Introduction
Vascular inflammation is a severe complication in
many countries around the world. This condition requires attention
as it is usually associated with other diseases, including
diabetes, hypertension and lipid disorders (1,2). In
the clinic, vascular inflammation may also accompany endothelial
dysfunctions, and may lead to cardiovascular diseases over time. In
a typical situation, inflammatory responses usually begin in the
vascular system or vascular endothelium and may lead to endothelial
cell dysfunction, which is one of the main causes for
cardiovascular diseases (3,4).
With the cascade of vascular inflammation, endothelial cells
express inflammatory cytokines that recruit immune cells (for
example, macrophages and leukocytes) to the inflammatory sites,
thereby amplifying the inflammatory response that may lead to
subsequent tissue injuries (2,5).
Wogonin (5,7-dihydroxy-8-methoxyflavone) is a
flavonoid-like chemical compound that received extensive attention
in recent years owing to its numerous potential therapeutic effects
(6). Wogonin is extracted from
Scutellaria baicalensis Georgi, and previous studies have
reported that this compound may inhibit cancer progression and
reduce the production of oxidants in cells and tissues (7–11).
For example, wogonin may inhibit proliferation in several cancer
cell lines, including hepatocellular carcinoma, ovarian and
promyeloleukemic cancer cells (12–16).
In anti-oxidative applications, wogonin decreased nitric oxide (NO)
production in macrophage cells that were stimulated with
lipopolysaccharide (LPS) (17). In
another study, wogonin inhibited hydrogen peroxide-induced
oxidative stress in SH-SY5Y neuroblastoma cells (18). Similar studies also demonstrated
the antioxidant and free radical scavenging effects of wogonin as
well as its analogues such as baicalein (19).
The immune modulatory effects of wogonin in
different in vitro and in vivo disease models have
been reported previously (10–12,17).
In one study, it was reported that wogonin upregulated
immunoglobulin A levels in mice with colitis (20). The study also reported that wogonin
downregulated the concentration levels of interleukin (IL)-4, IL-5
and IL-10 cytokines in mice. Thus, this study demonstrated that
wogonin may be able to alleviate the inflammatory response in mice
with colitis through T helper 2 cell responses. In another study,
wogonin inhibited the inflammation induced by toll-like receptor 4
adjuvants, such as LPS, in root ganglion neurons by downregulating
the nuclear factor-κB and mitogen-activated protein kinase
signaling pathways (7,10). These previous studies also revealed
that wogonin suppressed the secretion of pro-inflammatory
mediators, including cyclooxygenase (COX)-2, inducible NO
synthases, IL-1β, IL-6 and tumor necrosis factor-α (TNF-α)
(7,10). Other studies have reported that
wogonin may inhibit the production of NO, prostaglandin E2,
monocyte chemoattractant protein-1 and COX-2, all of which are
indicators of inflammation in cells or tissues (11,20,21).
The anti-inflammatory functions of wogonin have also been
demonstrated in several in vivo animal models, such as mouse
colitis model, mouse skin inflammation model and LPS-induced mouse
lung injury model (20–22). The present study examined the
effects of wogonin in modulating the inflammatory immune responses
in vascular inflammation. To facilitate the strategic delivery and
release of wogonin, this therapeutic component was encapsulated
into a biodegradable carrier, poly (lactic-co-glycolic) acid
(PLGA), a biocompatible material approved by the US Food and Drug
Administration (FDA).
Materials and methods
Ethics statement
All the experiments that involve the use of animals
were approved by the Animal Care and Use Committee in Shandong
Provincial Hospital Affiliated to Shandong University (Jinan,
China), and followed the institute's regulations and the local and
governmental laws on animal care and protection.
Materials
Bacterial LPS (from Escherichia coli O55:B5)
was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
RPMI-1640 cell culture medium was purchased from VWR International
(Leicestershire, UK). Cell culture supplements, including fetal
bovine serum FBS, penicillin and streptomycin were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). PLGA
(lactide:glycolide, 50:50; MW, 30,000–60,000) was from
Sigma-Aldrich (Merck KGaA).
Cell culture
Macrophages were raised in RMPI-1640 medium
supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin. The cells were cultured in an environment enriched
with 5% CO2 at 37°C at a density of 4×105
cells per petri dish (4 in. plastic petri dishes), and maintained
with a certain degree of humidity by adding water in the bottom
layer of a cell culture incubator. For the viability study, cells
from mice with no treatment were used as a control (CTRL). Cells
from mice induced with vascular inflammation were used as a
negative control. These two groups of cells received no wogonin
treatment. In other three groups, cells were treated with different
dose of wogonin (10, 25 and 100 µg/ml), either in soluble form or
carried in the biomaterial carrier. The cells were treated for 48
hours before being tested for viability. For soluble wogonin, the
wogonin cargo was added into PBS buffer and used for in
vitro and in vivo.
Animal experiments
A total of 32 female DBA/1J mice (age, 5–8 weeks,
~18 g each) were obtained from The Animal Center at Shandong
University. Streptozotocin was used to induce the disease model
with the method established and reported previously (22,23,24).
All the mice were raised in a room with a 12-h light/dark cycle.
Temperature of the room was kept at 23°C and 47% humidity, with
free access to food and water for all the mice. For the in
vivo cytokines study, the mice were divided randomly into 4
groups (8 mice/group): Mice with no treatment (i.e., No treat),
mice induced with LPS (1 mg/kg) daily for 3 days (Diseased), mice
treated with soluble wogonin (275 µg wogonin per mouse; Soluble)
and mice treated with materials carrying wogonin daily (Wog/NP).
The injections were performed via tail vein. Mice were anesthetized
by using ketamine and medetomidine (50 and 1 mg/kg, respectively)
if required.
Synthesis and characterization of
cargo loading nanoparticles
A previously described emulsion method was used to
synthesize nanoparticles to carry wogonin (25). Briefly, wogonin (3 mg; cat. no.
102987-206; VWR, Radnor, PA, USA) and PLGA polymer (20 mg) were
dissolved together in the dichloromethane (DCM) solvent at room
temperature. The mixture was sonicated for 1 min to make a
monodispersed solution on ice. The mixture was then added into 5 ml
polyvinyl alcohol (PVA) solution in water (2% w/w). Following
sonication for 20 min on ice, the mixture of aqueous solution and
organic solvent was stirred with a magnetic bar for 90 min at 750
rpm. Following the reaction, the particles were centrifuged at
6,200 × g for 15 min for collection at 4°C. Deionized water was
used to wash the particles twice, followed by lyophilizing the
particles to obtain dry powder by using a freeze dryer (Labconco,
Kansas City, MO, USA). The particles were stored at −20°C until
use. A JEOL-JSM 7500F Scanning Electron Microscope (SEM; JEOL Ltd.,
Tokyo, Japan) was used to analyze the particle morphology and
demonstrate particle size. Particle size was also measured by
dynamic light scattering with a Zetasizer Nano ZS system (Malvern
Instruments, Ltd., Malvern, UK). Samples for SEM characterization
were collected by dropping 50 µl particles (200 µg/ml in DI water)
onto a glass substrate, and the glass substrate with particles were
coated with a thin layer of gold (Emitech K550X; Energy Beam
Sciences Inc., CT, USA; 45 sec coating). During particle size
assessment, the incident beam was scattered at 90°, and each
measurement was taken 3 times to obtain an average value.
Enzyme-linked immunosorbent assay
(ELISA) assays
ELISA was performed according to the manufacturer's
protocols. Briefly, 75–90 µl peripheral blood was collected from
mice 3 days following treatment. The blood was maintained on ice;
serum was obtained by centrifugation at 20,000 × g for 5 min at
4°C. Serum was stored at −80°C prior to use to maintain its
bioactivity. The expression levels of IL-6 (cat. no. KMC0061;
Thermo Fisher Scientific, Inc.), TNF-α (cat. no. KMC3011; Thermo
Fisher Scientific, Inc.) and granulocyte macrophage
colony-stimulating factor (GM-CSF; cat. no. ab46078; Abcam,
Cambridge, UK) were measured. To test the level of cytokines
secreted by macrophages, the cells were treated with different
formulations for 60 h and then tested with ELISA kit by following
the manufacturer's protocols.
Macrophage viability measurement
The effects of the particles and the cargo (Wog./NP)
on cell viability were measured by co-culturing macrophages with
soluble wogonin or wogonin carried in the nanoparticles (Wog./NP).
Macrophages were collected from bone marrow as previously described
(26,27). The cells with different treatments
(i.e., macrophages with no treatment, macrophages with 10, 25 and
100 µg/ml, respectively) were cultured for 48 h and then collected
for analysis. Cells were cultured with 5% CO2 at 37°C at
a density of 1×105 cells per well in a 96-well plate.
Following two washes with PBS, the cells were stained with trypan
blue (1:1,000 dilution in PBS) for viability test; the number of
viable cells was measured with a NanoEnTek Automated Cell Counter
(NanoEnTek, Inc., Seoul, Korea).
Cell staining and flow cytometry
Macrophages were treated for 24 h; cells were
collected from 96 well plates (2×105 per well) and
washed with PBS twice. Subsequently, cells were treated with
Fc-block to reduce non-specific binding during the staining. The
cells were stained with the following fluorescence-labeled
antibodies for 25 min at room temperature: Fluorescein
isothiocyanate-conjugated CD80 (1:200 in PBS plus 1% BSA, cat. no.
561954; BD Biosciences, Franklin Lakes, NJ, USA);
phycoerythrin-conjugated CD86 (553692; 1:200; BD Biosciences); and
allophycocyanin-conjugated CD40 (124610; BioLegend, Inc., San
Diego, CA, USA). All the antibodies were used at 1:200 dilution in
PBS. Following antibody staining, the cells were collected and
washed with PBS for 1 min to remove non-specific staining. To
analyze the relative ratio of M1 and M2 macrophages, CD80 and CD86
was selected as M1 macrophage markers and were stained with
fluorescein isothiocyanate-conjugated CD80 (561954; 1:200; BD
Biosciences, Franklin Lakes, NJ, USA); phycoerythrin-conjugated
CD86 (553692; 1:200; BD Biosciences); and
allophycocyanin-conjugated CD40 (1:200; BioLegend, Inc.). CD71 and
CD206 were selected as M2 macrophage surface markers. These two
markers were stained with monoclonal antibody [R17217 (RI7
217.1.4), APC labeled, eBioscience; Thermo Fisher Scientific, Inc.]
and CD206 (MMR) monoclonal antibody (MR6F3; PE labeled; both at
1:200; eBioscience; Thermo Fisher Scientific, Inc.), respectively.
The cells were dispersed and counterstained in PBS with 0.1% DAPI
at room temperature and analyzed by flow cytometry for 45 min.
FlowJo version 10.1 (FlowJo LLC, Ashland, OR, USA) was used to
analyze the macrophage staining.
Statistical analysis
Statistical analysis was conducted with analysis of
variance (ANOVA) to determine the statistical difference between
different groups. If ANOVA indicated a significant difference, then
a t-test with Tukey's correction for multiple comparisons was
employed to examine the individual differences. GraphPad Prism
version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used
for analysis. The data are presented as the mean ± standard error
of the mean. P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
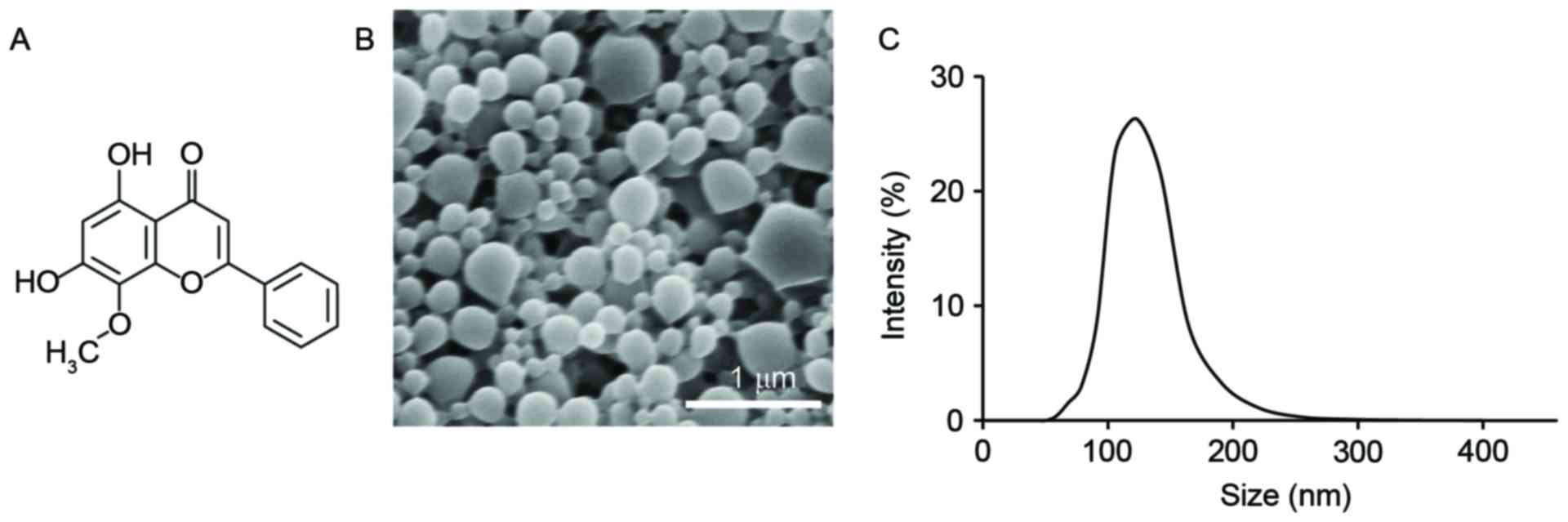
For a complete understanding of the study, the
chemical structure of wogonin is provided (Fig. 1A). Wogonin was loaded into a
biocompatible carrier, PLGA; the polymer was produced by an
emulsion method, which generated spherical PLGA particles that
carried wogonin. SEM imaging demonstrated that the particles had a
spherical shape (Fig. 1B), and
dynamic light scattering indicated that the particles had a
diameter of 107±39 nm (Fig. 1C).
This nanoscale size of the particles is appropriate for in
vivo usage, and the loading level of wogonin within the
particles was assessed by dissolving the Wog./NP powder in NaOH
(3M) and measuring the absorbance at 275 nm. The loading of wogonin
in the nanoparticles was ~12.6 µg/mg PLGA. These particles were
used in subsequent in vivo experiments to examine their
effects on controlling inflammatory responses. PLGA is an ideal
material for delivering immune and therapeutic cargos. The
advantages of using PLGA as a delivery vehicle include co-delivery
of different cargo, targeting of specific tissues or cells and
controlled release. In a previous study, the inflammatory function
of PLGA to the immune system has been reported to be minor
(26); several additional studies
demonstrated that PLGA microparticles, instead of PLGA
nanoparticles, were able to promote the production of inflammatory
cytokines such as TNF-α (28,29).
However, considering that PLGA is one of several existing materials
approved by the FDA for use in humans, and that PLGA at the
nanoscale level does not induce notable inflammation in
vitro (29), this material was
used in the present study to facilitate the delivery of
wogonin.
The effects of wogonin were examined in vivo,
using both a soluble form and one carried in PLGA. Mice with
streptozotocin-induced vascular inflammation were treated with 275
µg wogonin daily. Peripheral blood was collected from mice 3 days
post-treatment and analyzed for the levels of cytokines in the
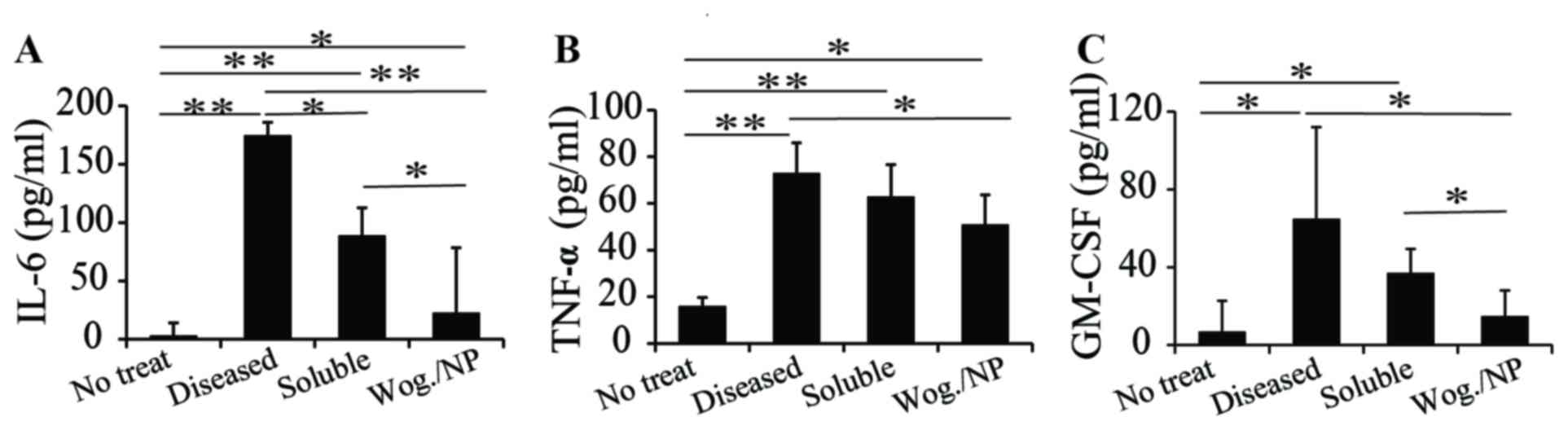
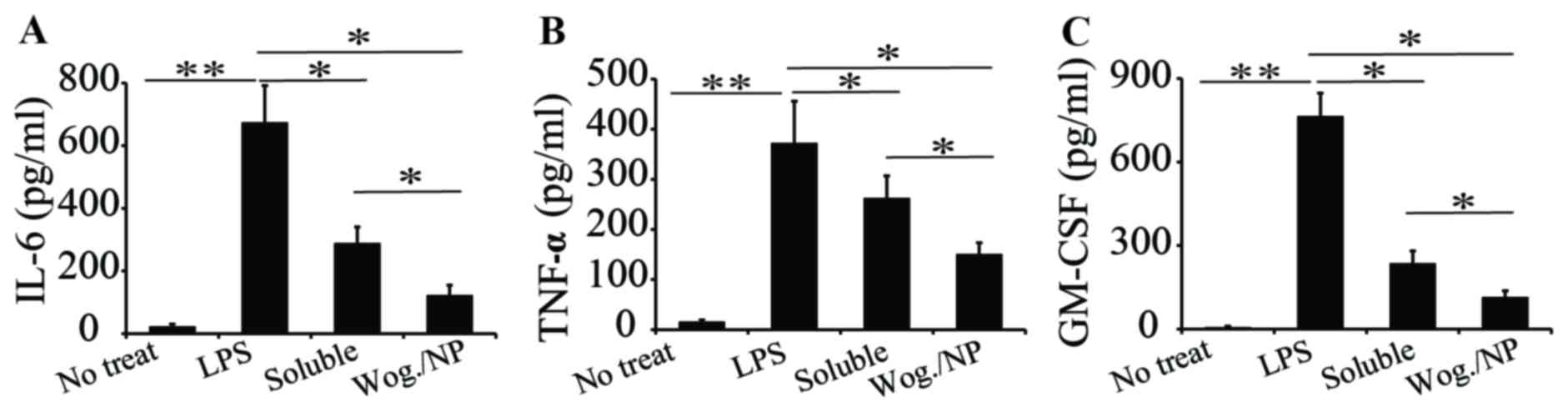
serum, including IL-6, TNF-α and GM-CSF (Figs. 2A-C, respectively). The mice with
vascular inflammation exhibited a higher level of inflammatory
cytokines in their serum compared with mice in the other groups.
The two wogonin treatments both reduced the production of IL-6
cytokines in mice with vascular inflammation. Compared with the
soluble formulation, PLGA-carried wogonin appeared to be more
effective in reducing IL-6 production (Fig. 2A), probably because the
nanoparticles improved the bioavailability of wogonin. Wogonin
treatment, either in a soluble form or carried in PLGA
nanoparticles, had a slight effect in reducing the production of
TNF-α, and no statistically significant difference was identified
between the soluble and nanoparticle forms (Fig. 2B). For GM-CSF production, a similar
trend as IL-6 level was observed; that is, both wogonin treatments
reduced the production of GM-CSF, with the PLGA-wogonin form more
effective in reducing GM-CSF production compared with the soluble
form (Fig. 2C). This may be
because wogonin in the nanoparticle form may have a controlled
release of wogonin, which may last for longer periods of time in
vivo, compared with wogonin in soluble form. In blood vessels,
inflammatory responses include vasodilation, enhanced permeability
of vessels and blood stasis (1,4). The
production of inflammatory cytokines in the blood vessels may
result in cytoskeletal alterations in the endothelial cells, which
may eventually disrupt and increase the permeability of vessels
(30). At the beginning of
inflammation in blood vessels, the inflammatory cells (monocytes,
neutrophils, lymphocytes and macrophages) respond by activating a
number of different inflammatory pathways and promoting the
production of cytokines (5,28,30).
Macrophages are one of major resources for producing inflammatory
cytokines (30). The present study
illustrated that regulating macrophage functions with wogonin
reduced the production of inflammatory cytokines. This indicated
that the vascular inflammation, especially the inflammation induced
by the cytokines produced by macrophages, can be controlled by
using wogonin. Comparing the soluble wogonin and wogonin carried in
PLGA nanoparticles, the cargo carried in PLGA nanoparticles
(Wog./NP) was more effective due to the improved functionality of
the biomaterial carrier.
The effects of wogonin on macrophage functions were
also examined. Macrophages were collected from the bone marrow of
untreated mice or mice induced with disease (i.e., diseased) and
incubated with the different forms of wogonin in a range of doses.
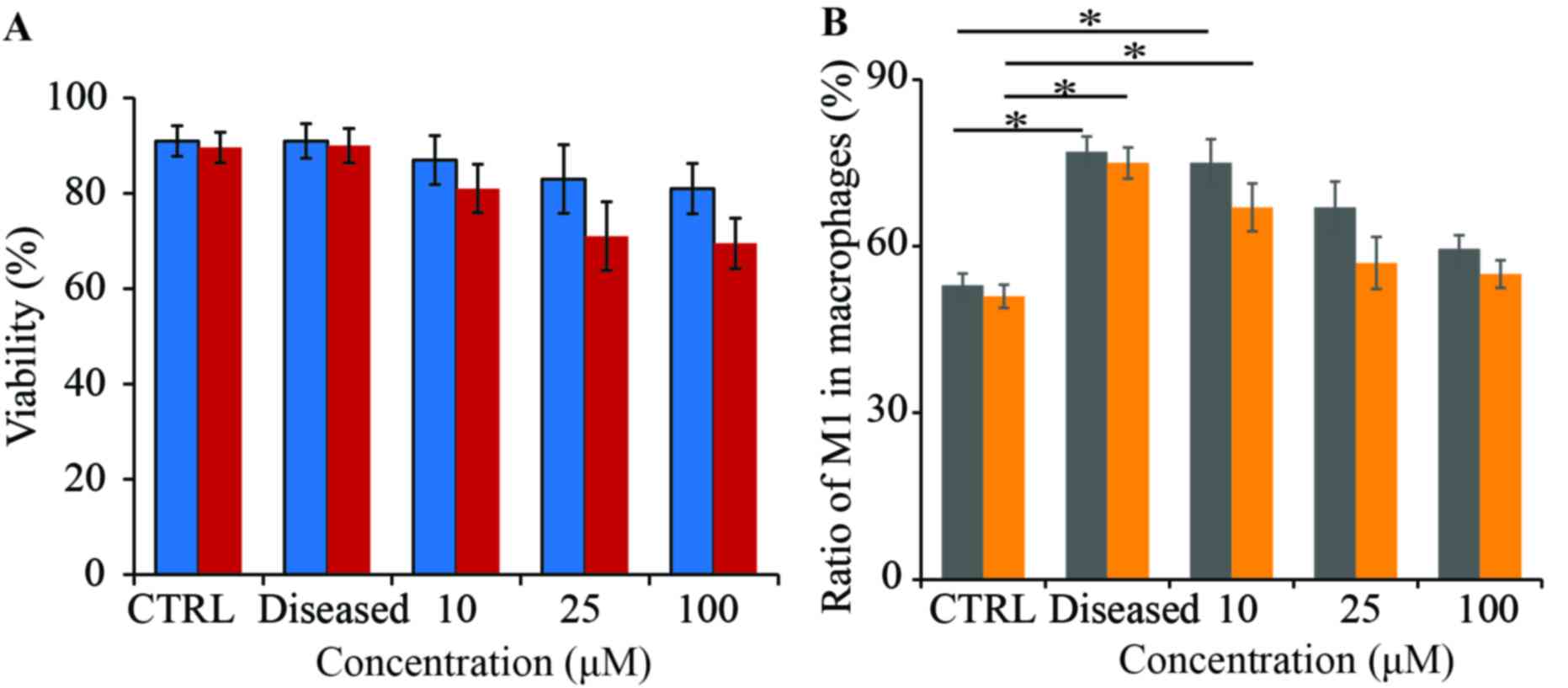
Macrophages obtained from diseased mice were treated with 10, 25 or
100 µg/ml wogonin to test the effect of the cargo on cellular
viability (Fig. 3A). The results
demonstrated that wogonin in nanoparticle form did not
significantly affect macrophage viability over 48 h incubation
(Fig. 3A). By contrast,
macrophages treated with soluble wogonin had a slightly lower
viability when the dose of wogonin was increased, although no
statistical difference was identified (Fig. 3A). The macrophages from diseased
mice also maintains viability comparable to untreated mice,
indicating the disease induction did not affect macrophage
viability in mice (Fig. 3A.). The
ratio of M1 macrophage cells to M2 macrophage cells was also
examined; following streptozotocin-induced vascular inflammation,
there was a higher M1/M2 ratio compared with Control (P<0.05;
Fig. 3B). Wogonin treatments in
particle form (Fig. 3B; gray bar)
were more effective led to a slightly higher ratio of M1
macrophages compared to mice receiving soluble wogonin (Fig. 3B; orange bar). The M1/M2 ratio
indicated the level of inflammation; M1 macrophages are responsible
for the inflammatory response, whereas M2 macrophages are in
involved in tissue repair and regeneration (31–33).
The present results also revealed that the concentration of wogonin
treatment regulated the relative ratio of M1 macrophages. In both
soluble and nanoparticle wogonin treatments, the relative level of
M1 macrophages decreased as the dose increased, which indicated
that wogonin was able to downregulate macrophage function in a
dose-dependent manner. The current study illustrated that wogonin
can change the relative ratio of M1 and M2 macrophages. More
studies are needed to illustrate the mechanism by which wogonin
affects macrophages differentiation. That M1 macrophage level
decreased with increased wogonin dose demonstrated that wogonin
controls vascular inflammation by regulating the relative ratio of
M1 and M2 macrophages.
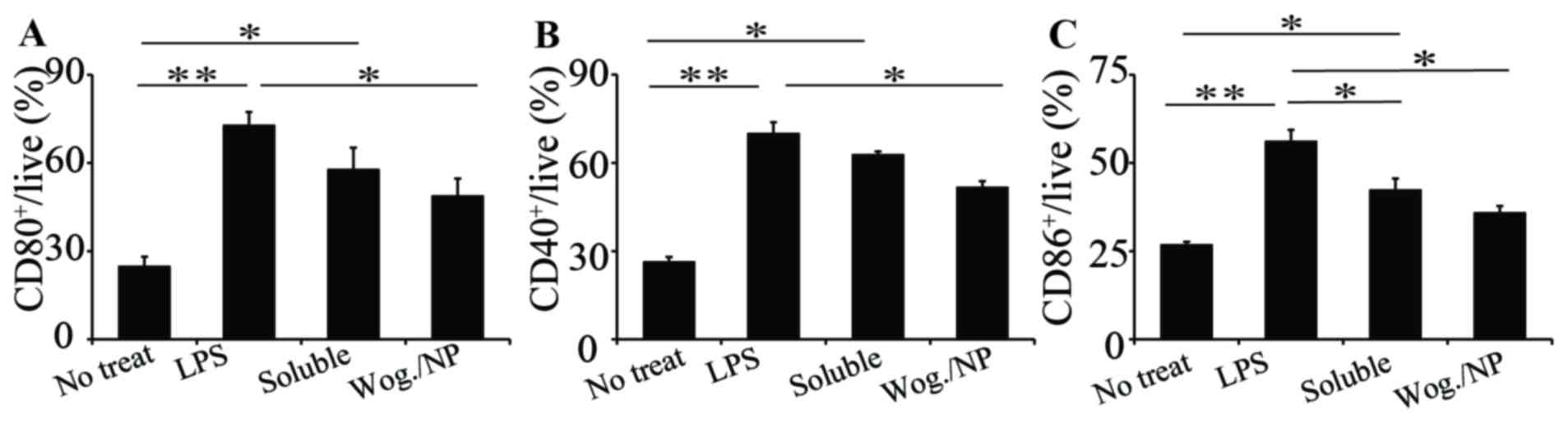
The activation of macrophage surface markers by
wogonin treatments was also examined. Macrophages were stimulated
with LPS to promote the inflammatory response. The cells were
treated for 24 h and collected for analysis. Expression levels of
all three macrophage activation surface markers, CD80+,
CD40+ and CD86+, significantly increased upon
LPS treatment (Fig. 4A-C,
respectively). Both soluble and particulate wogonin treatments
reduced the expression of CD80+ (Fig. 4A); compared to soluble wogonin,
wogonin loaded in nanoparticles was more effective in reducing the
expression of CD80+ markers. A similar trend was
observed in the expression of CD40+ and CD86+
markers (Fig. 4B and C,
respectively). These results indicated that wogonin reduced the
activation of macrophage markers, and wogonin loaded in PLGA was
more effective compared with soluble wogonin. This was probably
because the PLGA polymer carrier is biodegradable both in
vitro and in vivo, releasing wogonin cargo continuously
over time and providing the cells with consistent treatment;
instead, soluble formulation had less efficacy as wogonin did not
dissolve very well in culture medium. Wogonin has low solubility in
aqueous solutions, and this is one of the advantages of using a
biomaterials carrier to deliver wogonin. The present study
demonstrated that wogonin can reduce macrophage surface marker
expression and so reduce macrophage activation in the case of
vascular inflammation. A previous study indicated that wogonin
exhibits anti-inflammation functions by inhibiting vascular
hyperpermeability and expression of CAMs (6). The results from the present study
explain the regulation of the roles of wogonin in vascular
inflammation.
To connect the activation of macrophage surface
markers with their inflammatory immune responses, the cell culture
supernatant was collected and analyzed for the secretion of
inflammatory cytokines, including IL-6, TNF-α and GM-CSF, from the
macrophages in the different treatment groups (Fig. 5A-C, respectively). Upon LPS
stimulation, the expression level of each inflammatory cytokine was
significantly increased (Fig. 5).
Both soluble and particle forms of wogonin co-treatment
significantly reduced the production of IL-6 (Fig. 5A); wogonin in nanoparticle form
appeared to be more potent in reducing IL-6 production compared
with wogonin in soluble form. Similarly, TNF-α and GM-CSF
expression levels were significantly reduced in macrophages
co-treated with either form of wogonin compared with LPS-only
(Fig. 5B and C, respectively). In
both cases, nanoparticle wogonin was more effective in reducing the
expression of these cytokines compared with the soluble form; this
is probably due to the use of a particle carrier, which improved
the bioavailability of wogonin to the cells, thus providing better
therapeutic effects. Previous studies focused on using free wogonin
for treating different diseases including inflammation (7–10).
The present study demonstrated that the use of a PLGA carrier can
improve the efficacy by changing the bioavailability of wogonin. As
to the anti-inflammatory functions of wogonin, a previous study
indicated that wogonin can regulate the production of IL-6 gene,
another important inflammatory cytokine (8). Another study demonstrated that
wogonin can inhibit toll-like receptor 4 and MARK inflammatory
pathways (10). Compared to these
existing studies, the current study confirmed the anti-IL6
functions of wogonin. It also demonstrated that wogonin can
regulate another two inflammatory cytokines, TNF-α and GM-CSF. This
result indicated that other anti-inflammatory pathways might be
also involved in wogonin treatment, which will probably be the
subject of our future studies.
In conclusion, The present study examined the
effects of wogonin in regulating immune responses in vascular
inflammation. In the in vivo experiments, wogonin
downregulated the level of inflammatory cytokines. Wogonin loaded
in the biomaterial carrier had an improved therapeutic effect
compared with that in soluble form. The results also revealed that
wogonin did not affect macrophage viability, but did regulate the
relative ratio of M1 macrophages to M2 macrophages. In addition,
wogonin regulated the expression of macrophage surface activation
markers CD86+, CD80+ and CD40+.
Analysis of the level of inflammatory cytokines in the cell culture
supernatant demonstrated that wogonin was able to inhibit the
production of inflammatory cytokines from the stimulated
macrophages. The current study demonstrated both inflammatory
cytokines and macrophage activation were involved in the use of
wogonin against vascular inflammation. Future wogonin therapy
against vascular inflammation may benefit from the use of a
biodegradable carrier, which can improve the therapeutic efficacy
by improved bio-availability and other advantages brought by a
degradable carrier, including targeting and controlled release.
Acknowledgements
This study was supported by The Shandong Provincial
Hospital Affiliated to Shandong University (Jinan, China).
References
|
1
|
Virdis A and Schiffrin EL: Vascular
inflammation: A role in vascular disease in hypertension? Curr Opin
Nephrol Hypertens. 12:181–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tedgui A and Mallat Z: Anti-inflammatory
mechanisms in the vascular wall. Circ Res. 88:877–887. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taube A, Schlich R, Sell H, Eckardt K and
Eckel J: Inflammation and metabolic dysfunction: Links to
cardiovascular diseases. Am J Physiol Heart Circ Physiol.
302:H2148–H2165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schenkein HA and Loos BG: Inflammatory
mechanisms linking periodontal diseases to cardiovascular diseases.
J Clin Periodontol. 40 Suppl 14:S51–S69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ait-Oufella H, Taleb S, Mallat Z and
Tedgui A: Recent advances on the role of cytokines in
atherosclerosis. Arterioscler Thromb Vasc Biol. 31:969–979. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao JR, Do CW and To CH: Potential
therapeutic effects of baicalein, baicalin, and wogonin in ocular
disorders. J Ocul Pharmacol Ther. 30:605–614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee W, Ku SK and Bae JS: Anti-inflammatory
effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo.
Inflammation. 38:110–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Yao J, Wu XP, Zhao L, Zhou YX,
Zhang Y, You QD, Guo QL and Lu N: Wogonin suppresses human alveolar
adenocarcinoma cell A549 migration in inflammatory microenvironment
by modulating the IL-6/STAT3 signaling pathway. Mol Carcinog. 54
Suppl 1:E81–E93. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YM, Cheng PY, Chen SY, Chung MT and
Sheu JR: Wogonin suppresses arrhythmias, inflammatory responses,
and apoptosis induced by myocardial ischemia/reperfusion in rats. J
Cardiovasc Pharmacol. 58:133–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Xiong J, Zhan Y, Liu W and Wang X:
Wogonin inhibits LPS-induced inflammatory responses in rat dorsal
root ganglion neurons via inhibiting TLR4-MyD88-TAK1-mediated NF-κB
and MAPK signaling pathway. Cell Mol Neurobiol. 35:523–531. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen LG, Hung LY, Tsai KW, Pan YS, Tsai
YD, Li YZ and Liu YW: Wogonin, a bioactive flavonoid in herbal tea,
inhibits inflammatory cyclooxygenase-2 gene expression in human
lung epithelial cancer cells. Mol Nutr Food Res. 52:1349–1357.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YC, Shen SC, Lee WR, Lin HY, Ko CH,
Shih CM and Yang LL: Wogonin and fisetin induction of apoptosis
through activation of caspase 3 cascade and alternative expression
of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch
Toxicol. 76:351–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee WR, Shen SC, Lin HY, Hou WC, Yang LL
and Chen YC: Wogonin and fisetin induce apoptosis in human
promyeloleukemic cells, accompanied by a decrease of reactive
oxygen species, and activation of caspase 3 and
Ca2+-dependent endonuclease. Biochem Pharmacol.
63:225–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li DR, Hou HX, Zhang W and Li L: Effects
of wogonin on inducing apoptosis of human ovarian cancer A2780
cells and telomerase activity. Ai Zheng. 22:801–805.
2003.PubMed/NCBI
|
|
15
|
Wang W, Guo QL, You QD, Zhang K, Yang Y,
Yu J, Liu W, Zhao L, Gu HY, Hu Y, et al: The anticancer activities
of wogonin in murine sarcoma S180 both in vitro and in vivo. Biol
Pharm Bull. 29:1132–1137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CC, Kuo CL, Lee MH, Lai KC, Lin JP,
Yang JS, Yu CS, Lu CC, Chiang JH, Chueh FS, et al: Wogonin triggers
apoptosis in human osteosarcoma U-2 OS cells through the
endoplasmic reticulum stress, mitochondrial dysfunction and
caspase-3-dependent signaling pathways. Int J Oncol. 39:217–224.
2011.PubMed/NCBI
|
|
17
|
Lee JY and Park W: Anti-Inflammatory
effect of wogonin on RAW 264.7 mouse macrophages induced with
polyinosinic-polycytidylic acid. Molecules. 20:6888–6900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wakabayashi I and Yasui K: Wogonin
inhibits inducible prostaglandin E2 production in
macrophages. Eur J Pharmacol. 406:477–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shieh DE, Liu LT and Lin CC: Antioxidant
and free radical scavenging effects of baicalein, baicalin and
wogonin. Anticancer Res. 20:2861–2865. 2000.PubMed/NCBI
|
|
20
|
Lim BO: Efficacy of wogonin in the
production of immunoglobulins and cytokines by mesenteric lymph
node lymphocytes in mouse colitis induced with dextran sulfate
sodium. Biosci Biotechnol Biochem. 68:2505–2511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chi YS, Lim H, Park H and Kim HP: Effects
of wogonin, a plant flavone from Scutellaria radix, on skin
inflammation: In vivo regulation of inflammation-associated gene
expression. Biochem Pharmacol. 66:1271–1278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao J, Pan D, Zhao Y, Zhao L, Sun J, Wang
Y, You QD, Xi T, Guo QL and Lu N: Wogonin prevents
lipopolysaccharide-induced acute lung injury and inflammation in
mice via peroxisome proliferator-activated receptor gamma-mediated
attenuation of the nuclear factor-κB pathway. Immunology.
143:241–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen-Sela E, Chorny M, Koroukhov N,
Danenberg HD and Golomb G: A new double emulsion solvent diffusion
technique for encapsulating hydrophilic molecules in PLGA
nanoparticles. J Control Release. 133:90–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozcelikay AT, Pekiner C, Ari N, Ozturk Y,
Ozuari A and Altan VM: The effect of vanadyl treatment on vascular
responsiveness of streptozotocin-diabetic rats. Diabetologia.
37:572–578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nasri S, Roghani M, Baluchnejadmojarad T,
Rabani T and Balvardi M: Vascular mechanisms of
cyanidin-3-glucoside response in streptozotocin-diabetic rats.
Pathophysiology. 18:273–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weischenfeldt J and Porse B: Bone
marrow-derived macrophages (BMM): Isolation and applications. CSH
Protoc 2008. 2008.
|
|
27
|
Huang AY, Golumbek P, Ahmadzadeh M, Jaffee
E, Pardoll D and Levitsky H: Role of bone marrow-derived cells in
presenting MHC class I-restricted tumor antigens. Science.
264:961–965. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Lee Y, Park H, Hong D, Khang G and
Lee D: Reduced inflammatory responses to poly(lactic-co-glycolic
acid) by the incorporation of hydroxybenzyl alcohol releasing
polyoxalate. Macromol Res. 19:1242–1249. 2011. View Article : Google Scholar
|
|
29
|
Nicolete R, dos Santos DF and Faccioli LH:
The uptake of PLGA micro or nanoparticles by macrophages provokes
distinct in vitro inflammatory response. Int Immunopharmacol.
11:1557–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dinh QN, Drummond GR, Sobey CG and
Chrissobolis S: Roles of inflammation, oxidative stress, and
vascular dysfunction in hypertension. Biomed Res Int.
2014:4069602014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Italiani P and Boraschi D: From monocytes
to M1/M2 macrophages: Phenotypical vs. functional differentiation.
Front Immunol. 5:5142014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Graziano F, Vicenzi E and Poli G: Plastic
restriction of HIV-1 replication in human macrophages derived from
M1/M2 polarized monocytes. J Leukoc Biol. 100:1147–1153. 2016.
View Article : Google Scholar : PubMed/NCBI
|