Introduction
Schizophrenia is a severe psychiatric disorder with
a lifetime prevalence of around 1% (1). Cognitive impairments are the core
symptoms of the disease which is characterized by disturbances in
sensory information processing, attention, working memory and
executive functions deficits (2)
which are all hippocampus-dependent functions (3,4).
Schizophrenia imposes a heavy financial burden and many potential
safety hazards to families of sufferers and society as a whole.
Recent studies focusing on the pathogenesis and
treatment of schizophrenia have made significant progress in
understanding the biology underpinning the disease. The
glutamatergic hypothesis of schizophrenia states that dysfunction
of the glutamatergic system induces imbalance between the
glutamatergic and dopaminergic systems in the central nervous
system (CNS) and induces some symptoms of schizophrenia (5). N-methyl-D-aspartate receptors
(NMDARs) may be a key factor in the pathogenesis of schizophrenia
(6–8). NMDAR is one of the ionotropic
glutamatergic receptors (5), is a
tetrameric structure of seven subunits including at least one
obligatory subunit, N-methyl-D-aspartate receptor R1 (NR1; it
includes eight functional splice variants), and varying numbers of
a family of NR2 (NR2A-D) or NR3 (NR3A-B) subunits (9,10).
The properties of NMDARs are both complicated and diverse owing to
their complex subunit compositions. The characteristics of NMDAR
include high Ca2+ permeability and Mg2+
suppression closely associated with neurogenesis, neuronal
survival, synaptic plasticity and the formation of learning and
memory abilities (2,4,11–13).
Studies have demonstrated that schizophrenia patients show abnormal
synaptic plasticity, LTP and cognitive dysfunction (11,13–15).
The hippocampus is involved with cognitive functions, particularly
in learning and memory and so was chosen as the area of particular
interest for the current study. NR1 subunits are essential
components of NMDARs. NR1 is very important for understanding the
distribution and functions of NMDARs (8), however, the expression and regulation
of NR1 in the hippocampus of schizophrenia-like mice are not
known.
To investigate these issues, our study examined the
expression of the NR1 subunit in the granule cell layer CA1, CA3
and DG region, and discussed regulation of the NR1 subunit
expression by NMDA. We also characterized the expression of the NR1
subunit mRNA and protein, and levels of apoptosis in the
hippocampal nerve cells of schizophrenia-like mice. In conclusion,
we demonstrated that NMDA can regulate the expression of NR1 and
suppress apoptosis in the hippocampal nerve cells of
schizophrenia-like mice.
Materials and methods
Materials
MK-801 (3 mg/ml in 0.9% saline) and NMDA (1.5 µg/ml
in 0.9% saline) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The primary antibody of NR1 was purchased from
Abcam (Cambridge, MA, USA), and β-actin antibody purchased from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). The secondary
antibody of goat anti-rabbit fluorescein isothiocyanate (FITC) was
purchased from CWBIO (Beijing, China), and the HRP-conjugated goat
anti-rabbit antibody purchased from ZSGB-BIO (Beijing, China).
Annexin V-FITC/PI kit was purchased from Yeasen (USA). TRIzol
reagent was purchased from Life Technologies (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). All PCR primers were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
RevertAid First Strand cDNA Synthesis kit and Maxima SYBR Green
qPCR Master Mix were purchased from Thermo Fisher Scientific,
Inc.
Animals
Male C57BL/6 mice (23–25 grams, at 3-month old) were
used in this study and provided by the Experimental Animal Center
of the Ningxia Medical University. Mice were housed in groups of
five in cages with free access to food and tap water for 7 days.
The animal room temperature was maintained at 23±2°C, with a 12/12
dark/light cycle (lights on 7:00-19:00) and 50% humidity. All
animals were handled in accordance with the standards established
by the institutional animal care and use committee of Ningxia
Medical University.
Experimental groups and models
Male C57BL/6 mice were divided into 3 groups (n=12
in each group): A blank group, a MK-801 treated group and a
MK-801+NMDA treated group. In the MK-801+NMDA treated group, mice
were treated intra-peritoneally with MK-801 at dose of 0.6 mg/kg at
the same time every day for 14 days (16). After 14 days, mice were
anesthetized by intra-peritoneal injection of chloral hydrate and
mounted on a stereotaxic frame for intra-cerebroventricular
injection (mm from bregma: A −0.5, L −1.0, V-2.5) of NMDA (25
ng/µl, 3 ul) once each mouse (17). Brains were harvested 3 days after
the operation (n=12). In the MK-801 group (schizophrenia-like
group), saline was used instead of NMDA and all other operations
were the same as intra-cerebroventricular injection groups (n=12).
In the blank group, an equal volume of 0.9% saline was injected
instead of MK-801 and NMDA (n=12).
Immunofluorescence (IF) staining
Mice (n=3 in each group) were transcardially
perfused with ice-cold 0.01 M PBS (pH 7.4, 150 ml/mouse) and 4%
paraformaldehyde solution (200 ml/mouse). Brains were harvested,
and post-fixed for 24 h in 4% paraformaldehyde, followed by
immersion in 20%, 30% sucrose solution overnight. Brains were
washed once for 10 min with ice cold 0.01 M PBS, embedded in 1%
gelose solution and 30 µm-thick coronal sections cut and used for
immunofluorescent staining of NR1 in the hippocampal granule cell
layer. Sections were permeabilized and blocked with blocking
solution (7% BSA and 0.3% Triton X-100 in 0.01 M PBS) for 1 h at
room temperature, and incubated overnight at 4°C with rabbit
anti-NR1 (1:800). Sections were washed three times for 30 min with
0.01 M PBS followed by second antibody incubation with goat
anti-rabbit fluorescein isothiocyanate (FITC, 1:200) for 2 h at
room temperature. Sections were washed three times for 30 min with
0.01 M PBS. Finally, the number of NR1 positive cells in granule
cell layer of hippocampus was measured using confocal laser
scanning microscope.
Western blotting
Mice (n=3 in each group) were decapitated and
hippocampi were harvested. The hippocampi were put into glass
homogenates with ice-cold buffer containing protease inhibitor for
45 min. The homogenates were centrifuged at 12,000 × g for 20 min
and the supernatant was collected (stored at −80°C). The protein
levels were detected using the BCA method. Total protein in each
group was separated (NR1, β-actin) by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrophoretically transferred onto polyvinylidene difluoride
(PVDF) membranes. PVDF membranes were immersed in blocking buffer
(5% fat-free milk in PBST) for 1 h at room temperature and
incubated overnight at 4°C with the primary rabbit antibodies
respectively (NR1, 1:500; β-actin, 1:3,000). The membranes were
then washed three times for 30 min using PBST and incubated
HRP-conjugated goat anti-rabbit antibody (1:5,000) for 2 h at room
temperature and washed three times for 30 min in PBST.
Immuno-reactive proteins were visualized using enhanced
chemiluminescence (ECL) detection and the signals were quantified
by densitometry using a western blotting detection system.
Real-time PCR
Total RNA from the hippocampal tissue (n=3 in each
group) was extracted using TRIzol reagent. One microgram of the
purified total RNA was reverse transcribed using a RevertAid First
Strand cDNA Synthesis kit. The mRNA level of NR1 was determined
using the Maxima SYBR Green qPCR Master Mix. All PCR primers were
synthesized by Sangon Biotech (Shanghai, China). The forward and
reverse primer sequences for NR1 were 5′-CTTCCTCCAGCCACTACCC-3′ and
5′-AGAAAGCACCCCTGAAGCAC-3′, respectively; for β-actin, forward and
reverse primer sequence were 5′-CCTAAGGCCAACCGTGAAAAG−3′and
5′-ACCAGAGGCATACAGGGACAAC-3′, respectively. β-actin was used as an
internal reference to standardize each gene. Each sample was
investigated in triplicate. The relative amount of mRNA was
measured using the comparative threshold (Ct) method by normalizing
target cDNA Ct values to that of β-actin, and the fold expression
changes were calculated according to the 2−ΔΔCt method
(18,19).
Flow cytometry analysis
Analysis of apoptosis was performed by flow
cytometry of the hippocampi (n=3 in each group) isolated from each
mouse. Samples were digested with 0.25% trypsin at 37°C for 20 min
and cell suspension were then filtered through a cell strainer
(20) and the number of apoptotics
cells was determined by flow cytometry assay using Annexin
V-FITC/PI kit (21) and analyzed
using the FACS express v2.0 software.
Statistical analysis. Data are presented as the mean
± standard deviation. Statistical analysis was carried out in
SPSS11.5 (SPSS, Inc., Chicago, IL, USA) using one-way ANOVA.
P<0.05 was considered as statistically significant.
Results
Expression of NR1 subunits in the CA1,
CA3 and DG of the hippocampus
The distribution patterns of NR1 positive cells
(white arrows) in the CA1, CA3 and dentate gyrus (DG) of the
hippocampus are shown in Fig. 1A.
The number of NR1 positive cells is shown in Fig. 1B. The results showed that the
number of NR1 positive cells in the MK-801 group increased in the
CA1 (P>0.05) and DG (P<0.05) regions and this change was
reversible by NMDA. The MK-801 group showed a decrease in NR1
compared with the Blank group in the CA3 region (P<0.05).
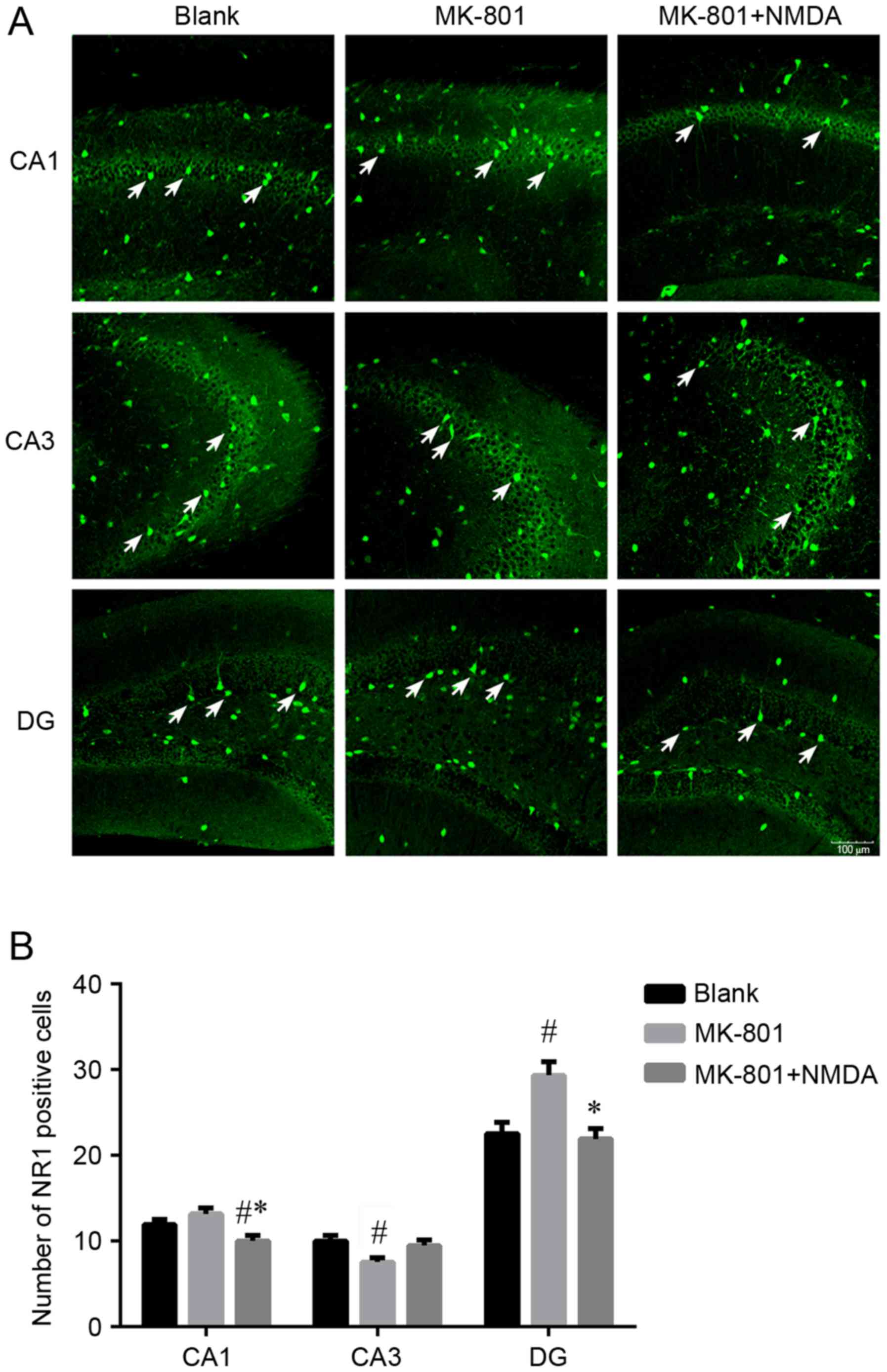 | Figure 1.The NR1 positive cells distribution in
different regions in hippocampus. (A) The distribution pattern of
NR1 positive cells. Bars, 20 µm. (B) The number of NR1 positive
cells. Three brain region of hippocampus: CA1, CA3, DG. Three
groups: Blank, MK-801 and MK-801+NMDA. MK-801, NMDA receptor
antagonist, MK-801 group (schizophrenia-like group); NMDA,
N-methyl-D-aspartate; NR1, N-methyl-D-aspartate receptor R1. Data
are presented as the mean ± standard deviation.
#P<0.05 vs. blank group, *P<0.05 vs. MK-801 group.
The number of NR1 positive cells in the MK-801 group increased in
the CA1 (P=0.053) and DG (P<0.001) regions and this change was
reversible by NMDA (P<0.001, P<0.001). The MK-801 group
showed a decrease in NR1 compared with the blank group in the CA3
region (P<0.005) and again these changes were not reversible
with NMDA (P=0.054). |
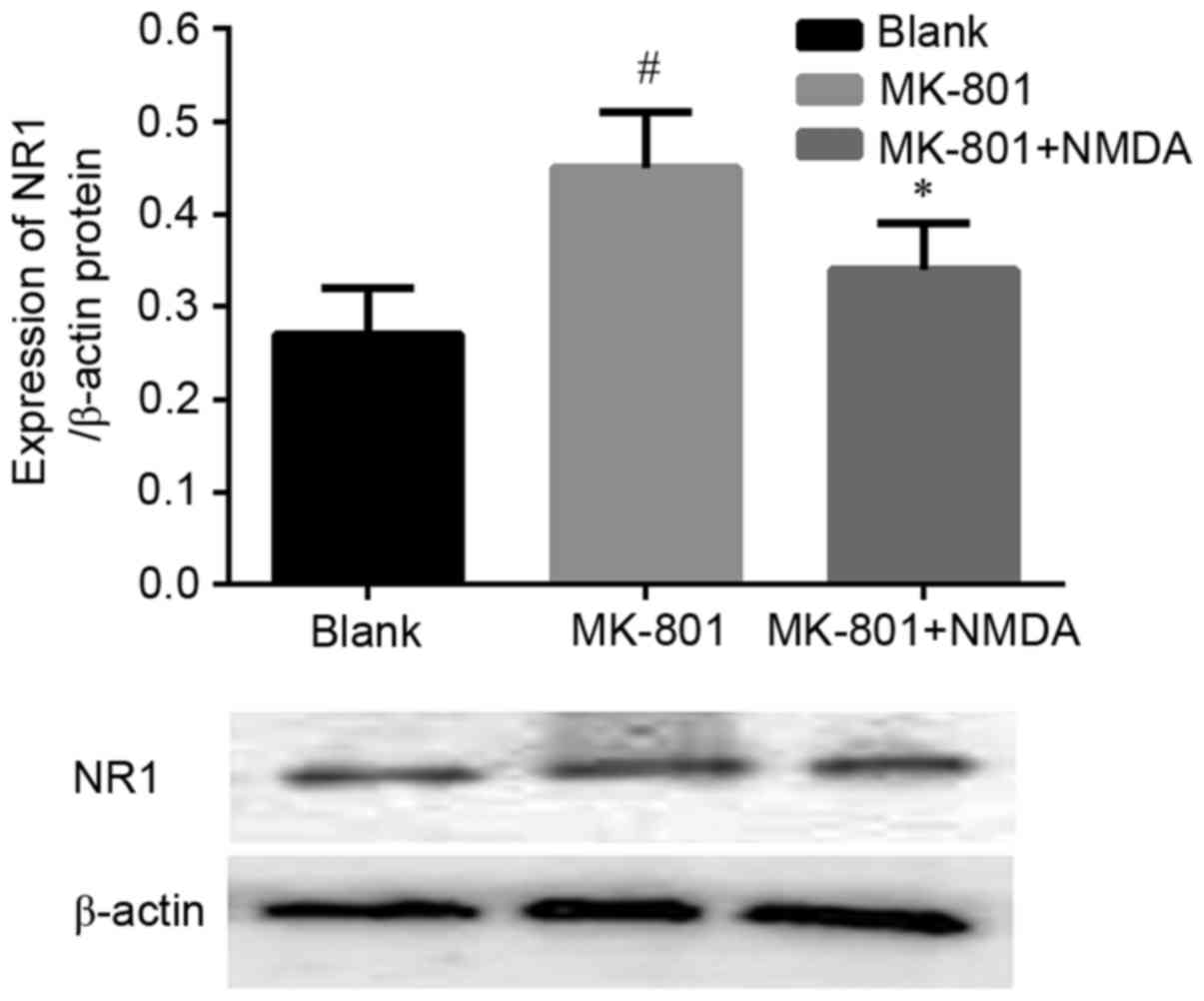
Expression of NR1 subunit protein
levels in the hippocampus
Fig. 2 shows the
expression of NR1 subunit protein levels in the hippocampus of
experimental mice. The data showed the expression of NR1 subunit
increased in the MK-801 treated group (P<0.05) compared with to
the Blank group and again this trend was reversible by NMDA.
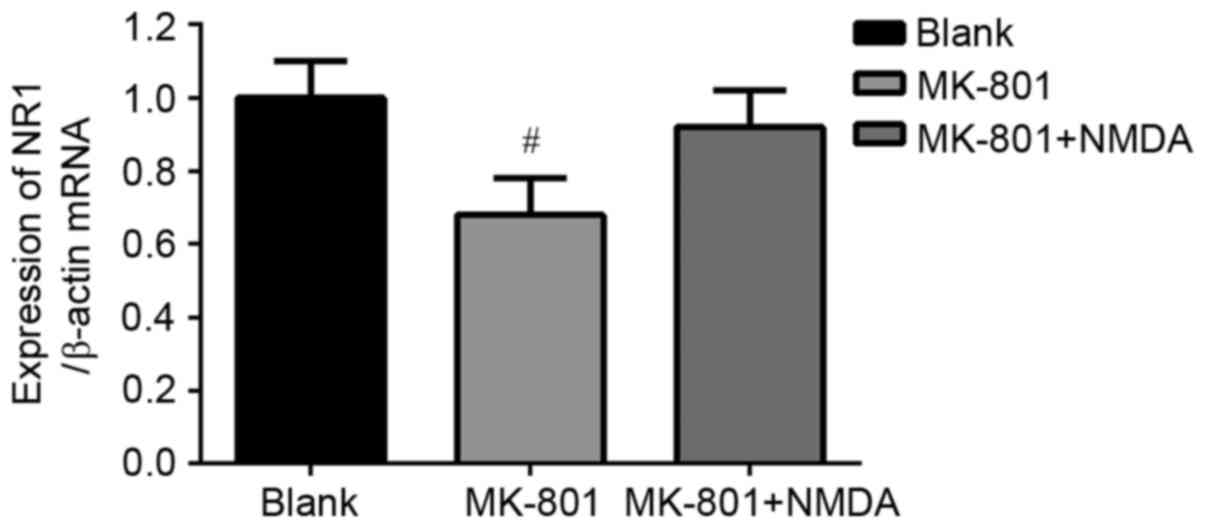
Expression of NR1 subunit mRNA levels
in hippocampus
The mRNA levels of the NR1 subunit normalized to
β-actin in hippocampus of mice are shown in Fig. 3. The data show that the mRNA
expression of NR1 subunit decreased in the MK-801 treated group
(P<0.05) compared to the Blank group. This change was reversed
by NMDA although no statistical significance was detected
(P>0.05).
Apoptosis of hippocampal nerve
cells
From Fig. 4 it can
be seen that the early and total levels of apoptosis in the
hippocampal nerve cells significantly increased (P<0.05) in the
MK-801 group compared to the Blank group. In contrast, these
changes were reversible by NMDA (P<0.05). The normal live cells
(NLC) are representative of the survival rate of hippocampus nerve
cells. The number of NLC decreased in the MK-801 group compared to
the blank group (P<0.05) and again these changes were reversible
with NMDA.
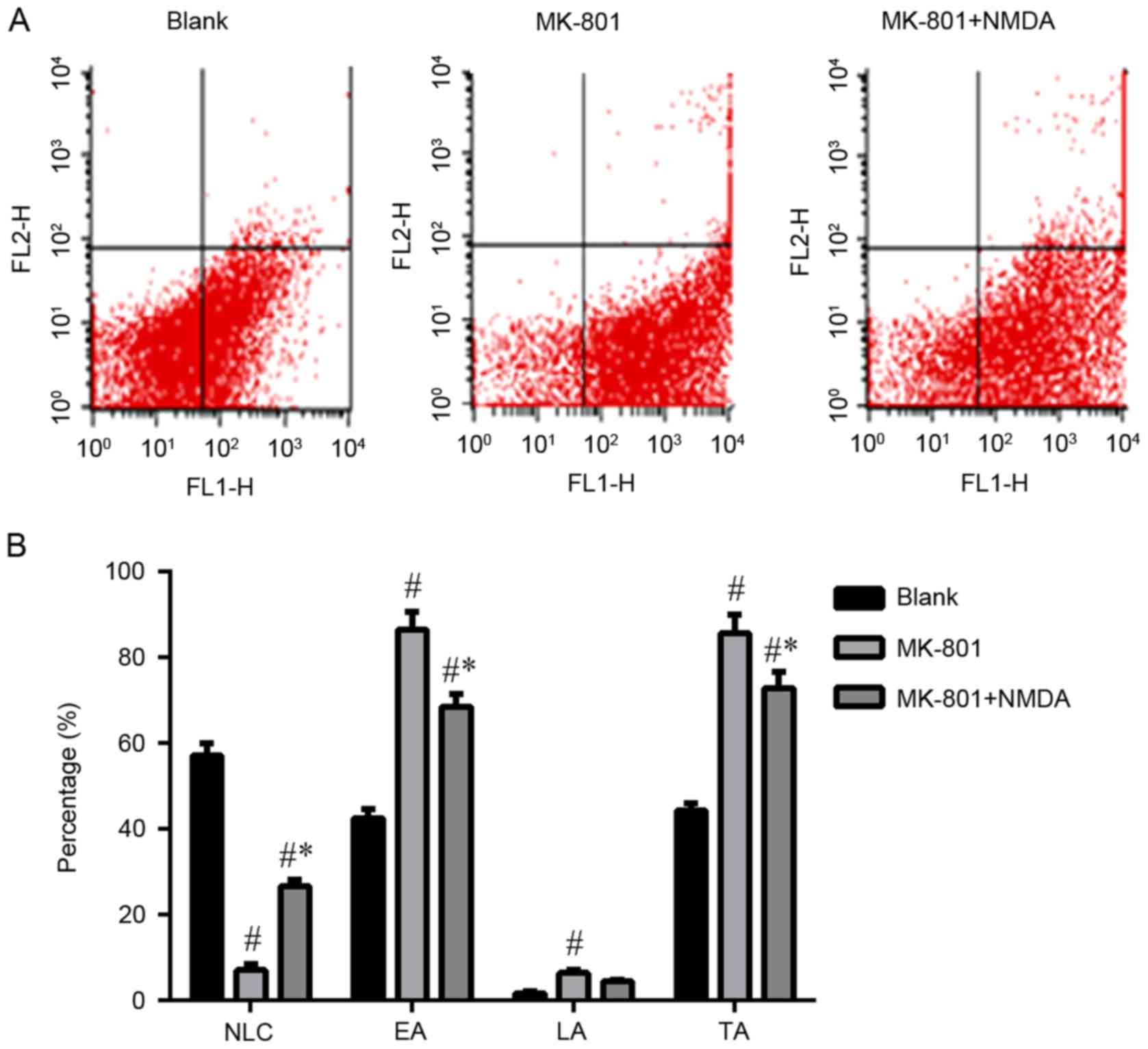 | Figure 4.Apoptosis of hippocampal nerve cells.
(A) Flow cytometry with apoptosis markers (FITC-Annexin V and PI).
Three brain region of hippocampus: CA1, CA3, DG. Three groups:
Blank, MK-801 and MK-801+NMDA. MK-801, NMDA receptor antagonist,
MK-801 group (schizophrenia-like group); NMDA,
N-methyl-D-aspartate; NR1, N-methyl-D-aspartate receptor R1. NLC,
normal live cells; EA, early apoptosis; LA, late apoptosis; TA,
total apoptosis. (B) Data are presented as the mean ± standard
deviation. #P<0.05 vs. blank group, *P<0.05 vs.
MK-801 group. The early and total levels of apoptosis in the
hippocampal nerve cells significantly increased (P<0.001,
P<0.001) in the MK-801 group compared to the Blank group. In
contrast, these changes were reversible by NMDA (P<0.001,
P<0.005). The number of NLC decreased in the MK-801 group
compared to the blank group (P<0.001) and again these changes
were reversible with NMDA (P<0.005). The number of LA increased
in the MK-801 group compared to the blank group (P=0.046) and again
these changes were not reversible with NMDA (P=0.308). |
Discussion
The prevalence of schizophrenia increased to 1.0%
between 1990 and 2010 all over the world (22). The underlying pathophysiological
mechanisms of the disease are complex and remain to be fully
elucidated. NMDAR is a major glutamate receptor subtype that is
known to play a key role in learning and memory. NMDARs include
NR1, NR2 (2A-2D), NR3 (3A-3B). NR1 subunits are the essential and
obligatory components of NMDARs. NR1 subunits play an important
role in determining the properties of NMDARs. Recently, NMDAR has
received great attention on schizophrenia research. Dysfunction of
NMDAR in the hippocampus is very important for the formation of
schizophrenia (8).
We adopted an MK-801 administrated mouse model to
investigate NR1 expression pattern and hippocampal neuron survival
deficit in schizophrenia. MK-801 is a NMDAR antagonist per se. We
worried changes released in this model may not be
schizophrenia-related, but just a classical receptor-ligand
feedback response of physiological process. Actually, no animal
model can accurately reproduce all aspects of schizophrenia but
mouse models can mimic several important aspects of the disease.
MK-801 has been used for inducing a schizophrenia-like phenotype in
rodents (23,24) which leads to different degrees of
cognitive impairment (25–27). Some studies have shown hypofunction
of the NMDA receptor by chronic treatment with MK-801 (28) or cell death in the hippocampus
following NMDAR hypofunction (29). NMDAR subunits are known to play an
important role in determining neurotoxic (30), functional and psychiatric effects
in the hippocampus (31,32).
In this study, the results showed that the number of
NR1 positive cells significantly increased in the hippocampal
granule cell layer of the DG and CA1 regions, but decreased in the
CA3 region of schizophrenia-like mice induced by MK-801. NMDA was
shown to reverse these changes. The NR1 total protein levels
significantly increased, whilste the mRNA levels were reduced in
the hippocampus of schizophrenia-like mice induced by MK-801. NMDA
could also reverse these changes. Other studies of NMDAR expression
in schizophrenia have shown variable changes at the transcript and
protein expression levels in different areas of the brain. For
example, study have shown that the protein expression of NR1 in the
anterior cingulate cortex increased (33) or was found to be unchanged in
hippocampus (34). However,
another study found that NR1 protein expression was reduced in the
prefrontal cortex, hippocampus and hippocampal DG region (35). Furthermore, at the mRNA level,
expression has been shown to be decreased in the hippocampus
(36,37), and the expression of the NR1
protein and mRNA levels were contradictory in the cortex of
schizophrenia mice (33).
Another study examined NR1 expression in postmortem
samples from patients with schizophrenia and comparison subjects.
The data showed that at the transcriptional level, NR1 levels were
lower in the thalamus of schizophrenia patients compared to control
subjects (38). These studies
support our experimental results to a certain extent. The current
study has demonstrated that transcriptional changes of the NMDAR
subunit expression in cortical areas appear to be associated with
specific regions (33). These data
may be explained by three supporting hypotheses. Firstly, the
regional specificity expression of NR1 protein levels comes from
the mRNA and the differential expressions in the CA1, CA3 and DG
region contribute to the function of different areas (4). Secondly, it is possible that the peak
of expression is not the same point at the protein and mRNA levels.
Finally, NR1 may be involved in regulation of other complex
components of glutamatergic pathways that are associated with
schizophrenia (33).
The levels of apoptosis in the hippocampal nerve
cells increased in schizophrenia-like mice induced by MK-801 which
could be reversed by NMDA. Studies have shown that hyperfunction of
glutamate receptors such as NMDA receptors increases intracellular
Ca2+ levels. The continual influx of Ca2+
through the open NMDA receptors results in mitochondrial stress
which attempts to sequester and buffer Ca2+. As
mitochondrial membrane potential decreases due to the overflow of
Ca2+ and mitochondria reverse their ATP synthase in an
attempt to restore Ca2+ homeostasis. Eventually, the
excess Ca2+ uptake causes loss of mitochondrial membrane
potential, mitochondrial swelling, opening of the mitochondrial
permeability transition pore (39,40),
outer membrane rupture and loss of Ca2+, and apoptogenic
factors into the cytoplasm. This process ultimately results in
neuronal cell death (38). Our
experimental data show that the apoptosis of hippocampal nerve
cells significantly increased in schizophrenia-like mice induced by
MK-801 and these changes could be reversed by NMDA.
We demonstrated that NR1 significantly contributes
to the acquisition and apoptosis of hippocampal nerve cells in
schizophrenia-like mice. NMDA can effectively regulate the
expression of NR1 and levels of apoptosis in hippocampal nerve
cells in schizophrenia-like mice, whereas the exactly underprinning
molecular mechanisms need to be further clarified.
In summary, we have demonstrated that the expression
of NR1 markedly increased in the hippocampus of schizophrenia-like
mice, but showed differential trends in the CA1, CA3 and DG
regions. NMDAR hyperfunction induced apoptosis in hippocampal nerve
cells. NMDA contributes to positive function in schizophrenia, and
may be offer an important molecular pathway for therapeutic
intervention. NR1 might hold much potential as target in the
therapy of schizophrenia.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (nos. 81160169, 81460214, 31460255,
31660270) and the Natural Science Foundation of Ningxia (NZ14058)
and West China Top Class Discipline Project in Basic Medical
Sciences, Ningxia Medical University.
References
|
1
|
de Candia TR, Lee SH, Yang J, Browning BL,
Gejman PV, Levinson DF, Mowry BJ, Hewitt JK, Goddard ME, O'Donovan
MC, et al: Additive genetic variation in schizophrenia risk is
shared by populations of African and European descent. Am J Hum
Genet. 93:463–470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sánchez-Blázquez P, Rodríguez-Muñoz M and
Garzón J: The cannabinoid receptor 1 associates with NMDA receptors
to produce glutamatergic hypofunction: Implications in psychosis
and schizophrenia. Front Pharmacol. 4:1692014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakashiba T, Cushman JD, Pelkey KA,
Renaudineau S, Buhl DL, McHugh TJ, Barrera V Rodriguez, Chittajallu
R, Iwamoto KS, McBain CJ, et al: Young dentate granule cells
mediate pattern separation, whereas old granule cells facilitate
pattern completion. Cell. 149:188–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tannenholz L, Jimenez JC and Kheirbek MA:
Local and regional heterogeneity underlying hippocampal modulation
of cognition and mood. Front Behav Neurosci. 8:1472014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaspar PA, Bustamante ML, Silva H and
Aboitiz F: Molecular mechanisms underlying glutamatergic
dysfunction in schizophrenia: Therapeutic implications. J
Neurochem. 111:891–900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Howes OD and Murray RM: Schizophrenia: An
integrated sociodevelopmental-cognitive model. Lancet.
383:1677–1687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Penzes P, Cahill ME, Jones KA, VanLeeuwen
JE and Woolfrey KM: Dendritic spine pathology in neuropsychiatric
disorders. Nat Neurosci. 14:285–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju P and Cui D: The involvement of
N-methyl-d-aspartate receptor (NMDAR) subunit NR1 in the
pathophysiology of schizophrenia. Acta Biochim Biophys Sin
(Shanghai). 48:209–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghasemi M, Phillips C, Trillo L, De Miguel
Z, Das D and Salehi A: The role of NMDA receptors in the
pathophysiology and treatment of mood disorders. Neurosci Biobehav
Rev. 47:336–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Henson MA, Roberts AC, Pérez-Otaño I and
Philpot BD: Influence of the NR3A subunit on NMDA receptor
functions. Prog Neurobiol. 91:23–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hardingham GE and Bading H: Synaptic
versus extrasynaptic NMDA receptor signalling: Implications for
neurodegenerative disorders. Nat Rev Neurosci. 11:682–696. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lau CG and Zukin RS: NMDA receptor
trafficking in synaptic plasticity and neuropsychiatric disorders.
Nat Rev Neurosci. 8:413–426. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Volianskis A, France G, Jensen MS,
Bortolotto ZA, Jane DE and Collingridge GL: Long-term potentiation
and the role of N-methyl-D-aspartate receptors. Brain Res.
1621:5–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bliss TV, Collingridge GL and Morris RG:
Synaptic plasticity in health and disease: Introduction and
overview. Philos Trans R Soc Lond B Biol Sci. 369:201301292013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soriano FX, Papadia S, Hofmann F,
Hardingham NR, Bading H and Hardingham GE: Preconditioning doses of
NMDA promote neuroprotection by enhancing neuronal excitability. J
Neurosci. 26:4509–4518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones KS, Corbin JG and Huntsman MM:
Neonatal NMDA receptor blockade disrupts spike timing and
glutamatergic synapses in fast spiking interneurons in a NMDA
receptor hypofunction model of schizophrenia. PLoS One.
9:e1093032014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad AS, Saleem S, Ahmad M and Doré S:
Prostaglandin EP1 receptor contributes to excitotoxicity and focal
ischemic brain damage. Toxicol Sci. 89:265–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zapatero-Solana E, García-Giménez JL,
Guerrero-Aspizua S, García M, Toll A, Baselga E, Durán-Moreno M,
Markovic J, García-Verdugo JM, Conti CJ, et al: Oxidative stress
and mitochondrial dysfunction in Kindler syndrome. Orphanet J Rare
Dis. 9:2112014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiao Y, Liu C, Cui FM, Xu JY, Tong J, Qi
XF, Wang LL and Zhu W: Long intergenic non-coding RNA induced by
X-ray irradiation regulates DNA damage response signaling in the
human bronchial epithelial BEAS-2B cell line. Oncol Lett.
9:169–176. 2015.PubMed/NCBI
|
|
20
|
Zhang Z, Zhao C, Liu B, Liang D, Qin X, Li
X, Zhang R, Li C, Wang H, Sun D and Cao F: Inositol pyrophosphates
mediate the effects of aging on bone marrow mesenchymal stem cells
by inhibiting Akt signaling. Stem Cell Res Ther. 5:332014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pervaiz N and Hoffman-Goetz L: Immune cell
inflammatory cytokine responses differ between central and systemic
compartments in response to acute exercise in mice. Exerc Immunol
Rev. 18:142–157. 2012.PubMed/NCBI
|
|
22
|
Whiteford HA, Degenhardt L, Rehm J, Baxter
AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD,
Johns N, et al: Global burden of disease attributable to mental and
substance use disorders: Findings from the global burden of disease
study 2010. Lancet. 382:1575–1586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Qi D, Xing M, Li R, Jiang K, Peng Y
and Cui D: MK-801 induces schizophrenic behaviors through
downregulating Wnt signaling pathways in male mice. Brain Res.
1385:281–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim TW, Kang HS, Park JK, Lee SJ, Baek SB
and Kim CJ: Voluntary wheel running ameliorates symptoms of
MK-801-induced schizophrenia in mice. Mol Med Rep. 10:2924–2930.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knott AB and Bossy-Wetzel E: Nitric oxide
in health and disease of the nervous system. Antioxid Redox Signal.
11:541–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mandillo S, Rinaldi A, Oliverio A and Mele
A: Repeated administration of phencyclidine, amphetamine and MK-801
selectively impairs spatial learning in mice: A possible model of
psychotomimetic drug-induced cognitive deficits. Behav Pharmacol.
14:533–544. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohn AR, Gainetdinov RR, Caron MG and
Koller BH: Mice with reduced NMDA receptor expression display
behaviors related to schizophrenia. Cell. 98:427–436. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rujescu D, Bender A, Keck M, Hartmann AM,
Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Möller HJ and
Grunze H: A pharmacological model for psychosis based on
N-methyl-D-aspartate receptor hypofunction: Molecular, cellular,
functional and behavioral abnormalities. Biol Psychiatry.
59:721–729. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe Y, Müller MK, von Engelhardt J,
Sprengel R, Seeburg PH and Monyer H: Age-dependent degeneration of
mature dentate gyrus granule cells following NMDA receptor
ablation. Front Mol Neurosci. 8:872016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anastasio NC, Xia Y, O'Connor ZR and
Johnson KM: Differential role of N-methyl-D-aspartate receptor
subunits 2A and 2B in mediating phencyclidine-induced perinatal
neuronal apoptosis and behavioral deficits. Neuroscience.
163:1181–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kocsis B: Differential role of NR2A and
NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced
aberrant cortical gamma oscillations. Biol Psychiatry. 71:987–995.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inta D, Vogt MA, Luoni A, Filipović D,
Lima-Ojeda JM, Pfeiffer N, Gasparini F, Riva MA and Gass P:
Significant increase in anxiety during aging in mGlu5 receptor
knockout mice. Behav Brain Res. 241:27–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kristiansen LV, Huerta I, Beneyto M and
Meador-Woodruff JH: NMDA receptors and schizophrenia. Curr Opin
Pharmacol. 7:48–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toro C and Deakin JF: NMDA receptor
subunit NRI and postsynaptic protein PSD-95 in hippocampus and
orbitofrontal cortex in schizophrenia and mood disorder. Schizophr
Res. 80:323–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JK, Lee SJ and Kim TW: Treadmill
exercise enhances NMDA receptor expression in schizophrenia mice. J
Exerc Rehabil. 10:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Law AJ and Deakin JF: Asymmetrical
reductions of hippocampal NMDAR1 glutamate receptor mRNA in the
psychoses. Neuroreport. 12:2971–2974. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao XM, Sakai K, Roberts RC, Conley RR,
Dean B and Tamminga CA: Ionotropic glutamate receptors and
expression of N-methyl-D-aspartate receptor subunits in subregions
of human hippocampus: Effects of schizophrenia. Am J Psychiatry.
157:1141–1149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ibrahim HM, Hogg AJ Jr, Healy DJ,
Haroutunian V, Davis KL and Meador-Woodruff JH: Ionotropic
glutamate receptor binding and subunit mRNA expression in thalamic
nuclei in schizophrenia. Am J Psychiatry. 157:1811–1823. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gono T, Kawaguchi Y, Kaneko H, Nishimura
K, Hanaoka M, Kataoka S, Okamoto Y, Katsumata Y and Yamanaka H:
Anti-NR2A antibody as a predictor for neuropsychiatric systemic
lupus erythematosus. Rheumatology (Oxford). 50:1578–1585. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Faust TW, Chang EH, Kowal C, Berlin R,
Gazaryan IG, Bertini E, Zhang J, Sanchez-Guerrero J, Fragoso-Loyo
HE, Volpe BT, et al: Neurotoxic lupus autoantibodies alter brain
function through two distinct mechanisms. Proc Natl Acad Sci USA.
107:pp. 18569–18574. 2010; View Article : Google Scholar : PubMed/NCBI
|