Introduction
Esophageal cancer (EC) is the sixth most common
cause of cancer-associated deaths worldwide (1) and esophageal squamous cell carcinoma
(ESCC) accounts for 70% of EC cases (2). Once diagnosed, the prognosis is poor
in the majority of patients with advanced EC, regardless of the
stage (3). Despite advances in the
traditional treatments for EC, the mortality from EC has not
improved greatly (4). Therefore,
it is necessary to develop novel therapeutic methods that may
improve the survival rate of patients with advanced EC.
Sporamin, a trypsin inhibitor, is the primary
soluble storage protein in sweet potato tuberous roots, and has
been identified as an antitumor agent that induces antitumor
effects in certain types of tumor cells (5,6).
However, to the best of our knowledge, the mechanisms underlying
its diverse biological effects have not previously been
investigated in ESCC cells. Therefore, the effects of sporamin on
cell viability, cell proliferation activity and apoptosis were
investigated in EC9706 and EC109 human ESCC cell lines.
Nuclear factor (NF)-κB has been associated with the
promotion of esophageal tumorigenesis by protecting tumor cells
from cell death (7). AKT is a
protein kinase that transduces signals from oncogenes or growth
factors, such as epidermal growth factor receptor (EGFR), to
downstream targets in tumor cells (8). NF-κB signaling may be activated via
the AKT signaling pathway (9).
Therefore, in the present study, the effects and underlying
mechanisms of sporamin treatment on NF-κB and AKT signaling
pathways in human ESCC cells were investigated.
The authors' previous studies have demonstrated that
sporamin treatment suppresses the growth of human tongue carcinoma
cells in vitro (6).
However, the detailed effects and mechanisms of sporamin on ESCC
cells have not been well characterized. To improve the
understanding of the effects and underlying mechanisms of sporamin
on ESCC cells, an MTT assay, [3H] thymidine
incorporation assay, morphological and flow cytometry analysis,
western blotting and electrophoretic mobility shift assay (EMSA)
were performed in the present study.
Materials and methods
Cell line and materials
EC109 and EC9706 cells (Type Culture Collection of
the Chinese Academy of Sciences, Shanghai, China), which are well
and poorly differentiated human ESCC cell lines, respectively, were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS), 10 U/ml penicillin and 10 µg/ml
streptomycin at 37°C and 5% CO2. Sporamin, a primary
soluble protein in sweet potatoes with trypsin inhibitory activity,
was purified from fresh sweet potato tuberous roots (Ipomoea
batatas cv. Tainong 57; Hangzhou Lianhua Huashang Group Co.,
Zhejiang, China), as described previously (6). The primary antibodies against Bcl-2
(catalog no. sc-509), Bcl-2 like 1 (Bcl-XL; catalog no. sc-7122),
Bcl-2-associated X (Bax; catalog no. sc-23959) and NF-κB p65
(catalog no. sc-8008) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). AKT (catalog no. 9272), phosphorylated
(p)-AKT (Thr308; catalog no. 4056), p70 S6 kinase (catalog no.
9202), p-p70 S6 kinase (Thr421/Ser424; catalog no. 9204), NF-κB
inhibitor α (IκBα; catalog no. 9242) and p-IκBα (Ser32; catalog no.
2859) primary antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA), and tubulin (catalog no.
ab59680), histone (H)2A (catalog no. ab13923) and β-actin (catalog
no. ab8227) primary antibodies were purchased from Abcam
(Cambridge, UK).
MTT assay
The in vitro cell viability of EC109 and
EC9706 cells treated with sporamin was measured using the MTT
assay. A total of 1×103 cells/well were cultured in
96-well plates and incubated with 0, 12.5, 25, 50 or 100 µM
sporamin for 24, 48 or 72 h at 37°C. Dimethyl sulfoxide (DMSO) was
cells treated with as a control instead of sporamin. Medium was
discarded and wells were replaced with 100 µl fresh medium
containing 10% MTT solution (5 mg/ml stock) at 37°C for 4 h, 100 µl
DMSO was subsequently added to each well before agitating the
plates at room temperature for 10 min. Colorimetric determination
of MTT reduction was determined at a wavelength of 570 nm using a
microplate ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
[3H] thymidine
incorporation assay
The in vitro cell proliferation activity of
EC109 and EC9706 cells treated with sporamin was measured by a
[3H] thymidine incorporation assay (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). A total of 2.5×105 cells/well
were cultured in 24-well plates in DMEM with 10% dialyzed and
charcoal-stripped FBS. When cells reached 80% confluence, the
cultures were rinsed in phenol-free DMEM medium and incubated with
0, 12.5, 25, 50 or 100 µM sporamin in phenol-free and serum-free
DMEM for 24, 48 and 72 h. [3H] thymidine
(1.35×104 Bq/l) was added and cultured with cells for a
further 12 h at 37°C. Subsequently, the supernatant was aspirated
and excess [3H] thymidine was removed by washing with
PBS. Finally, 0.2 mol/l NaOH was added at 37°C and radioactivity
([3H] thymidine incorporation activity) was determined
by scintillation counting (LS6500; Beckman Instruments, Inc.,
Fullerton, CA, USA).
Morphological determination and
quantification of apoptosis
Cells were cultured in 12-well plates at a density
of 2×104 cells per well and incubated with 25 µM
sporamin or DMSO for 48 h at 37°C. Cells were then fixed with 4%
cold paraformaldehyde at 4°C for 15 min (pH 7.4) and stained with
0.5 µg/ml DAPI (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for
5 min at room temperature. The cells were subsequently visualized
under a fluorescence microscope (model DMI4000B; Olympus
Corporation, Tokyo, Japan) using a 360–370 nm excitation light and
a 420–460 nm emission filter. Cells undergoing apoptosis exhibit
profound structural alterations, including apoptotic chromatin
condensation, nuclear envelope shrinkage and fragmentation.
Apoptotic morphological changes in the nucleus were distinguished
from intact nuclei and counted, and the percentages were
subsequently calculated. A total of six randomly chosen fields of
view were examined with a minimum number of 500 cells scored in
each condition.
Flow cytometry analysis for
apoptosis
Apoptosis was measured using an Annexin
V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) Apoptosis
Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) according
to the manufacturer's protocol. Briefly, 1.5×106
cells/well were incubated with 25 µM sporamin or DMSO for 48 h at
37°C. Both attached and floating cells were pooled, pelleted by
centrifugation at 100 × g for 5 min, 4°C, washed twice with cold
PBS and 1×106 cells/ml were resuspended in 1X binding
buffer [10 mM hydroxyethyl piperazine ethanesulfonic acid, (pH
7.4), 2.5 mM CaCl2, and 140 mM NaOH]. A total of
1×105 cells were transferred to a 5-ml tube and stained
with 5 µl annexin V-FITC and 5 µl PI for 15 min at room temperature
in the dark. Finally, 400 µl 1X binding buffer was added to each
tube and apoptosis was determined using a BD FACSCanto II flow
cytometer with BD Accuri™ C6 software (BD Biosciences, San Jose,
CA, USA).
Western blotting
Total protein was extracted from treated cells using
M-Per Mammalian Protein Extraction reagent (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Nuclear
fractions were prepared using a Nuclear Protein Extraction kit
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's protocol. The protein concentrations were
determined using a Bradford assay. Cell protein extracts were
either used immediately or stored at −70°C.
For SDS-PAGE, a total of 20 µg protein was loaded
into each well of 10–12% gel and electrophoresed at 98 V. Proteins
on the gel were transferred onto a polyvinylidene fluoride membrane
by wet electrotransfer for 2 h. The membrane was blocked in 5%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) in TBS containing
0.1% Tween-20 at room temperature for 90 min. The primary
antibodies (all used at a 1:200 dilution) against Bcl-2, Bcl-XL,
Bax, NF-κB p65, IκBα, AKT, p70 S6 kinase, p-IκBα (Ser32), p-AKT
(Thr308), p-p70 S6 kinase (Thr421/Ser424), tubulin, H2A and β-actin
were added separately and incubated with the membrane overnight at
4°C. The membranes were subsequently washed three times with
TBS/0.1% Tween-20. The following day, membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit (1:2,000; cat. no.
7074; Cell Signaling Technology, Inc.), anti-mouse (1:2,000; cat.
no. 7076; Cell Signaling Technology, Inc.), and anti-goat (1:2,000;
cat. no. sc-2350; Santa Cruz Biotechnology) immunoglobulin G
secondary antibodies. The membrane was subsequently washed four
times with TBS/0.1% Tween-20, 5 min per wash. All bands were
visualized using Pierce™ enhanced chemiluminescence Western
Blotting Substrate (Merck KGaA, Darmstadt, Germany) and scanned
using an LAS-4000 imaging system (Fujifilm, Tokyo, Japan).
EMSA
For EMSA, cells were treated with 0, 12.5, 25 or 50
µM sporamin for 48 h at 37°C. EMSA was performed using a commercial
kit (Gel Shift Assay System; Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol. Nuclear Protein
Extraction kit (Beyotime Institute of Biotechnology, Haimen, China)
was adopted to prepare nuclear fractions, according to the
manufacturer's protocol. A bicinchoninic assay was used to
determine the protein concentration. Nuclear protein (2 µg) was
preincubated in binding buffer [10 mM Tris-Cl, (pH 7.5), 50 mM
NaCl, 1 mM MgCl2, 0.5 mM EDTA, 4% glycerol, 0.5 mM DTT
and 0.05 g/l poly (deoxyinosinic deoxycytidylic acid)] for 15 min
at room temperature. After addition of 1 µl 32P-labeled
oligonucleotide probe (1.75 pmol/µl), the incubation was continued
for 30 min at room temperature. The reaction was stopped by adding
1 µl gel loading buffer and the mixture was subjected to 4%
nondenaturing polyacrylamide gel electrophoresis in 0.5X
Tris/Borate/EDTA buffer. The gel was vacuum-dried and exposed to
X-ray film at −70°C. The dried gels were visualized by
autoradiography using x-ray film.
Statistical analysis
Data are presented as the mean ± standard deviation.
A one-way analysis of variance followed by Tukey's post hoc test,
or student's t-test were used to compare differences between groups
using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sporamin inhibits cell viability and
proliferation activity in ESCC cells
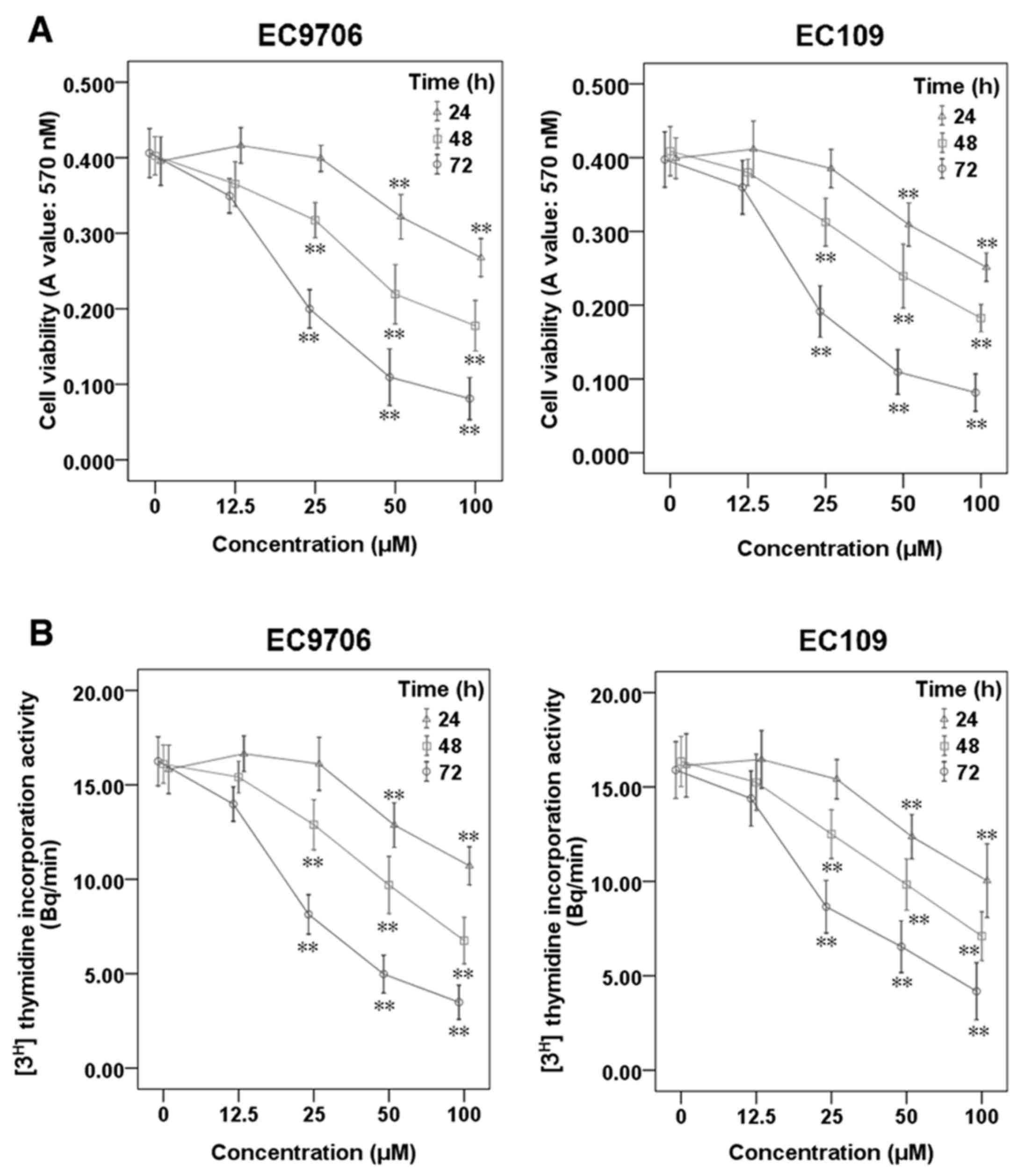
To investigate the effects of sporamin on cell
viability and the proliferation of ESCC cells, EC9706 and EC109
cells were treated with sporamin at increasing concentrations
(12.5, 25, 50 and 100 µM) and for varying durations (24, 48 and 72
h), and cell viability and proliferation were determined using an
MTT assay (Fig. 1A) and a
[3H] thymidine incorporation assay (Fig. 1B), respectively. The results
demonstrated that sporamin significantly inhibited cell viability
and proliferation activity in a concentration- and time-dependent
manner.
Sporamin induces cell apoptosis in
ESCC cells
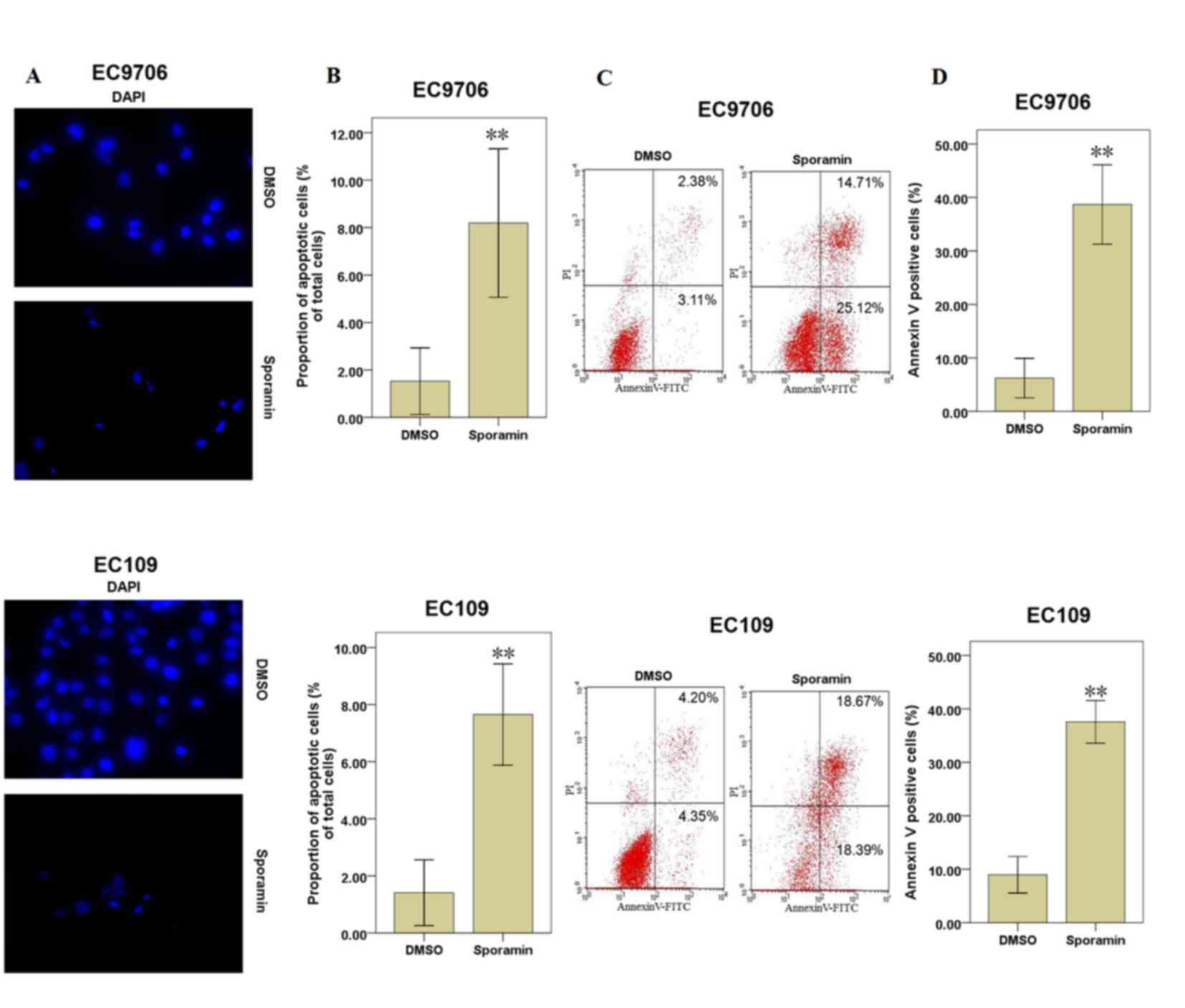
When EC9706 and EC109 cells were treated with 25 µM
sporamin for 48 h, the inhibition of cell viability and
proliferation activity were significant. Therefore, cells were
treated with 25 µM sporamin for 48 h for further experiments.
Apoptotic cells were examined under fluorescence microscopy
following DAPI staining. Following exposure to sporamin, the cell
volume reduced, the chromatin became condensed and the nuclei
became fragmented, compared with DMSO-treated cells (Fig. 2A). Compared with DMSO-treated
control cells, the percentage of apoptotic cells was increased
significantly following sporamin treatment in EC9706 and EC109
cells (Fig. 2B).
As sporamin induced morphological changes associated
with apoptosis at a concentration of 25 µM for 48 h, annexin V and
PI staining was additionally performed to determine cell apoptosis.
The results demonstrated that the percentages of apoptotic cells
(annexin V positive cells) were increased significantly following
culture with sporamin, compared with the DMSO control cells
(Fig. 2C and D). These results
indicate that sporamin may induce cell apoptosis in EC9706 and
EC109 cells.
Sporamin induces the downregulation of
Bcl-2 and Bcl-XL, and upregulation of Bax in ESCC cells
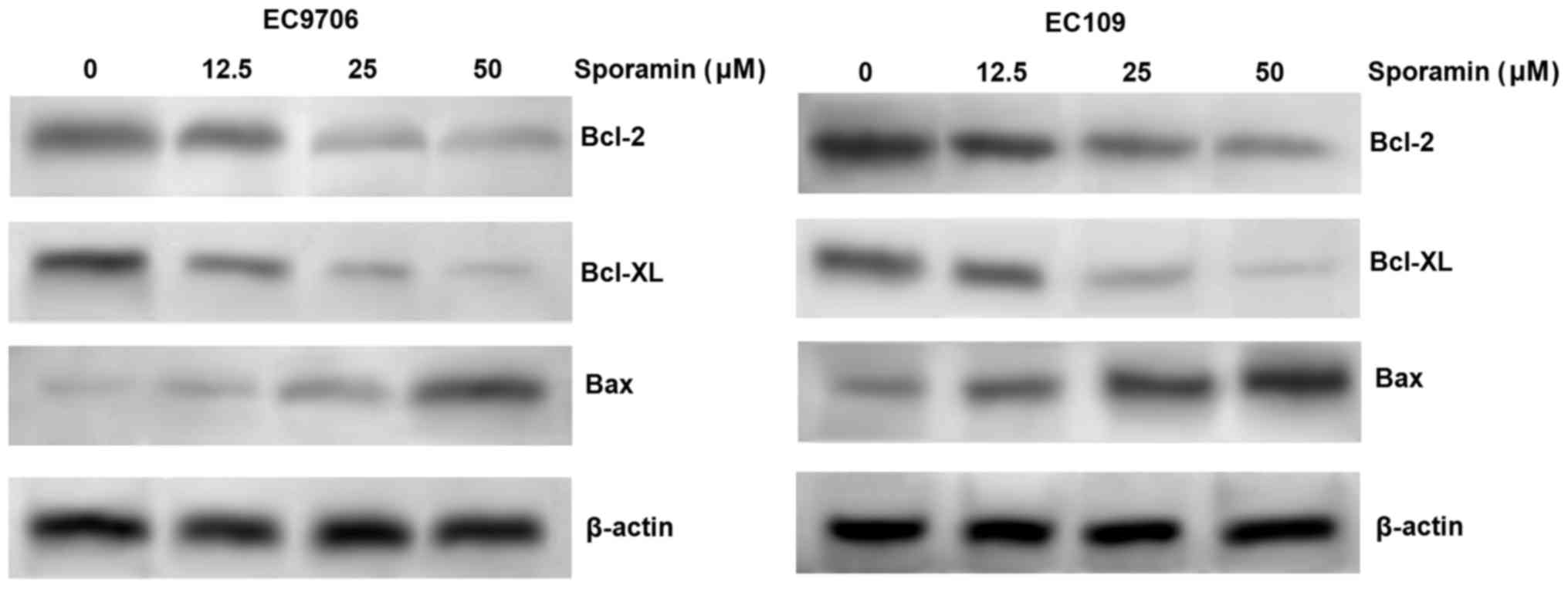
As Bcl-2, Bcl-XL and Bax are involved in the
promotion of apoptosis, the expression levels of Bcl-2, Bcl-XL and
Bax in EC9706 and EC109 cells were measured by western blotting, to
identify the potential underlying molecular basis for the effects
of sporamin on ESCC cells. The results indicated that sporamin
inhibited the expression of the anti-apoptotic proteins, Bcl-2 and
Bcl-XL, and induced the expression of the proapoptotic protein Bax,
in a concentration-dependent manner in EC9706 and EC109 cells
(Fig. 3). The results indicate
that sporamin may induce apoptosis via the regulation of Bcl-2,
Bcl-XL and Bax expression.
Sporamin inhibits NF-κB DNA-binding
activity in ESCC cells
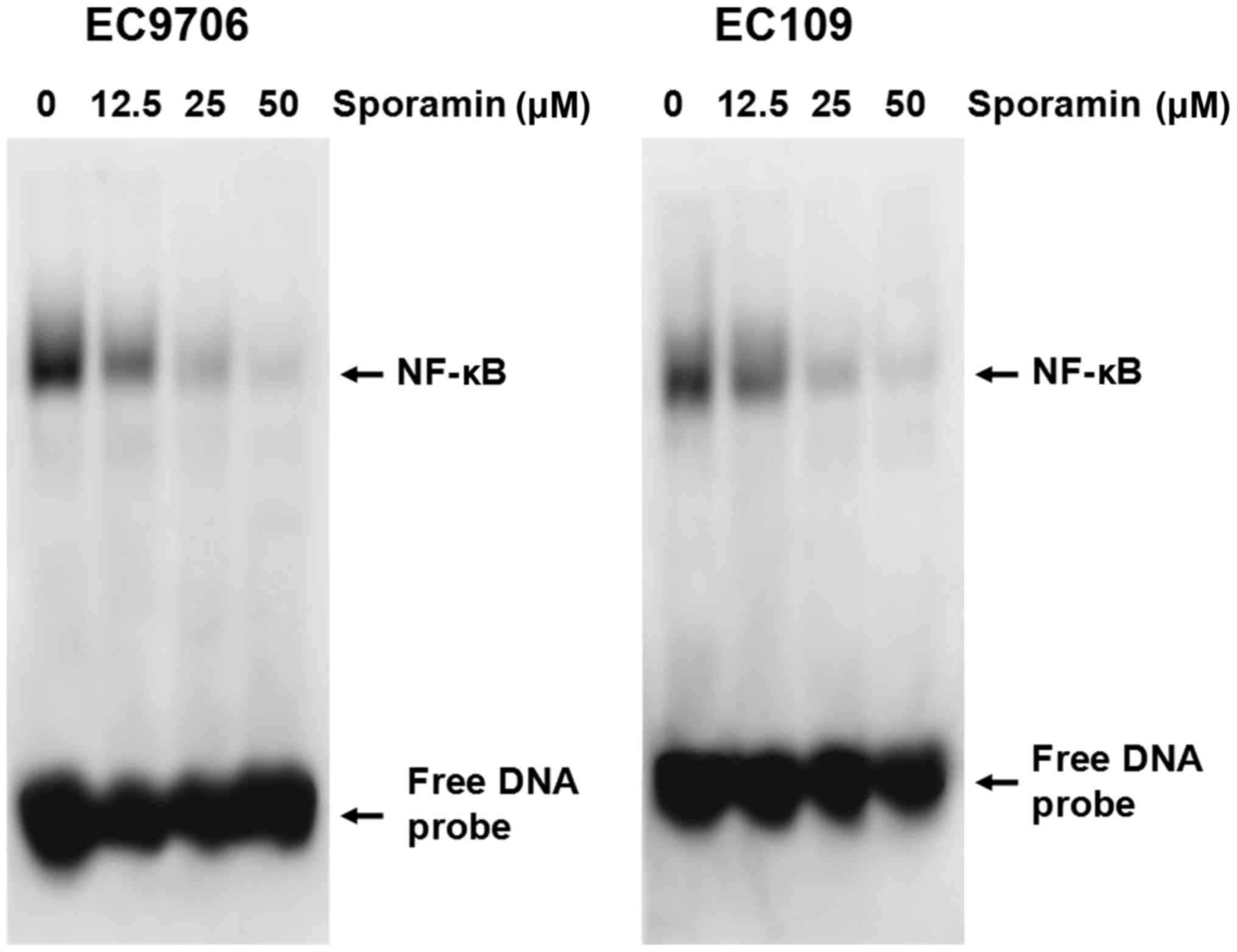
As Bcl-2 and Bcl-XL may be regulated by NF-κB
(10), it was hypothesized that
sporamin may mediate its effects by regulating NF-κB. Therefore,
nuclear extracts of EC9706 and EC109 cells treated with various
concentrations of sporamin (12.5, 25 and 50 µM) or control diluent
were prepared and NF-κB DNA-binding activity was examined by EMSA.
As demonstrated in Fig. 4, a
marked reduction of NF-κB DNA-binding activity was observed in
sporamin-treated cells in a concentration-dependent manner. These
results indicate that sporamin may inhibit NF-κB DNA-binding
activity.
Sporamin inhibits NF-κB activation via
an AKT-independent mechanism in ESCC cells
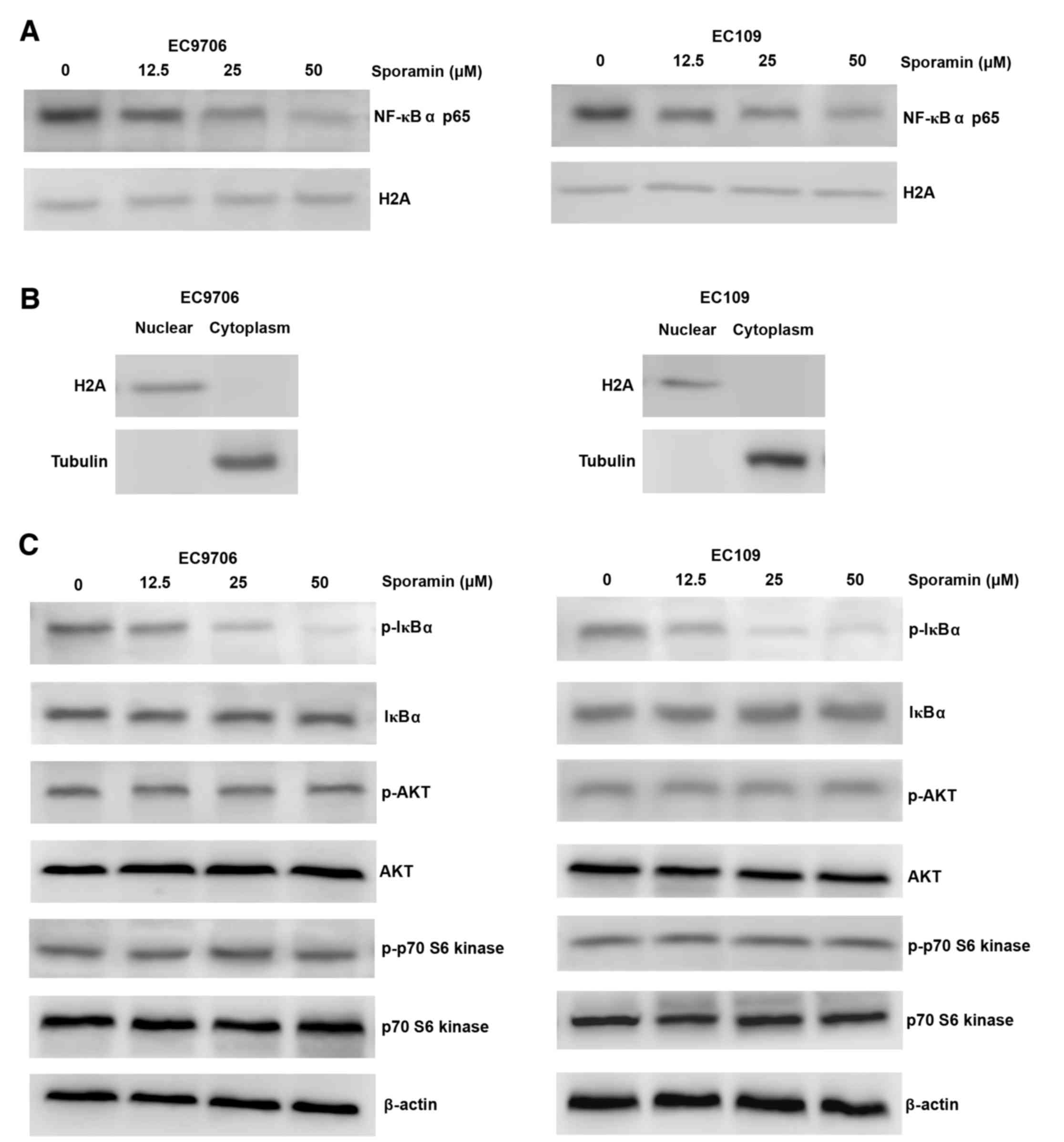
Treatment with sporamin resulted in reduced nuclear
expression of NF-κB p65 in a concentration-dependent manner in
EC9706 and EC109 cells (Fig. 5A and
B). Consistently, there was a marked reduction in the
expression levels of p-IκBα (Fig.
5C). However, the expression and phosphorylation levels of AKT,
or its downstream target, p-p70 S6 kinase, were not altered
following sporamin treatment (Fig.
5C). These results indicate that NF-κB inhibition by sporamin
may be partially regulated via the dephosphorylation of IκBα,
without inhibition of AKT.
Discussion
In the present study, human ESCC cell lines (EC9706
and EC109) were treated with sporamin. The results demonstrated
that sporamin suppressed the cell growth of ESCC cells in
vitro, which may be mediated via NF-κB inhibition. In addition,
NF-κB DNA-binding activity, phosphorylation of IκBα and nuclear
expression of NF-κB were reduced in sporamin-treated ESCC
cells.
To the best of our knowledge, the present study is
the first to demonstrate that sporamin exhibits inhibitory effects
on cell viability and proliferation in ESCC cells, and the
inhibitory effects were positively associated with treatment
duration and concentration. Apoptosis is a process that eliminates
unwanted cells in various physiological processes. Dysregulation of
apoptosis is implicated in carcinogenesis, and inhibition of cell
viability and proliferation activity may indicate the induction of
apoptosis (11). Therefore, the
present study investigated whether apoptosis contributes to the
inhibition of cell viability and proliferation following sporamin
treatment in EC9706 and EC109 cells. Following sporamin treatment,
EC9706 and EC109 cells exhibited features of apoptosis. Typical
morphological features of apoptosis and percentages of apoptotic
cells were demonstrated by DAPI nuclear staining and flow
cytometric analysis, respectively. In addition, the results
revealed that sporamin induced the downregulation of Bcl-2 and
Bcl-XL protein expression, and upregulation of Bax protein levels.
This indicates that sporamin may induce apoptosis via the
regulation of Bcl-2, Bcl-XL and Bax protein expression.
As it is established that NF-κB enhances tumor cell
growth via the regulation of Bcl-2 and Bcl-XL (10), the present study investigated
whether sporamin may inhibit NF-κB DNA-binding activity using EMSA,
and the results indicated that sporamin may suppress the cell
growth of human ESCC cells, at least partially, via the inhibition
of NF-κB activation. NF-κB is a heterodimer consisting of p50 and
p65 subunits, and, when inactive, is sequestered in the cytoplasm
as it is bound to its endogenous inhibitor, IκB, which consists of
α and β subunits (12,13). IκBα is phosphorylated by the IκB
kinase complex, and this phosphorylation leads to the
ubiquitination and subsequent degradation of p-IκBα, which allows
NF-κB to migrate from the cytoplasm to the nucleus, where it
subsequently induces target gene expression (12,13).
In the present study, sporamin inhibited NF-κB activation, which
may, at least partially, occur via reduced phosphorylation of
IκBα.
NF-κB is additionally activated via various other
signaling pathways (14). Of
these, AKT signaling induces NF-κB activation in human
hepatocellular carcinoma cells (15). Overexpression of EGFR and
inactivation of the tumor suppressor gene phosphatase and tensin
homolog may result in activation and/or expression of AKT and its
downstream targets, including p70 pS6 kinase (16–18).
However, in the present study, sporamin did not affect the
expression and phosphorylation levels of AKT, or its downstream
target p-p70 S6 kinase, indicating that sporamin may suppress cell
growth of ESCC cells via an AKT-independent mechanism.
In conclusion, sporamin may inhibit cell viability
and proliferation, and induce apoptosis in ESCC cells. In addition,
inhibition of NF-κB activation was accompanied by reduced Bcl-2 and
Bcl-XL expression, and increased Bax expression levels in
sporamin-treated ESCC cells. A better understanding of the
underlying mechanisms and further identification of additional
downstream effectors of sporamin will help guide the development of
more effective agents to treat human ESCC.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Zhejiang Province (grant no.
LY16H160033), the Public Welfare Technical Applied Research Project
of Zhejiang Province (grant no. 2016C33189), the Science and
Technology Plans of Taizhou City (grant no. 1701yw07), and the
National College Students' Innovation and Entrepreneurship Training
Program (grant no. 201710350008).
Glossary
Abbreviations
Abbreviations:
|
EC
|
esophageal cancer
|
|
ESCC
|
esophageal squamous cell carcinoma
|
References
|
1
|
Thrift AP: The epidemic of oesophageal
carcinoma: Where are we now? Cancer Epidemiol. 41:88–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohashi S, Miyamoto S, Kikuchi O, Goto T,
Amanuma Y and Muto M: Recent advances from basic and clinical
studies of esophageal squamous cell carcinoma. Gastroenterology.
149:1700–1715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowie A, Noble F and Underwood T:
Strategies to improve outcomes in esophageal adenocarcinoma. Expert
Rev Anticancer Ther. 14:677–687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li PG, Mu TH and Deng L: Anticancer
effects of sweet potato protein on human colorectal cancer cells.
World J Gastroenterol. 19:3300–3308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao J and Qian C: Sporamin induce
apoptosis in human tongue carcinoma cells by down-regulating
Akt/GSK-3 signaling. Fundam Clin Pharmacol. 25:229–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdel-Latif MM, Kelleher D and Reynolds
JV: Potential role of NF-kappaB in esophageal adenocarcinoma: As an
emerging molecular target. J Surg Res. 153:172–180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng HY, Ko JM, Yu VZ, Ip JC, Dai W, Cal S
and Lung ML: DESC1, a novel tumor suppressor, sensitizes cells to
apoptosis by downregulating the EGFR/AKT pathway in esophageal
squamous cell carcinoma. Int J Cancer. 138:2940–2951. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi YJ, Moon KM, Chung KW, Jeong JW, Park
D, Kim DH, Yu BP and Chung HY: The underlying mechanism of
proinflammatory NF-κB activation by the mTORC2/Akt/IKKα pathway
during skin aging. Oncotarget. 7:52685–52694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim C, Cho SK, Kim KD, Nam D, Chung WS,
Jang HJ, Lee SG, Shim BS, Sethi G and Ahn KS: β-Caryophyllene oxide
potentiates TNFα-induced apoptosis and inhibits invasion through
down-modulation of NF-κB-regulated gene products. Apoptosis.
19:708–718. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olechowska-Jarząb A, Ptak-Belowska A and
Brzozowski T: Terapeutic importance of apoptosis pathways in
pancreatic cancer. Folia Med Cracov. 56:61–70. 2016.
|
|
12
|
Fullard N, Wilson CL and Oakley F: Roles
of c-Rel signalling in inflammation and disease. Int J Biochem Cell
Biol. 44:851–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanarek N and Ben-Neriah Y: Regulation of
NF-κB by ubiquitination and degradation of the IκBs. Immunol Rev.
246:77–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghosh S and Dass JF: Study of pathway
cross-talk interactions with NF-κB leading to its activation via
ubiquitination or phosphorylation: A brief review. Gene.
584:97–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang G, Li Z, Wan X, Zhang Y, Zhu R, Liu
Z, Ji D, Zhang H, Wu F, Tian H, et al: Repression of Human
Hepatocellular Carcinoma Growth by Regulating
Met/EGFR/VEGFR-Akt/NF-κB Pathways with Theanine and Its Derivative,
(R)-2-(6,8-Dibromo-2-oxo-2H-chromene-3-carboxamido)-5-(ethylamino)-5-oxopentanoic
Ethyl Ester (DTBrC). J Agric Food Chem. 64:7002–7013. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooper JB and Cohen EE: Mechanisms of
resistance to EGFR inhibitors in head and neck cancer. Head Neck.
31:1086–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuge K, Kikuchi E, Hagiwara M, Yasumizu Y,
Tanaka N, Kosaka T, Miyajima A and Oya M: Nicotine Induces tumor
growth and chemoresistance through activation of the PI3K/Akt/mTOR
pathway in bladder cancer. Mol Cancer Ther. 14:2112–2120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|