Introduction
Macrophages are involved in the occurrence,
development and digestion of inflammation and other stages. At
different stages of the inflammatory response, macrophages exhibit
different phenotypes (1).
Macrophages have at least two different polarizations, namely
M1-type polarization and M2-type polarization (1,2). The
former is characterized by elevation of pro-inflammatory cytokines,
antimicrobial and tumoricidal activity, whereas the latter is
linked to immunosuppression and tissue repair (1,2).
Activation of M1-type macrophages is typically
driven by Thl-type cytokine interferon-Y, bacterial-related
components such as Toll-like receptor (TLR) analogs (1,3).
Type M1 is also characterized by efficient generation of reactive
oxygen intermediates (ROI), inflammatory cytokines such as IL-1β,
TNF and IL-6, and cytotoxicity (phagocytosis of microorganisms and
necrotic cells) (4). In mice, M1
macrophage-associated markers include IL-12, MHC class II molecules
and nitric oxide synthase 2 (NOS2) (5,6).
More and more studies had shown that macrophage
polarization imbalance is the key pathological factors of a variety
of immune-related diseases such as autoimmune diseases, tumors and
other diseases (7). The study of
target molecules for macrophage polarization regulation has become
a new research hotspot. Although it is known that signal molecules
such as signal transducer and activator of transcription (Stat)1,
Stat3, Stat6, SOCS1, IRF4 and IRFS, as well as a variety of miRNAs,
are involved in the macrophage polarization regulation (8,9), but
different receptor molecules how to activate the polarization
regulation signal above-mentioned is not very clear. It is of great
theoretical and practical significance to find a new type of
macrophage regulatory molecule and to clarify its regulatory
mechanism for the targeted immune intervention of macrophages.
Galectin is a family of lectin that specifically
binds to the glycoprotein-β-galactose residue and is present in a
wide variety of organisms and is widely distributed in the nucleus,
cytoplasm and extracellular matrix (10). Galectin has a variety of biological
functions, such as cell adhesion, cell growth regulation,
apoptosis, inflammatory response, immune regulation (10,11).
Recent report had showed that macrophages with upregulated
Galectin-3 participate in liver cirrhosis through production of
both M1- and M2-related factors (12). Another report revealed that the
inhibition of Gal-3 binding to integrin promoted macrophages
phenotype towards the M1 phenotype (13). Gal-9 is also a member of the
Galectin family and has a typical conserved region of the Galectin
family (14). It is widely
distributed in the liver, lung, tonsil, islet cells and various
immune cells (14). Gal-9 plays an
important role in the regulation of various diseases, including T
cell-mediated diseases (such as autoimmune diseases and asthma),
tumors (such as melanoma, cervical cancer, hepatic carcinoma)
(13,14). However, these biological effects of
Gal-9 are mostly activated by their binding to T cell
immunoglobulin mucin (Tim)-3 (14,15).
Tim-3 is an important member of the Tim gene family, mainly
expressed on the surface of activated Th1 cells, and plays an
important immunomodulatory role when combined with its ligand Gal-9
(14–16). Recently, the study found that Tim-3
is also expressed in natural immune cells (17). In addition, investigations showed
that the expression of Tim-3 in sepsis is related to the
overactivation of macrophages in vivo (18), suggesting that Tim-3 may be a new
regulatory target for macrophages via changing the levels of
Galectin-9 (Gal-9). However, the effect of Gal-9 on macrophage
polarization is poorly understood to date.
Since the polarization state of macrophages has a
crucial effect on its own function, and the mechanism of regulation
of polarization is not yet clear. Therefore, the aim of this study
was to investigate whether Gal-9 is involved in the regulation of
macrophage polarization.
Materials and methods
Cell culture
The mouse macrophage line RAW264.7 is a product of
ATCC. Cells were cultured in DMEM medium supplemented with 5% FBS,
100 U/ml penicillin and 100 µg/ml streptomycin and placed in a cell
culture chamber at 37°C in a humidified atmosphere with 5%
CO2. After the cells were grown to 80% confluence, the
culture medium was removed and washed with PBS and digested with
0.25% trypsin. Logarithmic growth phase cells were used for
experiments as follows.
Cell transfection
Gal-9 siRNA (Santa Cruz Biotechnology, Dallas, TX,
USA) and recombinant plasmid containing Gal-9 (Promocell,
Heidelberg, Germany) were transfected into RAW264.7 cells with
Invitrogen Lipofectamine 2000 according to the manufacturer's
instructions (Invitrogen, Carlsbad, CA, USA). Briefly, cells were
seeded in 6-well plates and grown to 60 to 70% confluence. Cells
were then suspended with transfection reagent and Gal-9 specific
siRNA (or scramble siRNA) or Gal-9 recombinant plasmid (or mock
plasmid) were added with a concentration of 10 nM into a 1.5 ml
centrifuge tube and incubated for 10 min. Lipofectamine 2000 (10
µl) was added to another 1.5 ml centrifuge tube and incubated at
room temperature for 10 min. The two tube solutions were then mixed
and incubated at room temperature for 20 min. The mixed solution
was then added to 6 wells and placed into the incubator at 37°C, 5%
C02 for 5 h. After transfection for 5 h, the transfected
cells were incubated for another 24 h in complete medium. The
transfected cells were divided into several groups as follows:
Control (without treatment), Scramble, pGal-9, mock, (negative
siRNA), Gal-9-siRNA and then the expression levels of Gal-9 mRNA
and protein were performed. After transfected for 48 h, the cells
were harvested to conduct analysis performed as follows.
Detection of cell proliferation
activity by CCK-8 assay
Cell proliferation activities from all groups were
determined by CCK-8 assay after cells transfected for 0, 6, 12, 24,
48 h. Briefly, cells were inoculated in 96-well plates at
1×105 cells per well. CCK-8 (20 µl; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was then added and the cells
were incubated for another 4 h. The absorption of cell solutions
was read at 450 nm using an ELISA reader (BioTek Instruments, Inc.,
Winooski, VT, USA) following the manufacturer's instructions.
Detection of cell polarization by flow
cytometry
Cells collected from all groups were fixed with 4%
paraformaldehyde at room temperature for 30 min and washed twice
with PBS, and then punched with 0.3% Triton X-100 for 5 min. After
washed twice with PBS, cells were blocked with 1% BSA 200 ml for 1
h. Cells solutions were then centrifuged and the supernatants were
discarded. Then, 0.125 µg of FITC-labeled CD206 and 0.25 µg of
PE-labeled NOS2 (both from BioLegend, Inc., San Diego, CA, USA)
were added, respectively, and incubated at 40°C for 30 min.
Followed by washing twice with PBS, and the cell polarity was
measured by flow cytometry.
Enzyme-linked immunosorbent assay
(ELISA) assay
Cell supernatant was isolated from all groups after
centrifugation at 14,000 rpm at 4°C for 20 min. Cell (20 µl)
supernatant from all groups were transferred to a 96-well plate and
NOS2, TNF-α, CD206, TGF-β levels were measured using specific ELISA
kits (Biosource International Inc., Camarillo, CA, USA). Briefly,
the ELISA assays were conducted using 96-well microplate coated
with anti-NOS2, TNF-α, CD206, TGF-β monoclonal antibody. Samples
and NOS2, TNF-α, CD206, TGF-β standard controls were added to the
plate, and then incubated at room temperature with shaking for 2 h
and 6 washes. The concentration of Gal-9 in supernatant was also
measured by a commercial ELISA kit (Cygnus, Southport, NC, USA)
according to manufacturer's instructions. Chemiluminescent signal
was subsequently measured at 450 nm.
Dual-luciferase reporter gene
assay
RAW264.7 cells were seeded on 24-well plates at a
density of 8X104 cells/well. To detect the transcription
activity of NF-κB, RAW264.7 cells were co-transfected with firefly
luciferase reporter gene, rennet luciferase reporter gene, and
Gal-9 plasmid with 1 ml Lipofectamine 2000 according to the
manufacturer's instructions (Invitrogen). After incubation for 48
h, the individual samples were collected and the cells were lysed
with the PLB lysate in the kit, and the protein was extracted and
used to detect luciferase activity. The substrate was added and the
activity of luciferase was determined. The change in NF-κB activity
in RAW264.7 cells was reflected by the ratio of firefly luciferase
activity/bloody luciferase activity.
Western blot assay
The cell lysates from all groups were extracted
using lysis Triton X-100 buffer containing 50 mM Tris (pH 7.6), 1%
Triton X-100, 150 mM NaCl, 0.1% sodium dodecylsulphate (SDS), 1%
deoxycholate, 0.2% aprotinin and1 mM PMSF (Sigma-Aldrich, St.
Louis, MO, USA). Protein content was detected using the BCA protein
assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The
equal protein samples were mixed into the wells of 7.5%
polyacrylamidegels. The samples were then resolved by 10% SDS-PAGE
gel and transferred on PVDF membrane (Roche Diagnostics, Penzberg,
Germany). Membranes were then blocked with 10% non-fat dried milk
in PBS. The blots were then incubated with anti-p-Stat1,
anti-Stat1, anti-p-Stat3 anti-Stat3, anti-Gal-9 antibody at 4°C
overnight. β-actin was used as an internal control. After washed
with PBS, the blots were incubated with appropriate HRP-conjugated
secondary antibodies at room temperature for 1 h. The blots were
visualized by using chemiluminescence and developed onto the film
(Kodak, Rochester, NY, USA) according to the manufacturer's
instructions.
RT-PCR assay
Total cellular RNA was isolated from all groups
using the RNeasy mini kit (Qiagen, Inc., Valencia, CA, USA)
according to manufacturer's protocol. Total RNA (1 µg) was reverse
transcribed into cDNA using the Omniscript™ Reverse Transcriptase
kit (Qiagen, Inc.). Real-time PCR was performed using a SYBR-Green
PCR Master mix (Applied Biosystems, Foster. City, CA, USA).
Analysis was performed on an ABI PRISM 7700 Sequence Detection
system (Applied Biosystem) according to the manufacturer's
protocol. The specificity of PCR products obtained was verified by
melting curve analysis and agarose gel electrophoresis. The
expression of Gal-9, NOS2, TNF-α, CD206 and TGF-β1 were normalized
against to β-actin and were calculated using delta-delta cycle
threshold (CT) method. Primers were designed by using ‘Primer3’ on
website. Briefly, we acquired the primers on website ‘Primer3 web
version 4.0.0’ according to the gene sequences obtained from
‘NCBI’. Primer sequences for Gal-9, NOS2, TNF-α, CD206, TGF-β are
listed as follows: Gal-9, 5′GGGCAGGAAGAGCGAAGTCT3′ (forward) and
5′GCTGGATATCACCCGCCACT3′ (reverse); TNF-α, 5′CCAGGCAGGTTCTGTCCCTT3′
(forward) and 5′ATAGGCACCGCCTGGAGTTC3′ (reverse); IL-10,
5′CCAGTACAGCCGGGAA GACA3′ (forward) and 5′GAAGGCAGTCCGCAGCTCTA3′
(reverse); IL-6, 5′CTGGAGCCCACCAAGAACGA3′ (forward) and
5′GCCTCCGACTTGTGAAGTGGT3′ (reverse); TGF-β1,
5′GCCACTGCCCATCGTCTACT3′ (forward) and 5′CACTTGCAGGAGCGCACAAT3′
(reverse); β-actin, 5′GCCGGGACCTGACAGACTAC3′ (forward) and
5′TGGCCATCTCCTGCTCGAAG3′ (reverse).
Statistical analysis
The data from three independent experiments was
expressed as means ± standard deviation. Statistical comparisons
between different groups were calculated by SPSS software (SPSS
version 20; IBM SPSS, Armonk, NY, USA). P<0.05 was considered
statistically significant.
Results
Recombinant Gal-9 and siRNA of Gal-9
are effectively transfected into RAW264.7 cells
In order to explore different expression levels of
Gal-9 on the regulation of macrophage polarization, we constructed
cell models transfected with recombinant Gal-9 genes or siRNA
targeting Gal-9. After cells transfected for 48 h, the expression
of Gal-9 was tested to confirm the transfection effectiveness. Our
results revealed that highly effective transfection of recombinant
Gal-9 and siRNA of Gal-9 were obtained in RAW264.7 cells. As showed
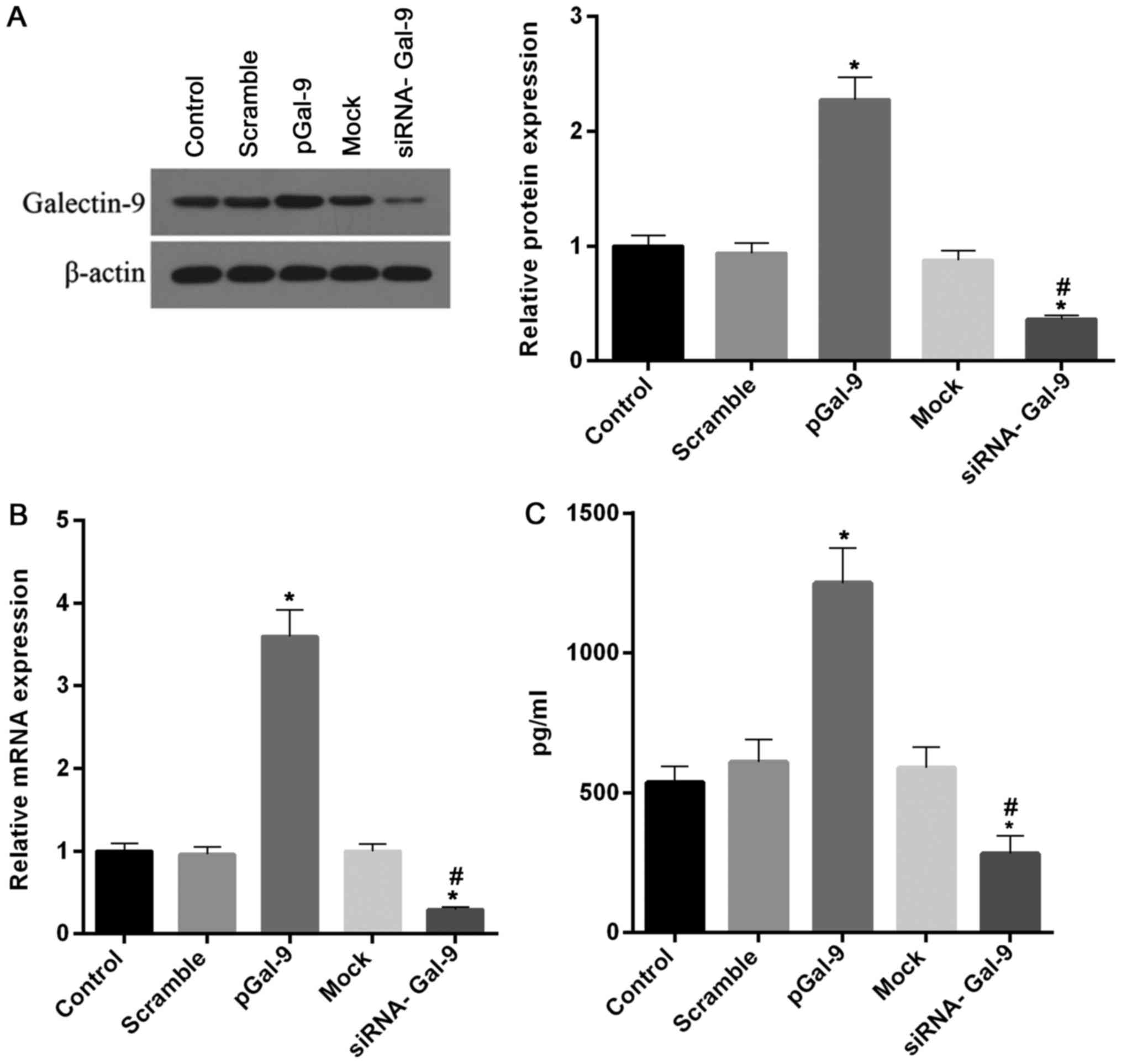
in Fig. 1, the levels of Gal-9
protein and mRNA were markedly increased and decreased in cells
treated with transfection of recombinant Gal-9 (pGal-9) and siRNA
of Gal-9 (siRNA-Gal-9), respectively, compared to cells from
control, negative control of recombinant Gal-9 (Scramble) or siRNA
of Gal-9 (Mock). Moreover, we tested the concentration of Gal-9 in
supernatant. The profile of Gal-9 concentration in the supernatant
is consistent with the intracellular level of Gal-9 protein
(Fig. 1C).
Cell viability of cells is reduced in
cells transfected with siRNA-Gal-9
To assess the effects of transfection treatment on
the cell proliferation activity, we detected the cell viabilities.
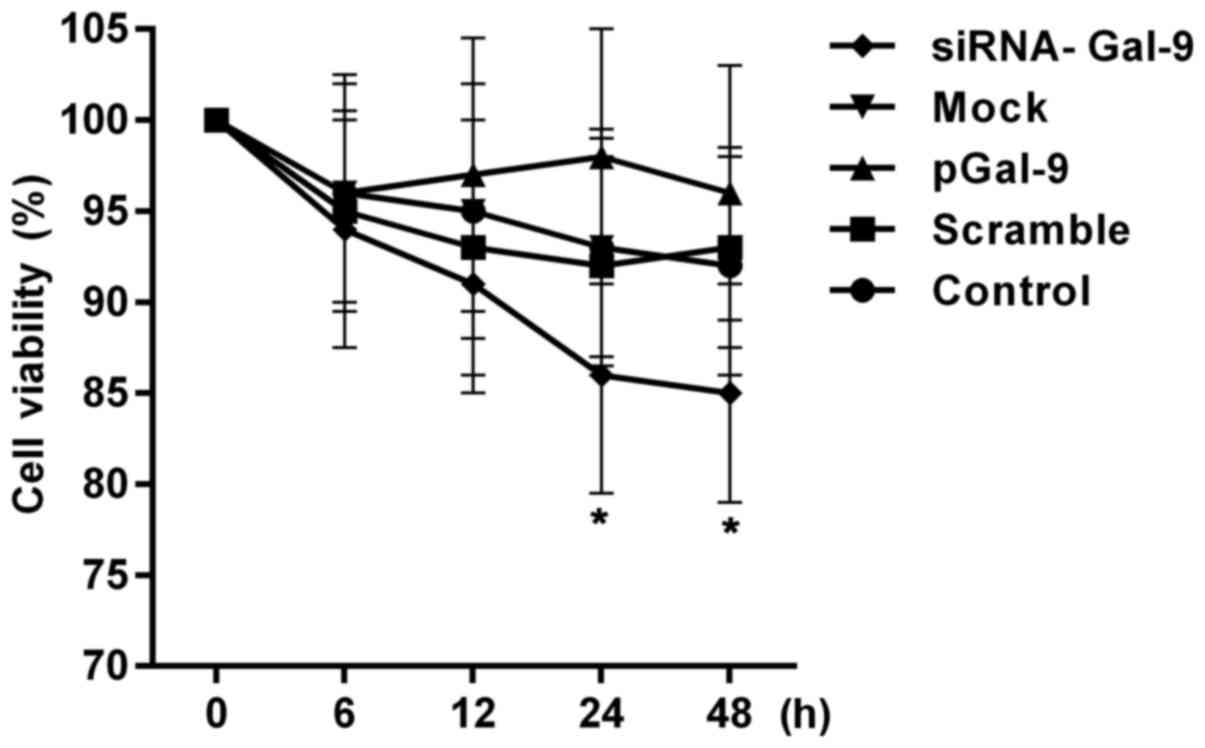
Fig. 2 showed a CCK-8 assay of
relative cell viability of cells at 0, 6, 12, 24, 48 h after cell
transfection. The cell viabilities were altered in cells from
pGal-9 and siRNA-Gal-9 group compared to Control, Scramble and Mock
group. The cell viability of cells from siRNA-Gal-9 was apparent
lower than that of the others, especially 24 h after the
transfection. Although a slight higher of cell viability was
observed in cells from pGal-9 group compared to Control, Scramble
and Mock group, there was no significant difference between them.
However, the cell viabilities were nearly equal among Control,
Scramble and Mock group.
Expression levels of NOS2 and CD206 in
cells are altered in the opposite way after regulation of Gal-9
level
To better understand the effect of elevated or
reduced expression of Gal-9 on the regulation of macrophage
polarization, we detected the levels of polarization biomarkers,
including NOS2 for M1-type and CD206 for M2-type. As showed in
Fig. 3, flow cytometry assay
revealed that NOS2 expression level was upregulated in cells
transfected with siRNA-Gal-9, whereas it was downregulated in cells
transfected with pGal-9, compared to cells from Control, Scramble,
and Mock group. By contrast, CD206 expression level was
downregulated in cells transfected with siRNA-Gal-9, whereas it was
upregulated in cells transfected with pGal-9, compared to cells
from Control, Scramble, and Mock group. In addition, the expression
levels of NOS2 and CD206 were no apparent variation among Control,
Scramble, and Mock group.
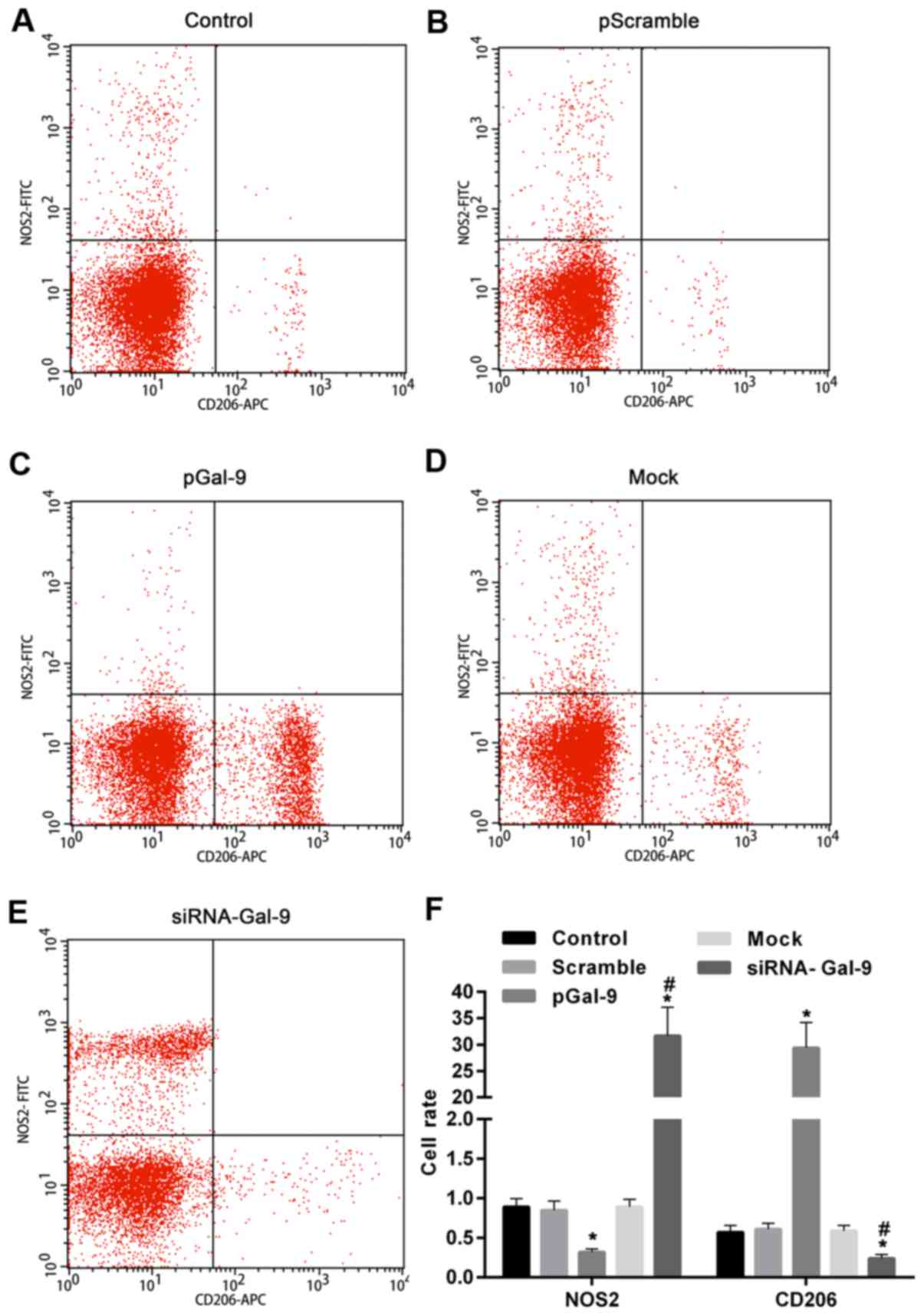 | Figure 3.NOS2 and CD206 expression in cells
were significantly changed by the transfection of pGal-9 and
siRNA-Gal-9. Detection of cell polarization was performed by flow
cytometry assay. Scatter plot of NOS2 and CD206 expression in (A)
Control, (B) pScramble, (C) pGal-9, (D) Mock and (E) siRNA-Gal-9
groups. (F) The expression level of NOS2 was downregulated and
upregulated in cells transfected with pGal-9 and siRNA-Gal-9,
respectively, compared to cells from Control, Scramble, and Mock
group. In contrast, CD206 expression in cells was altered in the
opposite way compared to NOS2. *P<0.05 vs. control;
#P<0.05, siRNA-Gal-9 vs. pGal-9. NOS2, nitric oxide
synthase 2; Gal-9, Galectin-9; pGal-9, recombinant Gal-9; siRNA,
small interfering RNA; CD, cluster of differentiation. |
Macrophage polarization-associated
genes are regulated by levels of Gal-9 in both extracellular and
intracellular
TNF-α and TGF-β were tightly related to M1-type
polarization and M2-type polarization, respectively. In order to
further confirm the effect of overexpression and down-regulation of
Gal-9 on RAW264.7 cell polarization, we detected the protein and
mRNA levels of IL-6, TNF-α, IL-10 and TGF-β using ELISA assay and
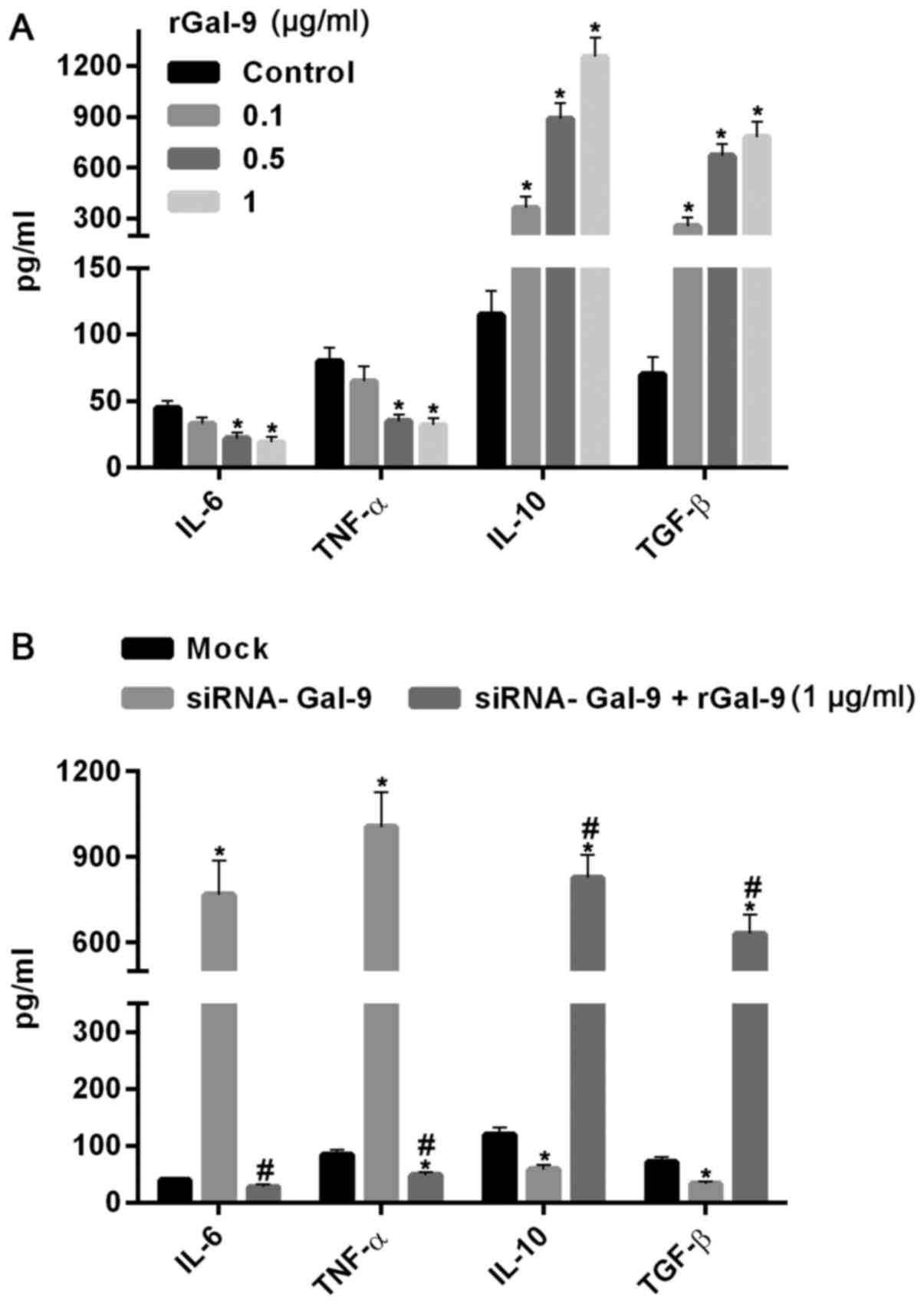
real-time RT-PCR assay, respectively. Fig. 4 showed the variations of macrophage
polarization-associated genes after transfection for 48 h. In cells
transfected with pGal-9, IL-6 and TNF-α protein and mRNA levels
were downregulated, whereas IL-10 and TGF-β protein and mRNA levels
were upregulated, compared to the control groups (Control, Scramble
and Mock). By contrast, in cells transfected with siRNA-Gal-9, IL-6
and TNF-α protein and mRNA levels were upregulated, whereas IL-10
and TGF-β protein and mRNA levels were downregulated, compared to
the control groups. These results confirmed the observations of
flow cytometry assay conducted above.
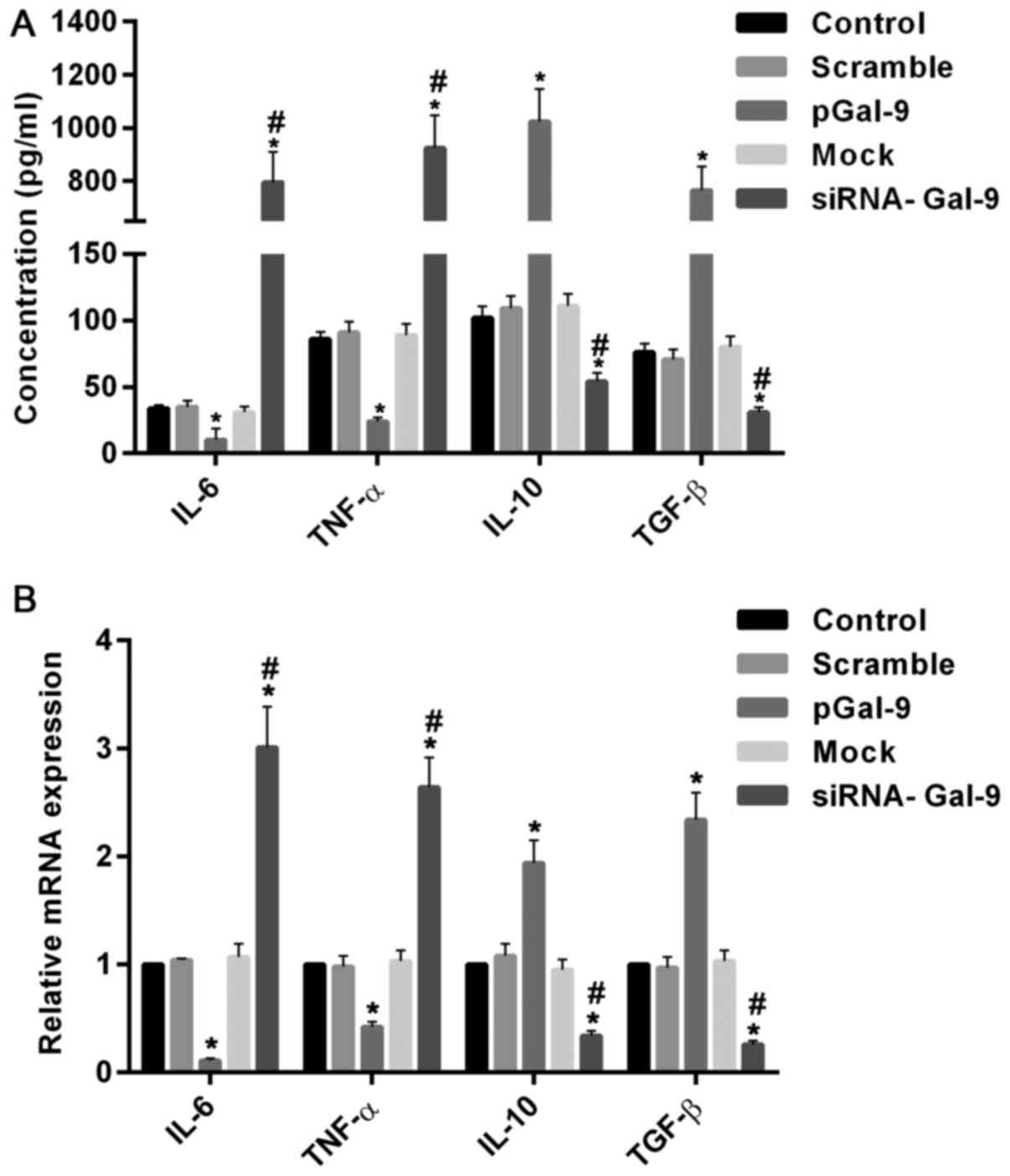 | Figure 4.Introduction or knockdown of Gal-9
simulated the variations of macrophage polarization-associated
genes. The protein and mRNA analysis conducted by ELISA assay (A)
and reverse transcription-quantitative polymerase chain reaction
assay (B) respectively. The results showed that in cells
transfected with pGal-9, IL-6 and TNF-α protein and mRNA levels
were reduced, whereas IL-10 and TGF-β protein and mRNA levels were
increased, compared to the control groups (Control, Scramble and
Mock). In contrast, in cells transfected with siRNA-Gal-9, IL-6 and
TNF-α protein and mRNA levels were elevated, whereas IL-10 and
TGF-β protein and mRNA levels were decreased, compared to the
control groups. *P<0.05 vs. control; #P<0.05,
siRNA-Gal-9 vs. pGal-9. Gal-9, Galectin-9; ELISA, enzyme-linked
immunosorbent assay; pGal-9, recombinant Gal-9; siRNA, small
interfering RNA; TGF, transforming growth factor; IL, interleukin;
TNF, tumor necrosis factor. |
To further confirm the effect of Gal-9 on the cells,
we added carrier-free recombinant Gal-9 (R&D Systems, Inc.,
Minneapolis, MN, USA) into the medium (19). After 24 h, the results showed that
IL-6 and TNF-α were dosage-dependently decreased, whereas IL-10 and
TGF-β were significantly increased with increasing levels of rGal-9
(0.1–1 µg/ml) (Fig. 5A). Moreover,
addition of rGal-9 (1 µg/ml) for 24 h evidently abolished the
effect of siRNA-Gal-9 on proteins expression (Fig. 5B).
Gal-9 can mediate the macrophage
polarization-associated transcription factors
Studies revealed that transcription factors,
including NF-κB, Stat1, and Stat3, are involved macrophage
polarization. To detect the potential pathway through which Gal-9
mediates the macrophage polarization, we determined the expression
levels of these factors after cells transfected with pGal-9 or
siRNA-Gal-9. The transcription activity of NF-κB detected by
dual-luciferase reporter gene assay was downregulated in cells
transfected with pGal-9, whereas it was upregulated in cells
transfected with siRNA-Gal-9, compared to the control groups. In
addition, there were nearly equal levels among the control groups
(Fig. 6A).
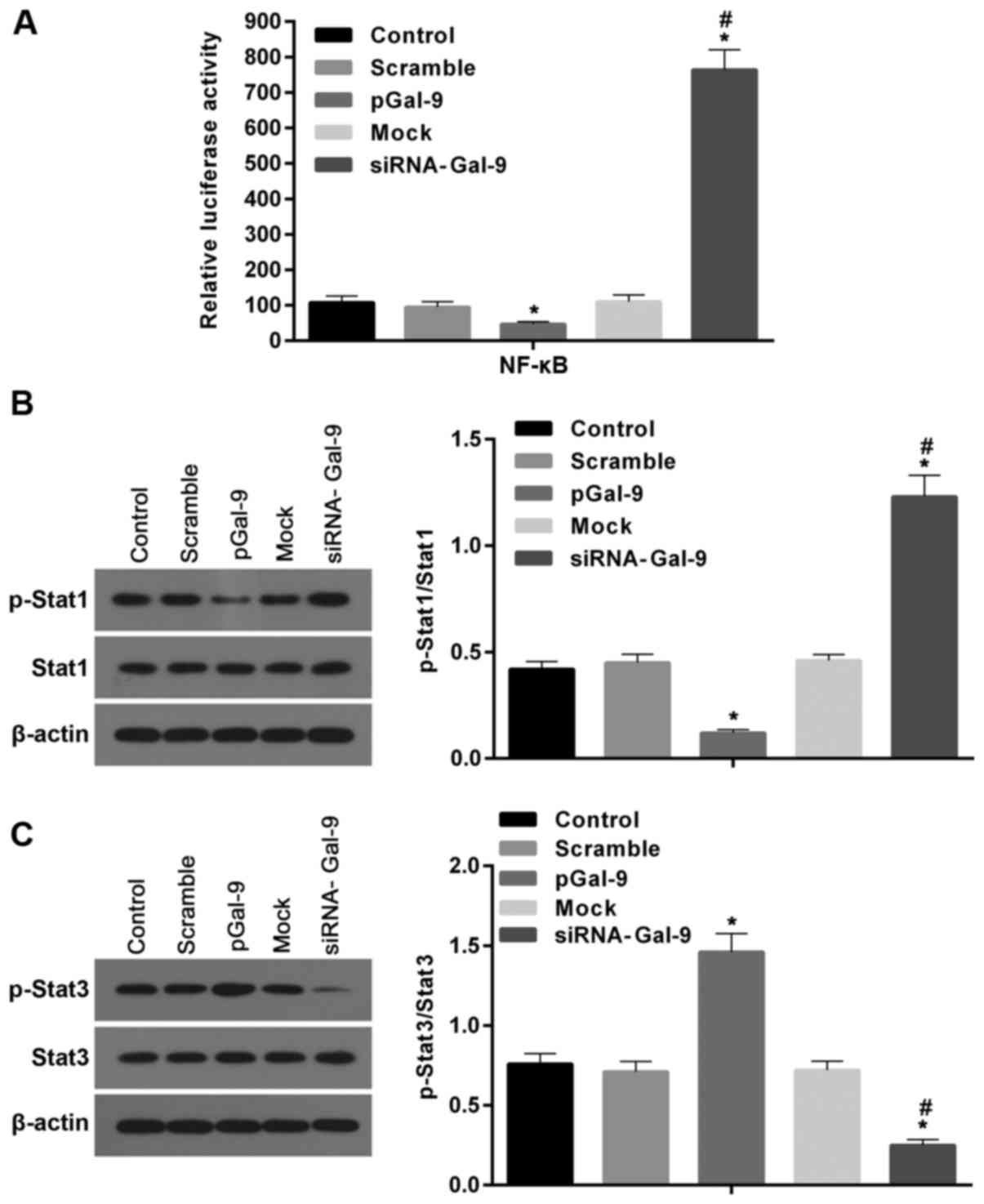 | Figure 6.Gal-9 can mediate the macrophage
polarization through transcription factors such as NF-κB, Stat1,
and Stat3. (A) The transcription activity of NF-κB was measured by
dual-luciferase reporter gene assay. Activity of NF-κB was
decreased in cells transfected with pGal-9, however it was
increased in cells transfected with siRNA-Gal-9, compared to the
control groups. (B and C) The protein levels of p-Stat1, Stat1,
p-Stat3 and Stat3 were detected by western blot assay. Both the
levels of p-Stat1 and the ratios of p-Stat1 to Stat1 were decreased
and increased in cells transfected with pGal-9 and siRNA-Gal-9,
respectively, compared to the control groups. By contrast, both
p-Stat3 levels and the ratios of p-Stat3 to Stat3 were elevated and
reduced in cells transfected with pGal-9 and siRNA-Gal-9,
respectively, compared to the control groups. However, no apparent
variations for the levels of Stat1 and Stat3 among groups were no
detected. *P<0.05 vs. control; #P<0.05,
siRNA-Gal-9 vs. pGal-9. Gal-9, Galectin-9; Stat, signal transducer
and activator of transcription; p-Stat, phosphorylated Stat;
pGal-9, recombinant Gal-9; siRNA, small interfering RNA. |
The western blot assay revealed that in cells
transfected with pGal-9, together with the level of p-Stat1
protein, the ratio of p-Stat1 to Stat1 were reduced, though Stat1
level was no apparent changes, compared to the control (Fig. 6B). By contrast, in cells
transfected with siRNA-Gal-9, both p-Stat1 protein level and the
ratio of p-Stat1 to Stat1 were elevated compared to control,
despite the Stat1 level was nearly equal compared with control
groups (Fig. 6B). However, the
Stat3 levels were altered in the opposite way compared to Stat1.
Fig. 6C showed that in cells
transfected with pGal-9, the level of p-Stat3 protein and the ratio
of p-Stat3 to Stat3 were upregulated, though Stat3 level was no
visible changes, compared to control groups. While in cells
transfected with siRNA-Gal-9, p-Stat3 protein levels and the ratio
of p-Stat3 to Stat3 were significantly decreased, and despite the
Stat3 level was slightly increased, compared with control
groups.
We further tested the role of Stat1, Stat3 and NF-κB
in regulations of M1-type and M2-type macrophages related
cytokines. As showed in Fig. 7A,
the levels of IL-6 and TNF-α were elevated, whereas the levels of
IL-10 and TGF-β were reduced in cells treated with siRNA-Gal-9,
compared to those in cells from Mock group. By contrast, these
cytokines were inversely varied in cells treated with sRNA-Gal-9
and Stat1 inhibitor Fludarabine (10 µM) (Selleck Chemicals,
Houston, TX, USA). These results suggested that Stat1 was a
positive transcription factor for M1-type macrophages. In Fig. 7B, the levels of IL-6 and TNF-α were
decreased, while the levels of IL-10 and TGF-β were dramatically
increased in cells treated with pGal-9, compared to those in cells
from Scramble group. However, these cytokines were inversely
changed in cells treated with pGal-9 and Stat3 inhibitor Stattic (7
µM) (Selleck Chemicals). These results indicated that Stat3
promotes the transcription of cytokines related to M2-type
macrophages. Moreover, similar effects of NF-κB and its inhibitor
JSH-23 (10 µM) (Selleck Chemicals) on levels of these cytokines as
Stat1 was showed in Fig. 7C, which
demonstrated that NF-κB can simulate expression of cytokines genes
related to M1-type macrophages and suppress expression of cytokines
associated with M2-type macrophages. Therefore, together with the
results discussed above, this study suggested that Gal-9 induces
the activation of NF-κB, Stat1 or Stat3 signaling molecules and
thereby promoting the M1-type or M2-type related genes expression,
resulting the shifting between M1-type and M2-type of macrophages
polarization.
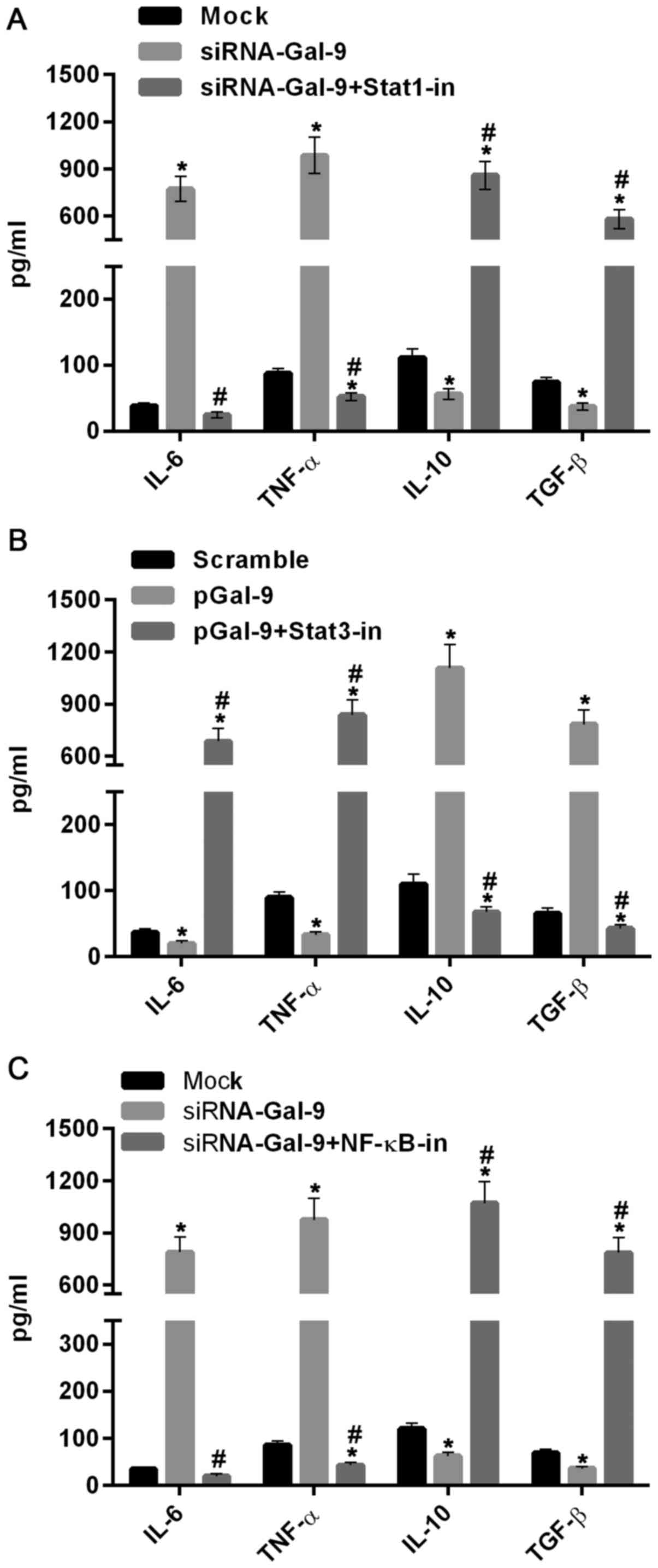 | Figure 7.Cytokines related to M1- and M2-type
macrophages were regulated by Gal-9 via activation of Stat1, Stat3
and NF-κB. (A) ELISA assay showed that the levels of IL-6 and TNF-α
were evidently increased, whereas the levels of IL-10 and TGF-β
were decreased in cells treated with siRNA-Gal-9, compared to those
in cells from Mock group. However, they were inversely changed in
cells treated with sRNA-Gal-9 and Stat1 inhibitor Fludarabine. (B)
The levels of IL-6 and TNF-α were reduced, whereas the levels of
IL-10 and TGF-β were clearly increased in cells treated with
pGal-9, compared to those in cells from Scramble group. However,
they were inversely varied in cells treated with pGal-9 and Stat3
inhibitor Stattic. (C) The levels of IL-6 and TNF-α were
dramatically elevated, whereas the levels of IL-10 and TGF-β were
decreased in cells treated with siRNA-Gal-9, compared to those in
cells from Mock group. However, these cytokines were inversely
altered in cells treated with NF-κB inhibitor JSH-23. *P<0.05
vs. control; #P<0.05, (A) siRNA-Gal-9 + Stat1-in vs.
siRNA-Gal-9, (B) pGal-9 + Stat3-in vs. pGal-9, (C) siRNA-Gal-9 +
NF-κB-in vs. siRNA-Gal-9. Gal-9, Galectin-9; Stat, signal
transducer and activator of transcription; ELISA, enzyme-linked
immunosorbent assay; pGal-9, recombinant Gal-9; siRNA, small
interfering RNA; TGF, transforming growth factor; IL, interleukin;
TNF, tumor necrosis factor; NF, nuclear factor; in, inhibitor. |
Discussion
Even though Gal-9 is involved in the regulation of a
variety of biological processes such as cell aggregation and
adhesion, chemotaxis of eosinophils, induction of thymocytes,
immune T cells and induction of melanoma cell apoptosis, the role
of it in macrophages is poorly understood. In our study, we had
explored the expression of macrophage polarization-associated
genes. Our results suggested that Gal-9 was a potential regulator
for the macrophage polarization. Firstly, we successfully
constructed cell models with upregulation or downregulation of
Gal-9, which confirmed by protein and mRNA analysis. The further
flow cytometry assay revealed that macrophage polarization
indicators for M1-type, NOS2, and for type-M2, CD206, were
downregulated and upregulated in cells with upregulated Gal-9. In
contrast, NOS2 and CD206 were reversed in cells with knockdown of
Gal-9 compared to cells with overexpression of Gal-9. These results
suggested that upregulated Gal-9 may induce M2-type macrophages,
while downregulated Gal-9 can simulate M1-type macrophages. In
addition, the expression levels of protein and mRNA of IL-6, TNF-α,
IL-10 and TGF-β confirmed our observation. Furthermore, our results
showed that the regulations of Gal-9 on the macrophage polarization
may be involved transcription factors NF-κB, Stat1, and Stat3. This
study showed a close association of Gal-9 with the regulation of
polarization in RAW264.7 cells.
Gal-9 is a member of the galactoside lectin family,
which was isolated from the mouse embryo for the first time in 1997
by Wada et al (20) and is
a sugar-binding protein. Tim-3 protein is an important member of
the Tim family, and plays an immunomodulatory role when combined
with its ligand Gal-9 (14–16).
Very recently, investigation showed that Tim-3 has a steady-state
regulation of macrophages polarization (18,21).
Therefore, we hypothesized that Gal-9 may be the target of
macrophage polarization.
Macrophage polarization is divided into two
categories: One is the classic activated macrophages, also known as
M1 macrophages; the other is the alternative activation of
macrophages, that is, M2 macrophages (1,2). M1
macrophages and M2 macrophages can exert their biological functions
by secreting different cytokines and effector molecules (4). Our results firstly showed that the
levels of Gal-9 are varied not only in intracellular but also in
extracellular after the cells treated with pGal-9 or siRNA-Gal-9
(Fig. 1). We then found that the
cell viability was obviously decreased in cells treated with
siRNA-Gal-9, while it was slightly increased in cells treated with
pGal-9, compared to control (Fig.
2). These results indicated that Gal-9 is associated with the
cytotoxicity or viability of macrophages. However, these findings
may be associated with macrophages polarization shifting between M1
and M2 (4). NOS2 is a marker
tightly connected with M1-type polarization of macrophages, where
as CD206 is a widely used marker for alternatively activated (M2)
macrophage (22,23). Our results showed that
overexpressed Gal-9 significantly inhibited the expression of NOS2,
but also increased the expression of CD206 in the flow cytometry
assay (Fig. 3C-F). Ours results
suggested that overexpressed Gal-9 can induce macrophage to
generate M2-type macrophages, and inhibit M1-type macrophages
polarization. By contrast, knockdown of Gal-9 is clearly associated
with M1-type macrophages since a markedly increased NOS2 expression
and reduction of CD206 expression was observed, suggesting that
Gal-9 downregulation can induce M1-type macrophages polarization
and inhibit M1-type macrophages polarization (Fig. 3C-F). Furthermore, M1-type
macrophages are characterized by IL-12high,
lL-23high, lL-10low, and efficient secretion
effector molecules (e.g., ROIs) and inflammatory cytokines such as
IL-6, TNF-α and IL-1β (24–26);
M2-type macrophages are characterized by IL-121ow,
IL-231ow, IL-10high, high expression of
galactose receptors, and cannot produce inflammatory factors, NO
and ROIs (27,28). In this study, we found that both
IL-6 and TNF-α were suppressed at level of transcription and
translation in cells with overexpressed Gal-9 in macrophages, where
IL-10 and TGF-β were markedly elevated (Fig. 4), suggesting that Gal-9
overexpression in macrophages can induce M2-type macrophages.
However, in cells with downregulated Gal-9 the transcriptional and
translational patterns of IL-6 and TNF-α or IL-10 and TGF-β were
inversely associated with that in cells with overexpressed Gal-9
(Fig. 4), suggesting that Gal-9
downregulation in macrophages can simulate M1-type macrophages.
Moreover, the effect of Gal-9 on cytokines related to M1-type or
M2-type macrophages was confirmed by addition of carrier-free
recombinant Gal-9 in extracellular (Fig. 5). These results were consistent
with the observations of decreased M1-type marker NOS2 and
increased M2-type marker CD206 mentioned above. It is exciting that
in another latest research, the expression levels of
pro-inflammatory cytokines in M1-differentiated THP-1 cells
co-cultured with hGal-9-transfected porcine kidney epithelial cells
were reduced (29). Besides, this
study also showed that hGal-9 has a decrease in M1-differentiated
THP-1 cell cytotoxic activity-related acute immune rejection in
pig-to-human xenotransplantation (29). Moreover, a similar study showed
that overexpression of Tim3 or exogenous recombinant Gal9 can
decrease inflammatory factor IFNγ, and elevate anti-inflammatory
factor IL-10 (19). These results
suggested that Gal-9 plays an important role in immunomodulatory of
macrophages.
Previous investigations revealed that NF-κB is
tightly connected with the cytokines exerted from M2-type
macrophages (30). In this study,
NF-κB activity was reduced and increased in cells with upregulated
and downregulated Gal-9 (Fig. 6A),
respectively, indicating that macrophages with Gal-9 overexpression
was tend to generate M2-type polarization and macrophages with
Gal-9 downregulation promote M1-type polarization. In addition,
Statl and Stat3 are members of the signal transduction and
transcription activator family (Stat), which are also involved in
macrophage polarization (8,9). The
Stat3 knockout mice showed that Stat3 in macrophages has an
important anti-inflammatory effect, and the bactericidal ability of
these mice is significantly decreased (31). Phosphorylated Stat (p-Stat)1
participates in M1-type polarization, and its expression is
regulated by NF-κB (32). While
p-Stat3 participates in M2-type polarization, and p-Stat3 inhibits
NF-κB activation and p-Stat1 expression, thereby inhibiting
macrophage M1 polarization and promoting M2-type polarization
(33,34). Consistent with these reports, our
results revealed that p-Stat1 as well as NF-κB activity were
inhibited and elevated in cells with upregulated and downregulated
Gal-9, respectively (Fig. 6B).
However, p-Stat3 level was increased and decreased in cells with
upregulated and downregulated Gal-9, respectively, which were
inversely associated with NF-κB activity (Fig. 6C). Interestingly, no apparent
variations on the levels of Stat1 and Stat3 were observed among all
groups in this study. Together with previous results, we suggested
that the regulation effect of Gal-9 in macrophages on the
expression of TNF-α, IL-6, TGF-β, IL-10 may be mediated by the
interactions between Gal-9 and NF-κB, Stat1 or Stat3. Moreover, we
further confirmed this hypothesis by suppressing the activation of
these signaling molecules with their specific inhibitors (Fig. 7). In a study by Jung et al
(29), M2 differentiation was
turned on while M1 differentiation was turned down in
M1-differentiated THP-1 cells co-cultured with hGal-9-transfected
porcine kidney epithelial cells via enhancing the phosphorylation
levels of Akt and PI3K and the expression level of PPAR-γ. Here,
the interactions between Akt/PI3K and NF-κB, Stat1 or Stat3 deserve
further investigations.
In summary, our results suggested that macrophage
polarization was tightly regulated with alterations of Gal-9
expression and significantly associated with Gal-9-mediated
cytokines, transcription factors and regulators, including TNF-α,
IL-6, TGF-β, IL-10, NF-κB, Stat1 and Stat3. Our results provide
insight into the mechanism of the effect of Gal-9 overexpression or
knockdown on the polarization and cytokines in macrophages.
Acknowledgements
This study was supported by The Natural Science Fund
of Zhejiang Province (LQ16H150001).
References
|
1
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Liu X, Liu Y, Zhang Q, Yao Z,
Huang B, Zhang P, Li N and Cao X: Zinc finger protein 64 promotes
Toll-like receptor-triggered proinflammatory and type I interferon
production in macrophages by enhancing p65 subunit activation. J
Biol Chem. 288:24600–24608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantovani A, Sica A and Locati M: New
vistas on macrophage differentiation and activation. Eur J Immunol.
37:14–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macatonia SE, Hsieh CS, Murphy KM and
O'Garra A: Dendritic cells and macrophages are required for Th1
development of CD4+ T cells from alpha beta TCR transgenic mice:
IL-12 substitution for macrophages to stimulate IFN-gamma
production is IFN-gamma-dependent. Int Immunol. 5:1119–1128. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Puddu P, Fantuzzi L, Borghi P, Varano B,
Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf SF, Belardelli
F and Gessani S: IL-12 induces IFN-gamma expression and secretion
in mouse peritoneal macrophages. J Immunol. 159:3490–3497.
1997.PubMed/NCBI
|
|
7
|
Bashir S, Sharma Y, Elahi A and Khan F:
Macrophage polarization: The link between inflammation and related
diseases. Inflamm Res. 65:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tymoszuk P, Charoentong P, Hackl H, Spilka
R, Müller-Holzner E, Trajanoski Z, Obrist P, Revillion F, Peyrat
JP, Fiegl H and Doppler W: High STAT1 mRNA levels but not its
tyrosine phosphorylation are associated with macrophage
infiltration and bad prognosis in breast cancer. BMC Cancer.
14:2572014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satoh T, Takeuchi O, Vandenbon A, Yasuda
K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh
T, et al: The Jmjd3-Irf4 axis regulates M2 macrophage polarization
and host responses against helminth infection. Nat Immunol.
11:936–944. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamura M, Watanabe T, Igarashi T, Takeuchi
T, Kasai K and Arata Y: Crosslinking of Cys-mutated human
galectin-1 to the model glycoprotein ligands asialofetuin and
laminin by using a photoactivatable bifunctional reagent. Biol
Pharm Bull. 37:877–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vicuña L, Pardo E, Curkovic C, Döger R,
Oyanadel C, Metz C, Massardo L, González A and Soza A: Galectin-8
binds to LFA-1, blocks its interaction with ICAM-1 and is
counteracted by anti-Gal-8 autoantibodies isolated from lupus
patients. Biol Res. 46:275–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wijesundera KK, Izawa T, Tennakoon AH,
Murakami H, Golbar HM, Katou-Ichikawa C, Tanaka M, Kuwamura M and
Yamate J: M1- and M2-macrophage polarization in rat liver cirrhosis
induced by thioacetamide (TAA), focusing on Iba1 and galectin-3.
Exp Mol Pathol. 96:382–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Markovic B Simovic, Nikolic A, Gazdic M,
Nurkovic J, Djordjevic I, Arsenijevic N, Stojkovic M, Lukic ML and
Volarevic V: Pharmacological inhibition of Gal-3 in mesenchymal
stem cells enhances their capacity to promote alternative
activation of macrophages in dextran sulphate sodium-induced
colitis. Stem Cells Int. 2016:26407462016.PubMed/NCBI
|
|
14
|
Elahi S, Niki T, Hirashima M and Horton H:
Galectin-9 binding to Tim-3 renders activated human CD4+ T cells
less susceptible to HIV-1 infection. Blood. 119:4192–4204. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang F, He W, Yuan J, Wu K, Zhou H, Zhang
W and Chen ZK: Activation of Tim-3-Galectin-9 pathway improves
survival of fully allogeneic skin grafts. Transpl Immunol.
19:12–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gooden MJ, Wiersma VR, Samplonius DF,
Gerssen J, van Ginkel RJ, Nijman HW, Hirashima M, Niki T, Eggleton
P, Helfrich W and Bremer E: Galectin-9 activates and expands human
T-Helper 1 cells. PLoS One. 8:e656162013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chabtini L, Mfarrej B, Mounayar M, Zhu B,
Batal I, Dakle PJ, Smith BD, Boenisch O, Najafian N, Akiba H, et
al: TIM-3 regulates innate immune cells to induce fetomaternal
tolerance. J Immunol. 190:88–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Z, Jiang X, Kang C, Xiao Y, Hou C, Yu
J, Wang R, Xiao H, Zhou T, Wen Z, et al: Blockade of the T cell
immunoglobulin and mucin domain protein 3 pathway exacerbates
sepsis-induced immune deviation and immunosuppression. Clin Exp
Immunol. 178:279–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Chen Y, Liu X, Zhang J, He X, Teng G
and Yu D: Tim3/Gal9 interactions between T cells and monocytes
result in an immunosuppressive feedback loop that inhibits Th1
responses in osteosarcoma patients. Int Immunopharmacol.
44:153–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wada J, Ota K, Kumar A, Wallner EI and
Kanwar YS: Developmental regulation, expression, and apoptotic
potential of galectin-9, a beta-galactoside binding lectin. J Clin
Invest. 99:2452–2461. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang X, Yu J, Shi Q, Xiao Y, Wang W, Chen
G, Zhao Z, Wang R, Xiao H, Hou C, et al: Tim-3 promotes intestinal
homeostasis in DSS colitis by inhibiting M1 polarization of
macrophages. Clin Immunol. 160:328–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mock BA, Krall MM, Byrd LG, Chin H, Barton
CH, Charles I, Liew FY and Blackwell J: The inducible form of
nitric oxide synthase (NOS2) isolated from murine macrophages maps
near the nude mutation on mouse chromosome 11. Eur J Immunogenet.
21:231–238. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu HF, Zhang HJ, Hu QX, Liu XY, Wang ZQ,
Fan JY, Zhan M and Chen FL: Altered polarization, morphology, and
impaired innate immunity germane to resident peritoneal macrophages
in mice with long-term type 2 diabetes. J Biomed Biotechnol.
2012:8670232012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kienast K, Knorst M, Müller-Quernheim J
and Ferlinz R: Modulation of IL-1beta, IL-6, IL-8, TNF-alpha, and
TGF-beta secretions by alveolar macrophages under NO 2 exposure.
Lung. 174:57–67. 1996.PubMed/NCBI
|
|
25
|
Gu Y, Hu X, Liu C, Qv X and Xu C:
Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and
tumour necrosis factor-alpha in aplastic anaemia. Br J Haematol.
142:109–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
An SJ, Pae HO, Oh GS, Choi BM, Jeong S,
Jang SI, Oh H, Kwon TO, Song CE and Chung HT: Inhibition of
TNF-alpha, IL-1beta and IL-6 productions and NF-kappa B activation
in lipopolysaccharide-activated RAW 264.7 macrophages by
catalposide, an iridoid glycoside isolated from Catalpa ovata G.
Don (Bignoniaceae). Int Immunopharmacol. 2:1173–1181. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Cheng S, Zhang M, Zhen L, Pang D,
Zhang Q and Li Z: High-infiltration of tumor-associated macrophages
predicts unfavorable clinical outcome for node-negative breast
cancer. PLoS One. 8:e761472013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bastos KR, Alvarez JM, Marinho CR, Rizzo
LV and Lima MR: Macrophages from IL-12p40-deficient mice have a
bias toward the M2 activation profile. J Leukoc Biol. 71:271–278.
2002.PubMed/NCBI
|
|
29
|
Jung SH, Hwang JH, Kim SE, Kim YK, Park HC
and Lee HT: Human galectin-9 on the porcine cells affects the
cytotoxic activity of M1-differentiated THP-1 cells through
inducing a shift in M2-differentiated THP-1 cells.
Xenotransplantation. 24:2017. View Article : Google Scholar
|
|
30
|
Cao S, Zhang X, Edwards JP and Mosser DM:
NF-kappaB1 (p50) homodimers differentially regulate pro- and
anti-inflammatory cytokines in macrophages. J Biol Chem.
281:26041–26050. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohkubo N, Suzuki Y, Aoto M, Yamanouchi J,
Hirakawa S, Yasukawa M and Mitsuda N: Accelerated destruction of
erythrocytes in Tie2 promoter-driven STAT3 conditional knockout
mice. Life Sci. 93:380–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cramer LA, Nelson SL and Klemsz MJ:
Synergistic induction of the Tap-1 gene by IFN-gamma and
lipopolysaccharide in macrophages is regulated by STAT1. J Immunol.
165:3190–3197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK
and Nam TJ: Pyropia yezoensis glycoprotein promotes the M1 to M2
macrophage phenotypic switch via the STAT3 and STAT6 transcription
factors. Int J Mol Med. 38:666–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tyagi A, Singh RP, Ramasamy K, Raina K,
Redente EF, Dwyer-Nield LD, Radcliffe RA, Malkinson AM and Agarwal
R: Growth inhibition and regression of lung tumors by silibinin:
Modulation of angiogenesis by macrophage-associated cytokines and
nuclear factor-kappaB and signal transducers and activators of
transcription 3. Cancer Prev Res (Phila). 2:74–83. 2009. View Article : Google Scholar : PubMed/NCBI
|