Introduction
Atopic dermatitis (AD) is a chronic, relapsing
inflammatory skin disease, which affects ~10–20% and 1–3% of
children and adults, respectively, in Western populations (1). Impaired epidermal barrier and immune
function defects are common in patients with AD (2,3).
AD is also characterized by a T helper type 2 (Th2)
dominance, mediated by pro-Th2 cytokines, thymic stromal
lymphopoietin and interleukin (IL)-33, which polarize dendritic
cells and promote Th2 responses (4). CD4+ T cells are the
primary mediators of cellular immunity and are found in the cell
infiltrate of the skin of patients with AD (5). Th17 cells, a distinct lineage of
CD4+ helper T cells, are important in the host defense
against extracellular fungal and bacterial pathogens, and the
pathogenesis of inflammatory and autoimmune disorders (6). IL-17, also known as IL-17A, is the
primary effector cytokine of Th17 cells and regulates the functions
of multiple cell types (7),
including the stimulation of keratinocytes to produce cytokines,
chemokines and vascular endothelial growth factor (6).
Another important component in AD is skin integrity
(8,9). Of note, skin barrier dysfunction in
patients with AD is associated with abnormal protein expression of
filaggrin (FLG), loricrin (LOR) and involucrin (IVL), which result
in skin impermeability by cross-linking (10,11).
FLG is a major structural protein in the stratum corneum of the
epidermis, with reduced levels altering the shape of skin
corneocytes (12). LOR comprises
80% of the total protein mass in the cornified layer (13), whereas IVL functions as a scaffold
to other cross-linked proteins (14). Patients with AD with an acquired
defect in the expression of FLG exhibit an atopic inflammatory
response (15). Therefore, it is
hypothesized that FLG and IVL can be regulated by AD-associated
cytokines, including IL-17, as the expression of IL-17 is enhanced
in acute lesions in AD skin, compared with that in normal skin,
with increased numbers of Th17 cells in the peripheral blood in
acute AD (16). IL-17 activates
mitogen-activated protein kinases (MAPKs), and the
P38/extracellular signal-regulated kinase (ERK) MAPK signaling
pathways are involved in the pathogenesis of inflammatory skin
diseases, including psoriasis (17). The present study aimed to examine
the effects of IL-17 on the expression of FLG and IVL in human
HaCaT keratinocytes, and investigate the regulatory mechanism.
Materials and methods
Cell culture
The HaCaT cells (JennioBioech Co., Ltd., Guangzhou,
China), a human keratinocyte cell line, were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 100 U/ml of penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.), at 37°C in a humid environment
containing 5% CO2. To examine the effects of cytokines
on the expression of FLG and IVL, the keratinocytes were
differentiated for 5 days by treatment with CaCl2 at 1.3
mmol/l. Cells seeded at 1×105 cells/ml were allowed to
grow to 70–80% confluence and were stimulated with medium
containing IL-4 (100 ng/ml) or different concentrations of IL-17
(50, 100 and 200 ng/ml) for 24 h at 37°C. IL-4 and IL-17 were
purchased from PeproTech, Inc. (Rocky Hill, USA). Following
treatment, the cells were harvested for protein extraction. Cells
in passages 2–5 were used for all experiments.
Treatment with MAPK inhibitors
The MAPK inhibitors directed against P38 (SB203580;
5 µM), ERK (PD98059; 20 µM), or c-Jun N-terminal kinase (SP600125,
1 µM), respectively, were added to the media for treatment of the
HaCaT cells 1 h prior to the addition of IL-17 (100 ng/ml) at 37°C.
The cells were cultured for 24 h prior to harvest for mRNA and
protein extraction.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis for quantitation of
mRNA expression
Total RNA was extracted from the HaCaT cells using
the RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA), according to
the manufacturer's protocol. cDNA was reverse transcribed from
total RNA using TaqMan RT reagents (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The mRNA levels were assessed using the
SYBR® Green ER™ qPCR Reagent system
(Invitrogen; Thermo Fisher Scientific, Inc.) on an ABI PRISM 7000
sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers used for RT-qPCR were as follows:
FLG forward, 5′-TGAAGCCTATGACACCACTGA-3′ and reverse,
5′-TCCCCTACGCTTTCTTGTCCT-3′; IVL forward,
5′-ACAAGGGAAGAGAGAGCCACTG-3′ and reverse,
5′-TGTAGAGGGACAGAGTCAAGTTCA-3′. The GAPDH gene was used as
endogenous control with the following sequences: Forward,
5′-ATCAAGAAGGTGGTGAAGCAGGC-3′ and reverse,
5′-TCAAAGGTGGAGGAGTGGGTGTC-3′. The cycling conditions were as
follows: 95°C for 2 min; followed by 45 cycles of denaturation at
95°C for 5 sec, annealing at 60°C for 10 sec and extension at 72°C
for 15 sec. Each PCR assay was run in triplicate. The relative gene
expression levels were analyzed using the 2−ΔΔCq method
(18).
Western blot analysis
The cells were washed three times with cold 1X
phosphate-buffered saline and harvested with
radioimmunoprecipitation buffer comprising 50 mM Tris-HCl (pH 8.0),
150 mM NaCl, 1% (v/v) Nonidet P-40, 0.5% (w/v) deoxycholate and
0.1% (w/v) SDS, a protease inhibitor cocktail (1:100; Roche Applied
Science, Penzberg, Germany) and a phosphatase inhibitor (sodium
orthovanadate, 0.5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Protein concentrations were measured using a
bicinchoninic Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc.). The proteins (20–40 mg) were first resolved by 12.5%
SDS-PAGE, and transferred onto an Immobilon-P1 transfer membrane
(Merck KGaA). The membrane was then blocked in 5% milk in
Tris-buffered saline-Tween TBST for 30 min at room temperature.
Following blocking, the membrane was incubated overnight at 4°C
with anti-FLG, anti-IVL, anti-ERK, anti-phosphorylated (p)-ERK
(1:500; cat. nos. SC30229, SC28557, SC135900 and SCSC7383,
respectively) from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA), anti-p38, anti p-p38 (diluted 1:500; cat. nos. AB7952 and
AB4822, respectively) from Abcam (Cambridge, MA, USA), or
anti-GAPDH (1:1,000; cat. no. 10494-1-AP; ProteinTech Group, Inc.,
Chicago, IL, USA) antibodies. The membranes were washed 3 times (5
min each) with PBS containing 0.1% Tween-20 and incubated with
horseradish peroxidase-conjugated secondary antibody (cat. no.
p0448; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) at
dilution of 1:5,000 in TBST for 1 h at room temperature. The blots
were quantified by densitometry using Quantity One software
(version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analyses are presented as the mean ±
standard deviation of the mean. Data were analyzed using GraphPad
Prism software (version 4.03; GraphPad Software, Inc., La Jolla,
CA, USA). Differences among multiple groups were determined using
one-way analysis of variance; differences between two groups were
assessed using the Tukey-Kramer test. P<0.05 was considered to
indicate a statistically significant difference.
Results
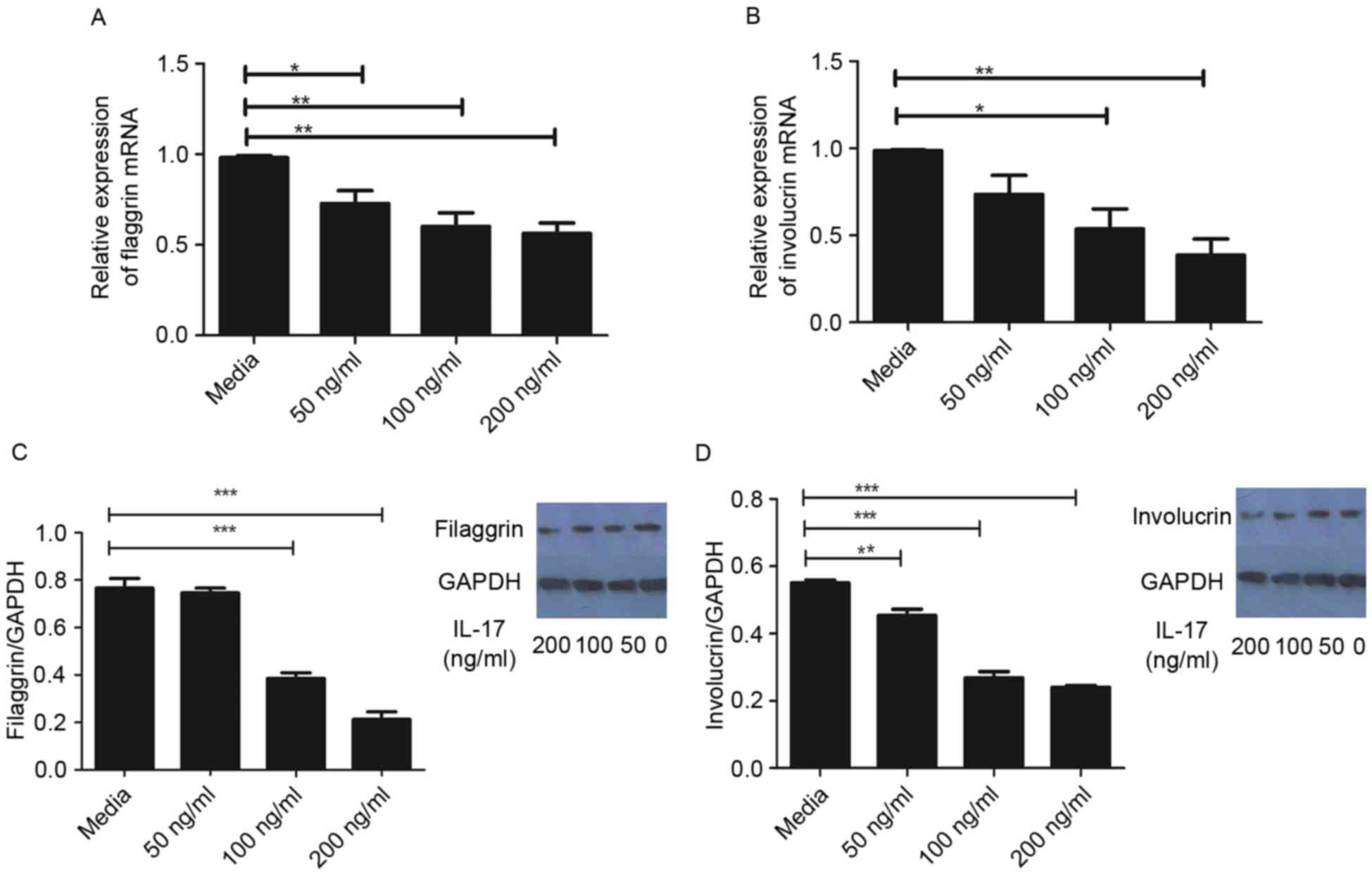
Effect of IL-17 on expression levels
of FLG and IVL in HaCaT cells
As demonstrated in Fig.
1A, the mRNA levels of FLG were significantly reduced following
treatment with IL-17, compared with that in the control group.
Similarly, the gene expression of IVL was significantly reduced by
IL-17 at concentrations ≥100 ng/ml, compared with that in the
control group (Fig. 1B). In
agreement, the protein levels of FLG and IVL were significantly
reduced following treatment with IL-17, as determined using western
blot analysis (Fig. 1C and D).
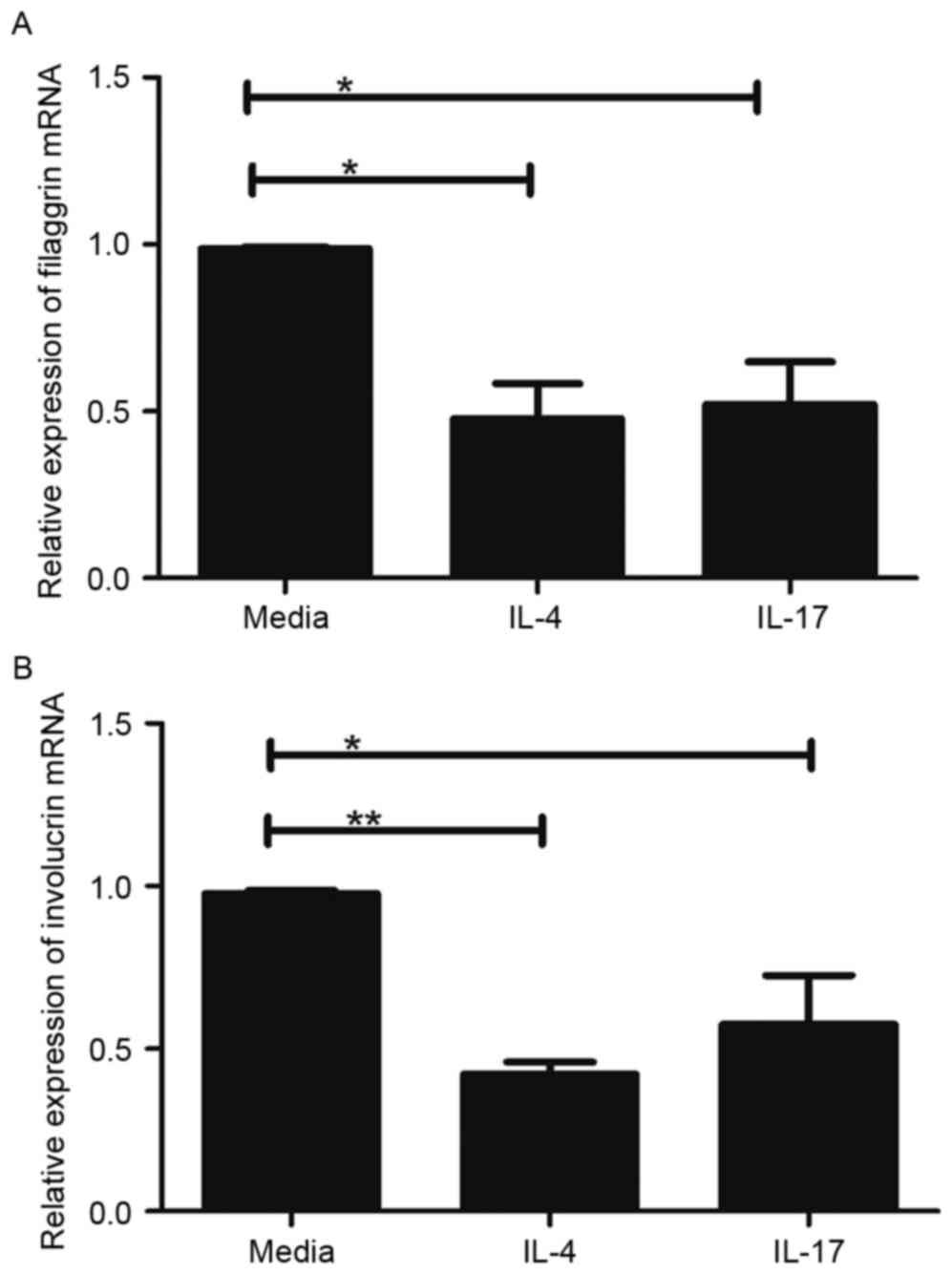
IL-17 is similar to Th2 cytokines in
regulating skin-barrier proteins
Th2 cytokines can downregulate the expression of
FLG, LOR and IVL (15). Therefore,
the present study comparatively assessed the effects of IL-4, a Th2
cytokine, and IL-17 on the expression levels of FLG and IVL in the
HaCaT cells. In this experiment, cells were treated with 100 ng/ml
IL-4 or IL-17 for 24 h, and the gene expression levels of FLG and
IVL were evaluated. As exhibited in Fig. 2, the mRNA levels of FLG and IVL
were significantly decreased in the HaCaT cells treated with IL-4
or IL-17, compared with the levels in the untreated HaCaT cells
(P<0.05). However, no significant differences were found between
IL-4 and IL-17 in terms of their ability to reduce the expression
of FLG and IVL (Fig. 2A and
B).
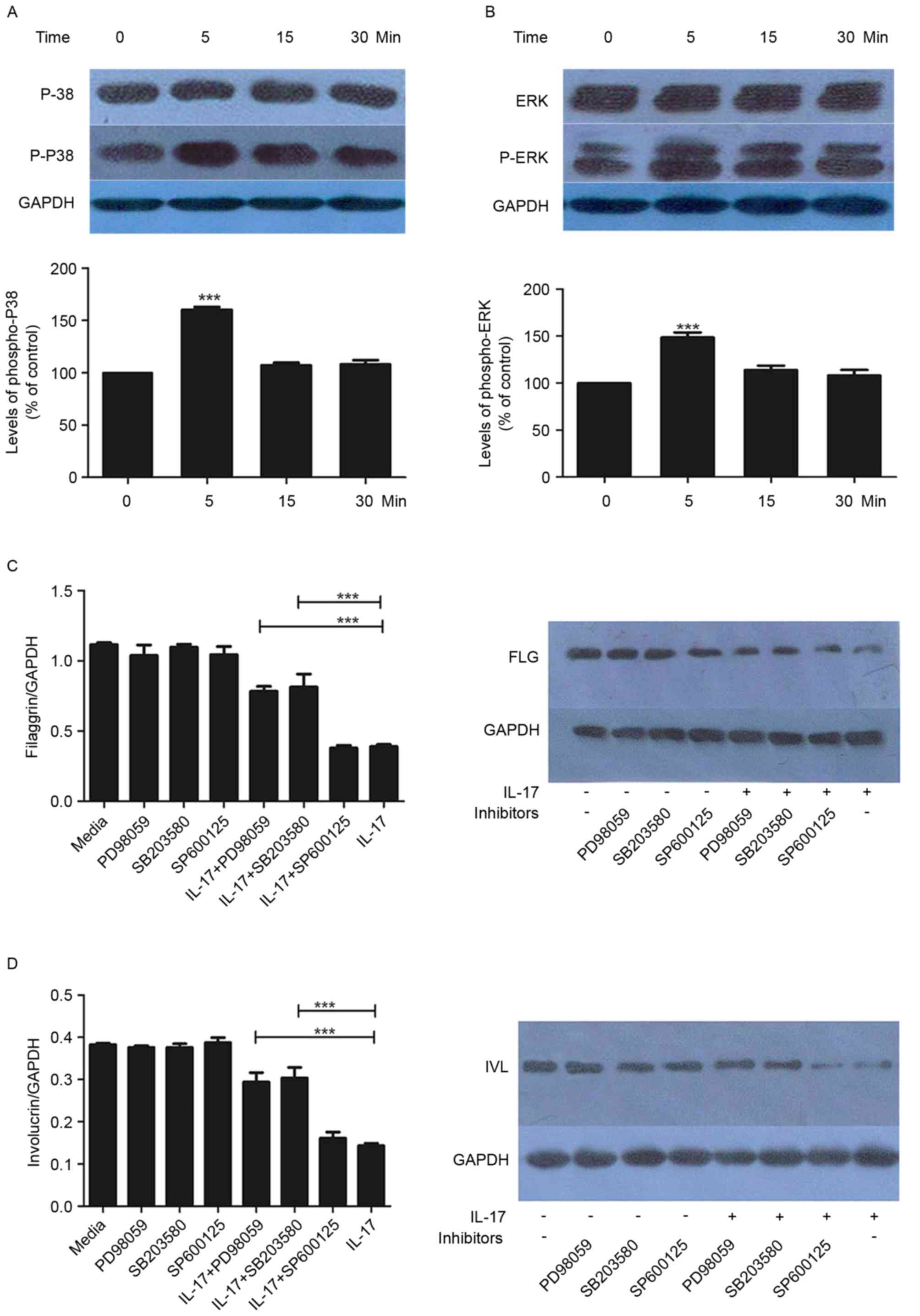
P38/ERK MAPK signaling is upregulated
by IL-17
Subsequently, the present study examined the effects
of IL-17 (100 ng/ml) on the activation of P38/ERK MAPK effectors
p38 and ERK. The phosphorylation of p38 and ERK was increased by
IL-17, with statistical significance at the 5-min time point
(Fig. 3A and B). This effect was
not observed at later time points (15 and 30 min).
Effect of MAPK inhibitors on the
expression of FLG and IVL
To further examine the mechanisms underlying the
regulation of FLG and IVL by IL-17, the HaCaT cells were treated
with SP600125, SB203580 or PD98059 in the presence or absence of
IL-17. As demonstrated in Fig. 3C and
D, SB203580 and PD98059 had significant inhibitory effects on
the IL-17-mediated reduction in the expression of FLG and IVL. This
was observed at the gene and protein levels (Fig. 3C and D).
Discussion
The present study demonstrated that IL-17 reduced
the levels of two skin barrier proteins, FLG and IVL, and that this
effect was partially inhibited by the addition of ERK and P38
inhibitors. Therefore, the downregulation of skin barrier proteins
by IL-17 may contribute to the pathogenesis of AD.
AD is a genetic disease caused by defects in skin
barrier proteins. The disruption of the skin barrier results in
contact between epidermal immune cells and antigens from the
external environment, leading to intense itching, scratching and
inflammation (19). FLG and IVL
are proteins belonging to the epidermal differentiation complex,
encoded by a cluster of genes on chromosome 1q21, which includes a
number of genes important for barrier function (20). The protein levels of FLG and IVL in
the skin of patients with AD are reduced (21), suggesting that patients with AD
have defects in these two proteins, which are important in skin
barrier function.
The IL-17 cytokine family, including IL-17A-F
(22), is involved in acute and
chronic inflammatory responses (23). IL-17 is the most potent Th17
cytokine, which stimulates the production of chemokines, cytokines
and other mediators by upregulating various genes associated with
inflammation in target cells, including keratinocytes (24). The number of Th17 cells has been
reported to be increased in the peripheral blood and skin tissue
samples from patients with AD and psoriasis (16). It is known that keratinocytes are
pivotal in skin barrier formation and maintenance (25). IL-17 affects the expression of
genes associated with cellular adhesion between keratinocytes,
resulting in skin barrier disruption (26), and skin barrier disruption
increases the penetration of allergens and the atopic inflammatory
response (27,28). In turn, the enhanced atopic immune
responses can worsen skin barrier defects in AD. IL-17 has been
shown to downregulate FLG and other genes involved in cellular
adhesion, with positive effects on the expression of IVL in primary
keratinocytes (26,29). In the present study, following
treatment of primary keratinocytes with IL-17, reduced mRNA and
protein levels of FLG and IVL were observed. The skin in patients
with AD at the acute phase is characterized by the overexpression
of Th2 cytokines IL-4 and IL-13, which can further downregulate the
expression of IVL and LOR through signal transducer and activator
of transcription-6 (21). Taken
together, the results of the present study and others (26) suggest that IL-17 is important in
the skin barrier dysfunction present in patients with AD.
IL-17 is known to activate MAPKs, and P38/ERK MAPK
signaling is involved in the pathogenesis of inflammatory skin
diseases (17). As described
above, increased phosphorylation levels of the ERK and P38 proteins
were observed following IL-17 treatment, and this effect was
alleviated by MAPK inhibitors. These findings suggested that IL-17
regulated FLG and IVL through the P38 and ERK pathways. Therefore,
inhibiting the activity of IL-17 may be a treatment option for
patients with chronic inflammatory diseases (30), including AD, restoring barrier
function (31). Other cytokines,
including the IL-27, IL-21 and IL-10 cytokines, are important
factors in the counter regulatory mechanism, which eliminates the
immune response and protects from excessive immune responses
(32). In addition, previous data
revealed the importance of the histamine 4-receptor for the
treatment of itching symptoms, suggesting that a multifaceted
approach may assist in AD therapy (33).
In conclusion, the present study demonstrated that
IL-17 is important in the pathogenesis of AD. Inhibiting the
downstream effectors of IL-17 offers a potential therapeutic
strategy for AD.
Acknowledgements
This study was supported by Natural Science
Foundation Project of CQ CSTC (grant no. cstc2012jjA10017).
Glossary
Abbreviations
Abbreviations:
|
AD
|
atopic dermatitis
|
|
FLG
|
filaggrin
|
|
GAPDH
|
glyceraldehydes-3-phosphate
dehydrogenase
|
|
IL
|
interleukin
|
|
IVL
|
involucrin
|
|
LOR
|
loricrin
|
|
MAPK
|
mitogen-activated protein kinase
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
Th2
|
T helper type 2
|
References
|
1
|
Williams H and Flohr C: How epidemiology
has challenged 3 prevailing concepts about atopic dermatitis. J
Allergy Clin Immunol. 118:209–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flohr C, England K, Radulovic S, McLean
WH, Campbel LE, Barker J, Perkin M and Lack G: Filaggrin
loss-of-function mutations are associated with early-onset eczema,
eczema severity and transepidermal water loss at 3 months of age.
Br J Dermatol. 163:1333–1336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Guo Y, Wang W, Shi M, Chen X and
Yao Z: Mutations in the filaggrin gene in Han Chinese patients with
atopic dermatitis. Allergy. 66:420–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lloyd CM and Hessel EM: Functions of T
cells in asthma: More than just T (H)2 cells. Nat Rev Immunol.
10:838–848. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai HC, Velichko S, Hung LY and Wu R:
IL-17A and Th17 cells in lung inflammation: An update on the role
of Th17 cell differentiation and IL-17R signaling in host defense
against infection. Clin Dev Immunol. 2013:2679712013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koga C, Kabashima K, Shiraishi N,
Kobayashi M and Tokura Y: Possible pathogenic role of Th17 cells
for atopic dermatitis. J Invest Dermatol. 128:2625–2630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nograles KE, Zaba LC, Guttman-Yassky E,
Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, Khatcherian A,
Gonzalez J, Pierson KC, White TR, et al: Th17 cytokines interleukin
(IL)-17 and IL-22 modulate distinct inflammatory and
keratinocyte-response pathways. Br J Dermatol. 159:1092–1102.
2008.PubMed/NCBI
|
|
8
|
Boguniewicz M and Leung DY: Atopic
dermatitis: A disease of altered skin barrier and immune
dysregulation. Immunol Rev. 242:233–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leung DY: New insights into atopic
dermatitis: Role of skin barrier and immune dysregulation. Allergol
Int. 62:151–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Candi E, Schmidt R and Melino G: The
cornified envelope: A model of cell death in the skin. Nat Rev Mol
Cell Biol. 6:328–340. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noh M, Yeo H, Ko J, Kim HK and Lee CH:
MAP17 is associated with the T-helper cell cytokine-induced
down-regulation of filaggrin transcription in human keratinocytes.
Exp Dermatol. 19:355–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elias PM, Hatano Y and Williams ML: Basis
for the barrier abnormality in atopic dermatitis:
Outside-inside-outside pathogenic mechanisms. J Allergy Clin
Immunol. 121:1337–1343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steven AC, Bisher ME, Roop DR and Steinert
PM: Biosynthetic pathways of filaggrin and loricrin-two major
proteins expressed by terminally differentiated epidermal
keratinocytes. J Struct Biol. 104:150–162. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalinin A, Marekov LN and Steinert PM:
Assembly of the epidermal cornified cell envelope. J Cell Sci.
114:3069–3070. 2001.PubMed/NCBI
|
|
15
|
Howell MD, Kim BE, Gao P, Grant AV,
Boguniewicz M, DeBenedetto A, Schneider L, Beck LA, Barnes KC and
Leung DY: Cytokine modulation of atopic dermatitis filaggrin skin
expression. J Allergy Clin Immunol. 124(3 Suppl 2): R7–R12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma L, Xue HB, Guan XH, Shu CM, Wang F,
Zhang JH and An RZ: The Imbalance of Th17 cells and CD4 (+) CD25
(high) Foxp3 (+) Treg cells in patients with atopic dermatitis. J
Eur Acad Dermatol Venereol. 28:1079–1086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johansen C, Kragballe K, Westergaard M,
Henningsen J, Kristiansen K and Iversen L: The mitogen-activated
protein kinases p38 and ERK1/2 are increased in lesional psoriatic
skin. Br J Dermatol. 152:37–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maintz L and Novak N: Getting more and
more complex: The pathophysiology of atopic eczema. Eur J Dermatol.
17:267–283. 2007.PubMed/NCBI
|
|
20
|
de Guzman Strong C, Conlan S, Deming CB,
Cheng J, Sears KE and Segre JA: A milieu of regulatory elements in
the epidermal differentiation complex syntenic block: Implications
for atopic dermatitis and psoriasis. Hum Mol Genet. 19:1453–1460.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim BE, Leung DY, Boguniewicz M and Howell
MD: Loricrin and involucrin expression is down-regulated by Th2
cytokines through STAT-6. Clin Immunol. 126:332–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aggarwal S and Gurney AL: IL-17: Prototype
member of an emerging cytokine family. J Leukoc Biol. 71:1–8.
2002.PubMed/NCBI
|
|
23
|
Iwakura Y, Ishigame H, Saijo S and Nakae
S: Functional specialization of interleukin-17 family members.
Immunity. 34:149–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirkham BW, Kavanaugh A and Reich K:
Interleukin-17A: A unique pathway in immune-mediated diseases:
Psoriasis, psoriatic arthritis and rheumatoid arthritis.
Immunology. 141:133–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feingold KR and Elias PM: Role of lipids
in the formation and maintenance of the cutaneous permeability
barrier. Biochim Biophys Acta. 1841:280–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gutowska-Owsiak D, Schaupp AL, Salimi M,
Selvakumar TA, McPherson T, Taylor S and Ogg GS: IL-17
downregulates filaggrin and affects keratinocyte expression of
genes associated with cellular adhesion. Exp Dermatol. 21:104–110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spergel JM, Mizoguchi E, Brewer JP, Martin
TR, Bhan AK and Geha RS: Epicutaneous sensitization with protein
antigen induces localized allergic dermatitis and
hyperresponsiveness to methacholine after single exposure to
aerosolized antigen in mice. J Clin Invest. 101:1614–1622. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Benedetto A, Rafaels NM, McGirt LY,
Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S,
Yoshida T, et al: Tight junction defects in patients with atopic
dermatitis. J Allergy Clin Immunol. 127:773–786. e1-e7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen JQ, Man XY, Li W, Zhou J, Landeck L,
Cai SQ and Zheng M: Regulation of involucrin in psoriatic epidermal
keratinocytes: The roles of ERK1/2 and GSK-3β. Cell Biochem
Biophys. 66:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simon D and Lang K Kernland: Atopic
dermatitis: From new pathogenic insights toward a barrier-restoring
and anti-inflammatory therapy. Curr Opin Pediatr. 23:647–652. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Noh G and Lee J: Atopic dermatitis and
cytokines: The immunoregulatory and therapeutic implications of
cytokines in atopic dermatitis-part II: Negative regulation and
cytokine therapy in atopic dermatitis. Recent Pat Inflamm Allergy
Drug Discov. 6:248–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roesner LM, Werfel T and Heratizadeh A:
The adaptive immune system in atopic dermatitis and implications on
therapy. Expert Rev Clin Immunol. 12:787–796. 2016. View Article : Google Scholar : PubMed/NCBI
|