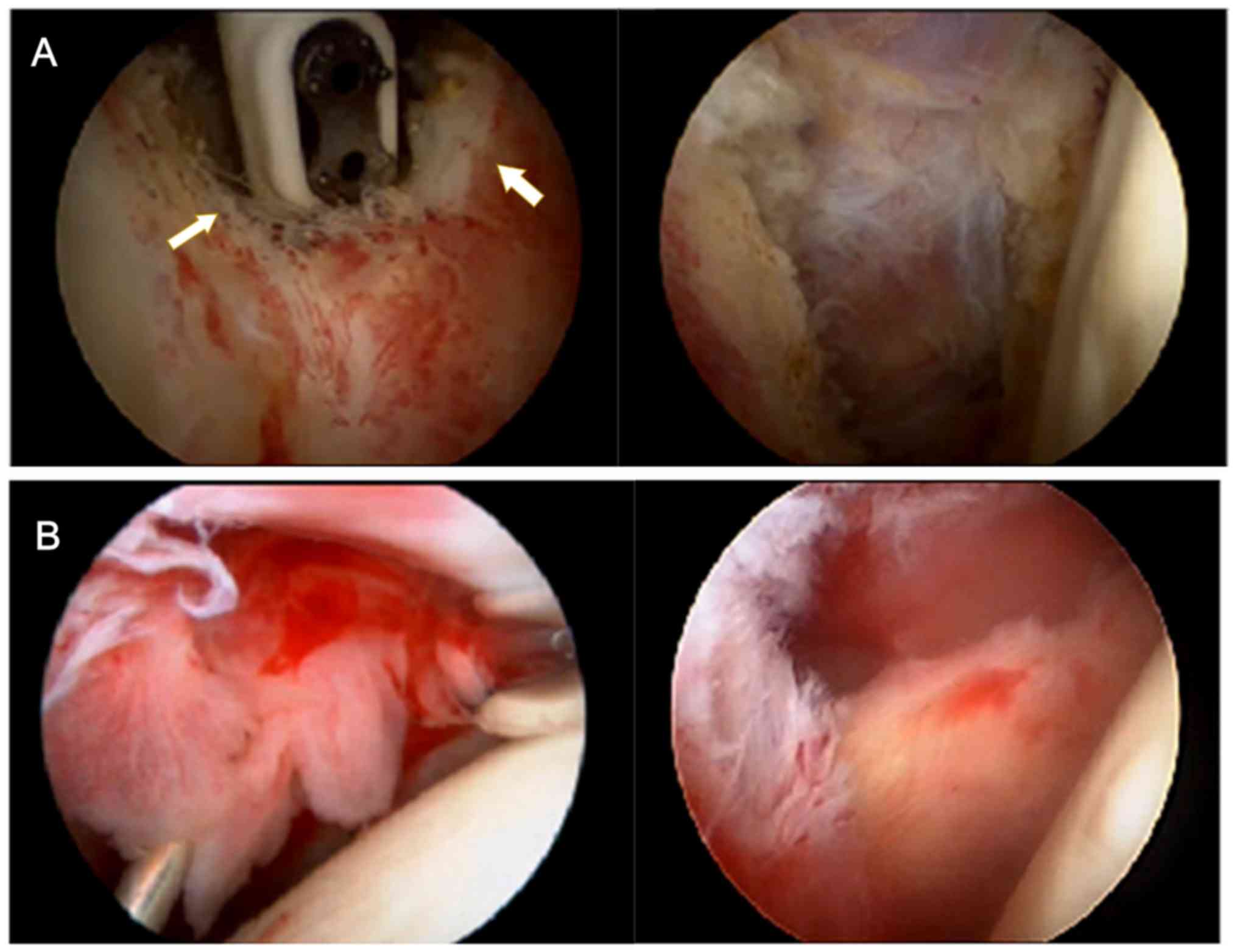
Our previous clinical work found that patients with
idiopathic adhesive capsulitis have a huge number of thick fibrous
adhesive bands generated by the proliferation of fibrous connective
tissue formed in the shoulder joint capsule (Fig. 1A left, the yellow arrows show the
adhesive band being ablated by radio frequency). After arthroscopic
release and debridement of these adhesive bands (Fig. 1A right), symptoms of limited
mobility and shoulder pain has significantly improved. Our previous
clinical work found that patients with idiopathic adhesive
capsulitis show increased areas of bright red synovium. The
synovium, which is rich in blood vessels, is more fragile and
bleeds easier when touched than normal synovium (Fig. 1B left). After arthroscopic
debridement of the synovium with blood vessels (Fig. 1B right), the symptoms of shoulder
pain are significantly relieved.
We generated an average of 12,899,579 clean reads,
and 88.17% of the reads mapped to the human genome (Table I). After a series of analyses (see
Materials and methods), 188 genes were identified as differentially
expressed gene (DEG) compared to control sample according to our
criteria (P>0.7, abs (log2 (case/control))>=1) (Table II).
 | Table I.Summary of the RNA-seq results. |
Table I.
Summary of the RNA-seq results.
| Sample name | Clean reads | Genome map
rate | Gene map rate | Expressed gene |
|---|
| Control | 12,833,093 | 0.882 | 0.8093 | 17,567 |
| FS2_JAS | 12,748,683 | 0.8865 | 0.7744 | 17,605 |
| FS2D_JAS | 13,116,962 | 0.8842 | 0.737 | 17,831 |
| F2D | 12,073,217 | 0.8864 | 0.808 | 17,826 |
| F3C | 12,153,733 | 0.8691 | 0.8389 | 17,651 |
| F4 | 12,122,251 | 0.8921 | 0.7875 | 17,468 |
| N2Y | 12,148,799 | 0.8712 | 0.8348 | 17,679 |
 | Table II.Top 20 DEGs (exclude microRNA and
small nucleolar RNA). |
Table II.
Top 20 DEGs (exclude microRNA and
small nucleolar RNA).
| Gene ID | Means of control
groupa | Means of case
groupb | log2Ratio
(case/control group) |
Up-/down-regulation | Probability | Symbol |
|---|
| 58 | 67.798 | 1.045 | −6.01966787 | Down | 0.95680825 | ACTA1 |
| 1158 | 40.484 | 0.23 | −7.45957417 | Down | 0.95341977 | CKM |
| 4632 | 26.866 | 0.225 | −6.89971273 | Down | 0.93373733 | MYL1 |
| 3039 | 1,486.478 | 157.21 | −3.24113322 | Down | 0.91165282 | HBA1 |
| 4151 | 30.018 | 1.085 | −4.79006091 | Down | 0.91126009 | MB |
| 7138 | 18.974 | 0.2 | −6.56788004 | Down | 0.91012755 | TNNT1 |
| 3040 | 375.082 | 41.745 | −3.16753072 | Down | 0.90738297 | HBA2 |
| 3043 | 885.424 | 107.95 | −3.03607206 | Down | 0.90423652 | HBB |
| 5346 | 52.46 | 4.19 | −3.64619566 | Down | 0.90008996 | PLIN1 |
| 7125 | 27.208 | 1.275 | −4.41546176 | Down | 0.89780662 | TNNC2 |
| 6029 | 45.314 | 352.66 | 2.960228742 | Up | 0.89624024 | RN7SL1 |
| 6288 | 13.144 | 0.01 | −10.3601887 | Down | 0.89516251 | SAA1 |
| 125 | 36.534 | 2.465 | −3.88958017 | Down | 0.89502094 | ADH1B |
| 4604 | 14.354 | 0.125 | −6.84338092 | Down | 0.88850427 | MYBPC1 |
| 4322 | 44.41 | 4.12 | −3.43016833 | Down | 0.8870064 | MMP13 |
| 8557 | 23.91 | 1.22 | −4.29266108 | Down | 0.88602456 | TCAP |
| 2354 | 212.662 | 30.965 | −2.77985191 | Down | 0.88517059 | FOSB |
| 4620 | 11.978 | 0.02 | −9.22617132 | Down | 0.88275938 | MYH2 |
| 729359 | 38.198 | 4.09 | −3.22332435 | Down | 0.87474484 | PLIN4 |
| 4633 | 17.344 | 0.695 | −4.64127987 | Down | 0.87408267 | MYL2 |
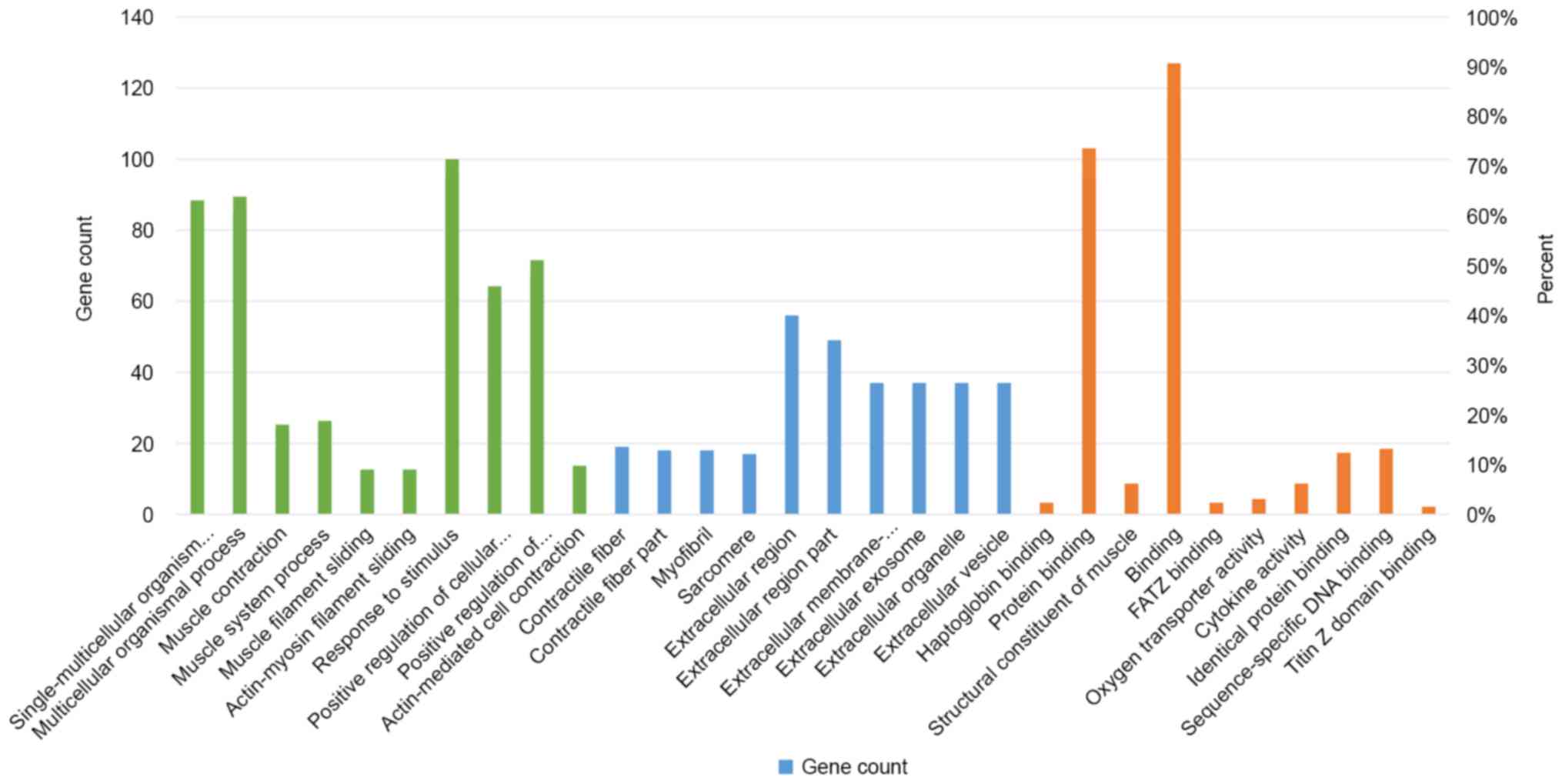
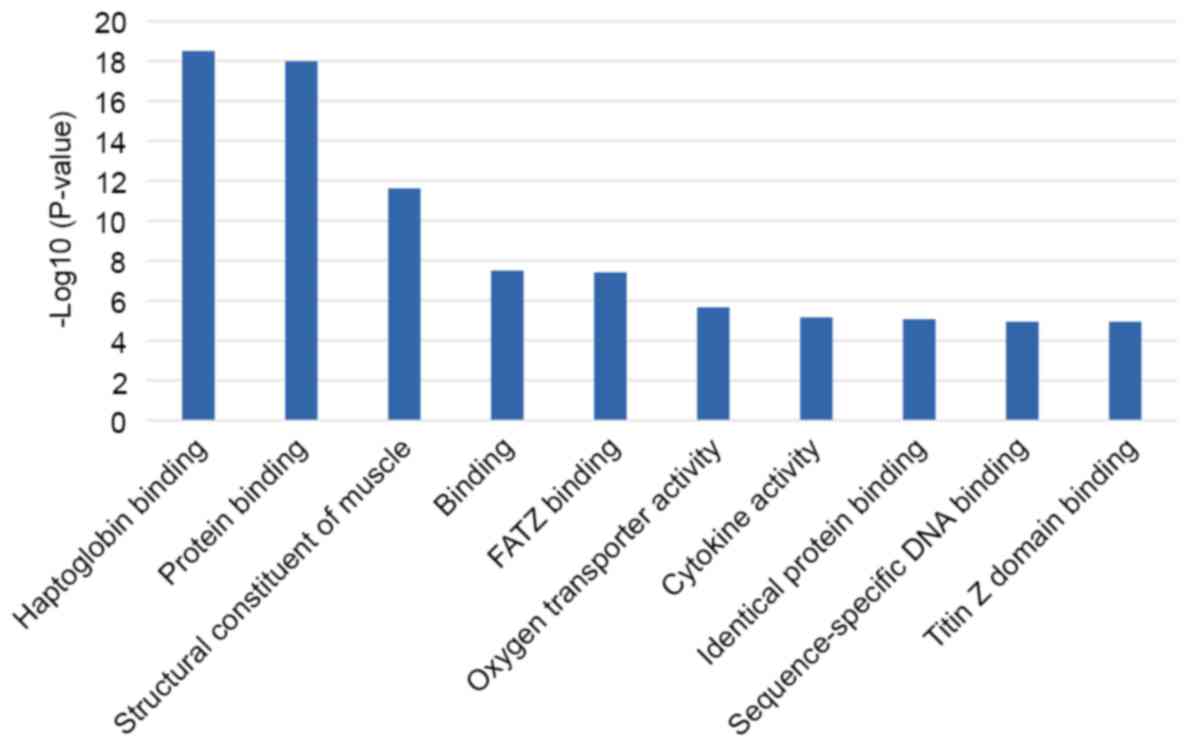
Gene ontology analysis
We analyzed gene ontology using up to 10
significantly enriched terms in BP, CC, and MF categories,
respectively. The cut-off of P-value was set to 0.05, and terms
under the same category were ordered by P-values. The terms on the
left side are more significant. Information on the percentage and
number of involved genes/proteins in a term are shown on the left
and right y-axis (Fig. 2). There
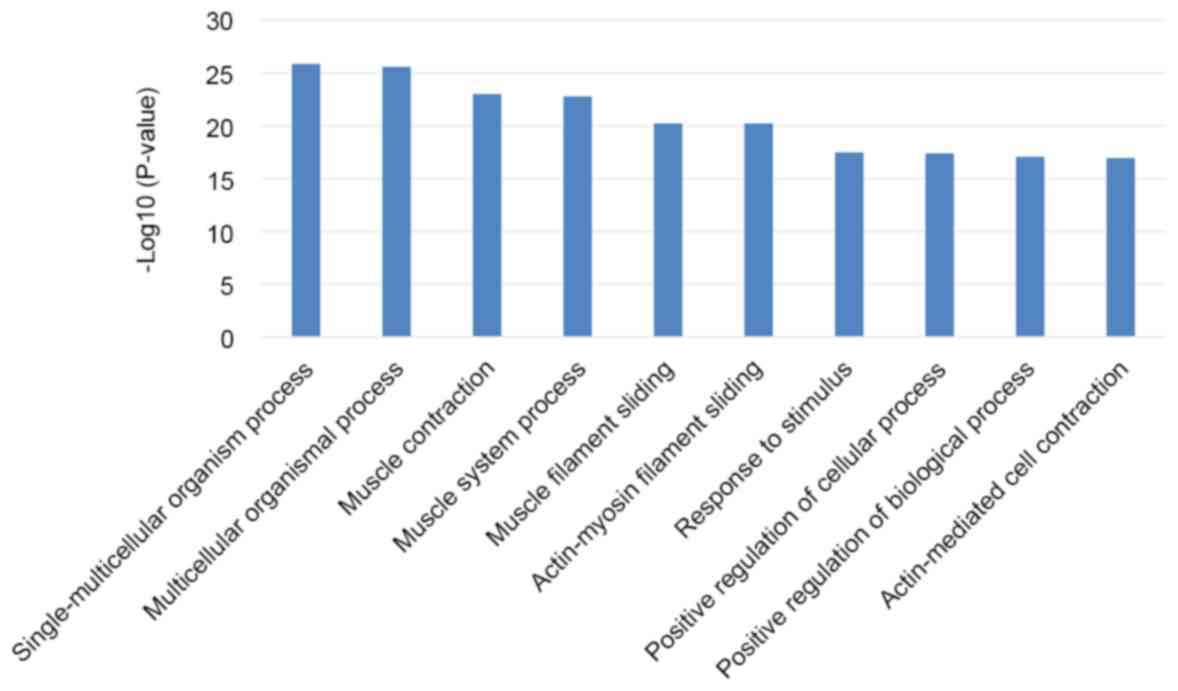
are 1,802 biological processes (BPs) that are statistically
significant among the whole enriched dataset of 3,309 BPs (Fig. 3). There are 318 cell components
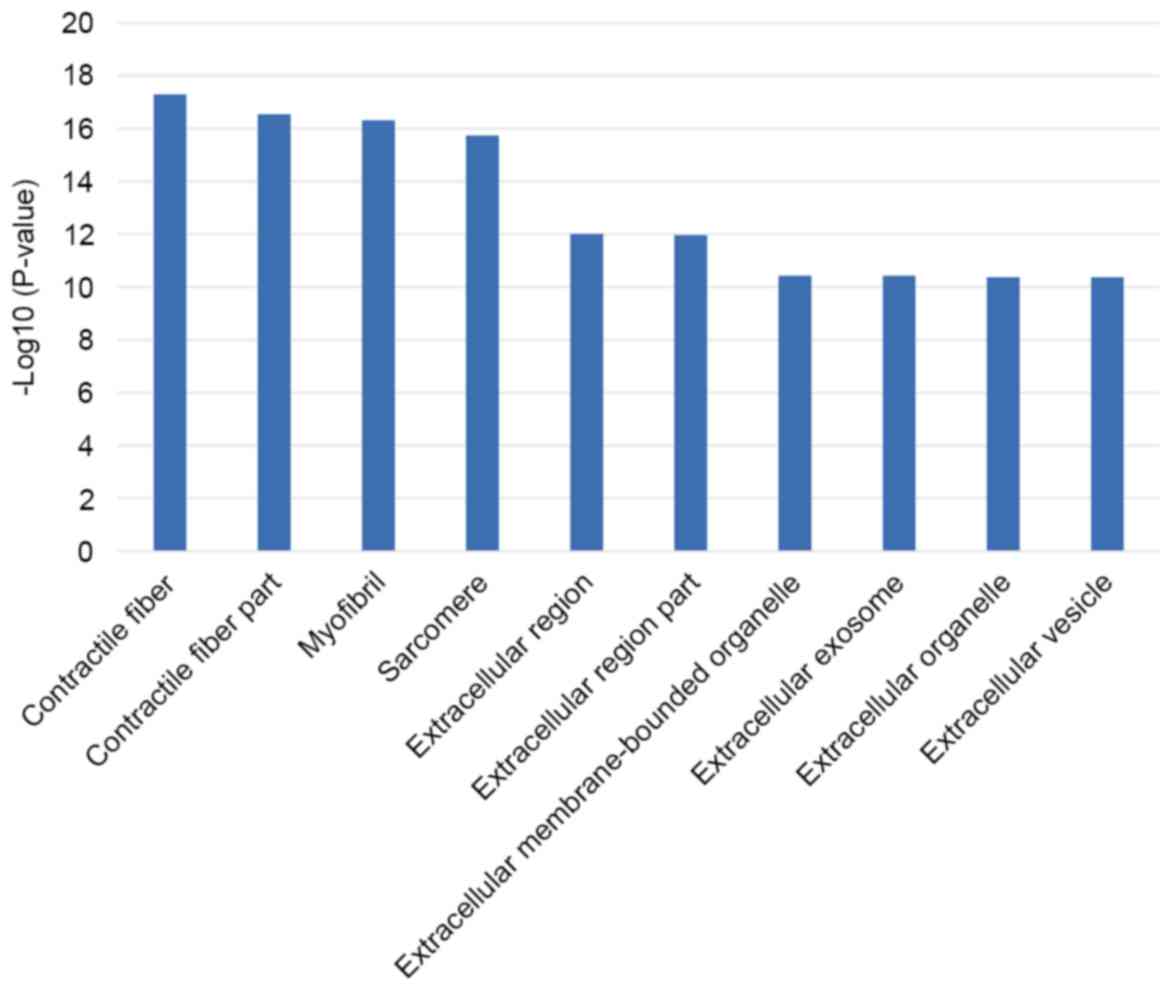
(CCs) enriched for this dataset and 120 of those are statistically
significant (Fig. 4). There are
443 molecular functions (MFs) were enriched for this dataset and
among that, 178 are statistically significant (Fig. 5).
Pathway enrichment analysis of
DEGs
The 188 differentially expressed genes were found to
be involved in 143 KEGG terms, such as PPAR signaling pathway,
rheumatoid arthritis, osteoclast differentiation, regulation of
lipolysis in adipocytes, p53 signaling pathway, and so on (Table III).
 | Table III.Top10 significantly pathway
enrichment. |
Table III.
Top10 significantly pathway
enrichment.
| Pathway ID | Pathway name | P-value | Genes count |
|---|
| hsa03320 | PPAR signaling
pathway | 5.27E-08 | 9 |
| hsa05219 | Bladder cancer | 6.59E-05 | 5 |
| hsa05144 | Malaria | 0.000157 | 5 |
| hsa05323 | Rheumatoid
arthritis | 0.000384 | 6 |
| hsa05143 | African
trypanosomiasis | 0.00048 | 4 |
| hsa04380 | Osteoclast
differentiation | 0.00268 | 6 |
| hsa04923 | Regulation of
lipolysis in adipocytes | 0.00285 | 4 |
| hsa04710 | Circadian
rhythm | 0.00419 | 3 |
| hsa04145 | Phagosome | 0.00589 | 6 |
| hsa04115 | p53 signaling
pathway | 0.00604 | 4 |
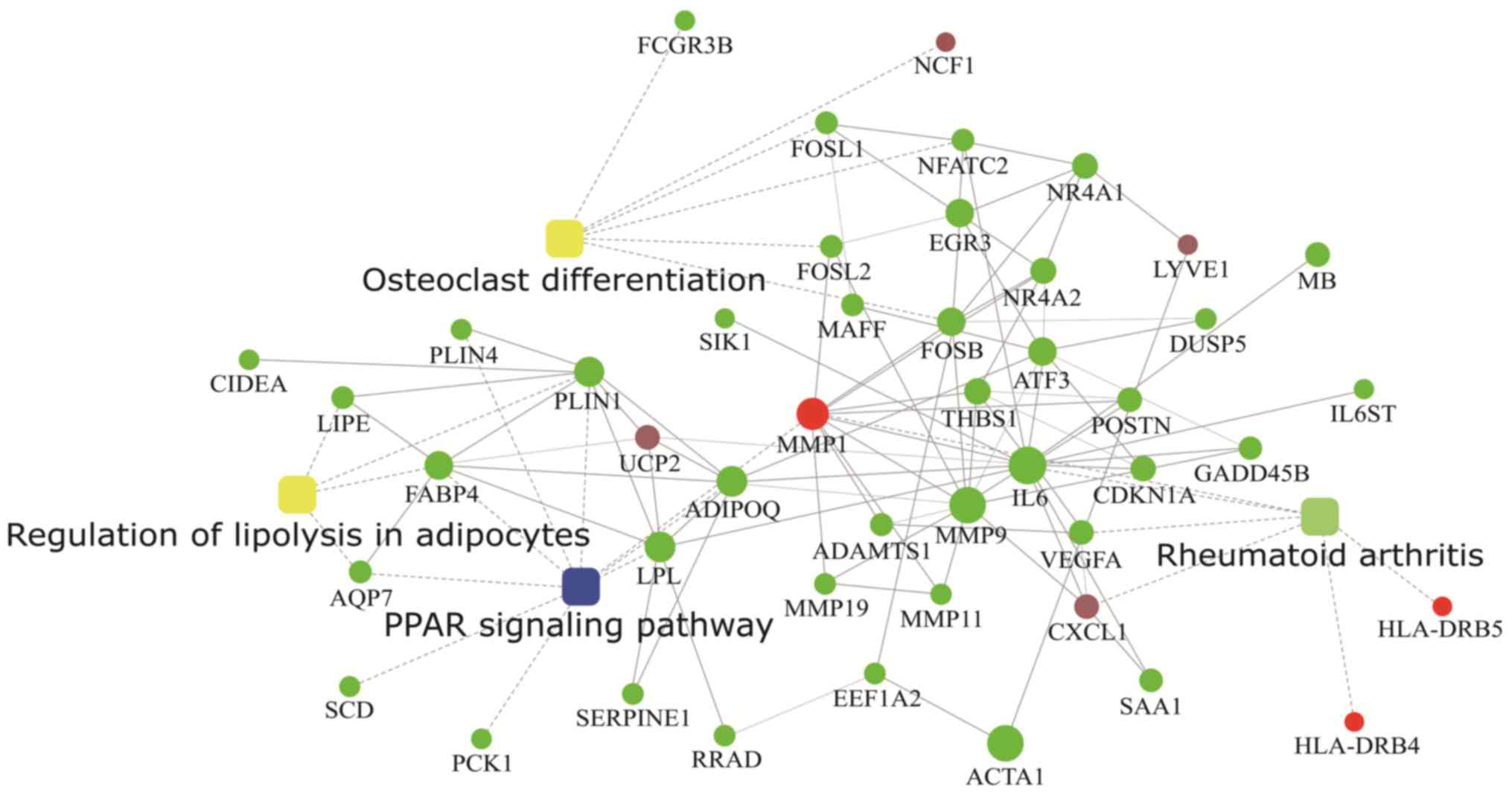
Protein-protein interaction (PPI)
analysis
Protein-protein interaction analysis by STRING
database and cytoscape web application provided 4 levels of
functional analysis: Fold change of gene/protein, protein-protein
interaction, KEGG pathway enrichment, and biological process
enrichment (Fig. 6).
Discussion
Idiopathic adhesive capsulitis is a common disease
of unclear cause and significant morbidity, which can last up to 2
years and longer. The diagnosis is still based upon the recognition
of the characteristic features initially described by Codman
(14) in 1934. The macroscopic and
histological features of the idiopathic adhesive capsulitis have
been described, but the underlying pathological processes and
molecular pathogenesis remain poorly understood. Therefore, the
identification and functional analysis of specific expression genes
involved in idiopathic adhesive capsulitis are necessary to
elucidate the disease molecular pathogenesis and the strategies of
precision medicine in idiopathic adhesive capsulitis.
The use of RNA-seq to assess the level of gene
expression of idiopathic adhesive capsulitis is novel in the field
of idiopathic adhesive capsulitis research. Cohen et al
(33) observed that the
synovium/capsule samples from the patients with adhesive capsulitis
had significantly higher TNC and FN1 expression than those from the
controls. They targeted the following proteins; TGFβ1, TGFβR1, LOX,
PLOD1, PLOD2, COMP, FN1, TNC, TNXB, B2 M and HPRT1 (34). We did not find these genes elevated
or changed in our RNA-seq study.
In the present study, we identified a total of 188
genes to be differentially expressed. These code for proteins of
the matrix metalloproteinase (MMP) family (MMP-9, MMP19, ADAMTS),
serum amyloid A1 (SAA1), glutathione S-transferase θ 1 (GSTT1),
myosin heavy chain family (MYH1, MYH2), amphiregulin (AREG), major
histocompatibility complex (HLA-DRB4), interleukin 6 (IL6), and
CD248 molecules.
Matrix turnover is a dynamic equilibrium between
synthesis and degradation and controlled by Matrix
metalloproteinases and other related proteins. Disruption of this
equilibrium may lead to fibrosis (35). The using of MMP inhibitors in
clinical trials, reported to be associated with idiopathic adhesive
capsulitis, rapidly resolved after cessation of therapy (36). Johnston et al (37) found that the level of MMP19 in the
Dupuytren's nodule is increased compared to cord, while the level
of ADAMTS is decreased. The dynamic equilibrium turnover of
extracellular matrix can be catalyzed by matrix metalloproteinases
and other related enzymes at neutral pH (38). A previous study showed that the
levels of MMP-8 and −9 in the systemic circulation are
representative of the levels of these enzymes in the inflamed
joint, and suggested that MMP-9 and MMP-1 may be involved in
degradation of the joint collagen (39).
The IL6 gene encodes a cytokine that acts as
a mediator in inflammatory and immune responses. The SAA1 gene
encodes a member of the serum amyloid family of apolipoproteins.
These differentially expressed genes are reported to be associated
with chronic inflammatory diseases such as atherosclerosis and
rheumatoid arthritis, which suggest that idiopathic adhesive
capsulitis is an inflammatory condition. These findings are similar
to those of Kabbabe et al (40) and Asleh et al (41).
The GO enrichment analysis revealed that DEGs are
enriched for a total of 3309 BP terms, 318 CC terms, and 443 MF
terms. Among these, 1802 BPs, 120 CCs, and 178 MFs are
statistically significant. The top 10 BPs mainly referred to
various processes including those related to to the muscular system
and actin-mediated cell contraction. The top 10 CCs are mostly
located in the extracellular region. The top 10 MFs mainly are
related to protein binding, cytokine activity, and oxygen
transporter activity.
The KEGG signaling pathway analysis showed that the
DEGs are possibly involved in 179 pathways including pathways
related PPAR signaling, regulation of lipolysis in adipocytes,
circadian rhythm, Phagosomes, p53 signaling, malaria, bladder
cancer, rheumatoid arthritis, African trypanosomiasis, and
osteoclast differentiation.
PPAR signaling pathway plays an important role not
only in the regulation of lipid and carbohydrate metabolism but
also in many signaling pathways (immunity, inflammation, apoptosis
and cell differentiation) (42,43).
Many studies have found that PPAR signaling pathway is involved in
many diseases related to prolonged nutrient excess such as type II
diabetes, hyperlipoproteinemia, and hyperalphalipoproteinemia
(44). Growing evidence suggests
that idiopathic adhesive capsulitis is associated with glucose and
lipid metabolic diseases, but not much is known beyond this
correlation. Our study found that PPAR signaling pathway and fatty
acid degradation might play a significant role in the pathogenesis
of idiopathic adhesive capsulitis.
Osteoclasts are responsible for bone resorption.
NFATC2, FOSB, FOSL2, FOSL1, and
FCGR3B are expressed at higher levels in disease samples
than in control samples. This suggests that bone resorption is
enhanced in idiopathic adhesive capsulitis. This is consistent with
some studies (45–47) looking at bone mineral density of
the shoulder joint in idiopathic adhesive capsulitis. Waldburger
et al (48) obtained
satisfactory results after treatment with calcitonin to increase
bone mass.
We also performed protein-protein interaction
analysis with PPAR signaling pathway, cytokine-cytokine receptor
interaction, rheumatoid arthritis and osteoclast differentiation
(Fig. 5) and found that MMP-9
seems to be a node that directly or indirectly connects these
pathways. The down regulation of MMP-9, which is a protein involved
in the degradation of extracellular matrix collagen, leads to
degeneration of collagen accumulation, which can facilitate
development of idiopathic adhesive capsulitis. Further
investigations are necessary to validate the molecular mechanism
(s) underlying the development of idiopathic adhesive capsulitis
and its link to glucose or lipid metabolism disorders.
Meanwhile, in the present study, the differentially
expressed genes were found to be involved in the regulation of
lipolysis in adipocytes. Currently, some articles reported that
idiopathic adhesive capsulitis is significantly correlated with
diabetes mellitus (49) and
hyperlipidaemia (50). A
nationwide population-based cohort study (49) found that hyperlipidemia is an
independent risk factor for idiopathic adhesive capsulitis. In
addition, hyperlipidemia can have cumulative detrimental effects to
tendon properties. For example, some studies have revealed that the
risk of rotator cuff disease is increased in patients with
hypercholesterolemia (51) and can
eventually lead to secondary idiopathic adhesive capsulitis
(52). Moreover, patients taking
hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors
have an increased risk of shoulder stiffness (53) that may predispose these patients to
idiopathic adhesive capsulitis. Proteins such as adiponectin,
leptin, resistin, and adipokines, which are normally involved in
metabolism, have recently been implicated in the development of
idiopathic adhesive capsulitis, as reviewed by Gómez et al
(54) and Schäffler et al
(55). Leptin has been shown to
have proinflammatory and catabolic roles in OA (54,56,57).
Thus, idiopathic adhesive capsulitis may correlate with the
metabolism in adipose tissue. Adiponectin in human synovial
fibroblasts appear to act as a mediator of arthritis
pathophysiology. Based on these observations, we presume that
proliferation of synovium and fibrosis of shoulder capsule is
because of the metabolic abnormalities in lipids.
In conclusion, this is a novel study investigating
the transcriptome of idiopathic adhesive capsulitis. The data have
provided important insights into the transcriptional regulation of
gene expression. We found 24 genes to be downregulated and 147
genes to be up-regulated in disease tissues vs. controls, and this
finding may be used to identify therapeutic targets. However, it is
still necessary to validate the DEGs identified in this study in
large patient populations and elucidate their specific functions in
the pathogenesis of idiopathic adhesive capsulitis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81672234); the Guangdong
Provincial Science and Technology Department (grant no.
2015A020212001) and the Shenzhen Science Technology Innovation
Council (grant nos. GCZX2015043017241191 and
JCYJ20160226192924528).
Glossary
Abbreviations
Abbreviations:
|
DEGs
|
differentially expressed genes
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PPI
|
protein-protein interaction
|
|
BP
|
biological process
|
References
|
1
|
Dias R, Cutts S and Massoud S: Frozen
shoulder. BMJ. 331:14532005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neviaser AS and Neviaser RJ: Adhesive
capsulitis of the shoulder. J Am Acad Orthop Surg. 19:536–542.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uppal HS, Evans JP and Smith C: Frozen
shoulder: A systematic review of therapeutic options. World J
Orthop. 6:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jayson MI: Frozen shoulder: Adhesive
capsulitis. Br Med J (Clin Res Ed). 283:1005–1006. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tasto JP and Elias DW: Adhesive
capsulitis. Sports Med Arthrosc. 15:216–221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnes CP, Lam PH and Murrell GA:
Short-term outcomes after arthroscopic capsular release for
adhesive capsulitis. J Shoulder Elbow Surg. 25:e256–e264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hand C, Clipsham K, Rees JL and Carr AJ:
Long-term outcome of frozen shoulder. J Shoulder Elbow Surg.
17:231–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bunker TD: Time for a new name for ‘frozen
shoulder’. Br Med J (Clin Res Ed). 290:1233–1234. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomas SJ, McDougall C, Brown ID, Jaberoo
MC, Stearns A, Ashraf R, Fisher M and Kelly IG: Prevalence of
symptoms and signs of shoulder problems in people with diabetes
mellitus. J Shoulder Elbow Surg. 16:748–751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arkkila PE, Kantola IM, Viikari JS and
Rönnemaa T: Shoulder capsulitis in type I and II diabetic patients:
Association with diabetic complications and related diseases. Ann
Rheum Dis. 55:907–914. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balci N, Balci MK and Tüzüner S: Shoulder
adhesive capsulitis and shoulder range of motion in type II
diabetes mellitus: Association with diabetic complications. J
Diabetes Complications. 13:135–140. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sung CM, Jung TS and Park HB: Are serum
lipids involved in primary frozen shoulder? A case-control study. J
Bone Joint Surg Am. 96:1828–1833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Won KS, Kim DH, Sung DH, Song BI, Kim HW,
Song KS, Lee SW and Cho CH: Clinical correlation of metabolic
parameters on 18F-FDG PET/CT in idiopathic frozen shoulder. Ann
Nucl Med. 31:211–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Codman EA: The shoulder rupture of the
supraspinatus tendon and other lesions in or about the subacromial
bursa. 2007.
|
|
15
|
Simmonds FA: Shoulder pain with particular
reference to the frozen shoulder. J Bone Joint Surg. 31B:1–432.
1949.
|
|
16
|
Hannafin JA and Chiaia TA: Adhesive
capsulitis. A treatment approach. Clin Orthop Relat Res. 1–109.
2000.
|
|
17
|
Ozaki J, Nakagawa Y, Sakurai G and Tamai
S: Recalcitrant chronic adhesive capsulitis of the shoulder. Role
of contracture of the coracohumeral ligament and rotator interval
in pathogenesis and treatment. J Bone Joint Surg Am. 71:1511–1515.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lundberg BJ: The frozen shoulder. Clinical
and radiographical observations. The effect of manipulation under
general anesthesia structure and glycosaminoglycan content of the
joint capsule. Local Bone Metabolism. Acta Orthop Scand. Suppl
119:S1–S59. 1969. View Article : Google Scholar
|
|
19
|
Bunker TD and Anthony PP: The pathology of
frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br.
77:677–683. 1995.PubMed/NCBI
|
|
20
|
Depalma AF: Loss of scapulohumeral motion
(frozen shoulder). Ann Surg. 135:193–204. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kay NR and Slater DN: Fibromatoses and
diabetes mellitus. Lancet. 2:3031981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bunker TD, Reilly J, Baird KS and Hamblen
DL: Expression of growth factors, cytokines and matrix
metalloproteinases in frozen shoulder. J Bone Joint Surg Br.
82:768–773. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson CM, Seah KTM, Chee YH, Hindle P
and Murray IR: Frozen shoulder. Bone Joint J. 94:1–9. 2012.
View Article : Google Scholar
|
|
24
|
Peirson SN and Butler JN: RNA extraction
from mammalian tissues. Methods Mol Biol. 362:315–327. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li B and Dewey CN: RSEM: Accurate
transcript quantification from RNA-Seq data with or without a
reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tarazona S, García-Alcalde F, Dopazo J,
Ferrer A and Conesa A: Differential expression in RNA-seq: A matter
of depth. Genome Res. 21:2213–2223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huntley RP, Binns D, Dimmer E, Barrell D,
O'Donovan C and Apweiler R: QuickGO: A user tutorial for the
web-based Gene Ontology browser. Database (Oxford).
2009:bap0102009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanehisa M, Araki M, Goto S, Hattori M,
Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T
and Yamanishi Y: KEGG for linking genomes to life and the
environment. Nucleic Acids Res. 36(Database issue): D480–D484.
2008.PubMed/NCBI
|
|
32
|
Jensen LJ, Kuhn M, Stark M, Chaffron S,
Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et
al: STRING 8-a global view on proteins and their functional
interactions in 630 organisms. Nucleic Acids Res. 37(Database
issue): D412–D416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cohen C, Leal MF, Belangero PS, Figueiredo
EA, Smith MC, Andreoli CV, de Castro Pochini A, Cohen M, Ejnisman B
and Faloppa F: The roles of Tenascin C and Fibronectin 1 in
adhesive capsulitis: A pilot gene expression study. Clinics (Sao
Paulo). 71:325–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui J, Lu W, He Y, et al: Molecular
Biology of Frozen Shoulder-Induced Limitation of Shoulder Joint
Movements. J Res Med Sci. 22:612017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mandal M, Mandal A, Das S, Chakraborti T
and Chakraborti S: Clinical implications of matrix
metalloproteinases. Mol Cell Biochem. 252:305–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hutchinson JW, Tierney GM, Parsons SL and
Davis TR: Dupuytren's disease and frozen shoulder induced by
treatment with a matrix metalloproteinase inhibitor. J Bone Joint
Surg Br. 80:907–908. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnston P, Chojnowski AJ, Davidson RK,
Riley GP, Donell ST and Clark IM: A complete expression profile of
matrix-degrading metalloproteinases in Dupuytren's disease. J Hand
Surg Am. 32:343–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: An overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tchetverikov I, Ronday HK, Van El B, Kiers
GH, Verzijl N, TeKoppele JM, Huizinga TW, DeGroot J and Hanemaaijer
R: MMP profile in paired serum and synovial fluid samples of
patients with rheumatoid arthritis. Ann Rheum Dis. 63:881–883.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kabbabe B, Ramkumar S and Richardson M:
Cytogenetic analysis of the pathology of frozen shoulder. Int J
Shoulder Surg. 4:75–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Asleh R, Marsh S, Shilkrut M, Binah O,
Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, et
al: Genetically determined heterogeneity in hemoglobin scavenging
and susceptibility to diabetic cardiovascular disease. Circ Res.
92:1193–1200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujii H: PPARs-mediated intracellular
signal transduction. Nihon Rinsho. 63:565–571. 2005.PubMed/NCBI
|
|
43
|
Cipolletta D, Feuerer M, Li A, Kamei N,
Lee J, Shoelson SE, Benoist C and Mathis D: PPAR-γ is a major
driver of the accumulation and phenotype of adipose tissue Treg
cells. Nature. 486:549–553. 2012.PubMed/NCBI
|
|
44
|
Saito T, Hasegawa-Moriyama M, Kurimoto T,
Yamada T, Inada E and Kanmura Y: Resolution of inflammation by
resolvin D1 is essential for peroxisome proliferator-activated
receptor-γ-mediated analgesia during postincisional pain
development in type 2 diabetes. Anesthesiology. 123:1420–1434.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Okamura K and Ozaki J: Bone mineral
density of the shoulder joint in frozen shoulder. Arch Orthop
Trauma Surg. 119:363–367. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leppälä J, Kannus P, Sievänen H, Järvinen
M and Vuori I: Adhesive capsulitis of the shoulder (frozen
shoulder) produces bone loss in the affected humerus, but long-term
bony recovery is good. Bone. 22:691–694. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Müller LP, Müller LA, Happ J and
Kerschbaumer F: Frozen shoulder: A sympathetic dystrophy? Arch
Orthop Trauma Surg. 120:84–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Waldburger M, Meier JL and Gobelet C: The
frozen shoulder: Diagnosis and treatment. Prospective study of 50
cases of adhesive capsulitis. Clin Rheumatol. 11:364–368. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lo SF, Chu SW, Muo CH, Meng NH, Chou LW,
Huang WC, Huang CM and Sung FC: Diabetes mellitus and accompanying
hyperlipidemia are independent risk factors for adhesive
capsulitis: A nationwide population-based cohort study (version 2).
Rheumatol Int. 34:67–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hwang KR, Murrell GA, Millar NL, Bonar F,
Lam P and Walton JR: Advanced glycation end products in idiopathic
frozen shoulders. J Shoulder Elbow Surg. 25:981–988. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Abboud JA and Kim JS: The effect of
hypercholesterolemia on rotator cuff disease. Clin Orthop Relat
Res. 468:1493–1497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zuckerman JD and Rokito A: Frozen
shoulder: A consensus definition. J Shoulder Elbow Surg.
20:322–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Harada K, Tsuruoka S and Fujimura A:
Shoulder stiffness: A common adverse effect of HMG-CoA reductase
inhibitors in women? Intern Med. 40:817–818. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gómez R, Conde J, Scotece M, Gómez-Reino
JJ, Lago F and Gualillo O: What's new in our understanding of the
role of adipokines in rheumatic diseases? Nat Rev Rheumatol.
7:528–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schäffler A, Ehling A, Neumann E, Herfarth
H, Tarner I, Schölmerich J, Müller-Ladner U and Gay S:
Adipocytokines in synovial fluid. JAMA. 290:1709–1710. 2003.
|
|
56
|
Gómez R, Scotece M, Conde J, Gómez-Reino
JJ, Lago F and Gualillo O: Adiponectin and leptin increase IL-8
production in human chondrocytes. Ann Rheum Dis. 70:2052–2054.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Koskinen A, Vuolteenaho K, Nieminen R,
Moilanen T and Moilanen E: Leptin enhances MMP-1, MMP-3 and MMP-13
production in human osteoarthritic cartilage and correlates with
MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp
Rheumatol. 29:57–64. 2011.PubMed/NCBI
|