Introduction
Colorectal cancer (CRC) has become a major global
threat to health. According to a 2012 report, 1.4 million people
were diagnosed with colorectal cancer worldwide; these cases
accounted for 9.7% of the total number of cancer cases globally
(1). Therefore, it is of
importance that a novel and effective therapy for CRC be
developed.
As a promising therapy, oncolytic viruses, including
conditional replication oncolytic adenovirus (OA) and the
OncoPox, replicate in and eventually lyse tumor cells
(2). Cancer-targeting
gene-viro-therapy (CTGVT), using oncolytic viral vectors carrying
an antitumor gen, has been demonstrated to enhance the antitumor
effects of oncolytic viruses (3–7).
A CTGVT using the carcinoembryonic antigen (CEA)
promoter to regulate early region 1A (E1A) and carrying tumor
necrosis factor ligand superfamily member 10 (TRAIL) was previously
developed. TRAIL belongs to the tumor necrosis factor superfamily,
which display antitumor effects by activating the death receptors
in tumor cells to induce apoptosis (8,9).
Oncolytic virus-mediated TRAIL has displayed antitumor effects in
hepatocellular carcinoma (10,11),
CRC (12–15), lung adenocarcinoma (16) and gastric carcinoma (17). Based on the tumor specific
carcinoembryonic antigen (CEA), Ad·CEA·E1A·E1B (Δ55)-TRAIL
[complement decay-accelerating factor (CD55)-TRAIL] was
constructed, which used the CEA promoter instead of the native E1A
promoter to improve tumor cell targeting and carried the TRAIL gene
(11).
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a
natural substance obtained from fruits, vegetables and certain
medicinal plants (18). Previous
studies have reported that luteolin is able to suppress tumor
growth by inducing tumor cell apoptosis, cell cycle arrest, and
anti angiogenesis (19–24). Luteolin may specifically inhibit
tumor growth in CRC by activating nuclear factor erythroid
2-related factor 2 signaling (25). The combination of luteolin and
TRAIL has been shown to promote apoptosis in bladder (26) and cervical cancer cells (27).
In the present study, the antitumor effect of
co-treatment with luteolin and CD55-TRAIL were examined in CRC
cells, and it was observed that the combination markedly decreased
CRC progression in vitro and in vivo.
Materials and methods
Cells and culture
293 cells were obtained from Microbix Biosystems,
Inc. (Mississauga, ONT, Canada). The human colorectal cancer cell
lines HT-29, SW620 and SW480, and the human normal lung/bronchial
normal epithelial cells Beas-2B, were acquired from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). 293 cells and Beas-2B cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
The other cell lines, HT-29, SW620 and SW480, were cultured in DMEM
supplemented with 5% FBS. All cells were cultured in a 5%
CO2 humidified incubator at 37°C.
Generation and purification of the
oncolytic virus
The OA CD55-TRAIL was constructed in a previous
study (11). The CD55-TRAIL was
amplified by infecting 293 cell cultures. The virus was collected
by CsCl gradient centrifugation, followed by dialysis. Viral titer
was determined by 50% tissue culture infective dose (TICD50)
analysis in 293 cells (11).
Cell viability assay
Cells (5,000) were seeded into 96-well plates and
cultured for 24 h. The culture medium was subsequently replaced
with medium containing CD55-TRAIL [5 multiplicity of infection
(MOI), 10 MOI, 15 MOI], luteolin (25 µM) (Beyotime Institute of
Biotechnology, Haimen, China), or a combination of CD55-TRAIL and
luteolin. Following incubation for approximately 24, 48, 72 and 96
h, MTT (0.5 mg/ml) was added to each well. Following 4 h
incubation, the cell supernatant was completely removed and 150 ml
dimethyl sulfoxide was added to each well. Following mixing
thoroughly, the 96-well plates were read by a microplate reader at
a wavelength of 490 nm (Tecan Group, Ltd., Mannedorf, Switzerland).
The effect of combination treatment was evaluated using CalcuSyn
version 2.1 (Biosoft, Cambridge, UK).
Detection of green fluorescent
protein
A total of 5×104 cells were seeded into
6-well plates and were left to attach overnight. The cells were
treated with CD55-EGFP (15 MOI) or a combination of CD55-TRAIL and
luteolin (25 µM). Following 48 h incubation, the cells were
subsequently observed under a fluorescence microscope (0.2 mm
fields; magnification, ×200; IX71-22FL/PH, Olympus Corporation,
Japan).
Apoptotic cell staining
A total of 5×104 cells were seeded into
6-well plates and were left to attach overnight. The cells were
treated with CD55-TRAIL (15 MOI), luteolin (25 µM), or a
combination of CD55-TRAIL and luteolin. Following 48 h incubation,
the cells were incubated in Hoechst 33342 (Beyotime Institute of
Biotechnology) for 10 min, and washed twice with PBS. The cells
were subsequently observed under a fluorescence microscope (0.2 mm
fields; magnification, ×200; IX71-22FL/PH, Olympus Corporation,
Japan).
Western blot analysis
A total of 5×105 cells were seeded into
6-well plates and were left to attach overnight. The cells were
treated with CD55-TRAIL (15 MOI), luteolin (25 µM), or a
combination of CD55-TRAIL and luteolin. After 72 h, the cells were
removed and lysed with radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Shanghai, China) containing
protease inhibiters to collect protein. The concentration of
proteins collected was determined using the Pierce bicinchoninic
acid protein assay kit (Thermo Fisher Scientific, Inc.).
Subsequently, the protein (20 µg) was separated using 6% SDS-PAGE
to detect cleaved poly-ADP-ribose polymerase (PARP; cat. no. 5625)
and PARP and the protein (20 µg) were separated using 12% SDS-PAGE
to detect GAPDH (cat. no. 51332), procaspase-9 (cat. no. 9508),
cleaved caspase-9 (cat. no. 7237), E3 ubiquitin-protein ligase XIAP
(XIAP; cat. no. 14334), E1A (cat. no. sc374663) and TRAIL (cat. no.
3219) and transferred onto a polyvinylidene difluoride membrane.
The membranes were blocked with 5% non-fat dried milk at room
temperature for 2 h and incubated in primary antibodies [GAPDH,
procaspase-9, cleaved caspase-9, cleaved poly-ADP-ribose polymerase
(PARP), PARP, E3 ubiquitin-protein ligase XIAP (XIAP), E1Aand
TRAIL] overnight at 4°C. The working dilution of all primary
antibodies was 1:1,000. The membranes of GAPDH, procaspase-9 and
E1A were subsequently incubated in the corresponding mouse
secondary antibody (cat. no. 10230269) and the membranes of cleaved
caspase-9 cleaved PARP, PARP and TRAIL were subsequently incubated
in corresponding rabbit secondary antibody (cat. no. 10245169). the
working dilution of all secondary are the ratio of 1:5,000 and the
all membranes are incubated at room temperature for 2 h. Protein
expression was detected using the Odyssey infrared imaging system
(LI-COR Biosciences, Lincoln, NE, USA). All antibodies, except the
E1A antibodies, were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The E1A antibodies were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). The mouse and rabbit
secondary antibodies were obtained from PerkinElmer, Inc. (Waltham,
MA, USA).
Flow cytometric analysis
A total of 5×105 cells were seeded into
6-well plates and were left to attach overnight. The cells were
treated with CD55-TRAIL (15 MOI), luteolin (25 µM), or a
combination of CD55-TRAIL and luteolin. After 48 h, the cells were
trypsinized and harvested. Aliquots of cells were resuspended in
500 ml binding buffer and stained with annexin V fluorescein
isothiocyanate/propidium iodide (BD Biosciences, San Jose, CA,
USA), according to the manufacturer's protocol. The cells were
immediately identified using fluorescence-activated cell sorting
(BD Biosciences).
Animals
Animal studies followed the regulations and
standards set by the US Department of Agriculture and the National
Institutes of Health. A total of 24 female BALB/c nude mice (4
weeks old; mean body weight 18 g) were purchased from the Zhejiang
Chinese Medical University (Hangzhou, China) and housed at 28°C,
40–60% humidity and a 12-h light/dark cycle. The experimental
protocol was approved by the Animal Care and Welfare Committee of
Zhejiang Chinese Medical University (Hangzhou, China).
Tumor xenograft
A total of 8×106 HT-29 cells were
injected subcutaneously into the right flank of nude mice. When the
tumor had grown to a diameter of 400–600 mm3, the mice
were divided randomly into 4 groups and were injected with PBS
(vehicle), luteolin (50 mg/kg) alone, CD55-TRAIL (2×109
plaque-forming units) alone or CD55-TRAIL and luteolin. Subsequent
to the injection, the tumor size was measured using a Vernier
calliper every 5 days.
Histopathology, immunohistochemistry
(IHC) and terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) assay
The liver, kidney, spleen and tumor tissues were
harvested and fixed in 5% paraformaldehyde at room temperature for
48 h, dehydrated with gradient increasing ethanol concentrations
and embedded in paraffin wax, and were cut into 5-mm sections. The
sections were stained with hematoxylin and eosin at room
temperature for 5 min for histological analysis. The sections were
blocked with 5% BSA at room temperature for 20 min and subsequently
incubated in anti-TRAIL antibodies (3219; Cell Signaling
Technology, Inc.) overnight at 4°C, followed by
avidin-biotin-peroxidase complex reagents (Vector Laboratories,
Inc., Burlingame, CA, USA) for the IHC analysis. The working
dilution of anti-TRAIL antibodies was 1:300. Hematoxylin was used
as a counterstain at room temperature for 5 min. The in-situ
apoptosis detection kit (Sino-American Biotechnology Co., Luoyang,
China) was used to stain apoptotic cell tumor tissue sections,
according to the manufacturer's protocol, for the TUNEL assay. All
sections were counterstained with hematoxylin at room temperature
for 5 min. All sections were subsequently observed under a
microscope (0.2 mm fields; magnification, ×200; IX71-22FL/PH,
Olympus Corporation, Japan).
Statistical analysis
All experiments were repeated three times and data
are presented as the mean ± standard deviation. Statistical
analysis was performed using GraphPad Prism 6 version is 6.01
(GraphPad Software, Inc., La Jolla, CA, USA) for a Student's t
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
CD55-TRAIL with luteolin reduces the
cell viability of CRC cells
The oncolytic virus CD55-TRAIL was constructed by
inserting a TRAIL gene-expressing cassette into CD55. TRAIL gene
expression was regulated by the human cytomegalovirus
immediate-early promoter. CEA protein expression in HT-29 and SW620
cells was detected via western blotting to ensure the replication
of CD55-TRAIL.
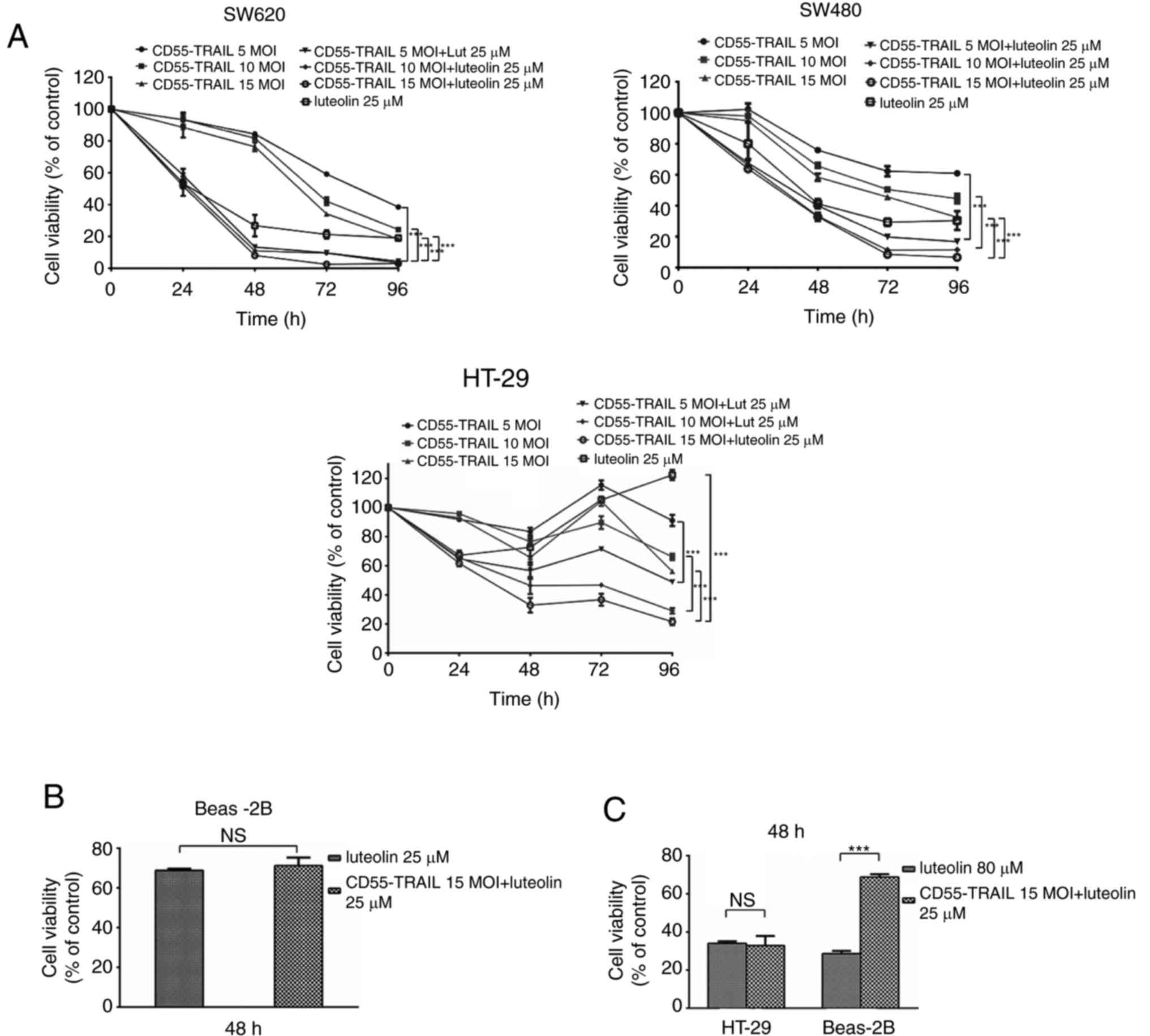
In order to evaluate the antitumor effect of
CD55-TRAIL and luteolin in vitro, CRC cell lines SW620,
SW480 and HT-29 were infected with CD55-TRAIL (at various
concentrations) and luteolin (25 µM) (Fig. 1A) for 48 h. It was observed that,
following treatment, cell viability significantly decreased in a
time-dependent manner. At 96 h post-infection, cell viability was
<30%. Treatment with CD55-TRAIL and luteolin was similar to
luteolin alone, illustrating the decreased toxicity of CD55-TRAIL
in normal BEAS-2B cells (Fig. 1B)
at 48 h. An increased concentration of luteolin (80 µM), and the
combination of CD55-TRAIL (15 MOI) with luteolin (25 µM), was used
to treat HT-29 and BEAS-2B cells; in HT-29 CRC cells, the same
cytotoxic effects were observed leading to a viability of <35%,
although the combination treatment induced less toxicity in normal
Beas-2B cells (>65% viability) (Fig. 1C). The results of the present study
suggested that the combination of CD55-TRAIL with luteolin
suppressed CRC cell proliferation with minimal toxic impact to
normal cells compared with luteolin alone.
Luteolin enhances CD55-mediated
transgene expression in CRC cells
In order to detect whether the luteolin was able to
enhance oncolytic adenovirus CD55-mediated gene expression, HT-29
cells were treated with CD55-enhanced green fluorescent protein
(EGFP) (15 MOI) or CD55-EGFP (15 MOI) and luteolin (25 µM). After
24 h, it was observed that the percentage of EGFP fluorescent cells
was increased in HT-29 cells treated with CD55-EGFP and luteolin
compared with CD55-EGFP alone (Fig.
2A), suggesting that luteolin promoted CD55-EGFP replication
and EGFP expression. In addition, E1A and TRAIL expression was
detected in CD55-TRAIL-infected HT-29 cells. The results
demonstrated that combination treatment with CD55-TRAIL and
luteolin led to increased E1A and TRAIL expression compared with
CD55-TRAIL alone or luteolin alone (Fig. 2B), indicating that luteolin was
able to enhance CD55-mediated associated protein expression.
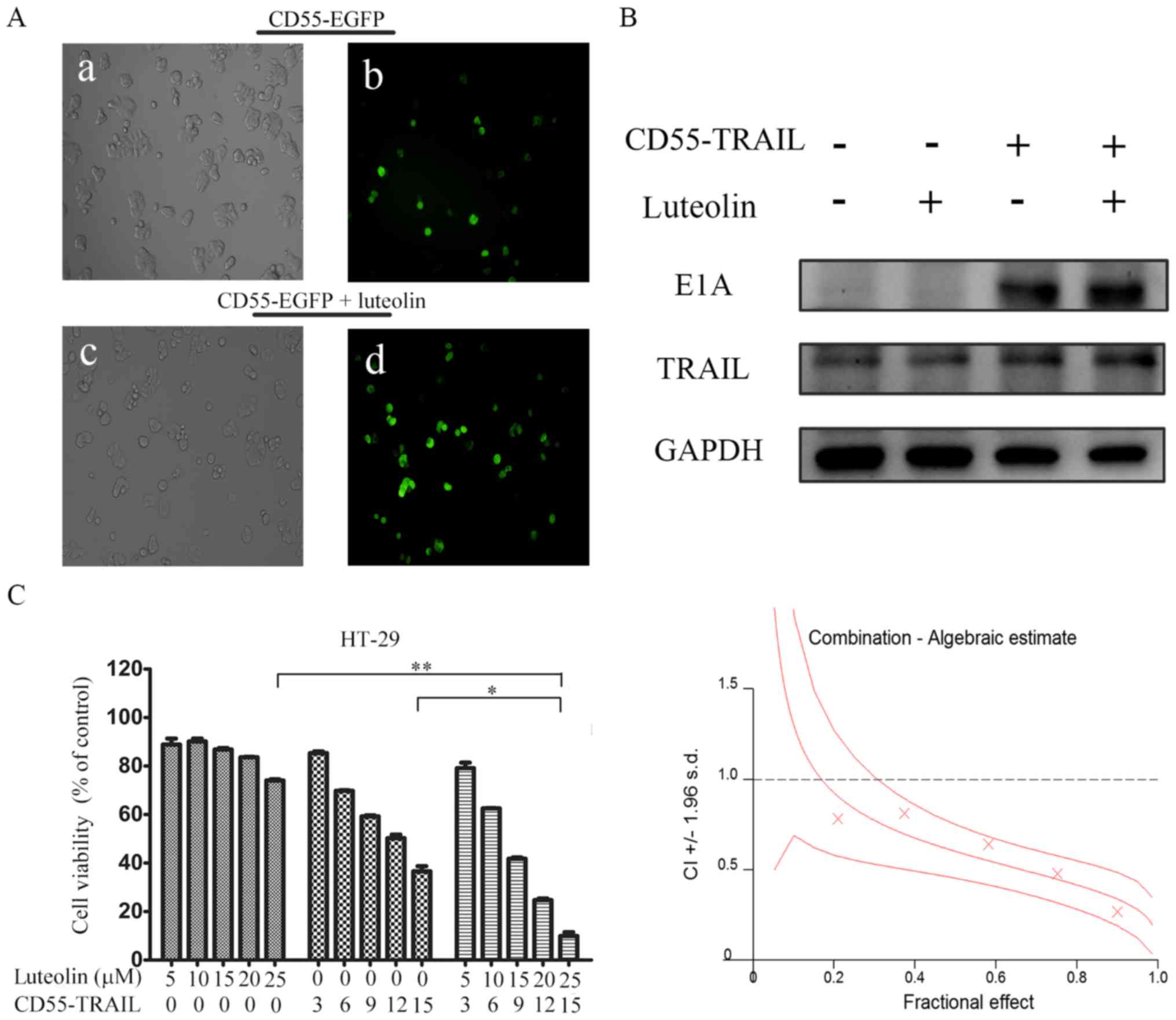 | Figure 2.Luteolin promotes CD55-TRAIL
replication and associated gene expression. (A) Colorectal cancer
HT-29 cells were treated with CD55-Trail (15 MOI; a and b), or with
CD55-Trail (15 MOI) plus luteolin (25 µM; c and d). Images a and c
were captured using a light microscope (0.2 mm fields;
magnification, ×200); b and d were captured using a fluorescence
microscope (0.2 mm fields; magnification, ×200). (B) E1A and TRAIL
expression was detected by western blot analysis. (C) Cell
viability was detected via an MTT assay. The quantitated data were
processed using CalcuSyn software, and the CI values of the
treatment groups are represented by X-marks. All data are presented
as the mean ± standard deviation. n=3. *P<0.05, **P<0.01.
CD55, complement decay-accelerating factor; TRAIL, tumor necrosis
factor ligand superfamily member 10; MOI, multiplicity of
infection; CI, combination index; E1A, early region 1A; EGFP,
enhanced green fluorescent protein. |
In order to determine whether the antitumor effect
of the combination therapy was synergistic or additive, the effect
of combination treatment was evaluated using CalcuSyn. HT-29 cells
were treated with CD55-TRAIL alone at various concentrations,
luteolin alone or a combination at the constant ratio of 3:5 for 48
h. The X-marks on the graph (Fig.
2C) were used as the combination index (CI) values. All the
experimental CI values were <1, illustrating that the
combination treatment had a synergistic effect (Fig. 2C).
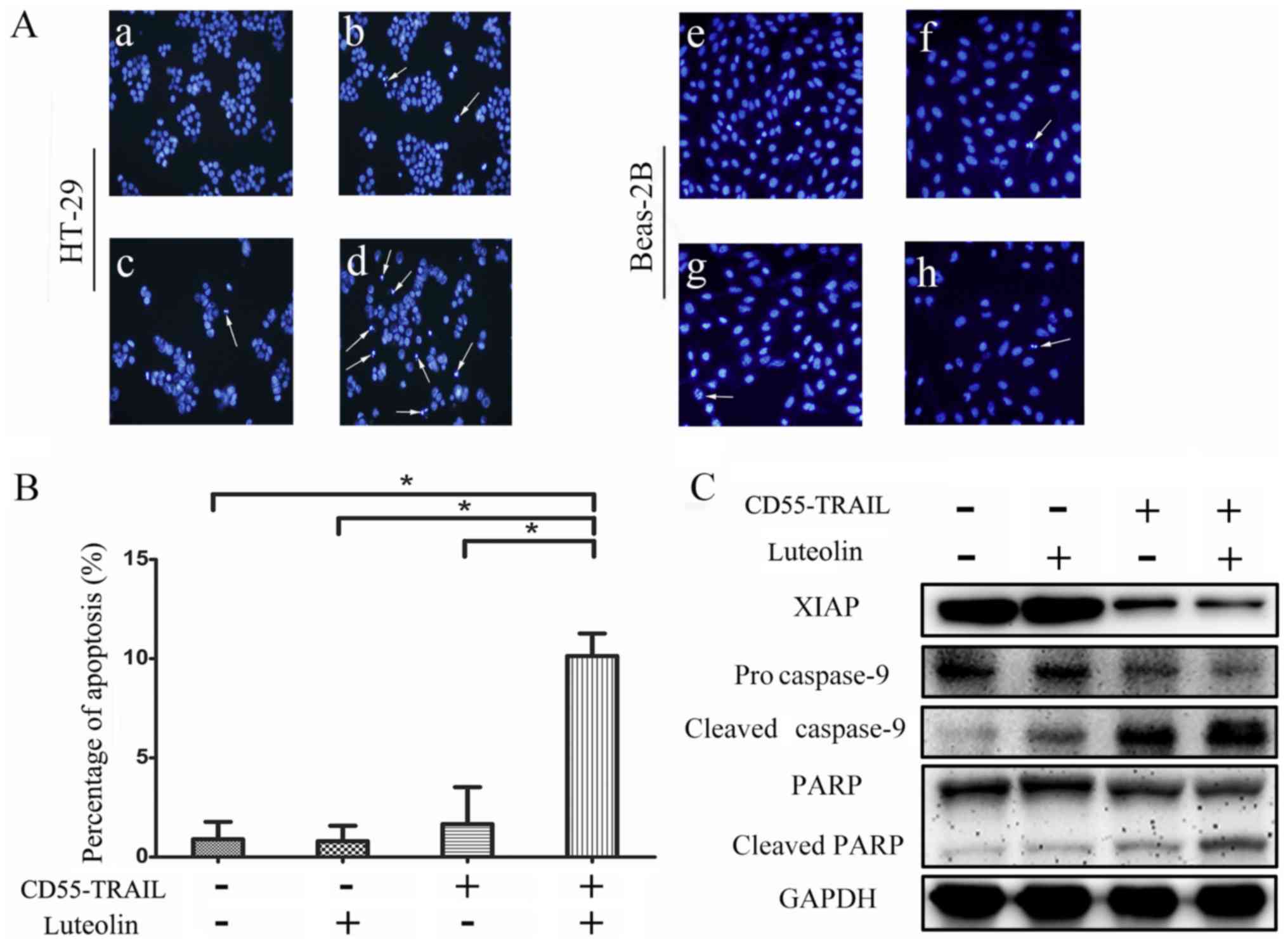
Combination of CD55-TRAIL with
luteolin induces apoptosis in cancer cells
In order to determine whether the antitumor effects
of the combination therapy were induced by an enhancement of
apoptosis in cancer cells, Hoechst 33342 analysis was used to
examine morphological alterations indicative of apoptosis in HT-29
cells. The results indicated that compared with CD55-TRAIL or
luteolin alone, the combination-treated cells displayed greater
chromatin condensation, nuclear fragmentation and apoptotic body
formation (Fig. 3A). Notably,
normal Beas-2B cells exhibited few signs of apoptosis (Fig. 3A). The examination of cell death by
flow cytometry of treated HT-29 cells revealed that compared with
1.7% for luteolin and 3.8% for CD55-TRAIL, treatment with
CD55-TRAIL with luteolin resulted in a significantly increased
percentage of apoptotic cells at 9.8% (Fig. 3B). Therefore, combination treatment
induced apoptosis to a greater degree compared with each treatment
alone.
In order to further elucidate the underlying
mechanism of cellular apoptosis, expression of the caspase
signaling pathway proteins was detected by western blot analysis.
Procaspase-9, cleaved caspase-9, PARP, cleaved PARP and XIAP were
detected in HT-29 cells treated for 48 h (Fig. 3C). There was a significant decrease
in the expression of anti-apoptosis proteins procaspase-9 and PARP,
and an increase in the pro-apoptosis proteins cleaved caspase-9 and
cleaved PARP, in cells treated with the combination therapy
compared with the other treatments. The decreased expression of
XIAP with the combination treatment compared with other apoptosis
treatment significantly increased the effect of apoptosis. The
results of the present study suggested that the combination
treatment enhanced cellular apoptosis via activation of the caspase
apoptosis pathway.
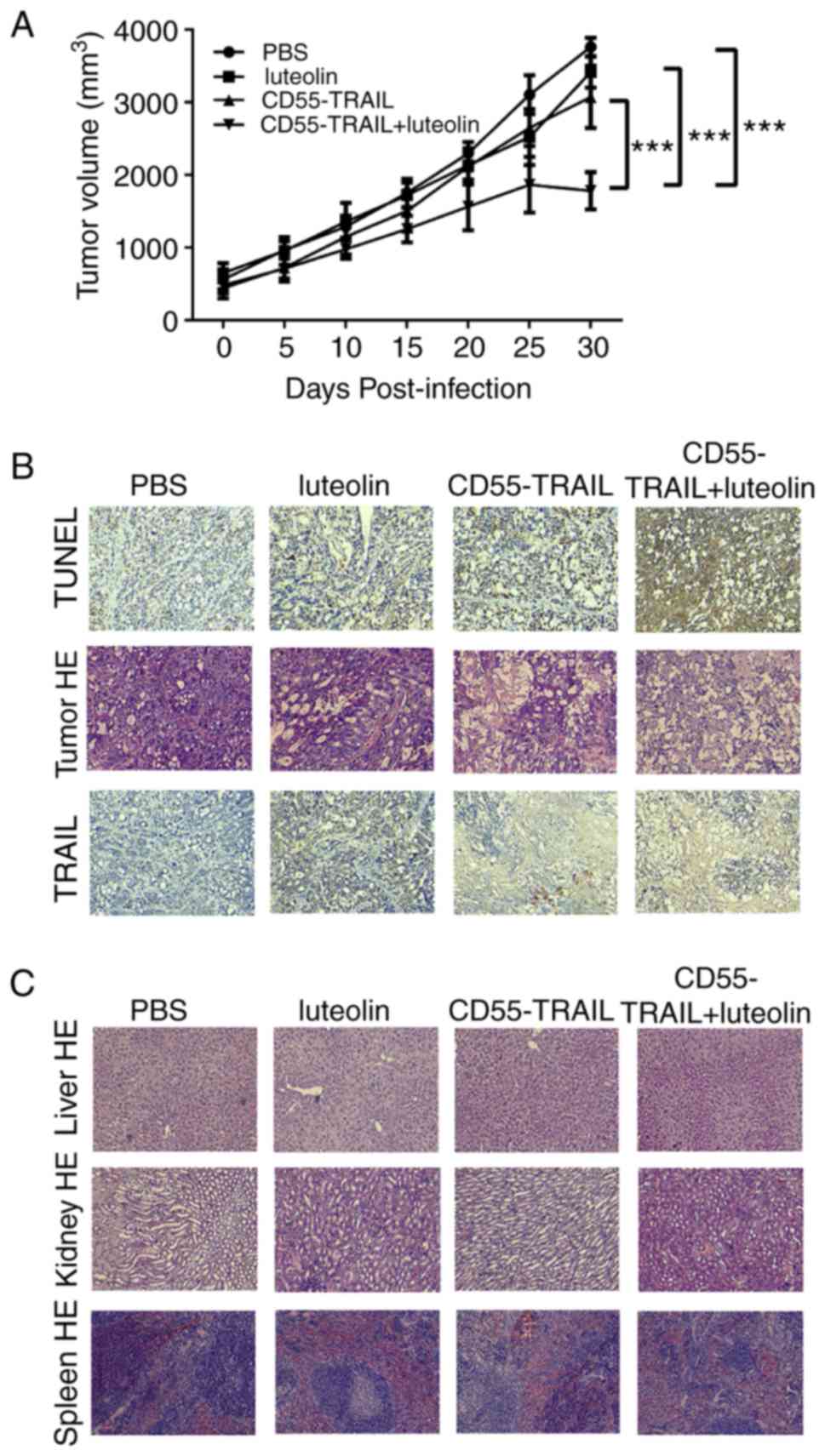
Antitumor efficacy of the combination
of CD55-TRAIL with luteolin in vivo
In order to further examine their antitumor efficacy
in vivo, the HT-29 xenograft model was established in nude
mice. Treatment with luteolin, CD55-TRAIL or a combination of the
two for 48 h was applied subcutaneously to the right flank of the
mice. As solid tumors may not exceed 20 mm diameter for ethical
reasons, animals were sacrificed at day 30. The results
demonstrated that the tumor volume in the groups receiving PBS,
luteolin or CD55-TRAIL reached ~3,761 mm3, ~3,415
mm3 and ~3,072 mm3, respectively, within 30
days; the combination of CD55-TRAIL with luteolin significantly
impeded the growth of the tumor, with a tumor volume of ~1,780
mm3 representing a 58% inhibition of tumor growth
(Fig. 4A). Additionally, after 25
days, the tumor volume in the combination treatment was decreased,
suggesting that the combination treatment has better anti-tumor
effect.
Examination of TRAIL and cell death-associated
protein TUNEL expression by IHC analysis of tumor tissues 20 days
subsequent to drug treatment revealed that the expression of TUNEL
was increased in animals receiving combination treated cells,
compared with other mice (Fig.
4B). Hematoxylin and eosin staining demonstrated that the
combination of CD55-TRAIL with luteolin caused more severe
cytopathic effects in tumor tissues compared with each therapy
alone (Fig. 4B). In addition, the
liver, kidney and spleen tissues without toxic effects were
indicated by hematoxylin and eosin staining, implying that little
to no cell damage or apoptosis had occurred in these tissues
(Fig. 4C). IHC staining
demonstrated that the TRAIL protein was highly expressed in tumor
tissue following treatment with the combination of CD55-TRAIL and
luteolin (Fig. 4B).
Discussion
Luteolin functions as a tumor suppressor by inducing
tumor cell apoptosis, cell cycle arrest, and antiangiogenesis
(19–24). However, these effects are
accompanied by severe side effects which may cause damage to normal
cells. In addition, long-term use of chemotherapy drugs may cause
drug resistance in the tumor, leading to an increase in the dose of
the drug, thereby resulting in more serious side effects. A number
of combined therapeutic strategies have demonstrated fewer negative
effects (28,29). Therefore, a combination strategy
which may markedly decrease these severe side effects may provide a
potentially promising treatment for CRC.
The present study revealed the potent antitumor
effect of oncolytic adenovirus CD55-TRAIL with luteolin on CRC
cells in vitro and in vivo, while minimizing
cytotoxicity to normal cells. The combination therapy significantly
and synergistically inhibited CRC cell proliferation. This
inhibition was induced in part by the enhancement of CD55-mediated
TRAIL expression in CRC cells by luteolin. The combination of
CD55-TRAIL with luteolin additionally decreased the toxicity of
luteolin in lung/bronchial normal epithelial cells. It was
demonstrated that the combination of CD55-TRAIL with luteolin
reduced the dose of luteolin required to elicit the same antitumor
effect as luteolin alone.
The results of the present study demonstrated that
the combination treatment induced antitumor effects by enhancing
apoptosis, evidenced by an increase in chromatin condensation,
nuclear fragmentation, the formation of apoptotic bodies and the
expression of caspase apoptotic pathway proteins in CRC cells. In
addition, compared with each treatment individually, the
combination of CD55-TRAIL with luteolin decreased the expression of
the caspase inhibitor gene XIAP in order to activate the caspase
apoptotic pathway. This finding was consistent with a previous
report showing that the combination of TRAIL with luteolin
inhibited the expression of the XIAP gene to activate the caspase
apoptotic pathway in HeLa cells (30). The distinct molecular mechanism of
the combination treatment strategy remains to be further elucidated
in future studies.
Progress has been made in the field of OAs,
including the CTGVT strategy and the use of specific promoters
instead of the E1A promoter to improve targeting. However, OAs, due
to their heterogeneity, may induce an inflammatory response
following infection of the tumor tissue and elicit antiviral
effects. In addition, the bioavailability of oncolytic adenoviruses
is low when administered by intravenous injection. Further studies
are required to resolve these issues. Thus, OAs have been
considered less as an individual treatment for tumors, and more as
a combination therapy, including OAs combined with nanomaterials
(31,32), immune cells (33,34)
and chemotherapy drugs (30,31),
which have exhibited an improved therapeutic effect compared with
oncolytic viruses alone. Additionally, previous studies have
focused on the identification of tumor biomarkers based on
microRNAs (35,36), which may provide the possibility of
improving the antitumor effect of oncolytic virotherapy.
The results of the present study demonstrated that
the combined use of luteolin and CD55-TRAIL had a potent antitumor
effect, and that the antitumor effect of CD55-TRAIL was synergized
by luteolin in CRC cells with reduced toxicity to normal epithelial
cells. Additionally, this combination resulted in a strong
apoptotic effect in CRC cells in vitro and in vivo.
In conclusion, the combination of CD55-TRAIL with luteolin provided
a potentially effective clinical therapy for CRC.
Acknowledgements
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant nos.
LY16H160056 and LZ13H160004), the Grant for 521 Talent Project of
Zhejiang Sci-Tech University and the Innovation Project of Zhejiang
Sci-Tech University (grant no. YCX15035).
References
|
1
|
Global battle against cancer won't be won
with treatment alone-effective prevention measures urgently needed
to prevent cancer crisis. Cent Eur J Public Health. 22:23–28.
2014.PubMed/NCBI
|
|
2
|
Breitbach CJ, Burke J, Jonker D,
Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R,
et al: Intravenous delivery of a multi-mechanistic cancer-targeted
oncolytic poxvirus in humans. Nature. 477:99–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu XY: A new anticancer
strategy-gene-virotherapy of cancer. Chin J Cancer Biother.
8:12001.
|
|
4
|
Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH,
Sun LY, Qian QJ and Liu XY: An armed oncolytic adenovirus system,
ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res.
13:481–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Liu T, Huang P, Zhao H, Zhang R,
Ma B, Chen K, Huang F, Zhou X, Cui C and Liu X: A novel Golgi
protein (GOLPH2)-regulated oncolytic adenovirus exhibits potent
antitumor efficacy in hepatocellular carcinoma. Oncotarget.
6:13564–13578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lei W, Liu HB, Wang SB, Zhou XM, Zheng SD,
Guo KN, Ma BY, Xia YL, Tan WS, Liu XY and Wang YG: Tumor suppressor
in lung cancer-1 (TSLC1) mediated by dual-regulated oncolytic
adenovirus exerts specific antitumor actions in a mouse model. Acta
Pharmacol Sin. 34:531–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He G, Lei W, Wang S, Xiao R, Guo K, Xia Y,
Zhou X, Zhang K, Liu X and Wang Y: Overexpression of tumor
suppressor TSLC1 by a survivin-regulated oncolytic adenovirus
significantly inhibits hepatocellular carcinoma growth. J Cancer
Res Clin Oncol. 138:657–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R,
Ebner R, Ni J and Dixit VM: The receptor for the cytotoxic ligand
TRAIL. Science. 276:111–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma H, Liu Y, Liu S, Kung HF, Sun X, Zheng
D and Xu R: Recombinant adeno-associated virus-mediated TRAIL gene
therapy suppresses liver metastatic tumors. Int J Cancer.
116:314–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang R, Zhang X, Ma B, Xiao B, Huang F,
Huang P, Ying C, Liu T and Wang Y: Enhanced antitumor effect of
combining TRAIL and MnSOD mediated by CEA-controlled oncolytic
adenovirus in lung cancer. Cancer Gene Ther. 23:168–177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao L, Dong A, Gu J, Liu Z, Zhang Y,
Zhang W, Wang Y, He L, Qian C, Qian Q and Liu X: The antitumor
activity of TRAIL and IL-24 with replicating oncolytic adenovirus
in colorectal cancer. Cancer Gene Ther. 13:1011–1022. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hang ZQ, Zheng MF and Huang JH: Detection
and diagnostic value of serum carcinoembryonic antigen and
cytokeratin 19 fragment in lung cancer patients. Zhonghua Zhong Liu
Za Zhi. 33:847–849. 2011.(In Chinese). PubMed/NCBI
|
|
14
|
Verberne CJ, Nijboer CH, de Bock GH,
Grossmann I, Wiggers T and Havenga K: Evaluation of the use of
decision-support software in carcino-embryonic antigen (CEA)-based
follow-up of patients with colorectal cancer. BMC Med Inform Decis
Mak. 12:142012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang YR, Yan JX and Wang LN: The
diagnostic value of serum carcino-embryonic antigen, alpha
fetoprotein and carbohydrate antigen 19-9 for colorectal cancer. J
Cancer Res Ther. 10 Suppl:S307–S309. 2014. View Article : Google Scholar
|
|
16
|
Shi J, Zheng D, Liu Y, Sham MH, Tam P,
Farzaneh F and Xu R: Overexpression of soluble TRAIL induces
apoptosis in human lung adenocarcinoma and inhibits growth of tumor
xenografts in nude mice. Cancer Res. 65:1687–1692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai H, Jin Q, Lin Y, Mo X, Li B, He K and
Chen J: Combined use of lysyl oxidase, carcino-embryonic antigen,
and carbohydrate antigens improves the sensitivity of biomarkers in
predicting lymph node metastasis and peritoneal metastasis in
gastric cancer. Tumour Biol. 35:10547–10554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ross JA and Kasum CM: Dietary flavonoids:
Bioavailability, metabolic effects, and safety. Annu Rev Nutr.
22:19–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang YT, Hwang JJ, Lee PP, Ke FC, Huang
JH, Huang CJ, Kandaswami C, Middleton E Jr and Lee MT: Effects of
luteolin and quercetin, inhibitors of tyrosine kinase, on cell
growth and metastasis-associated properties in A431 cells
overexpressing epidermal growth factor receptor. Br J Pharmacol.
128:999–1010. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko WG, Kang TH, Lee SJ, Kim YC and Lee BH:
Effects of luteolin on the inhibition of proliferation and
induction of apoptosis in human myeloid leukaemia cells. Phytother
Res. 16:295–298. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JH, Jin YR, Park BS, Kim TJ, Kim SY,
Lim Y, Hong JT, Yoo HS and Yun YP: Luteolin prevents
PDGF-BB-induced proliferation of vascular smooth muscle cells by
inhibition of PDGF beta-receptor phosphorylation. Biochem
Pharmacol. 69:1715–1721. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HJ, Wang CJ, Kuo HC, Chou FP, Jean LF
and Tseng TH: Induction apoptosis of luteolin in human hepatoma
HepG2 cells involving mitochondria translocation of Bax/Bak and
activation of JNK. Toxicol Appl Pharmacol. 203:124–131. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leung WC, Wu CH, Lin CH and Lee HZ:
Luteolin induced DNA damage leading to human lung squamous
carcinoma CH27 cell apoptosis. Eur J Pharmacol. 508:77–83. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bagli E, Stefaniotou M, Morbidelli L,
Ziche M, Psillas K, Murphy C and Fotsis T: Luteolin inhibits
vascular endothelial growth factor-induced angiogenesis; inhibition
of endothelial cell survival and proliferation by targeting
phosphatidylinositol 3′-kinase activity. Cancer Res. 64:7936–7946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pandurangan AK, Sadagopan SK Ananda,
Dharmalingam P and Ganapasam S: Luteolin, a bioflavonoid inhibits
Azoxymethane-induced colorectal cancer through activation of Nrf2
signaling. Toxicol Mech Methods. 24:13–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HS and Choi YH: Induction of
Apoptosis by Combination Treatment with Luteolin and TRAIL in T24
Human Bladder Cancer Cells. J Korean Society Food Sci Nut.
42:1363–1369. 2013. View Article : Google Scholar
|
|
27
|
Horinaka M, Yoshida T, Shiraishi T, Nakata
S, Wakada M, Nakanishi R, Nishino H and Sakai T: The combination of
TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa
cells. Biochem Biophys Res Commun. 333:833–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma BY and Wang Y, Zhou X, Huang P, Zhang
R, Liu T, Cui C, Liu X and Wang Y: Synergistic suppression effect
on tumor growth of hepatocellular carcinoma by combining oncolytic
adenovirus carrying XAF1 with cisplatin. J Cancer Res Clin Oncol.
141:419–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng PH, Lian S, Zhao R, Rao XM,
McMasters KM and Zhou HS: Combination of autophagy inducer
rapamycin and oncolytic adenovirus improves antitumor effect in
cancer cells. Virol J. 10:2932013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi RX, Ong CN and Shen HM: Protein kinase
C inhibition and x-linked inhibitor of apoptosis protein
degradation contribute to the sensitization effect of luteolin on
tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis in cancer cells. Cancer Res. 65:7815–7823. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Yao B, Li Q, Mei K, Xu JR, Li HX,
Wang YS, Wen YJ, Wang XD, Yang HS, et al: Gene therapy with
recombinant adenovirus encoding endostatin encapsulated in cationic
liposome in coxsackievirus and adenovirus receptor-deficient colon
carcinoma murine models. Hum Gene Ther. 22:1061–1069. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nigatu AS, Vupputuri S, Flynn N, Neely BJ
and Ramsey JD: Evaluation of cell-penetrating peptide/adenovirus
particles for transduction of CAR-negative cells. J Pharm Sci.
102:1981–1993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang SN, Choi IK, Huang JH, Yoo JY, Choi
KJ and Yun CO: Optimizing DC vaccination by combination with
oncolytic adenovirus coexpressing IL-12 and GM-CSF. Mol Ther.
19:1558–1568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Z, Zhang Q, Xu K, Shan J, Shen J, Liu
L, Xu Y, Xia F, Bie P, Zhang X, et al: Combined therapy with
cytokine-induced killer cells and oncolytic adenovirus expressing
IL-12 induce enhanced antitumor activity in liver tumor model. PLoS
One. 7:e448022012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang F, Zheng Z, Guo J and Ding X:
Correlation and quantitation of microRNA aberrant expression in
tissues and sera from patients with breast tumor. Gynecol Oncol.
119:586–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ding X, Ding J, Ning J, Yi F, Chen J, Zhao
D, Zheng J, Liang Z, Hu Z and Du Q: Circulating microRNA as a
potential biomarker for liver injury. Mol Med Rep. 5:1428–1432.
2012.PubMed/NCBI
|