Introduction
Dendropanax morbifera Leveille (D.
morbifera) is a member of the Araliaceae family. It is a
subtropical broad-leaved evergreen tree that has been used in
traditional medicine for the treatment of headache, infectious
disease, skin diseases, and neurological disorders (1,2). The
plant contains several components that exhibit various
pharmacological effects. One of these is the triterpenoid compound
Oleifolioside A, that inhibits nitric oxide (NO) and prostaglandin
E2 (PGE2) through the downregulation of nuclear factor (NF)-κB and
mitogen-activated protein kinase (MAPK) signaling in
lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophages
(3), and induces
caspase-independent cell death in HeLa human cervical carcinoma
cells (4). Ethanol extract of
D. morbifera induces apoptosis of human leukemia U937 cells
through the caspase dependent pathway (1). Anti-cancer activities of methanol
extracts have been reported in hepatocarcinoma, colon
adenocarcinoma, biliary tract cancer and human osteosarcoma cells
(5). Anti-inflammatory responses
by D. morbifera extracts involve the suppression of NF-κB
dependent pathways in LPS-stimulated macrophages (6) and microglia (7).
Helicobacter pylori (H. pylori) is a gram
negative-bacterium that is commonly located in the stomach of
individuals. In some people, H. pylori is a pathogen,
causing gastric inflammatory diseases including gastritis, peptic
ulcer, duodenal ulcer, and even mucosa-associated lymphatic tissue
lymphoma (8,9). Production of important inflammatory
mediators, such as interleukin (IL)-8 and vascular endothelial
growth factor (VEGF), via NF-κB and MAPK signaling in gastric
epithelial cells results in gastric inflammation (10) and tumor progression (11).
The present study aimed to investigate whether
1-tetradecanol (1-TD), which was recently isolated from the
n-hexane fraction of D. morbifera, has
anti-inflammatory activity in H. pylori-infected gastric
epithelial cells.
Materials and methods
Preparation of 1-TD
Water extract of Dendropanax morbifera leaves
was prepared at 100°C for 4 h. The extracted solution was filtered,
concentrated with an evaporator under a vacuum, and freeze-dried.
The preparation was suspended in water and successively divided
with n-hexane, chloroform, ethyl acetate and n-butanol (3×500 ml).
Tetradecanol used in this study was isolated from the n-hexane
fraction and the purity was confirmed to be >99% as described
previously (12).
H. pylori strain and culture
conditions
H. pylori strain 26695 (American Type Culture
Collection, Manassas, VA, USA) was cultured on Brucella broth (BD
Biosciences, Franklin Lakes, NJ, USA) containing 10% fetal bovine
serum (FBS; Corning Incorporated, Corning, NY, USA) and antibiotic
supplement in a micro-aerobic environment. The bacteria were grown
to an optical density at 600 nm (OD600) of 0.6 measured using an
enzyme-linked immunosorbent assay (ELISA) reader (BioTek
Instruments, Inc., Winooski, VT, USA), which corresponded to
~109 colony-forming units (CFU)/ml and were diluted to
the desired concentrations (13).
Cell culture and treatment
The AGS (KCLB; 21739) and MKN45 (KCLB; 80103) human
gastric epithelial cell lines were purchased from the Korean Cell
Line Bank (Seoul, Korea) and grown in RPMI-1640 medium (Welgene,
Inc., Daegu, Korea) supplemented with 10% FBS and 1X
penicillin/streptomycin (100 U/ml) (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in a humidified atmosphere of 5% CO2
at 37°C. Briefly, to determine the secretion of VEGF and IL-8, AGS
and MKN45 cells (1×105 cells/well in a 48-well plate)
were infected with H. pylori 26695 at the indicated
multiplicity of infection (MOI; 50) in the absence or presence
various quantities of 1-TD (30–300 µM; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 24 h in a humidified incubator at 37°C in
an atmosphere of 5% CO2. To measure the levels of
hypoxia-inducible factor-1α (HIF-1α), AGS cells (1×106
cells/well in a 6-well plate) were infected with H. pylori
26695 at MOI 50 with or without 1-TD (300 µM) for 6 h.
Determination of IL-8 and VEGF
The culture supernatants of H.
pylori-infected AGS and MKN45 cells were collected for ELISA
for IL-8 and VEGF. Commercial Duoset ELISA kits (DY208 for IL-8 and
DY293B for VEGF; R&D Systems, Minneapolis, MN, USA) were
performed according to the manufacturer's protocol.
MTT assay
An MTT assay was performed to determine the
cytotoxicity of 1-TD on gastric epithelial cell lines. The cells
(1×105 cells/well in a 48-well plate) were treated to
different concentrations of 1-TD (30, 100 and 300 µM) for 24 h.
Each well was incubated with MTT (4 mg/ml; Sigma-Aldrich; Merck
KGaA) in RPMI-1640 medium (Welgene, Inc.) for 4 h at 37°C. After 4
h, the MTT solution was removed and replaced with 200 µl of
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA). The plates were
agitated for 5 min to dissolve the formazan crystals. The OD were
determined at a wavelength of 570 nm using an ELISA plate reader
(BioTek Instruments, Inc.).
Western blotting
AGS cells were seeded into 35-mm dishes and
incubated for 24 h. Cells infected with H. pylori 26695 (MOI
50) were then pre-treated with 1-TD (300 µM) for 2 h. After 0, 15,
30 or 60 min of treatment, cells were lysed in a buffer containing
1% Nonidet-P40 supplemented with protease inhibitor (complete Mini
EDTA-free; Roche, Mannheim, Germany), phosphatase inhibitor
(Phosphatase Inhibitor Cocktail 2; Sigma-Aldrich; Merck KGaA) and 2
mM dithiothreitol. The extracted protein concentration was examined
by a Protein Assay kit (500–0006; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Lysates (30 µg) were separated by 12% SDS-PAGE
and transferred onto nitrocellulose membranes by electro-blotting.
The membranes were blocked with blocking buffer [5% skimmed milk in
PBS-Tween (0.05% Tween-20)] and incubated at room temperature for 1
h. The membranes were probed with primary antibodies against
regular and phosphorylated (p) forms of c-jun N-terminal kinase
(JNK; cat. no. 9252; 1:1,000; Cell Signaling Technology, Inc.,
Beverly, MA, USA), p38 (cat. no. sc 101759; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), extracellular
signal-regulated kinase (ERK; cat. no. sc 7383; 1:1,000; Santa Cruz
Biotechnology, Inc.), inhibitor of NF-κB kinase subunit α (IκB-α;
1:1,000; cat. no. 9242S; Cell Signaling Technology, Inc.), p-NF-κB
p65 (1:1,000; cat. no. 3033S; Cell Signaling Technology, Inc.) and
HIF-1α (1:1,000, cat. no. 610958; BD Transduction Laboratories; BD
Biosciences), followed by an incubation at 4°C overnight. A primary
antibody against β-actin (cat. no. sc-130656; 1:1,000;
Sigma-Aldrich; Merck KGaA) was used to verify equal loading of
protein samples. After immunoblotting with corresponding goat
anti-rabbit (cat. no. sc-2301; 1:4,000; Santa Cruz Biotechnology,
Inc.) or goat anti-mouse IgG (cat. no. sc-2031; 1:2,000; Santa Cruz
Biotechnology, Inc.) secondary antibodies for 2 h at room
temperature, signals were detected with a SuperSignal™ West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.). Images
of the blots were captured on a CP-BU new film (Agfa Gevaert N.V.,
Mortsel, Belgium).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression of VEGF was evaluated by RT-qPCR in
AGS cells infected with H. pylori strain 26695 (MOI 50) in
the absence or presence of 1-TD (300 µM) for 12 h. Total RNA was
isolated from cultured cells using the easy-BLUE™ Total RNA
Extraction kit (Intron Biotechnology, Inc., Seongnam, Korea). cDNA
was synthesized from 0.1 µg RNA using the ReverTra Ace®
qPCR RT Master Mix kit (Toyobo Life Science, Osaka, Japan)
according to the manufacturer's protocol. qPCR was performed using
the Qiagen SYBR Green PCR kit (Qiagen GmbH, Hilden, Germany),
according to the manufacturer's protocol. Primers were: VEGF
forward, 5′-CCTTGCTGCTCTACCTCCAC-3′ and reverse,
5′-TGGTGATGTTGGACTCCTCA-3′; and GAPDH forward,
5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′ and reverse,
5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′. qPCR was performed by using a
two-step cycle of 95°C for 10 sec followed by 58°C for 45 sec for
40 cycles in a Roter-GeenQ Real-time PCR system (Qiagen). Gene
expression was quantified using the comparative Ct method,
normalizing to GAPDH mRNA (14).
Anti-bacterial activity
For anti-bacterial testing, 50 µl bacterial
suspension (1×109 CFU/ml) was added to 2 ml Brucella
broth containing various doses of 1-TD. After 6 or 12 h of
incubation at 37°C under microaerobic conditions, bacterial growth
was determined by measuring the OD600 of the culture
broth with an ELISA reader (Epoch; BioTek Instruments, Inc.). The
experiment was repeated in triplicate.
Statistical analysis
Data are expressed as the mean ± standard error.
Statistical significances were analyzed by one-way analysis of
variance with a Bonferroni post hoc test, and analysis was
performed using GraphPad Prism version 5.00 (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.01 was considered to indicate a
statistically significant difference.
Results
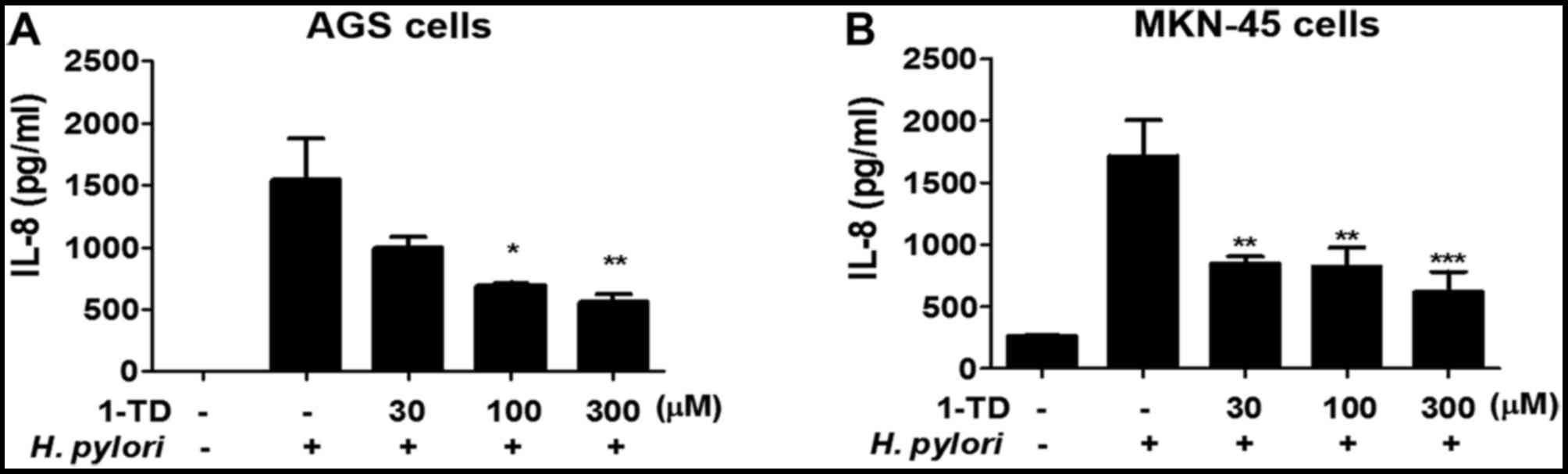
1-TD suppresses IL-8 production by H.
pylori-infected gastric epithelial cells
To understand the effects of 1-TD on the H.
pylori-induced inflammatory immune response in gastric
epithelial cells, IL-8 production in AGS cells co-incubated with
H. pylori (MOI 50) and 1-TD at concentrations of 30, 100 and
300 µM was analyzed. Following an incubation for 24 h, levels of
IL-8 significantly decreased in a dose-dependent manner in H.
pylori-infected AGS cells that were co-treated with 1-TD,
compared with H. pylori-infected AGS cells that were not
co-treated with 1-TD (Fig. 1A).
Similar inhibition in another gastric epithelial cell line (MKN45)
was also demonstrated (Fig. 1B).
In addition, 1-TD was not cytotoxic in either gastric epithelial
cell lines at 30, 100 and 300 µM using MTT assay (data not shown),
suggesting that 1-TD is a candidate agent to suppress H.
pylori-induced inflammation in gastric epithelial cells.
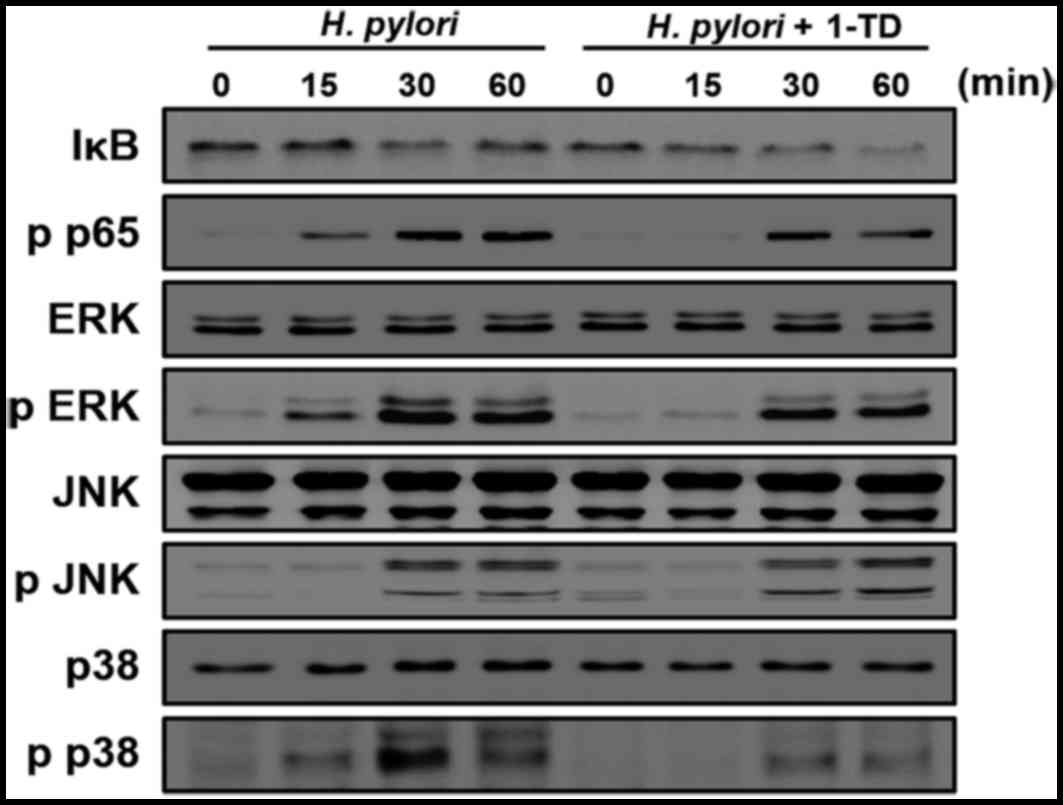
1-TD inhibits the activation of NF-κB,
ERK1/2 and p38 in H. pylori-infected gastric epithelial cells
The activation of NF-κB and MAPK signaling is
critical for the production of IL-8 in H. pylori-infected
gastric epithelial cells (11).
Therefore, the present study examined the impact of 1-TD treatment
on their expression in H. pylori-infected AGS cells using
western blotting. H. pylori induced decrease of IκB-α at 30
min after infection; however, this process was delayed by 1-TD
(Fig. 2). Notably, 1-TD decreased
the phosphorylation levels of p65, ERK1/2 and p38, whereas it had
no influence on the level of p-JNK (Fig. 2). These results indicated that 1-TD
potentially suppresses NF-κB and MAPK-dependent inflammation in
H. pylori-infected gastric epithelial cells.
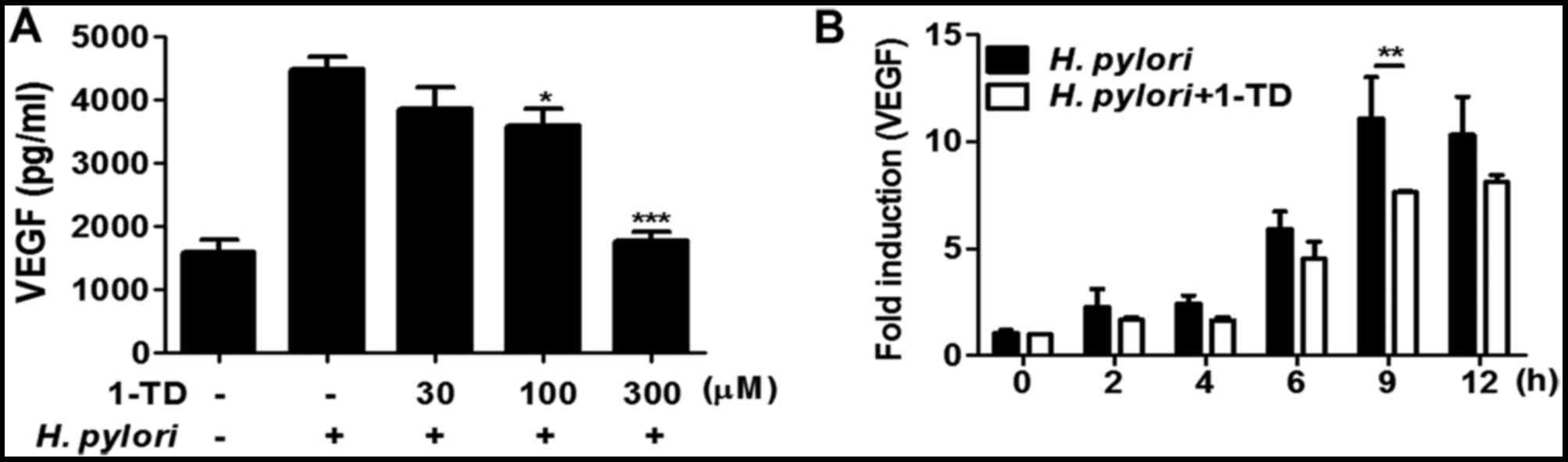
1-TD inhibits H. pylori-induced VEGF
production in gastric epithelial cells
VEGF production is regulated via NF-κB, ERK and p38
MAPK in the gastric mucosa (11,15).
Therefore, the present study examined the effect of 1-TD on
secretion and mRNA expression of VEGF in H. pylori-infected
AGS cells. The ELISA results demonstrated that the protein
expression level of VEGF was significantly decreased in AGS cells
co-treated with H. pylori (MOI 50) and 30, 100, and 300 µM
of 1-TD after 24 h incubation in a dose-dependent manner (Fig. 3A). Additionally, the mRNA
expression of VEGF tended to be lower in H. pylori-infected
AGS cells co-treated with 1-TD over time, compared with those in
the absence of 1-TD (Fig. 3B). The
difference reached statistical significance only at 9 h (Fig. 3B). These results indicated that
1-TD inhibited protein synthesis and transcription of H.
pylori-induced VEGF in gastric epithelial cells.
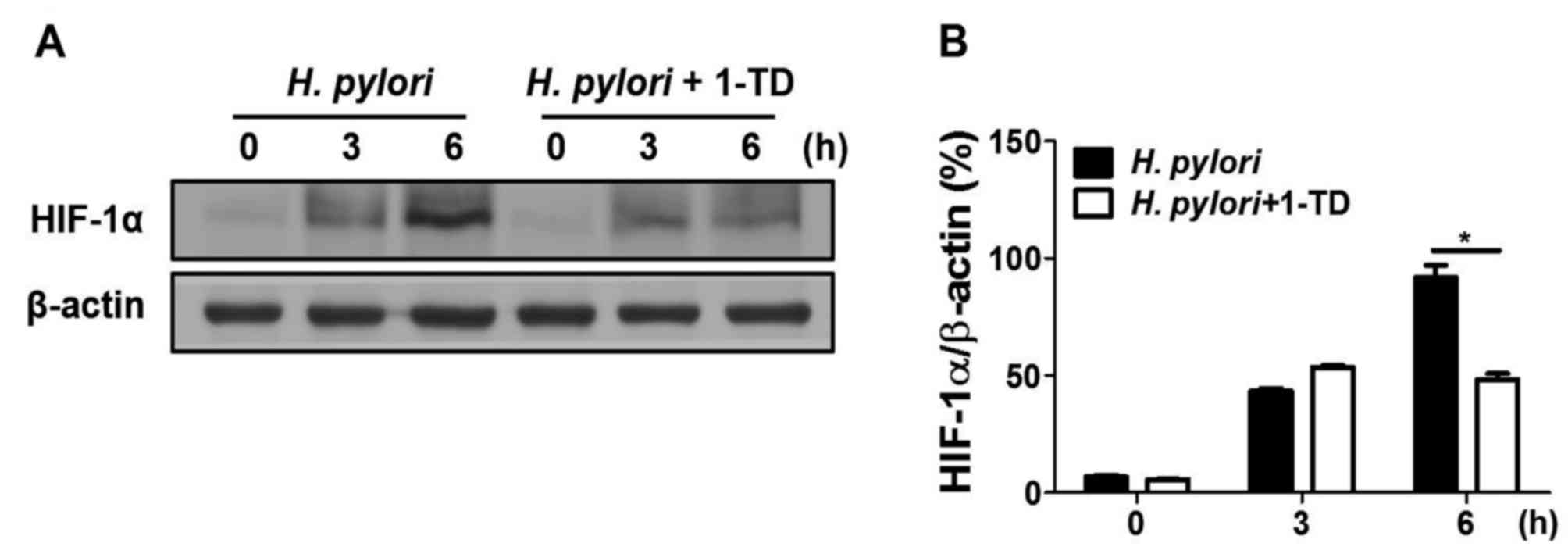
1-TD inhibits H. pylori-induced HIF-1α
stabilization in gastric epithelial cells
To determine whether 1-TD affects H.
pylori-mediated HIF-1α stabilization in AGS cells, western blot
analysis was performed to measure the expression of HIF-1α. 1-TD
treatment inhibited H. pylori-induced HIF-1α protein
expression levels at 6 h, compared with that at 0 and 3 h (Figs. 4A and B), suggesting that 1-TD has
an inhibitory effect on H. pylori-induced VEGF production
through HIF-1α stabilization.
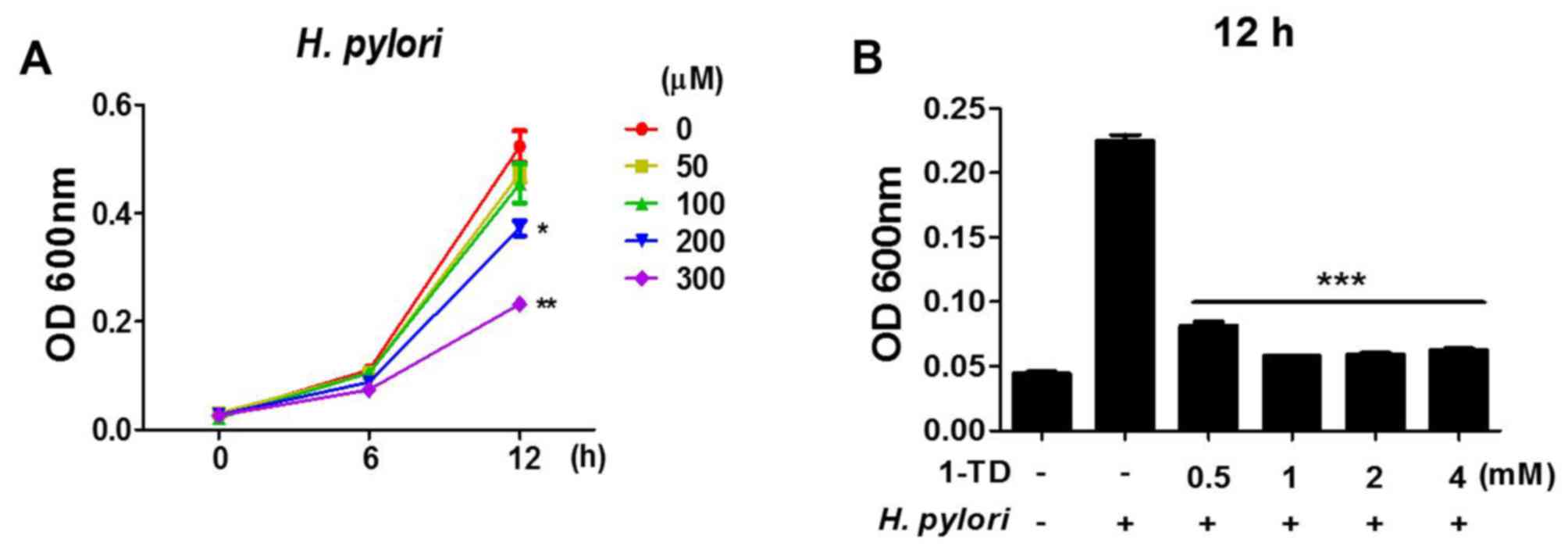
1-TD affects anti-bacterial activity
against H. pylori
To assess whether 1-TD has any direct effect on the
viability of H. pylori, bacterial growth curves in the
presence of 1-TD (0, 50, 100, 200 and 300 µM) for 12 h were
assessed. It was demonstrated that there was a dose-dependent
reduction in the growth of H. pylori at high doses (200 and
300 µM), while no effect was observed at lower doses (Fig. 5A). To determine a minimal
inhibitory concentration (MIC), the bacterial growth was determined
in the presence of much higher doses of 1-TD (>500 mM). More
than 1 mg of 1-TD completely inhibited the growth of H.
pylori (Fig. 5B), indicating
that the MIC of 1-TD on H. pylori is between 0.5 and 1 mg.
These data suggested a direct inhibitory effect of 1-TD on the
growth of H. pylori.
Discussion
Anti-oxidant, anti-complement, anti-plasmodium and
anti-cancer activities have been associated with extracts from the
leaves and stems of D. morbifera (5,16,17).
However, limited data are available about the physiological effects
of the compound 1-TD, isolated from the lower stem of D.
morbifera. Only a few studies have examined its
anti-inflammatory effects on cells such as microglial cells
(6,7) and macrophages (3). Furthermore, IL-8, a member of the CXC
chemokine family, is involved in inflammatory responses, leukocyte
chemotaxis and cancer development (18,19).
The high expression of IL-8 is markedly associated with the
proliferation, invasion and migration of gastric epithelial cells
(20,21). In the case of H. pylori
infection, IL-8 production is directly increased in gastric
epithelial cells (22,23). To the best of our knowledge, the
present study was the first to report that 1-TD has potent
inhibitory effects against H. pylori-induced inflammation.
1-TD significantly inhibited IL-8 production in AGS cells infected
with H. pylori, suggesting that 1-TD may act as a potential
agent to suppress H. pylori-induced inflammatory responses
involved in IL-8 production.
No cytotoxic effects of 0–300 µM 1-TD on H.
pylori-infected gastric epithelial cells were evident. In
contrast, previous studies have demonstrated that oleifolioside A
and B, ethanol and methanol extracts isolated from D.
morbifera have cytotoxic effects on human leukemia cells
(1), lung carcinoma cells
(24), cervical carcinoma cells
(4) and hepatocellular carcinoma
cells (5). Therefore, the results
of the present study suggested that the inhibition of H.
pylori-induced inflammation in gastric epithelial cells is not
due to cell toxicity. Furthermore, 1-TD restricted the growth of
H. pylori in Brucella broth, indicating direct antibacterial
activity, as was previously observed for other long-chain fatty
alcohols (25). However, the
mechanism through which 1-TD inhibits the bacterial growth is yet
to be elucidated.
MAPKs (ERK1/2, p38 and JNK) regulate oxidative
stress, gene expression, mitosis, metabolism and apoptosis
(26,27). NF-κB and MAPK activation are
required for transcription of IL-8 gene induced by H.
pylori-infected gastric epithelial cells (28). In the present study, pre-treatment
with 1-TD inhibited H. pylori-induced phosphorylation of p38
MAPK, ERK1/2, and NF-κB, suggesting that the inhibition of the
levels of IL-8 induced by H. pylori was MAPK- and
NF-κB-dependent. In contrast, the phosphorylation of JNK was not
affected. A similar observation was previously reported in
LPS-stimulated RAW 264.7 macrophages (3). The authors also reported the
proinflammatory mediators involved in NF-κB, p38 MAPK and ERK1/2
signaling by oleifolioside A (3).
The potential reduction of these mediators by 1-TD in H.
pylori-infected epithelial cells was not elucidated in the
present study.
During H. pylori infection, VEGF can be
produced. This is important in vascular remodeling in gastric
epithelial cells (29). Our
previous study revealed that this production is associated with the
activation of NF-κB and MAPK signaling, as well as HIF-1α
stabilization resulting from the generation of reactive oxygen
species induced by H. pylori (30,31).
In the present study, 1-TD significantly decreased H.
pylori-induced VEGF production and inhibited HIF-1α
stabilization in AGS cells. These findings suggested that 1-TD may
be a novel agent on the inhibition of inflammatory responses
involved in angiogenesis in H. pylori-infected gastric
epithelial cells.
In conclusion, 1-TD effectively inhibited the
production of inflammatory mediators (IL-8 and VEGF) through the
inhibition of p38 MAPK, ERK1/2, and NF-κB signaling in H.
pylori-infected gastric epithelial cells. Moreover, 1-TD
inhibited the production of VEG and HIF-1α stabilization induced by
H. pylori in gastric epithelial cells. Taken together, these
data suggested that 1-TD may serve as a novel anti-inflammatory
agent for H. pylori-infected gastric epithelial cells.
Further studies are warranted to evaluate the impact of this
anti-inflammatory effect in an in vivo model.
Acknowledgements
The present study was supported by the Basic
Research in Science and Engineering program, funded by the National
Research Foundation of Korea (NRF) in the Ministry of Science, ICT,
and Future Planning of Korea (MSIP) (grant no.
NRF-2015R1A2A2A01002360).
References
|
1
|
Lee JW, Park C, Han MH, Hong SH, Lee TK,
Lee SH, Kim GY and Choi YH: Induction of human leukemia U937 cell
apoptosis by an ethanol extract of Dendropanax morbifera Lev.
through the caspase-dependent pathway. Oncol Rep. 30:1231–1238.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim W, Kim DW, Yoo DY, Jung HY, Kim JW,
Kim DW, Choi JH, Moon SM, Yoon YS and Hwang IK: Antioxidant effects
of Dendropanax morbifera Léveille extract in the hippocampus of
mercury-exposed rats. BMC Complement Altern Med. 15:2472015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu HY, Kim KS, Lee YC, Moon HI and Lee JH:
Oleifolioside A, a new active compound, attenuates LPS-stimulated
iNOS and COX-2 expression through the downregulation of NF-kappaB
and MAPK activities in RAW 264.7 macrophages. Evid Based Complement
Alternat Med. 2012:6375122012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu HY, Jin CY, Kim KS, Lee YC, Park SH,
Kim GY, Kim WJ, Moon HI, Choi YH and Lee JH: Oleifolioside A
mediates caspase-independent human cervical carcinoma HeLa cell
apoptosis involving nuclear relocation of mitochondrial apoptogenic
factors AIF and EndoG. J Agric Food Chem. 60:5400–5406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hyun TK, Kim MO, Lee H, Kim Y, Kim E and
Kim JS: Evaluation of anti-oxidant and anti-cancer properties of
Dendropanax morbifera Léveille. Food Chem. 141:1947–1955. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akram M, Kim KA, Kim ES, Syed AS, Kim CY,
Lee JS and Bae ON: Potent anti-inflammatory and analgesic actions
of the chloroform extract of Dendropanax morbifera mediated by the
Nrf2/HO-1 pathway. Biol Pharm Bull. 39:728–736. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shim HJ, Park S, Lee JW, Park HJ, Baek SH,
Kim EK and Yu SW: Extracts from Dendropanax morbifera leaves have
modulatory effects on neuroinflammation in Microglia. Am J Chin
Med. 44:119–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strowski MZ, Cramer T, Schafer G, Schäfer
G, Jüttner S, Walduck A, Schipani E, Kemmner W, Wessler S, Wunder
C, Weber M, et al: Helicobacter pylori stimulates host vascular
endothelial growth factor-A (vegf-A) gene expression via
MEK/ERK-dependent activation of Sp1 and Sp3. FASEB J. 18:218–220.
2004.PubMed/NCBI
|
|
9
|
Brown LM: Helicobacter pylori:
Epidemiology and routes of transmission. Epidemiol Rev. 22:283–297.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sue S, Shibata W and Maeda S: Helicobacter
pylori-Induced signaling pathways contribute to intestinal
metaplasia and gastric carcinogenesis. Biomed Res Int.
2015:7376212015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim G, Kim TH, Kang MJ, Choi JA, Pack DY,
Lee IR, Kim MG, Han SS, Kim BY, Oh SM, et al: Inhibitory effect of
withaferin A on Helicobacter pylori induced IL8 production and
NF-κB activation in gastric epithelial cells. Mol Med Rep.
13:967–972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SY, Choi EJ, Bae DH, Lee DW and Kim
SO: Effects of 1-tetradecanol and β-sitosterol isolated from
Dendropanax morbifera Lev. on skin whitening, moisturizing and
preventing hair loss. J Soc Cosmet Sc. Korea. 41:73–83. 2015.
|
|
13
|
Kao JY, Zhang M, Miller MJ, Mills JC, Wang
B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS, et al: Helicobacter
pylori immune escape is mediated by dendritic cell-induced Treg
skewing and Th17 suppression in mice. Gastroenterology.
138:1046–1054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang MJ, Song EJ, Kim BY, Kim DJ and Park
JH: Helicobacter pylori induces vascular endothelial growth factor
production in gastric epithelial cells through hypoxia-inducible
factor-1α-dependent pathway. Helicobacter. 19:476–483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung IM, Kim MY, Park SD, Park WH and
Moon HI: In vitro evaluation of the antiplasmodial activity of
Dendropanax morbifera against chloroquine-sensitive strains of
Plasmodium falciparum. Phytother Res. 23:1634–1637. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park BY, Min BS, Oh SR, Kim JH, Kim TJ,
Kim DH, Bae KH and Lee HK: Isolation and anticomplement activity of
compounds from Dendropanax morbifera. J Ethnopharmacol. 90:403–408.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CJ, Kuo FC, Wang CL, Kuo CH, Wang SS,
Chen CY, Huang YB, Cheng KH, Yokoyama KK, Chen CL, et al:
Suppression of IL-8-Src signalling axis by 17β-estradiol inhibits
human mesenchymal stem cells-mediated gastric cancer invasion. J
Cell Mol Med. 20:962–972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi J, Li YJ, Yan B and Wei PK:
Interleukin-8: A potent promoter of human lymphatic endothelial
cell growth in gastric cancer. Oncol Rep. 33:2703–2710. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crabtree JE, Farmery SM, Lindley IJ,
Figura N, Peichl P and Tompkins DS: CagA/cytotoxic strains of
Helicobacter pylori and interleukin-8 in gastric epithelial cell
lines. J Clin Pathol. 47:945–950. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma SA, Tummuru MK, Miller GG and
Blaser MJ: Interleukin-8 response of gastric epithelial cell lines
to Helicobacter pylori stimulation in vitro. Infect Immun.
63:1681–1687. 1995.PubMed/NCBI
|
|
24
|
Jin CY, Yu HY, Park C, Han MH, Hong SH,
Kim KS, Lee YC, Chang YC, Cheong J, Moon SK, et al: Oleifolioside
B-mediated autophagy promotes apoptosis in A549 human non-small
cell lung cancer cells. Int J Oncol. 43:1943–1950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Togashi N, Shiraishi A, Nishizaka M,
Matsuoka K, Endo K, Hamashima H and Inoue Y: Antibacterial activity
of long-chain fatty alcohols against Staphylococcus aureus.
Molecules. 12:139–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ki YW, Park JH, Lee JE, Shin IC and Koh
HC: JNK and p38 MAPK regulate oxidative stress and the inflammatory
response in chlorpyrifos-induced apoptosis. Toxicol Lett.
218:235–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee KE, Khoi PN, Xia Y, Park JS, Joo YE,
Kim KK, Choi SY and Jung YD: Helicobacter pylori and interleukin-8
in gastric cancer. World J Gastroenterol. 19:8192–8202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JH, Kim TY, Jong HS, Kim TY, Chun YS,
Park JW, Lee CT, Jung HC, Kim NK and Bang YJ: Gastric epithelial
reactive oxygen species prevent normoxic degradation of
hypoxia-inducible factor-1alpha in gastric cancer cells. Clin
Cancer Res. 9:433–440. 2003.PubMed/NCBI
|
|
31
|
Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS,
Lin JT, Inoue H and Chen GH: Helicobacter pylori promote gastric
cancer cells invasion through a NF-kappaB and COX-2-mediated
pathway. World J Gastroenterol. 11:3197–3203. 2005. View Article : Google Scholar : PubMed/NCBI
|