Introduction
The regenerative repair of deep-degree (second
degree) burned skin remains a notable challenge in the treatment of
burn injuries, despite improvements being made to the treatment
modality and the emergence of novel therapies (1). The healing outcome of burned skin
depends on the depth and extent of the burn (2). Numerous patients suffer from
extensive scar formation following deep-degree burns; therefore,
these patients must undergo numerous surgeries over the years to
alleviate their disability (3).
When the degree of a burn injury is beyond the self-repairing and
structural reconstructive capabilities of the skin, a local
excessive inflammatory immune response is initiated, which is
characterized by a large increase in inflammatory cell infiltration
into the wound over a long period of time, resulting in the release
of cytokines that induce the excessive proliferation of repair
cells and scar formation (4).
However, the mechanism underlying this process remains unknown. To
avoid the adverse effects of scar formation, numerous studies have
focused on the mechanism underlying scarless healing of burned skin
(5–8). Fetal skin constitutes an attractive
target for investigating scarless healing of burned skin for
numerous reasons: i) Previous studies have reported that scarless
healing commonly occurs in early and middle mammalian embryos; this
healing ability relies not on the intrauterine environment, but on
the properties of the embryo itself (9–12).
ii) No apparent inflammatory immune cell infiltration into the skin
has been detected during the wound healing process in early and
middle embryos, since the inflammatory immune system is undeveloped
(13). iii) Skin cells from early
and middle embryos possess improved proliferative and migratory
abilities compared with late embryos and infants (14). iv) Numerous types of cytokines and
proteins are different in the skin of early and middle embryos,
which may aid scarless healing (15–18).
The present study aimed to establish an animal model carrying
burned human fetal skin, which could be used to investigate the
mechanisms underlying the scarless healing process of burned fetal
skin. In addition, the response of burned fetal skin to treatment
with human peripheral blood mononuclear cells (hPBMCs) was
determined.
Lane et al (19) generated an animal model, which
consisted of nude mice carrying human embryonic skin, in order to
investigate the features of scarless healing outside of the womb.
In addition, a previous study investigated scarless healing, which
mainly involves the healing process of incision wounds (13). To the best of our knowledge, the
healing mechanism differs greatly between incision wounds and burn
wounds. Therefore, the present study established an animal model,
which consisted of nude mice carrying burned human fetal skin,
based on the animal model described by Lane et al (19). Subsequently, the development
process of implanted human fetal skin and the healing process of
burned human fetal skin were characterized in terms of
histomorphology, and the expression levels of matrix
metalloproteinase (MMP)-9 and tissue inhibitor of
metalloproteinases (TIMP)-1 were detected during these processes.
The effects of hPBMCs on the healing process, and on MMP-9 and
TIMP-1 expression in burned human fetal skin were investigated, in
order to identify the immune mechanism underlying scarless healing
of burn wounds.
The present study investigated the mechanism
underlying scarless healing of burn wounds outside of the womb, and
may provide novel information regarding the treatment of
deep-degree burn injuries.
Materials and methods
Animals, human fetal skin and ethical
approval
All severe combined immunodeficient nude mice (n=54;
age, 6 weeks; weight 20±3.8 g) used in the present study were
obtained from the Animal Center of Peking University Health Science
Center (Beijing, China). The mice were maintained at 20–24°C and
50–60% humidity, under a 12-h light/dark cycle with ad
libitum access to animal chow and water in the animal quarters
at the Animal Laboratory of the Second Hospital of Shandong
University (Jinan, China). All experimental procedures were
conducted according to the criteria outlined in the Guide for the
Care and Use of Laboratory Animals, published by the National
Institutes of Health (NIH pub no. 85-23; revised 1996). All
experimental protocols were approved by the Animal Care and Use
Committee of the Second Hospital of Shandong University.
The present study was approved by the Ethics
Committee of the Second Hospital of Shandong University. All human
fetal skin specimens were obtained from abortions induced by
artificial abortion vacuum aspiration at the Department of
Obstetrics, The Second Hospital of Shandong University. Prior to
fetal skin collection, all of the pregnant women who were
undergoing the induced abortion were informed of the study and
provided written informed consent permitting the use of aborted
fetal tissue for scientific research and education purposes. The
present study conformed to the provisions of the 1975 Declaration
of Helsinki.
A total of 9 aborted fetuses (aged between 22 and 26
weeks) were obtained for use in the present study. Within 1 h of
abortion, fetal skin from the back of the shoulders was collected
under sterile conditions and washed twice with cold sterile
phosphate-buffered saline (PBS). The skin was maintained in Roswell
Park Memorial Institute-1640 (RPMI-1640) medium supplemented with
10% fetal bovine serum (both from Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA), 100 IU/ml penicillin and 100 mg/ml
streptomycin (both from Beyotime Institute of Biotechnology,
Haimen, China) on ice.
Establishment of a nude mouse model
carrying human fetal skin
Surgery was conducted in the laminar flow operating
room, which was specific-pathogen-free following disinfection with
ultraviolet light for 60 min prior to surgery. All operative
instruments were autoclaved, and the fetal skin samples were
trimmed to size (20 mm2).
Briefly, the nude mice were anesthetized by
intraperitoneal injection with 4% chloral hydrate (312 mg/kg). The
skin on the dorsum of the mice was sterilized with povidone iodine
solution, and was then cut to form a 20 mm2 pedicle skin
flap. Following separation of subcutaneous tissue, the pedicle skin
flap was opened. The trimmed fetal skin was fixed onto the dorsal
muscular mantle with 5-0 MERSILK® [Johnson & Johnson
Medical (China) Ltd., Shanghai, China] and covered with Vaseline
gauze. Following suturing of the skin flap, the nude mice were
maintained on a HICO-Polyurethane warming blanket (Hirtz & Co.,
KG, Köln, Germany) at 37°C until they awoke, and were then fed.
Each postoperative nude mouse was administered 1×105 IU
penicillin by intraperitoneal injection every 8 h for 3 days.
A total of 2, 4, 6 and 8 weeks after surgery, the
nude mice were anesthetized with 4% chloral hydrate as above and
the skin flap was opened. The secreta were removed from the surface
of the fetal skin, and a 5×10 mm2 piece of fetal skin
was collected. The Vaseline gauze was replaced with fresh gauze.
Once the skin flap was sutured, the nude mice were fed again. The
fetal skin sample was placed in 4% paraformaldehyde solution at 4°C
overnight for hematoxylin and eosin (H&E) and
immunohistochemical staining.
Establishment of a nude mouse model
carrying burned human fetal skin
The development of implanted human fetal skin was
observed, and after 2 weeks of implantation the fetal skin that had
survived and had developed stable skin appendages was chosen to
establish the model of deep-degree burned human fetal skin. The
temperature of the iron head of the constant temperature electric
heat apparatus (The Key Laboratory of Trauma Repair Department, The
First Affiliated Hospital of PLA General Hospital, Beijing, China)
was adjusted to 80°C. The nude mice were anesthetized as above and
the skin flaps were opened. After cleaning the secreta on the skin
surface, the heated iron head (20×20 mm2) was gently
placed on the surface of the implanted human fetal skin and kept
there for 4 sec. Subsequently, the burned fetal skin was quickly
covered with gauze, which was soaked with sterile PBS for cooling
purposes, sterilized with povidone iodine solution, and covered
with Vaseline gauze and sutured. Post-operation, the nude mice were
administered 1×105 IU penicillin by intraperitoneal
injection every 8 h for 3 days.
Following 3, 7, 10, 14 and 21 days, the nude mice
were anesthetized and the skin flaps were opened. The secreta were
removed from the surface of the fetal skin, and a piece of fetal
skin (5×10 mm2) was collected. Subsequently, the
Vaseline gauze was replaced with fresh gauze. Following the
suturing of the skin flap, the nude mice were fed again. The fetal
skin samples were placed in 4% paraformaldehyde solution at 4°C
overnight for H&E and immunohistochemical staining.
Separation of hPBMCs
The present study was approved by the Ethics
Committee of the Second Hospital of Shandong University. All blood
samples used for hPBMC separation were collected from healthy adult
male volunteers (23–26 years old). All volunteers were informed
about the study and provided written informed consent permitting
the use of their blood sample for scientific research and education
purposes. The present study conformed to the provisions of the
Declaration of Helsinki. Briefly, 10 ml blood was drawn through the
median cubital vein of each volunteer and was added to 50 IU/ml
heparin (Shanghai No. 1 Biochemical & Pharmaceutical Co., Ltd.,
Shanghai, China) for the prevention of coagulation.
Human PBMCs were separated using density gradient
centrifugation over Lymphocyte Separation Medium (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) according to
the manufacturer's protocol. Briefly, blood was diluted 1:1 with
RPMI-1640 medium (v/v), and was then precisely added to the surface
of the lymphocyte separation medium and centrifuged at 400 × g for
30 min at room temperature (25°C). Subsequently, the hPBMC layer,
which was between the upper layer (plasma and platelets) and the
middle layer (lymphocyte separation medium), was carefully
collected and transferred into a 15-ml Falcon tube. The hPBMC layer
was washed with RPMI-1640 medium and was further centrifuged at 200
× g for 10 min at room temperature (25°C). The separated cell
pellet was resuspended in RPMI-1640 medium for further
experiments.
Treatment with hPBMCs
During the establishment of a nude mouse model
carrying deep second-degree burned human fetal skin, hPBMCs
suspended in RPMI-1640 were subcutaneously injected into the burned
fetal skin immediately after the fetal skin was burned. The total
number of hPBMCs injected into each nude mouse was 1×106
cells. The nude mice in the control group were injected with the
same volume of RPMI-1640. Subsequently, fetal skin samples were
collected as aforementioned.
Histological staining
Skin specimens were embedded in paraffin blocks
following fixation with 4% paraformaldehyde solution. Subsequently,
5 µm sections were obtained, deparaffinized and stained with
H&E, 4 min each dye, at room temperature. The skin sections
were then examined and evaluated in random order under blinded
conditions using a light microscope (CX31; Olympus Corporation,
Tokyo, Japan).
Immunohistochemical staining
The MMP-9 and TIMP-1 monoclonal antibodies were
purchased from Abcam (Shanghai, China). The 3,3′-diaminobenzidine
tetrahydrochloride (DAB) staining kit and Polink-2 plus Polymer
horseradish peroxidase detection system for mouse primary antibody
were obtained from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. (Beijing, China).
Skin sections (5 µm) were deparaffinized, washed
with distilled water and immersed in PBS for 5 min. For antigen
retrieval, the sections were incubated in antigen retrieval
solution (Beijing Solarbio Science &Technology Co., Ltd.) at
93°C for 15 min. Subsequently, the sections were incubated in 3%
H2O2 at room temperature for 10 min, and the
sections were incubated with anti-MMP-9 (1:150) or anti-TIMP-1
(1:150) for 2 h at 37°C. The sections were then incubated with
polymer helper at room temperature for 30 min and were washed three
times with PBS (2 min/wash). Poly peroxidase-anti-mouse/rabbit
immunoglobulin G was added to the sections for 30 min at room
temperature. Color of the sections was developed using DAB.
Positively stained cells exhibited brownish yellow cytoplasmic
granules under the light microscope. Once the color of the positive
cells exhibited brownish yellow cytoplasmic granules under
microscopy, and the color of cytoplasmic granules were observable
yet not too dark, the sections were washed under water to terminate
color development. The sections were then counterstained with
hematoxylin and were mounted in glycerin jelly mounting medium.
Images of the stained skin sections were analyzed
using Image-Pro Plus 6.0 software (Media Cybernetics Inc.,
Rockville, MD, USA). Using immunohistochemical image gray-scale
analysis, integral optical density (IOD) was determined. IOD
divided by sum area of the whole image was used to calculate mean
optical density (MOD), which was used to evaluate the intensity of
the chemical reaction of cells in every specimen.
Statistical analysis
Each experiment was conducted in triplicate. All
data are presented as the mean ± standard deviation. Dual
comparisons between groups exhibiting significant values were
evaluated usingone-way analysis of variancefollowed by Dunnett's
test. Statistical analysis was performed using SPSS version 19.0
(IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
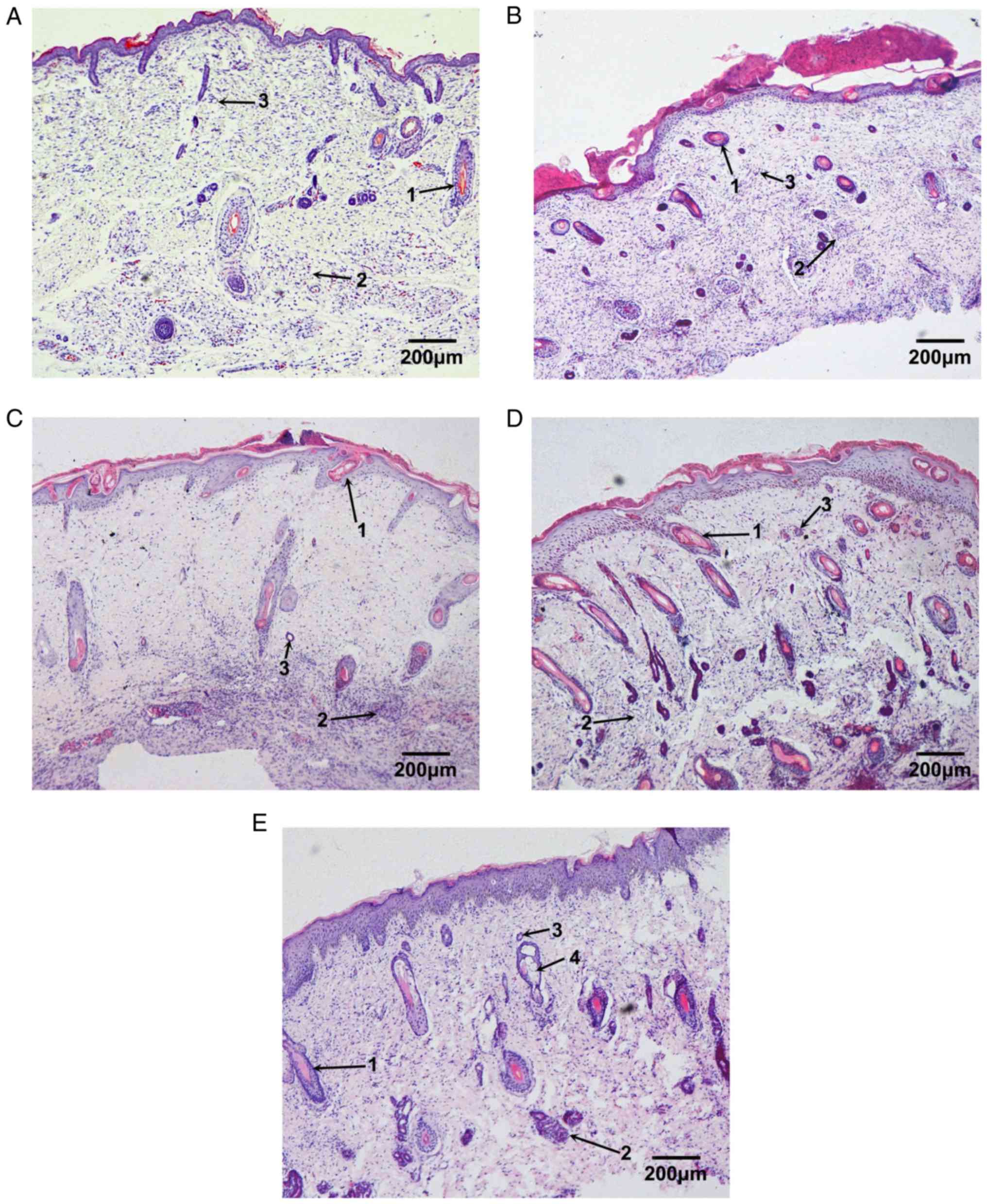
Development of fetal skin
subcutaneously implanted into nude mice
Following implantation under the skin of nude mice,
fetal skin was observed to develop similarly to in the intrauterine
environment. In the present study, skin from 23-week-old fetuses
was used as a control to determine the developmental process of
fetal skin in a nude mouse model (Fig.
1).
Under the light microscope, the epidermis of the
fetal skin exhibited slight keratinization, and was comprised of
four layers of cells that were regularly arranged. Cells in the
walls of the follicular cavity were well arranged. The hair shaft
in the follicular cavity had developed but did not grow out of the
epidermis. Some sweat glands could be observed, which possessed the
distinct duct and secretory portions; however, the cavity was still
narrow. In addition, some undeveloped sweat glands were observed
(Fig. 1A).
Following 2 weeks of implantation, the number of
cell layers in the fetal epidermis was increased. New blood vessels
were detected in the deep dermis and more skin appendages could be
observed. The hair follicles and sweat glands possessed complete
structures and larger cavities (Fig.
1B). Following 4 weeks of implantation, the number of cell
layers in the fetal epidermis was further increased. The hair shaft
could be seen clearly and had grown out of the epidermis. In
addition, the volume of hair follicles and sweat glands was larger
(Fig. 1C). Following 6 weeks of
implantation, the epidermis of the fetal skin became thicker, and
the papillary layer of the dermis was detected. Furthermore, the
volume and number of skin appendages was markedly increased
(Fig. 1D). Following 8 weeks of
implantation, the fetal skin was almost completely developed, with
the papillary layer apparent and obviously differentiated cuticle.
The skin appendages, including sweat glands, sebaceous glands and
follicles exhibited normal, complete structures and stable
quantity. Some secretions could be seen in the cavity of the
sebaceous glands (Fig. 1E).
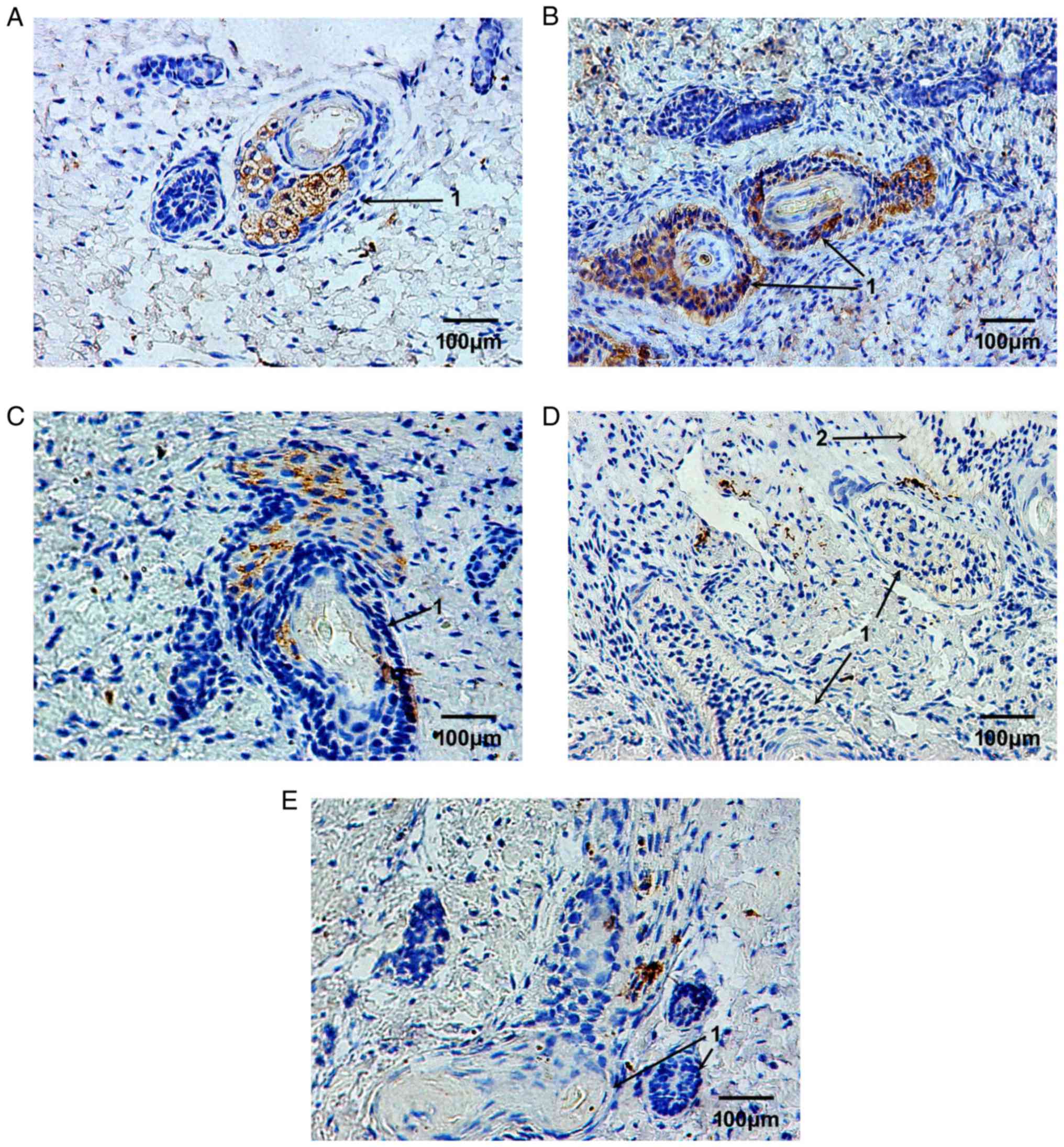
Using immunohistochemical staining, the expression
levels of MMP-9 (Fig. 2) and
TIMP-1 (Fig. 3) were detected
during the development of fetal skin outside the womb. In the skin
obtained from a 23-week-old fetus, the MMP-9-positive brownish
granules were widely distributed, and were mainly located in the
plasma of blastemal cells, in the deep epithelial cells, and in the
fibroblasts of developing skin appendages, such as follicles and
sweat glands (Fig. 2A). Following
2 weeks of implantation, the skin appendages survived and grew
well, their numbers were increased and their cavities became wider
and larger. The expression levels of MMP-9 were slightly increased
and were mostly located in the plasma of epithelial cells of skin
appendages and nearby fibroblasts (Fig. 2B). Following 4 weeks of
implantation, the expression levels of MMP-9 began to decrease. The
MMP-9-positive cells were mostly located in fibroblasts surrounding
hair follicles (Fig. 2C).
Following 6 and 8 weeks, the expression levels of MMP-9 were
significantly decreased and remained stable. The MMP-9-positive
cells were sporadically located in the plasma of fibroblasts
(Fig. 2D and E).
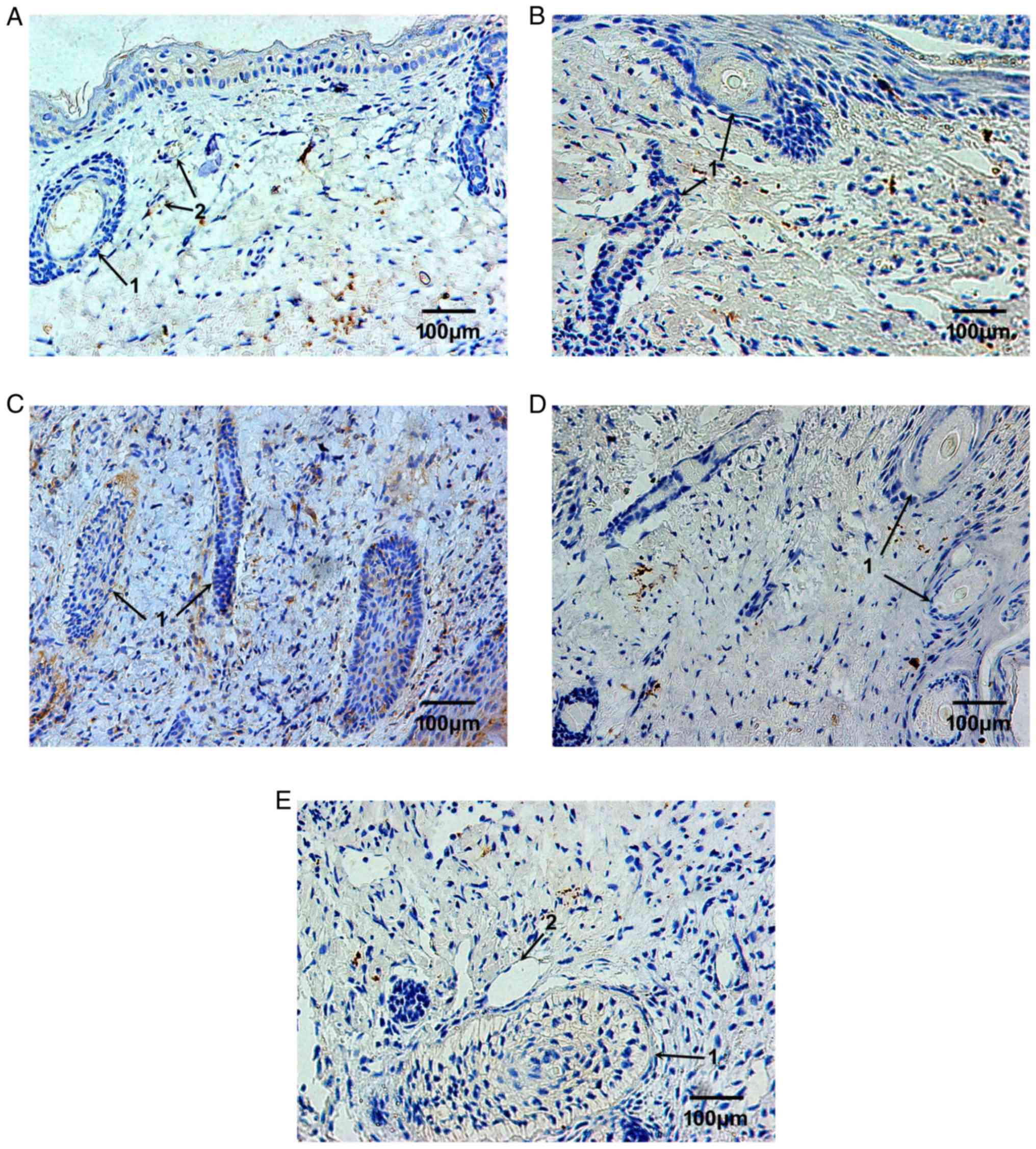
The expression levels of TIMP-1 were markedly lower
than MMP-9. A small number of TIMP-1-positive cells were observed
in the plasma of vascular epithelial cells in the dermis (Fig. 3A). Following 2 weeks of
implantation, TIMP-1 expression was reduced (Fig. 3B). Following 4 weeks of
implantation, TIMP-1 expression was markedly increased; the
TIMP-1-positive cells were mostly located around hair follicles
with narrow cavities (Fig. 3C).
Following 6 and 8 weeks, the expression of TIMP-1 was markedly
reduced to a very low level; therefore, TIMP-1-positive cells were
almost undetectable (Fig. 3D and
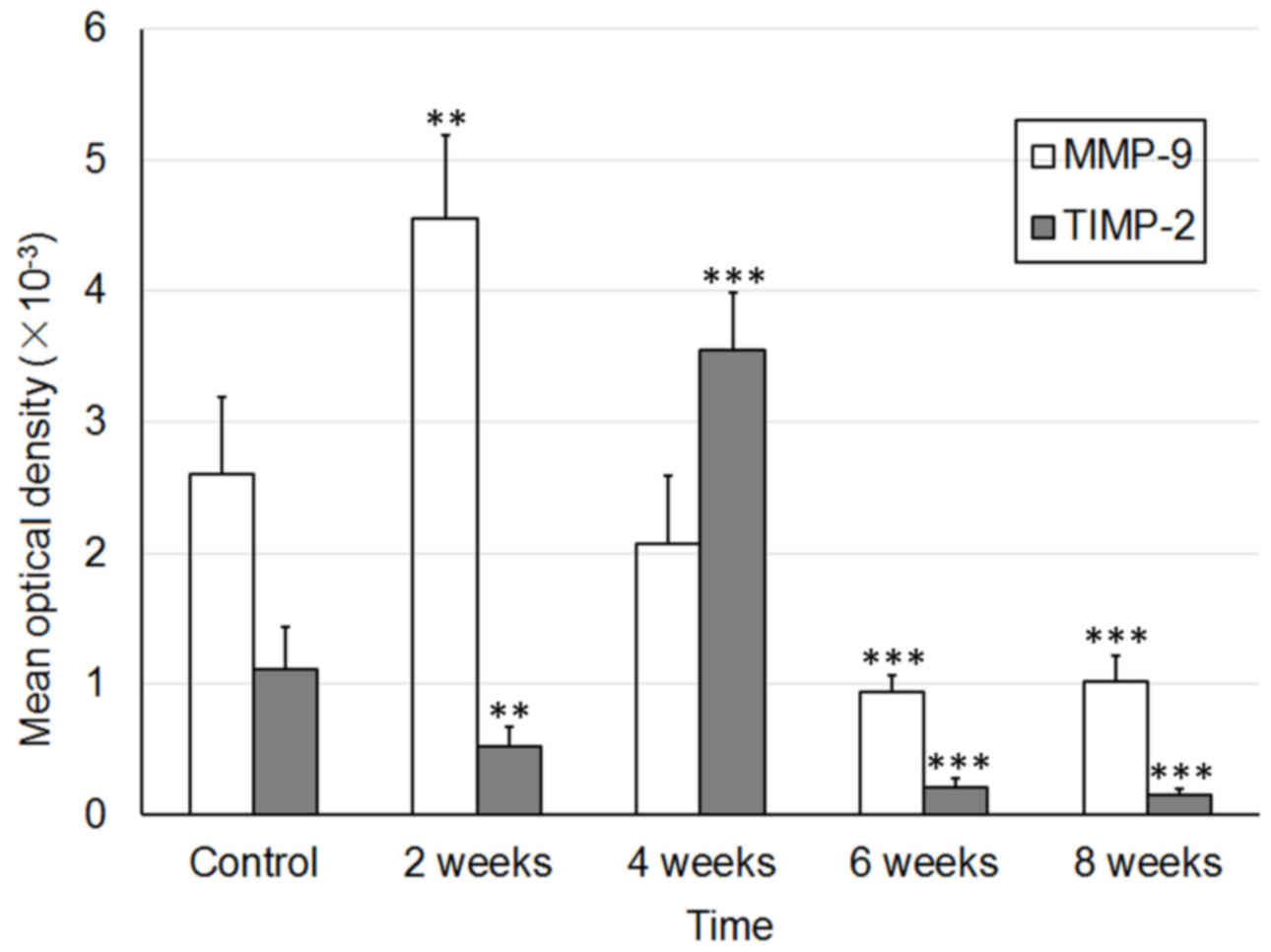
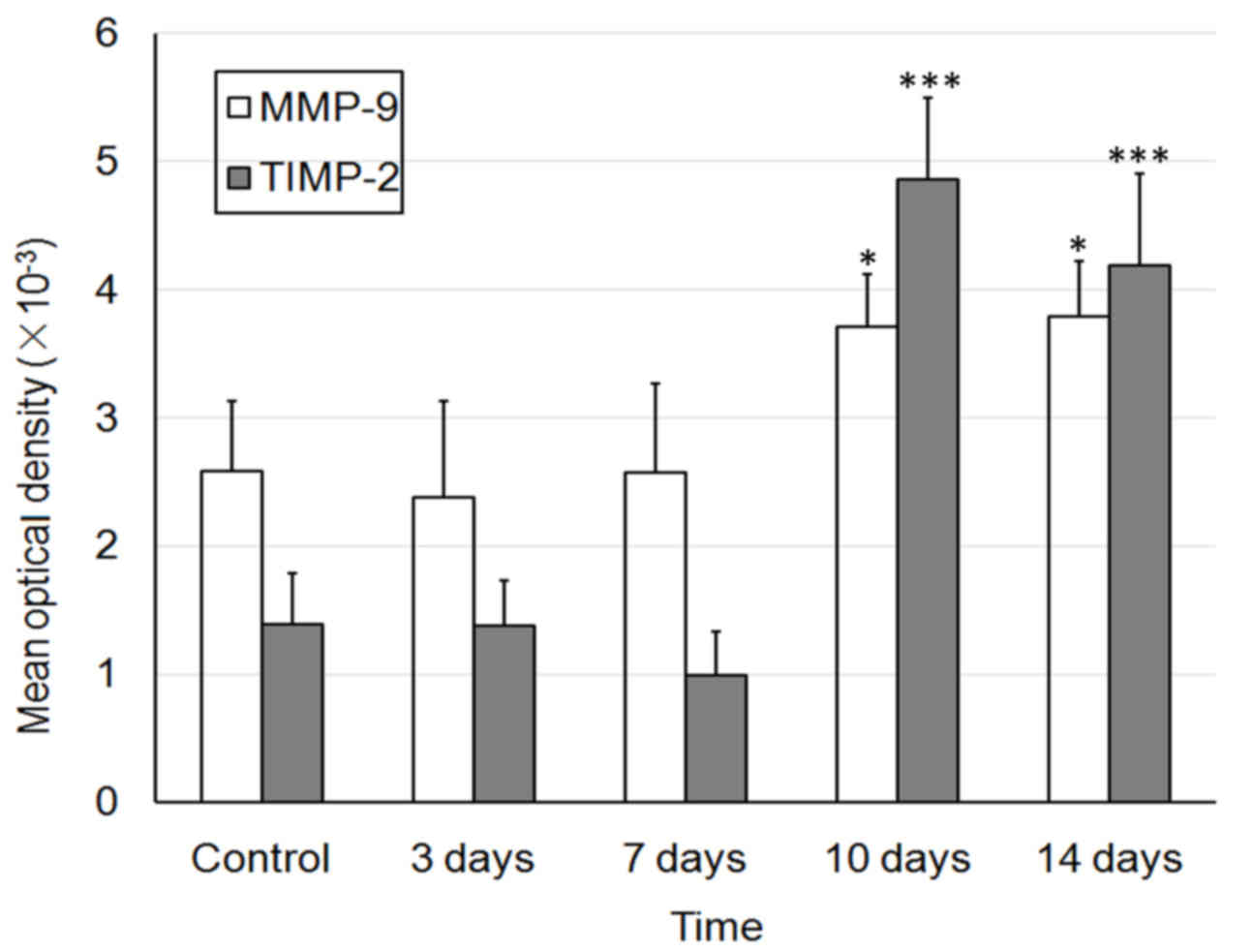
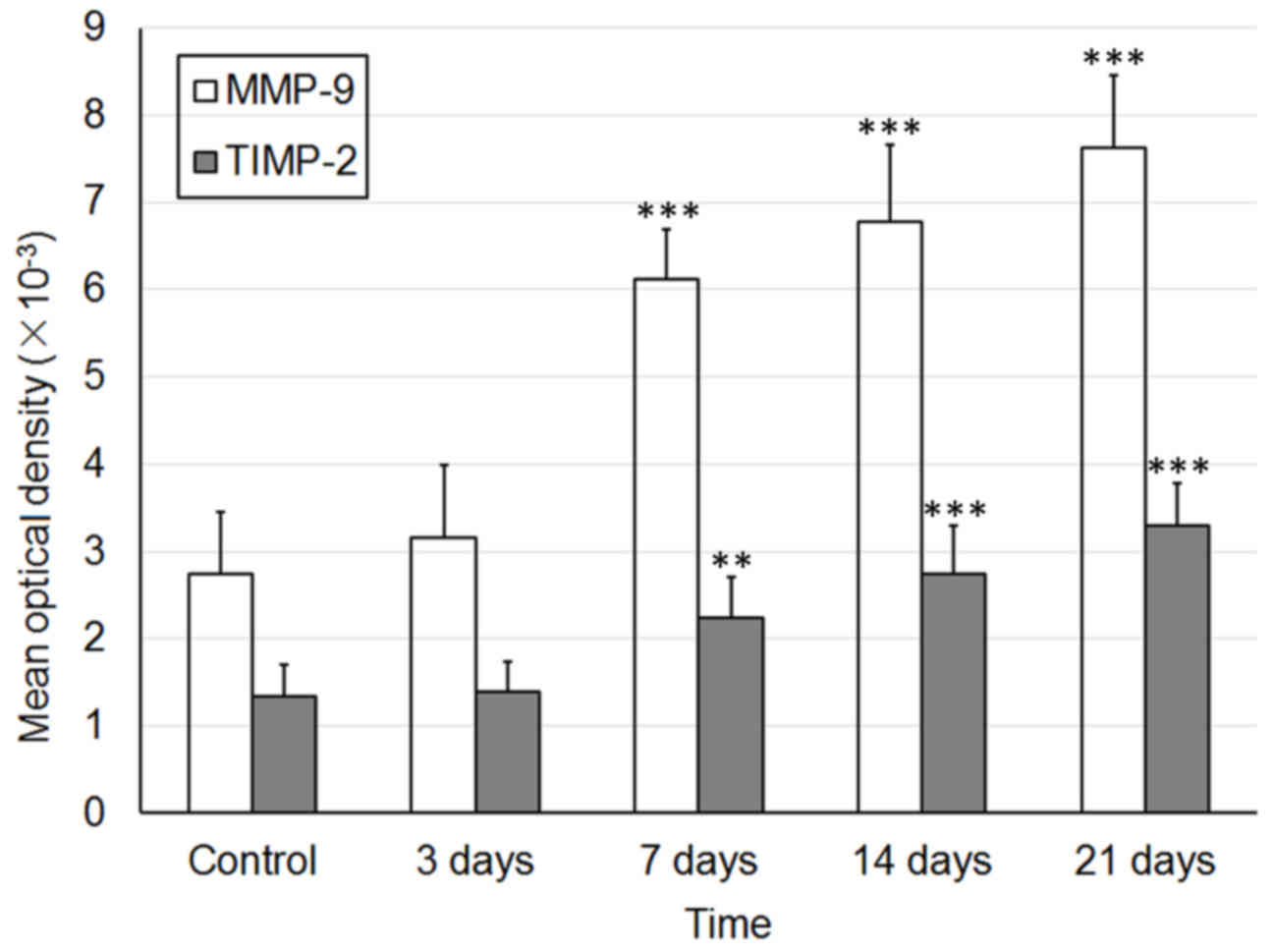
E). The MOD values of MMP and TIMP-1 are presented in Fig. 4.
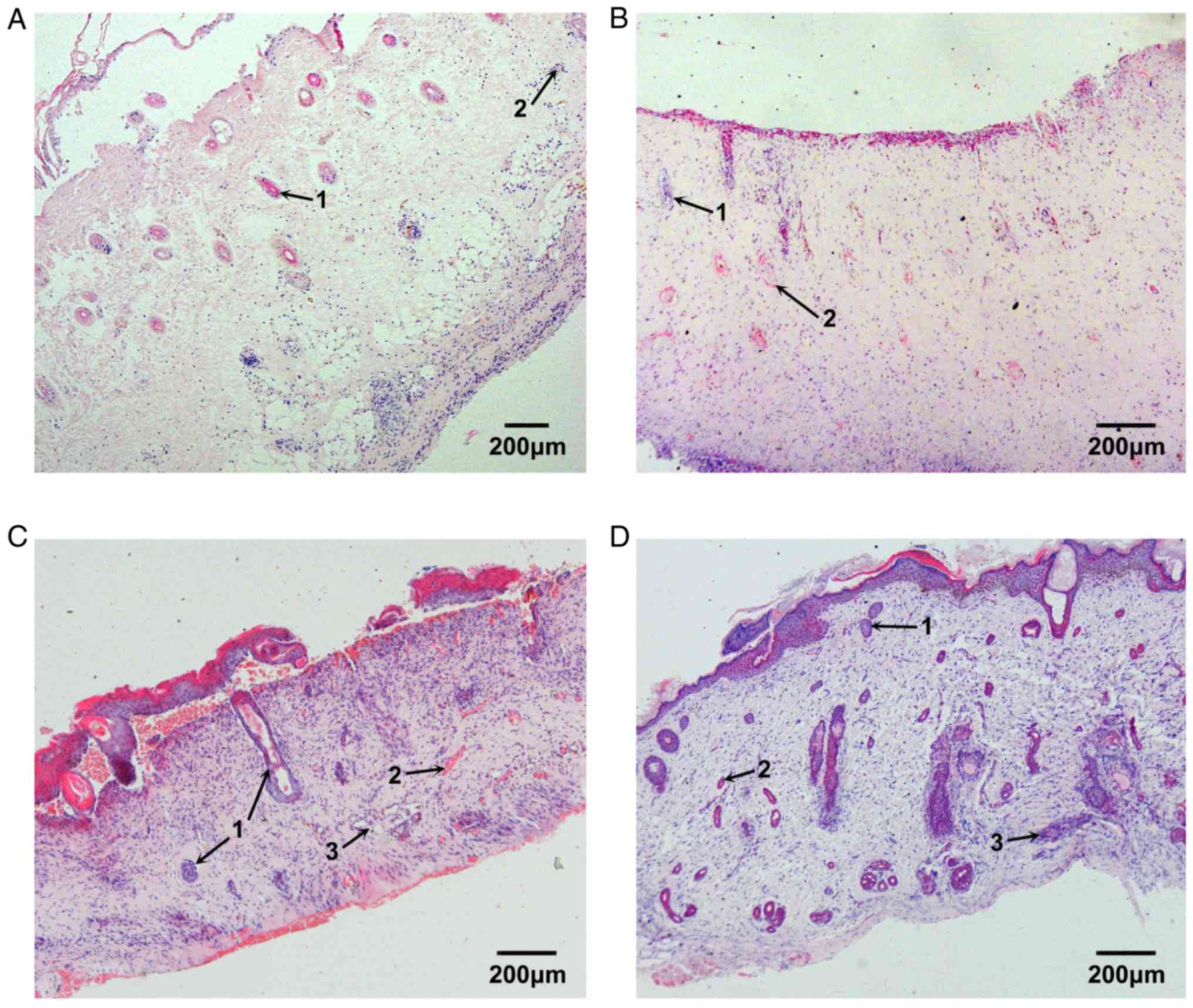
Repair process of burned fetal skin
implanted into nude mice
The epidermis and the superficial dermis of the
implanted fetal skin were removed 3 days after burn injury; the
basal cell layer was absent. Under microscopy, dilated blood
capillaries in the residual dermis were markedly congested. In
addition, the collagenous fibers of the deep dermis were well
arranged, and the structure of skin appendages, such as follicles
and blood vessels, were normal. A small number of inflammatory
cells infiltrated the burned tissue (Fig. 5A). Capillary hyperplasia in deep
tissue and fibroblast proliferation in superficial tissue were
apparent 7 days after burn injury and collagenous fibers were
deposited in the normal network structure. In addition, epithelial
cells of residual skin appendages migrated towards the burned
surface and began to proliferate (Fig.
5B). The proliferation of the epithelial cells of residual skin
appendages was markedly increased 10 days after burn injury, and
the epithelial cells that had reached the burn surface began to
regenerate the basal cell layer. The cavities of small blood
capillaries reopened, and were larger than the normal capillary
cavities. The collagenous fibers were better arranged, and sweat
glands and hair follicles began to develop (Fig. 5C). The basal cell layer had
completely differentiated and the integrity of repaired skin was
good 14 days after burn injury; more hair follicles were
developing, and appeared normal. Sweat glands continued to develop
and the structure of the collagenous fiber network was regular.
These observations suggested that the repair process of burned
fetal skin was complete (Fig.
5D).
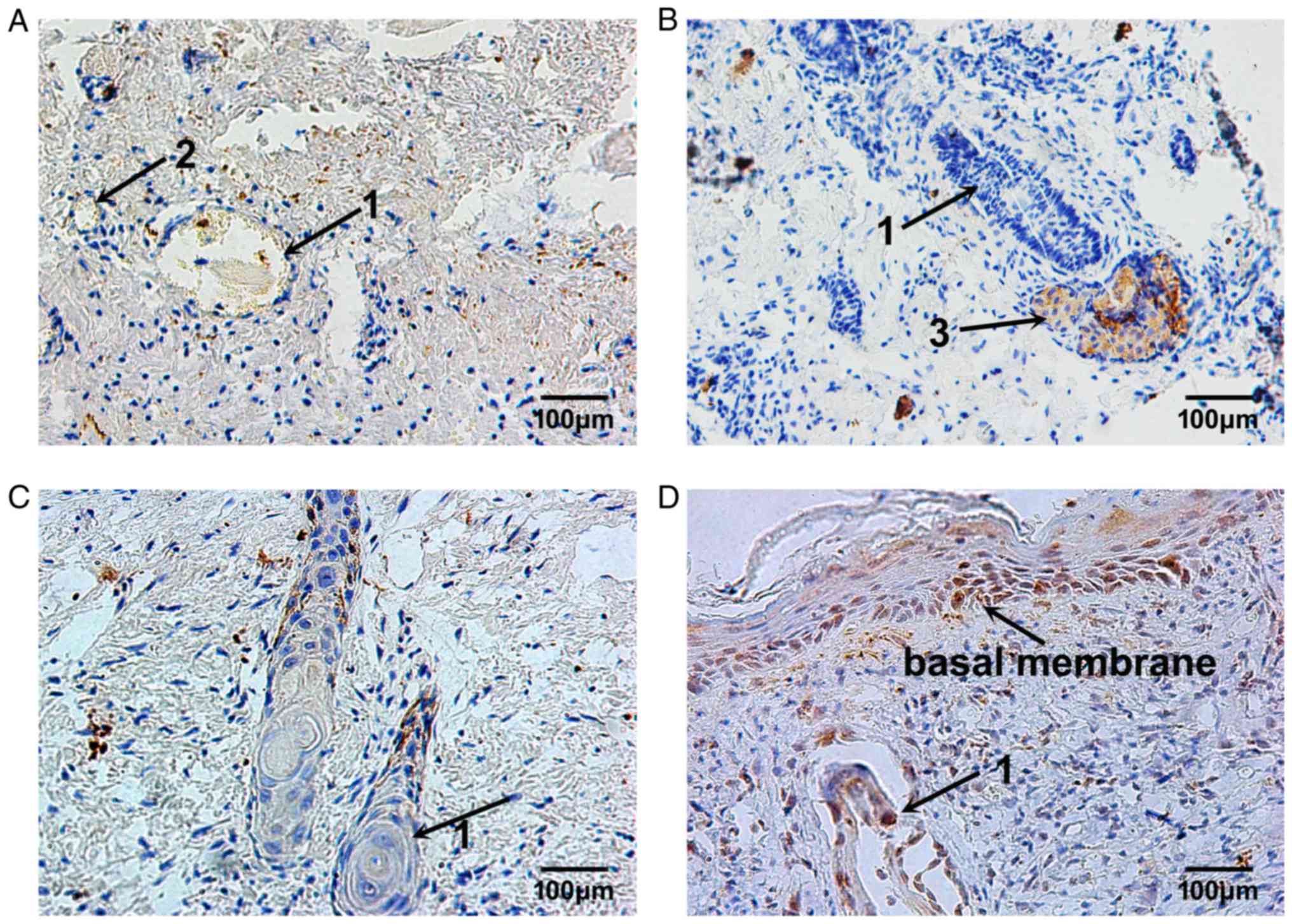
MMP-9 expression remained at a low level on day 3
following burn injury. The MMP-9-positive cells were located
sporadically in the superficial layer of the residual dermis
(Fig. 6A). With the elimination of
necrotic tissues and the migration of repairing cells, MMP-9
expression was continuously increased, from day 7 to day 10 after
burn injury, in the plasma of epithelial cells of residual hair
follicles and sweat glands, and in nearby fibroblasts (Fig. 6B and C). Then, 14 days following
burn injury, the MMP-9-positive cells were located in the plasma of
epithelial cells of hair follicles and in the cells of the basal
membrane, and the repair process of burned fetal skin was complete
(Fig. 6D).
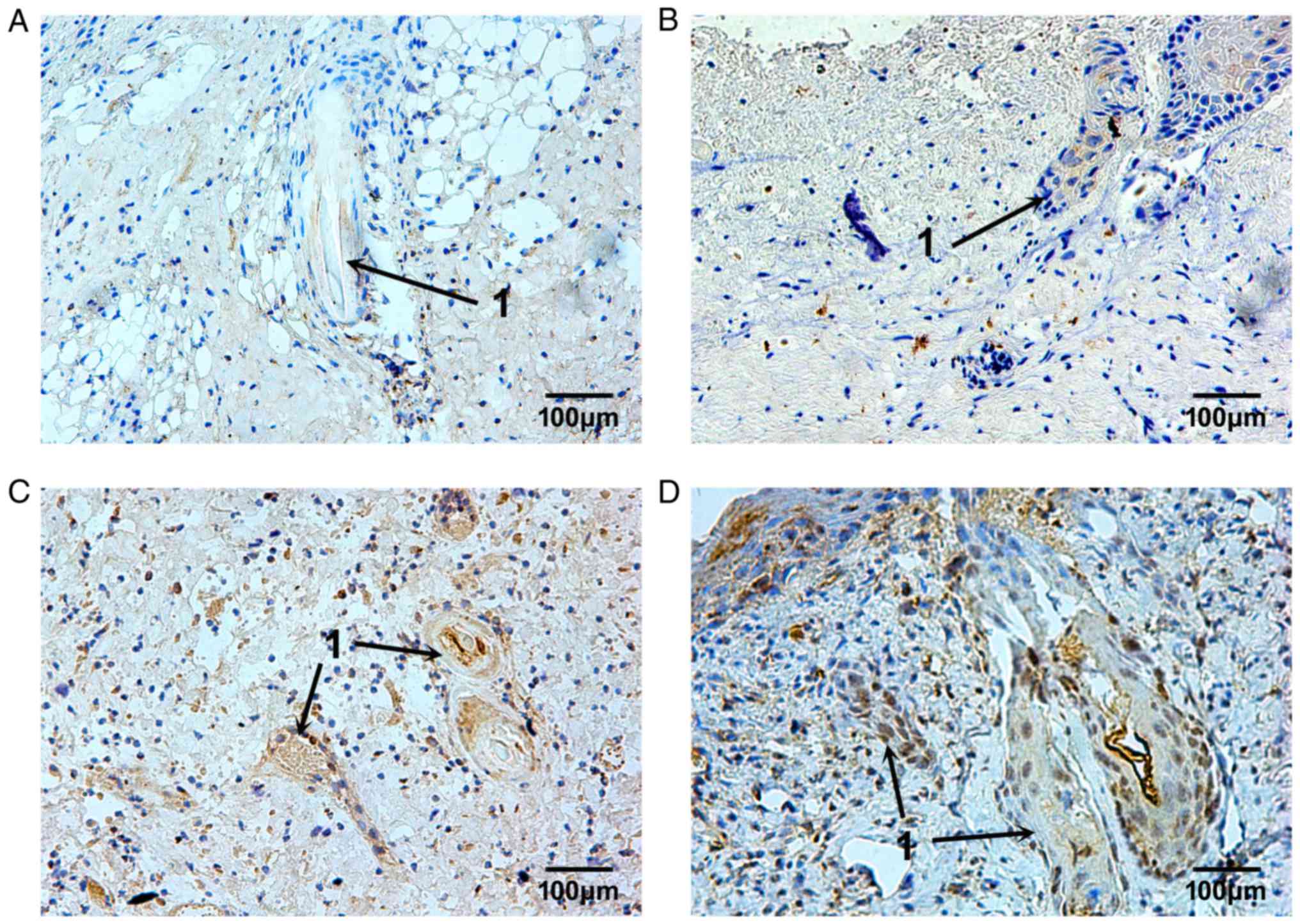
The expression pattern of TIMP-1 was similar to
MMP-9; however, the TIMP-1-positive granules were irregularly
distributed. Some of the TIMP-1-positive cells were located in the
plasma of epithelial cells of skin appendages 3 days after burn
injury, (Fig. 7A and B). From day
10 to 14 after burn injury, TIMP-1 expression in the plasma of
epithelial cells of skin appendages, and in fibroblasts in the
dermis, was markedly increased and remained so until the repair
process was complete (Fig. 7C and
D). The MOD values of MMP and TIMP-1 are presented in Fig. 8.
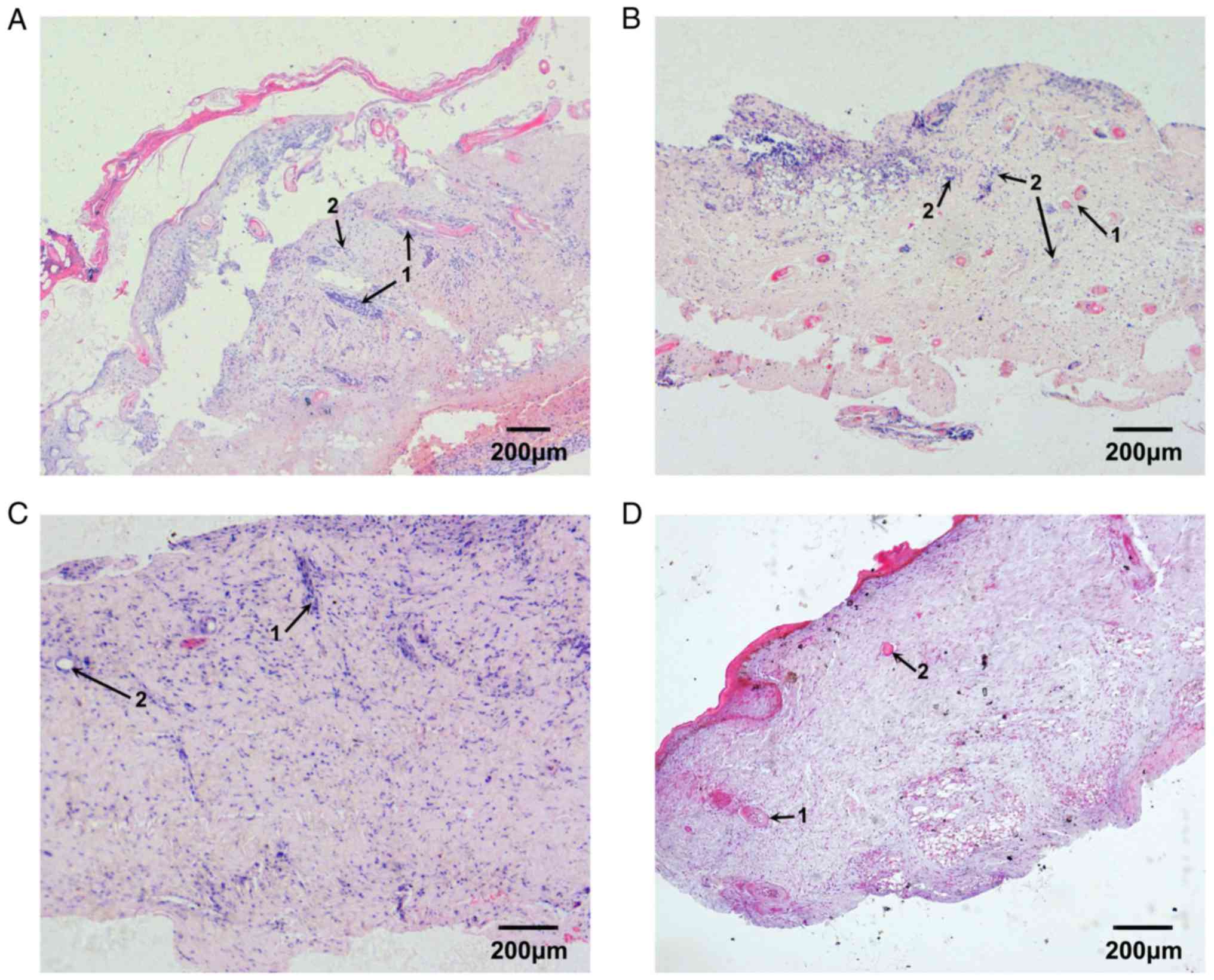
Effects of PBMCs on the repair process
of deep-degree burned fetal skin
Following 3 days of treatment with PBMCs, there was
no rejection reaction to the implanted PBMCs. Some inflammatory
cells were detected apparently infiltrating near the burn surface
and into the subcutaneous fatty layer (Fig. 9A). Following 7 days of treatment
with PBMCs, some new capillaries were revealed to be developing
near the burned surface and in the deep dermis. The inflammatory
reaction became more obvious and more inflammatory cells had
infiltrated into the fetal skin tissue. The arrangement of
collagenous fibers improved, but became tighter than that observed
in skin untreated with PBMCs (Fig.
9B). Following 14 days of treatment with PBMCs, the
inflammatory reaction was weaker; however, more fibroblasts began
to proliferate and infiltrate into the fetal skin and produce
excessive collagen, which resulted in scar formation. Although
collagenous fibers were deposited in the injured tissue, the
collagenous fiber network was disordered. In addition, the residual
skin appendages developed slowly and abnormally. The epithelial
cell layer was hardly repaired, and the basal and papillary layers
had not formed, and were replaced by an overgrowth of fibroblasts
and collagen-like scar tissue (Fig.
9C). Compared with untreated burned skin, the repair process of
burned skin treated with PBMCs was incomplete after 21 days, and
hyperplastic scar tissue was detected. Epithelial cells that
infiltrated near the burn surface regenerated the basal layer;
however, the epithelium was incomplete and dermal papilla could not
develop. The majority of skin appendages degenerated and could not
develop normally. At the end point of the study, the burned fetal
skin was wholly occupied by hyperplastic scar and possessed only
incomplete epithelium (Fig.
9D).
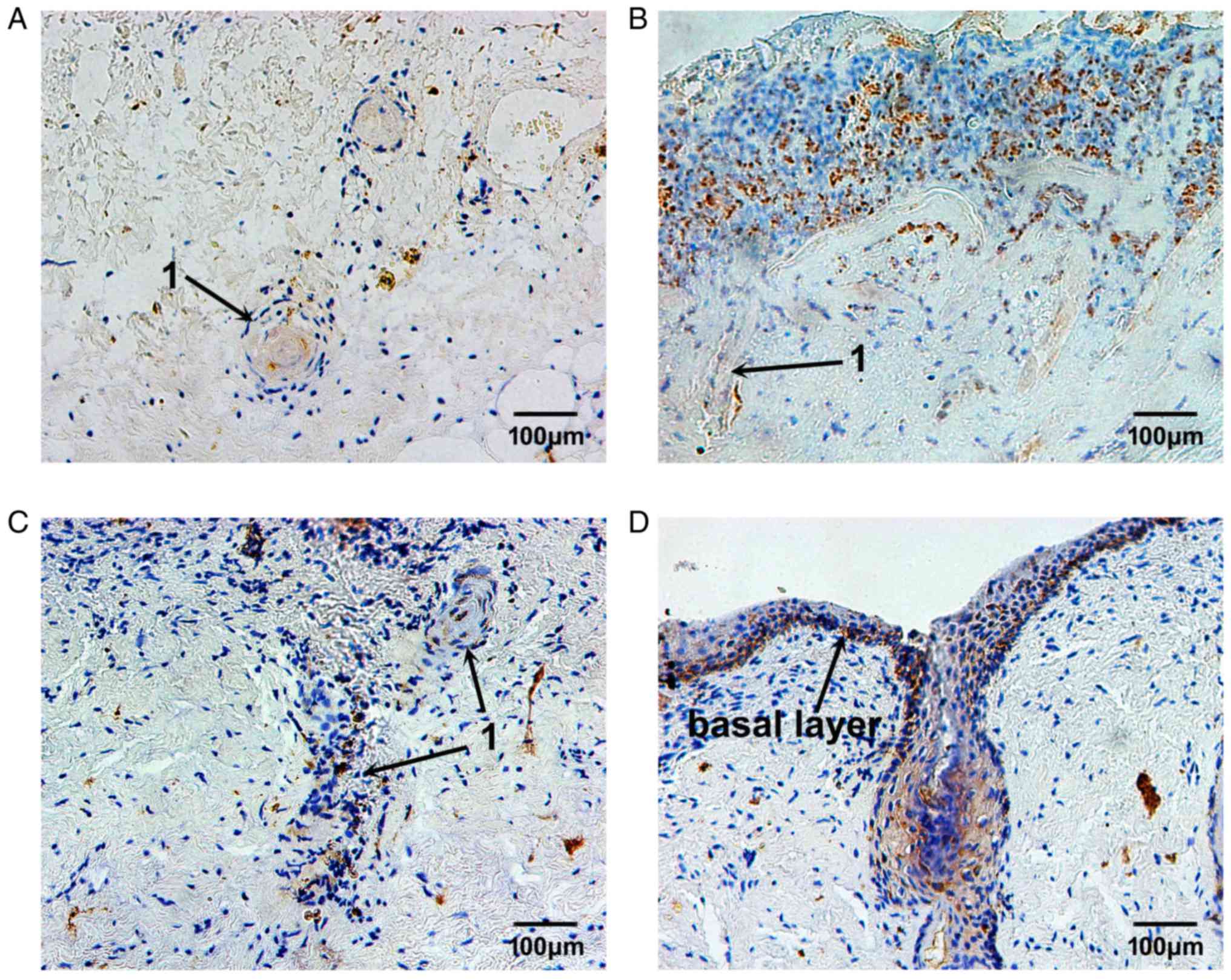
MMP-9 expression was not markedly altered after 3
days of burn and PBMCs treatment (Fig. 10A). However, after 7 days of PBMCs
treatment, MMP-9 expression was obviously increased in the plasma
of some inflammatory cells and fibroblasts near the burn surface.
In some epithelial cells of the residual skin appendages,
MMP-9-positive granules were also observed (Fig. 10B). Following 14 days of PBMCs
treatment, the MMP-9-positive granules were predominantly located
in the plasma of proliferating epithelial cells, which infiltrated
towards the burn surface. MMP-9 expression began to decrease in the
plasma of fibroblasts near the burn surface. In addition,
MMP-9-positive granules were hardly detected in subcutaneous tissue
(Fig. 10C). On day 21 following
PBMCs treatment, MMP-9 expression was only detected in the local
basal layer, which had completely differentiated (Fig. 10D).
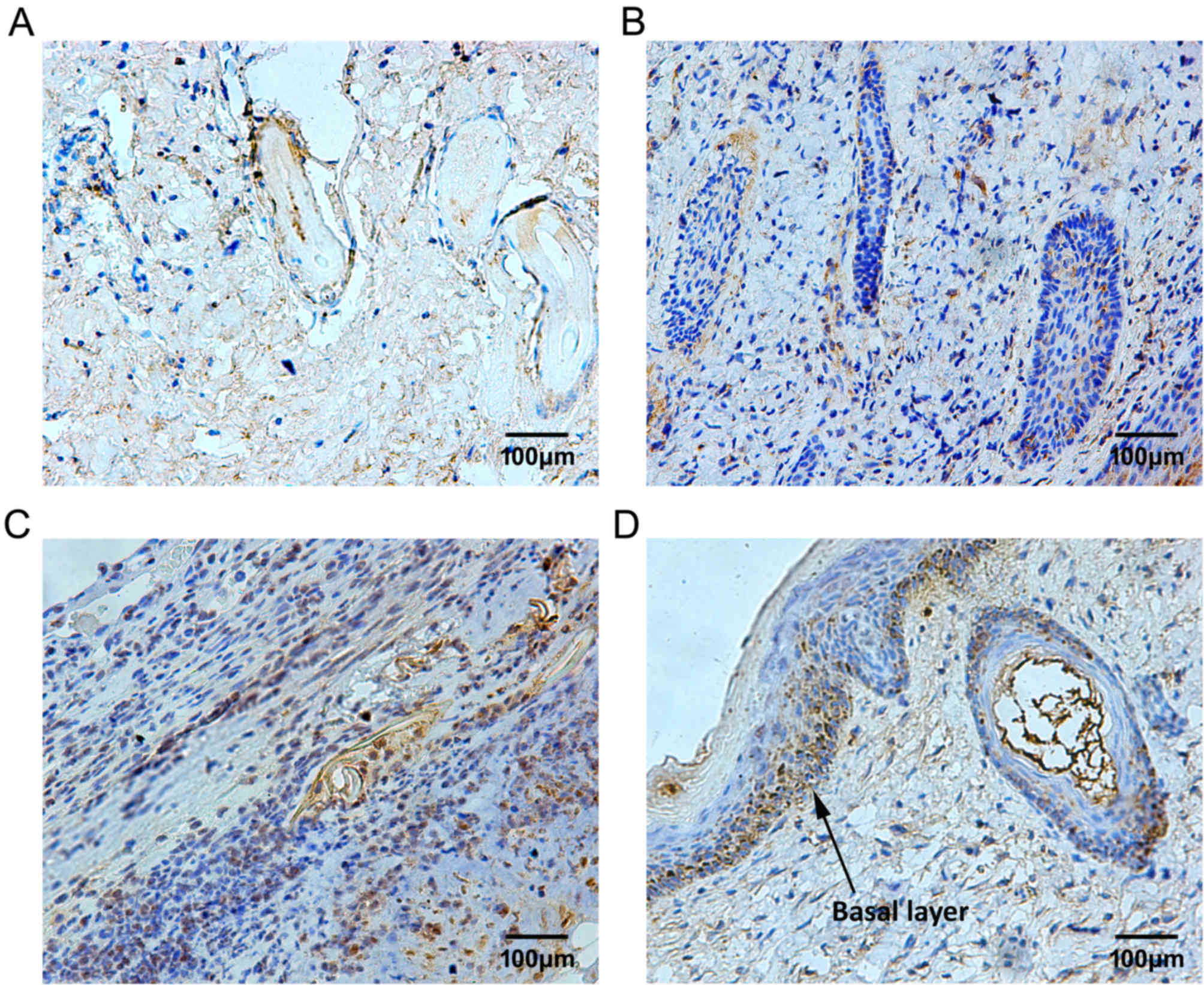
In response to PBMCs treatment, the expression
pattern of TIMP-1 was similar to that of MMP-9 during the repair
process of deep-degree burned fetal skin; however, it was
maintained at a relatively low level. Following 3 days of PBMCs
treatment, a small number of TIMP-1-positive granules were
sporadically distributed (Fig.
11A). On day 7 following PBMCs treatment, TIMP-1-positive cells
began to increase among fibroblasts and some cells in hair
follicles (Fig. 11B).
Subsequently, the expression levels of TIMP-1 were continuously
increased. Finally, the distribution of TIMP-1-positive cells was
located in the local basal layer, which was similar to
MMP-9-positive cells (Fig. 11C and
D). The MOD values of MMP and TIMP-1 are presented in Fig. 12.
Discussion
Hypertrophic scar formation and contracture
following deep-degree burn-induced extensive skin defects can
result in serious disability. Therefore, reducing scar formation is
considered a significant issue in burn injury management (2,20,21).
Scarless healing, the ideal subsequent to skin injury, exists in
human fetuses up to 24 weeks of pregnancy (12,22–24).
The amniotic fluid that surrounds the fetus is warm, sterile, and
rich in nutrients, growth factors and extracellular matrix (ECM)
elements, including hyaluronic acid and fibronectin, which are all
important for wound healing (12,25).
However, in a previous study, when wounded skin from adult goats
was transplanted into goat fetuses, and supplied with goat fetal
blood, scar formation still occurred (26). Conversely, scarless healing has
been observed in fetal skin outside the womb (19). Therefore, it may be suggested that
scarless healing of fetal skin depends on the characteristics of
the skin itself, and is not associated with the external
environment. Identifying the mechanisms that underlies scarless
healing may facilitate the clinical treatment of patients with
extensive burns and could be used to generate treatments that may
be applied to adult burn wounds. However, such mechanisms and
treatments are not currently available.
During the healing process of incision wounds, the
skin undergoes three stages: Inflammatory stage, hyperplasia stage
and reconstruction stage (27).
The healing process of burn wounds is complex and involves four
stages: Inflammatory response, neovascularization, granulation
tissue formation, and epithelium and connective tissue remodeling
(28,29). The healing process of deep-degree
burn wounds is markedly different compared with the healing process
of incision wounds. On one hand, there is apparent dermal necrosis
in burn wounds, which induces chemotaxis of inflammatory cells.
Inflammatory cells can aggregate together to secrete various
enzymes, which can lyse and phagocytose necrotic tissue, and
release numerous inflammatory factors and cytokines that can
facilitate infiltration of fibroblasts and vascular epithelial
cells into burned and defective tissues. On the other hand, in burn
wounds, the regeneration of epithelial cells depends on
proliferation, differentiation and infiltration of epithelial cells
of residual skin appendages. Conversely, in incision wounds,
regenerative epithelial cells are usually sourced from the wound
edge.
The present study generated a mouse model carrying
burned fetal skin. In the pre-experiment, a piece of dorsal skin
was cut from nude mice; the open wound was then covered and fetal
skin was fixed onto the wound surface. Initially, fetal skin was
superficially transplanted onto the wound, which was similar to the
method used to treat burns with tissue-engineered skin. However,
the transplanted fetal skin rarely survived or could not develop
normally. It was hypothesized that this failure may be because the
open environment was unlike the intrauterine environment;
therefore, the fetal skin was subcutaneously implanted into the
nude mice, which was successful.
The healing process was observed and the fetal skin
was revealed to require14 days to complete the healing process
following burn injury, which was less time than the same process in
burned adult skin (30). In
addition, the collagen fibers formed in the burned fetal skin were
well arranged and similar to the fibers in unwounded fetal skin.
Fetal skin has been demonstrated to possess strong proliferative
and differentiative capabilities, as characterized by positive
proliferating cell nuclear antigen (PCNA) expression in epithelial
cells and dermal fibroblasts (31). Following burn injury, fetal skin is
able to produce various cytokines, which may induce these
PCNA-positive cells to proliferate, differentiate and migrate
toward the wound surface, resulting in the reconstruction of
defective tissues.
The present study detected no inflammatory cell
infiltration during the acute inflammatory phase (2 weeks; Fig. 1B), which is similar to the healing
process of incision wounds in fetal skin (13). During the healing process of
wounded fetal skin, the aggregation of platelets is decreased
compared with in adult skin, which may result in a decrease in the
release of transforming growth factor (TGF)-β1, TGF-β2 and
platelet-derived growth factor (32). The lack of these inflammatory
factors could decrease the chemotaxis of inflammatory cells.
Consequently, the lack of neutrophils may reduce the release of
enzymes with the function of lysing necrotic tissues, and the
injured cells may therefore die in an apoptotic manner, instead of
by direct lysis. These apoptotic cells will be engulfed by
fibroblasts instead of macrophages (33). Furthermore, the lack of
inflammatory cells may reduce the stimulation of fibroblasts and
the vascular epithelial cells, thus avoiding excessive generation
of collagens and excessive hyperplasia of granulation tissue. The
present study hypothesized that the mechanism underlying necrotic
tissue removal in burned wound of fetal skin is similar to the
hypothesis outlined above; however, further study is required for
its full investigation.
The MMP family consists of collagenases, gelatinases
and stromelysins (34,35), all of which serve important roles
in ECM reconstruction, epithelial regeneration and
revascularization (36). TIMPs are
able to suppress the activity of MMPs. During the scarless healing
process, MMP expression is markedly increased and the expression of
TIMP is decreased. The increasing MMP/TIMP ratio promotes the
degradation of ECM and is associated with scarless healing in fetal
skin (37). During the wound
healing process, certain cells, including inflammatory cells and
epithelial cells of skin appendages, may exhibit enhanced MMP-9
expression (38). An increased
level of MMP-9 is often observed during the early inflammatory
phase of wound healing (39).
Repair cells, which regenerate tissues in the wound or repair
damaged tissue, have an important role in the wound healing
process, and proliferate, differentiate and migrate to the wound
surface where they reconstruct defective tissues. Once repair cells
are activated, the ECM around these cells and in their migratory
path is degraded, in order to increase the migration of these
repair cells. As the repair cells reach the required position, ECM
is reconstructed to provide an appropriate environment for the
repair cells (40). As an
endogenous inhibitor of MMP-9, TIMP-1 secretion by fibroblasts can
combine irreversibly with the active center of activated MMP-9,
resulting in the inhibition of MMP-9 activity, which can reduce the
excessive degradation of ECM molecules (41,42).
In the present study, the positive expression of
MMP-9 and TIMP-1 was observed in the cellular plasma of blastemal
cells in the sweat glands, as well as in the epithelial cells and
fibroblasts in immature skin appendages, including follicles and
sweat glands. During the process of fetal skin implantation, MMP-9
expression was markedly increased after 2 weeks. With the
proliferation of fibroblasts, TIMP-1 expression began to increase,
resulting in the suppression of MMP-9, which was clearly observed
after 4 weeks.
During the early stage of burn healing in fetal
skin, the expression levels of MMP-9 and TIMP-1 remained stable,
which indicated that the inflammatory response was inactivated.
However, 10 days after burn injury, with the development of the
skin appendages, the expression levels of MMP-9 were markedly
increased, in accordance with the number of proliferating repair
cells. This may have resulted in the degradation of ECM surrounding
fibroblasts and epithelial cells in skin appendages, and finally
the migration of proliferative repair cells to the wound surface.
As the repair cells migrated to the wound surface and
differentiated into a new layer of basal cells, MMP-9 expression
began to decrease. Meanwhile, the expression of TIMP-1 was
increased, which may further inhibit MMP-9 activity, facilitate the
deposition of ECM molecules and maintain stability of the new basal
cell layer (43).
Human PBMCs are a group of peripheral blood
mononuclear cells, including T lymphocytes, which are important for
regulating the inflammatory response (44,45).
During the process of hyperplastic scar formation in adults,
numerous T lymphocytes can be detected (46); however, during the process of
scarless wound healing in the oral mucosa, the number of
lymphocytes is very small (47). T
lymphocytes, including T helper (Th) and T suppressor cells, are
involved in wound healing (48). T
suppressor cells inhibit wound healing (49). Th1 cells are able to secrete
interleukin (IL)-2 and interferon-γ, which may suppress the
synthesis of collagens, and increase the expression and activity of
collagenase, subsequently resulting in degradation of collagen
(50). Th2 cells predominantly
secrete IL-4 and IL-13, which may act on fibroblasts to promote the
generation of collagen and fibronectin, thus facilitating scar
formation (51). Mononuclear cells
often become larger with a stronger phagocytic ability during the
wound healing process, and are finally transformed into macrophages
(52). Macrophages can secrete
collagenase, elastase and plasminogen activator, which facilitate
ECM degradation, and secrete certain cytokines, including TGF-β,
epidermal growth factor, to induce an inflammatory response
(53). Therefore, activated
mononuclear cells can facilitate wound healing and scar formation
(54).
The present study demonstrated that when PBMCs were
used to treat burned fetal skin, inflammatory cells were observed
near the wound surface on day 7 after burn injury. In the cellular
plasma of these cells, MMP-9-positive granules were observed; this
may be caused by the release of inflammatory factors from the
burned fetal skin, which could induce chemotaxis of mononuclear
cells and MMP-9 release. By day 14 following burn injury, the
inflammatory response had become stronger and the expression levels
of MMP-9 increased near the wound surface, but were reduced in the
subcutaneous layer, which may be due to macrophage-induced
chemotaxis of T lymphocytes to the wound surface. Subsequently, a
number of infiltrating fibroblasts generated collagens, which
exhibited a disordered arrangement, and the duration of the healing
process increased. These findings indicated that T suppressor cells
and Th2 cells may serve a dominant role in this process.
Furthermore, fibroblasts could secrete TIMP-1 to suppress ECM
degradation by inhibiting MMP-9, which resulted in the excessive
deposition of collagens. Finally, scar formation occurred in the
PBMCs treatment group.
In conclusion, fetal skin was subcutaneously
implanted into a dorsal skin flap in nude mice. The results
demonstrated that the skin exhibited similar development to that of
skin grown in the womb. Subsequently, a mouse model carrying burned
fetal skin was successfully established. In the mouse model,
scarless healing was observed, and was completed within 2 weeks.
However, following treatment with PBMCs, the burned fetal skin may
generate certain inflammatory factors to induce an inflammatory
response; finally, the healing process in the PBMCs-treated group
was slower and associated with scar formation. MMP-9 may be
associated with the proliferation of fibroblasts, whereas the
inhibitory effects of TIMP-1 onMMP-9 may serve an important role in
the process of scar formation. The results of the present study
demonstrated that exogenous immune cells may alter the lowered
immune response environment, which is required for scarless
healing, resulting in scar formation. Therefore, the results
suggested that the involvement of inflammatory cells is important
in the healing process of deep-degree burned skin; however, the
mechanism remains unclear and requires further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30772258, 81071560
and 81372074), the Special Ally Project of Natural Science
Foundation of Shandong Province (grant no. ZR2014HL060) and the
Jinan Young Star Project of Science and Technology (grant no.
2013031).
References
|
1
|
Reinke JM and Sorg H: Wound repair and
regeneration. Eur Surg Res. 49:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orgill DP and Ogawa R: Current methods of
burn reconstruction. Plast Reconstr Surg. 131:827e–836e. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu X: Wound care in China: From repair to
regeneration. Int J Low Extrem Wounds. 11:143–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu S, Xiang J, Qing C, Jin S, Liao Z and
Shi J: Effect of necrotic tissue on progressive injury in deep
partial thickness burn wounds. Chin Med J (Engl). 115:323–325.
2002.PubMed/NCBI
|
|
5
|
Galatz LM, Gerstenfeld L, Heber-Katz E and
Rodeo SA: Tendon regeneration and scar formation: The concept of
scarless healing. J Orthop Res. 33:823–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu H and Xu AA: Towards the Holy Grail:
What can we do for truly scarless surgery? World J Gastrointest
Endosc. 7:814–818. 2015.PubMed/NCBI
|
|
7
|
Choi JW, Park JK, Chang JW, Kim DY, Kim
MS, Shin YS and Kim CH: Small intestine submucosa and mesenchymal
stem cells composite gel for scarless vocal fold regeneration.
Biomaterials. 35:4911–4918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Srokowski EM and Woodhouse KA: Evaluation
of the bulk platelet response and fibrinogen interaction to
elastin-like polypeptide coatings. J Biomed Mater Res A.
102:540–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Namazi MR, Fallahzadeh MK and Schwartz RA:
Strategies for prevention of scars: What can we learn from fetal
skin? Int J Dermato. 50:85–93. 2011. View Article : Google Scholar
|
|
10
|
Longaker MT, Bouhana KS, Harrison MR,
Danielpour D, Roberts AB and Banda MJ: Wound healing in the fetus.
Possible role for inflammatory macrophages and transforming growth
factor-beta isoforms. Wound Repair Rege. 2:104–112. 1994.
View Article : Google Scholar
|
|
11
|
Armstrong JR and Ferguson MW: Ontogeny of
the skin and the transition from scar-free to scarring phenotype
during wound healing in the pouch young of a marsupial, Monodelphis
domestica. Dev Biol. 169:242–260. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larson BJ, Longaker MT and Lorenz HP:
Scarless fetal wound healing: A basic science review. Plast
Reconstr Surg. 126:1172–1180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burrington JD: Wound healing in the fetal
lamb. J Pediatr Surg. 6:523–528. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Estes JM, Adzick NS, Harrison MR, Longaker
MT and Stern R: Hyaluronate metabolism undergoes an ontogenic
transition during fetal development: Implications for scar-free
wound healing. J Pediatr Surg. 28:1227–1231. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whitby DJ and Ferguson MW:
Immunohistochemical localization of growth factors in fetal wound
healing. Dev Biol. 147:207–215. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu M, Peled ZM, Chin GS, Liu W and
Longaker MT: Ontogeny of expression of transforming growth
factor-beta 1 (TGF-beta 1), TGF-beta 3 and TGF-beta receptors I and
II in fetal rat fibroblasts and skin. Plast Reconstr Surg.
107:1787–1796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Colwell AS, Beanes SR, Soo C, Dang C, Ting
K, Longaker MT, Atkinson JB and Lorenz HP: Increased angiogenesis
and expression of vascular endothelial growth factor during
scarless repair. Plast Reconstr Surg. 115:204–212. 2005.PubMed/NCBI
|
|
18
|
Liechty KW, Kim HB, Adzick NS and
Crombleholme TM: Fetal wound repair results in scar formation in
interleukin-10-deficient mice in a syngeneic murine model of
scarless fetal wound repair. J Pediatr Surg. 35:866–873. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lane AT, Scott GA and Day KH: Development
of human fetal skin transplanted to the nude mouse. J Invest
Dermatol. 93:787–791. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eldad A, Din A, Weinberg A, Neuman A,
Lipton H, Ben-Bassat H, Chaouat M and Wexler MR: Cryopreserved
cadaveric allografts for treatment of unexcised partial thickness
flame burns: Clinical experience with 12 patients. Burns.
23:608–614. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kagan RJ, Peck MD, Ahrenholz DH, Hickerson
WL, J IV Holmes, Korentager R, Kraatz J and Pollock K: Surgical
management of the burn wound and use of skin substitutes: An expert
panel white paper. J Burn Care Res. 34:e60–e79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Julia MV, Albert A, Morales L, Miro D,
Sancho MA and Garcia X: Wound healing in the fetal period: The
resistance of the scar to rupture. J Pediatr Surg. 28:1458–1462.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colwell AS, Krummel TM, Longaker MT and
Lorenz HP: An in vivo mouse excisional wound model of scarless
healing. Plast Reconstr Surg. 117:2292–2296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lorenz HP, Lin RY, Longaker MT, Whitby DJ
and Adzick NS: The fetal fibroblast: The effector cell of scarless
fetal skin repair. Plast Reconstr Surg. 96:1251–1261. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rolfe KJ and Grobbelaar AO: A review of
fetal scarless healing. ISRN Dermatol. 2012:6980342012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Longaker MT, Whitby DJ, Ferguson MW,
Lorenz HP, Harrison MR and Adzick NS: Adult skin wounds in the
fetal environment heal with scar formation. Ann Surg. 219:65–72.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Jia S, Geoffrey R, Alemzadeh R,
Ghosh S and Hessner MJ: Identification of a molecular signature in
human type 1 diabetes mellitus using serum and functional genomics.
J Immunol. 180:1929–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Velnar T, Bailey T and Smrkolj V: The
wound healing process: An overview of the cellular and molecular
mechanisms. J Int Med Res. 37:1528–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tintinalli JE: Emergency Medicine: A
Comprehensive Study Guide. McGraw-Hill Companies; New York, NY: pp.
1374–1386. 2010
|
|
31
|
Kurki P, Ogata K and Tan EM: Monoclonal
antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as
probes for proliferating cells by immunofluorescence microscopy and
flow cytometry. J Immunol Methods. 109:49–59. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olutoye OO, Yager DR, Cohen IK and
Diegelmann RF: Lower cytokine release by fetal porcine platelets: A
possible explanation for reduced inflammation after fetal wounding.
J Pediatr Surg. 31:91–95. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin P, D'Souza D, Martin J, Grose R,
Cooper L, Maki R and McKercher SR: Wound healing in the PU.1 null
mouse-tissue repair is not dependent on inflammatory cells. Curr
Biol. 13:1122–1128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matrisian LM: The matrix-degrading
metalloproteinases. Bioessays. 14:455–463. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
36
|
Bullard KM, Cass DL, Banda MJ and Adzick
NS: Transforming growth factor beta-1 decreases interstitial
collagenase in healing human fetal skin. J Pediatr Surg.
32:1023–1027. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Soo C, Shaw WW, Zhang X, Longaker MT,
Howard EW and Ting K: Differential expression of matrix
metalloproteinases and their tissue-derived inhibitors in cutaneous
wound repair. Plast Reconstr Surg. 105:638–647. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manuel JA and Gawronska-Kozak B: Matrix
metalloproteinase 9 (MMP-9) is upregulated during scarless wound
healing in athymic nude mice. Matrix Biol. 25:505–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gillard JA, Reed MW, Buttle D, Cross SS
and Brown NJ: Matrix metalloproteinase activity and
immunohistochemical profile of matrix metalloproteinase-2 and −9
and tissue inhibitor of metalloproteinase-1 during human dermal
wound healing. Wound Repair Regen. 12:295–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Steffensen B, Häkkinen L and Larjava H:
Proteolytic events of wound-healing-coordinated interactions among
matrix metalloproteinases (MMPs), integrins and extracellular
matrix molecules. Crit Rev Oral Biol Med. 12:373–398. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao WQ, Li H, Yamashita K, Guo XK,
Hoshino T, Yoshida S, Shinya T and Hayakawa T: Cell
cycle-associated accumulation of tissue inhibitor of
metalloproteinases-1 (TIMP-1) in the nuclei of human gingival
fibroblasts. J Cell Sci. 111:1147–1153. 1998.PubMed/NCBI
|
|
42
|
Matsumoto H, Niimi A, Takemura M, Ueda T,
Minakuchi M, Tabuena R, Chin K, Mio T, Ito Y, Muro S, et al:
Relationship of airway wall thickening to an imbalance between
matrix metalloproteinase-9 and its inhibitor in asthma. Thorax.
60:277–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vu TH and Werb Z: Matrix
metalloproteinases: Effectors of development and normal physiology.
Genes Dev. 14:2123–2133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mesko B, Poliska S and Nagy L: Gene
expression profiles in peripheral blood for the diagnosis of
autoimmune diseases. Trends Mol Med. 17:223–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Van der Meide PH and Schellekens H:
Cytokines and the immune response. Biotherapy. 8:243–249. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boyce DE, Ciampolini J, Ruge F, Murison MS
and Harding KG: Inflammatory-cell subpopulations in keloid scars.
Br J Plast Surg. 54:511–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Glim JE, van Egmond M, Niessen FB, Everts
V and Beelen RH: Detrimental dermal wound healing: What can we
learn from the oral mucosa? Wound Repair Regen. 21:648–660. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Efron JE, Frankel HL, Lazarou SA,
Wasserkrug HL and Barbul A: Wound healing and T-lymphocytes. J Surg
Res. 48:460–463. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Barbul A, Breslin RJ, Woodyard JP,
Wasserkrug HL and Efron G: The effect of in vivo T helper and T
suppressor lymphocyte depletion on wound healing. Ann Surg.
209:479–483. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang R, Ghahary A, Shen YJ, Scott PG and
Tredget EE: Human dermal fibroblasts produce nitric oxide and
express both constitutive and inducible nitric oxide synthase
isoforms. J Invest Dermatol. 106:419–427. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujitsu Y, Fukuda K, Kumagai N and Nishida
T: IL-4-induced cell proliferation and production of extracellular
matrix proteins in human conjunctival fibroblasts. Exp Eye Res.
76:107–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Serbina NV, Jia T, Hohl TM and Pamer EG:
Monocyte-mediated defense against microbial pathogens. Annu Rev
Immunol. 26:421–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bettinger DA, Pellicane JV, Tarry WC,
Yager DR, Diegelmann RF, Lee R, Cohen IK and DeMaria EJ: The role
of inflammatory cytokines in wound healing: accelerated healing in
endotoxin-resistant mice. J Trauma. 36:810–814. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Danon D, Kowatch MA and Roth GS: Promotion
of wound repair in old mice by local injection of macrophages. Proc
Natl Acad Sci USA. 86:pp. 2018–2020. 1989; View Article : Google Scholar : PubMed/NCBI
|