Introduction
The effect of aging on atherosclerosis (AS) can be
explained by the observation that the prevalence of AS increases
progressively with age. Functional and morphological changes in
healthy aged vascular tissue lead to decreases in arterial
elasticity that affect reservoir function and pulse pressure in
older adults and are considered a predictor of several
cardiovascular outcomes, such as stroke and myocardial infarction
(1). Age-related oxidative damage
and vascular inflammation are the main causes of arterial
dysfunction. Nitric oxide (NO) is the most important regulator of
vascular endothelial function, and its bioavailability enables it
to play a prominent role during the initial stages of AS, which are
characterized by the combination of endothelial damage and lipid
infiltration (2). Reactive oxygen
species (ROS) induced activation of the transcription factor
nuclear factor-κB (NF-κB) promotes AS via the release of several
cytokines and adhesion molecules, which collectively contribute to
AS progression (3,4). Recently, increasing amounts of
evidence have linked vascular endothelial cell senescence to AS
development (5,6), but the biological mechanisms
underlying this relationship need to be studied further. Migliaccio
et al found that genetic p66Shc deletions
decrease ROS levels and prolong mouse lifespans by 30% and that p53
and p21 mediated stress responses are impaired in
p66shc-/− cells (7).
p66Shc is also involved in the pathology of AS and is a
key determinant of aging. p66Shc silencing attenuates
oxidized low-density lipoprotein (ox-LDL)-induced ROS production in
endothelial cells (8) and reduces
plaque formation in ApoE−/− mice subjected to a
high-cholesterol diet (9). Here,
we showed that ox-LDL-induced varieties in senescence-related
protein occurred in conjunction with senescence-related increases
in lipid deposition in EA.hy926 endothelial cell, but the
biological mechanisms underlying the promotion of cell senescence
have not been completely elucidated. Some studies have demonstrated
that p16 levels gradually increase in senescent human diploid
fibroblasts, reaching levels that are approximately 40-fold higher
than those of early passaged cells (10), and that increased cyclin-dependent
kinase inhibitor 2A, isoform INK4a (p16INK4a) expression leads to
retinal ganglion cell senescence in adult human retinas (11). It is known that p16 is the major
cyclin-dependent kinase (CDK) inhibitor and that p16 upregulation
is a key event in growth arrest-related cell senescence. It is now
considered that p21 was involved in regulation of cell
proliferation, differentiation, migration, senescence, and
apoptosis (12). And recent report
confirmed that p21-mediated cellular senescence was p53-independent
(13). In ox-LDL-induced senescent
EA.hy926 cells, the levels of p53, p21 and p16 mRNA and cellular
protein expression were increased dramatically, the phosphorylated
retinoblastoma (Rb) level was decreased, and the cell cycle was
arrested in G0/G1 phase. We confirmed that decreases in
phosphorylated Rb protein levels may underlie the development of
EA.hy926 cell senescence.
Salidroside, one of the active ingredients of
Rhodiolacrenulata, can be used as an effective anti-diabetes
agent (14), can protect against
Aβ-induced neurotoxicity in transgenic Drosophila
Alzheimer's disease (AD) models (15), and can also alleviate oxidative
stress (16) and inflammation
(17) in different cell and animal
models; however, there are little data suggesting that salidroside
protects against age-related AS. Xing et al showed that
salidroside plays a critical role in promoting NO production and
activating AMPK signaling pathway to improve endothelial function
in AS (18) and Some study
indicated that salidroside could attenuate
H2O2 induced cell death by inhibiting
caspase-3, 9 and cleavage of poly (ADP-ribose) polymerase (PARP)
activation (19). Our previous
study found that aging cells exist in atherosclerotic plaque of
high-fat diet ApoE−/− mice, and salidroside could
decrease the aging cells. In the present study, we investigated the
protective effects of salidroside against ox-LDL-induced EA.hy926
cell senescence and explored the mechanisms underlying ox-LDL
induced endothelial cell senescence. We showed that salidroside
significantly protected cultured EA.hy926 cells against
ox-LDL-induced cytotoxicity. Furthermore, we provided evidence that
these effects were induced by changes in the expression of
senescence-related molecular targets (p66, p53 and p21) in the
phosphorylated Rb protein signaling pathway.
Materials and methods
EA.hy926 cells culture and
proliferation assay
Human umbilical vein EA.hy926 cells (Cell bank,
Shanghai Institute for Biological Science, Shanghai, China) were
cultured in Dulbecco's modified Eagle medium (DMEM; Invitrogen, CA,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; Invitrogen, CA, USA) at 37°C in a humidified atmosphere of
95% air and 5% CO2. Cells were passaged according to the
number of cells required for different experiments. The second day
after passage, the cells were incubated with 10, 100, 250 and 500
µg/ml ox-LDL (Yuanye Biotechnology, Shanghai, China) and/or with
500 µM salidroside (purity, >98%; Yuanye Biotechnology; 50 µM
pravastatin was used as a positive control) for 48 h to establish
lipid oxidation and AS therapy models. Then, the cells were
incubated with 20 µl of CCK-8 (Beyotime, Shanghai, China) for 2 h,
according to the manufacturer's instructions. Cell proliferation
was analyzed using a microplatereader, and the absorbance was
measured at 450 nm (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany).
Oil Red O and senescence-associated
β-galactosidase (SA-β-gal) staining
Oil Red O staining was carried out to assess
cellular lipid accumulation. Briefly, cells were fixed with 4%
paraformaldehyde solution for 30 min and then stained with 0.05%
Oil Red O solution in 60% isopropanol for 30 min at room
temperature. The cells were subsequently decolorized with 75%
alcohol/60% isopropanol for 2 min before being photographed and
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics Inc.,
Rockville, MD, USA).
Senescent EA.hy926 cells express a β-gal, which was
detected using a Beta Galactosidase (β-gal) Activity Assay kit
(BioVison, Milpitas, CA, USA), according to the manufacturer's
instructions, based on the formation of a local blue precipitate
upon enzyme-mediated cleavage. Briefly, the cells were fixed in 4%
formaldehyde for 10 min at room temperature and washed in phosphate
buffered saline (PBS). Freshly prepared aged x-gal solution was
used to stain senescent cells for approximately 18–24 h at 37°C.
Then, the cells were observed via lightmicroscopy, and the
percentages of β-gal-positive cells were calculated.
RNA isolation and real-time PCR
assay
Total RNA from EA.hy926 cells was prepared using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer's instructions. Reverse transcription was performed
using a PrimeScript™ II 1st Strand cDNA Synthesis kit (Takara,
Shiga Prefecture, Japan), according to the manufacturer's
instructions, and cDNA was used as a template for PCR amplification
of the target mRNA fragments. A SYBR® Premix Ex Taq™
(TliRNaseH Plus; Takara, Shiga Prefecture, Japan) system was used
for all real-time PCR reactions. The comparative threshold cycle
(ΔCq) method was used to quantify target mRNA
levels at different time points. Target mRNA levels were normalized
to an endogenous reference 18S rRNA and a control. The following
primer pairs were used: p66 sense, 5′-GGTTCATGTTAACCAGGCCA-3′ and
anti-sense, 5′-TGAGACTGCCAGACACCAAG-3′; p53 sense,
5′-GCTTTCCACGACGGTGAC-3′ and anti-sense,
5′-GCTCGACGCTAGGATCTGAC-3′); p21 sense, 5′-AGTCAGTTCCTTGTGGAGCC-3′
and anti-sense, 5′-CATGGGTTCTGACGGACAT-3′; p16 sense,
5′-CCCAACGCACCGAATAGTTA-3′ and anti-sense,
5′-GGTCGGGTGAGAGTGGC-3′); and 18S rRNA sense,
5′-TAGTAGCGACGGGCGGTGTG-3′ and anti-sense,
5′-CAGCCACCCGAGTTGAGCA-3′).
Western blot analysis
Western blot analysis was performed as described
previously (20). Primary
polyclonal anti-p66, anti-p53, anti-p27, anti-p21, anti-p16,
anti-CDK4, anti-cyclin D1, anti-p-Akt, anti-Akt, anti-p-Erk,
anti-Erk, anti-p-Rb, anti-Rb and anti-β-actin antibodies were all
purchased from Abcam (Abcam, Massachusetts, USA). After the cells
were incubated with a secondary horseradish peroxidase
(HRP)-conjugated goat anti-rabbit or goat anti-mouse immunoglobulin
(Ig)G antibody (1:10,000; SABC) for 1 h at 37°C, signals were
detected using an enhanced chemiluminescence (ECL) kit (Pierce,
Rockford, IL, USA) and quantified via densitometric analysis using
Image-Pro Plus 6.0 software (Media Cybernetics).
Cell cycle analysis
Changes in EA.hy926 cell cycle progression induced
by ox-LDL treatment were investigated by flow cytometry. Briefly,
cells (1×105) were harvested and fixed with cold 75%
ethanol for 24 h at −20°C. Then, the cells were rinsed with PBS and
incubated with 150 µl of RNase A (100 µg/µl; Sigma-Aldrich, St.
Louis, MO, USA) and 0.05% Triton X-100 for 30 min at 37°C before
being resuspended in 2 ml of PBS and 100 µl of propidium iodide
(PI; 2.5 mg/ml) and incubated for 30 min at 4°C. Cell cycle
distribution (2×104 cells) was determined using a
CytoFLEX flow cytometer (Beckman Coulter, USA), and analysis was
performed using MultiCycle software (Beckman Coulter, Brea, CA,
USA).
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS Inc., Chicago, USA). The results are expressed as
the mean ± SD, as indicated in the figure. legends. One-way ANOVA
and Tukey HSD post hoc t-tests were used to determine the level of
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Salidroside attenuates lipid
accumulation and senescence in endothelial cells
In order to prepare AS cell model, ox-LDL at
different concentration was incubated with EA.hy926 cells, finally,
100 µg/ml ox-LDL was used to induce lipid damage in EA.hy926 cells
(Fig. 1A). At the same time,
EA.hy926 cells were treated with salidroside for 2 days. To
ascertain the effects of salidroside on lipid deposition and cell
senescence, Oil Red O staining and SA-β-gal staining were
performed, respectively. As shown in Fig. 1B, the relative percentage of Oil
Red O-positive cells was significantly lower in the salidroside
group than in the ox-LDL group, indicating that intracellular lipid
accumulation was successfully induced in EA.hy926 cells by ox-LDL
and that salidroside treatment prevents ox-LDL-induced lipid
accumulation in the cytoplasm. This finding suggests that
salidroside administration attenuates lipid accumulation in
endothelial cells. Histological analysis of SA-β-gal-stained cells
revealed significant reductions in the numbers of positively
stained cells in the salidroside-treated group compared with the
ox-LDL model group (Fig. 2A).
Therefore, we further examined the effect of salidroside on p66,
p53, p21 and p16 mRNA expression, results indicated that the levels
of p66 and p21 mRNA expression were significantly decreased in
ox-LDL plus salidroside-treated cells compared with ox-LDL-treated
cells (Fig. 2B). Therefore, we
hypothesized that ox-LDL-induced damage may lead to cell aging in
cultured EA.hy926 cells, and salidroside improved cell senescence
in ox-LDL induced cell AS model.
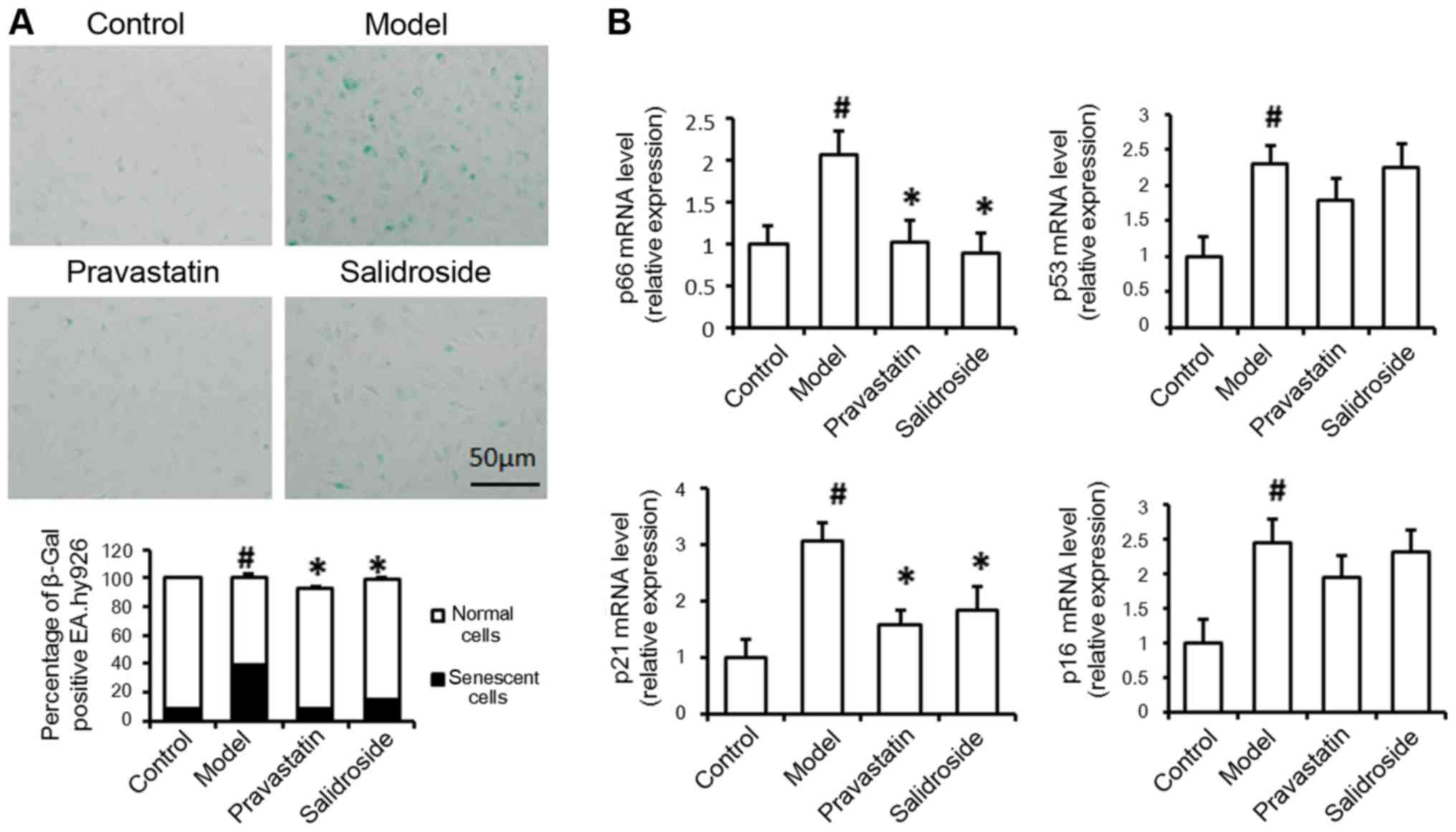 | Figure 2.Salidroside treatment represses cell
aging and senescence-related molecule mRNA expression. (A) EA.hy926
cells treated with 100 µg/ml ox-LDL (model) were subjected to
SA-β-gal analysis. (B) Quantitative polymerase chain reaction was
used to determine the relative levels of p66, p53, p21 and p16 mRNA
expression in normal cells without any treatment (control), cells
treated with 100 µg/ml ox-LDL alone (model), cells were treated
with ox-LDL and 500 µM salidroside (salidroside group) and
pravastatin (positive control). Data represent the mean ± standard
deviation from 3 independent experiments. #P<0.05 vs.
control and *P<0.05 vs. model. Scale bar, 50 µm. ox-LDL,
oxidized low-density lipoprotein; SA-β-gal, senescence-associated
β-galactosidase; p53, cellular tumor antigen 53; p21,
cyclin-dependent kinase inhibitor 1; p16, cyclin-dependent kinase
inhibitor 2A. |
Salidroside decreases p66, p53 and p21
expression in ox-LDL-treated endothelial cells
We examined the effect of salidroside on
senescence-related protein expression. As shown in Fig. 3, the levels of p66, p53 and p21
protein expression were significantly decreased in ox-LDL and
salidroside-treated cells compared with ox-LDL-treated cells, the
levels of p27, p16, CDK4, Cyclin-D1, p-Akt and p-Erk have no
differences between two groups. Western blot analysis indicated
that the increases in p66, p53 and p21 expression induced by ox-LDL
were all significantly reversed by salidroside. The above results
demonstrate that ox-LDL treatment induces EA.hy926 cell senescence
and that salidroside prevents cell senescence by decreasing the
expression of aging-related proteins. In order to further detect
the regulation mechanisms of senescence-related protein in ox-LDL
induced EA.hy926 damage, and according to previous study that the
senescence-related proteins p21 participate in cell cycle
regulation, therefore, we examined the effects of salidroside on
cell cycle protein Rb phosphorylation to determine whether Rb
protein is the target of salidroside. As shown in Fig. 3, phosphorylated Rb protein levels
were significantly increased by salidroside treatment compared with
ox-LDL treatment.
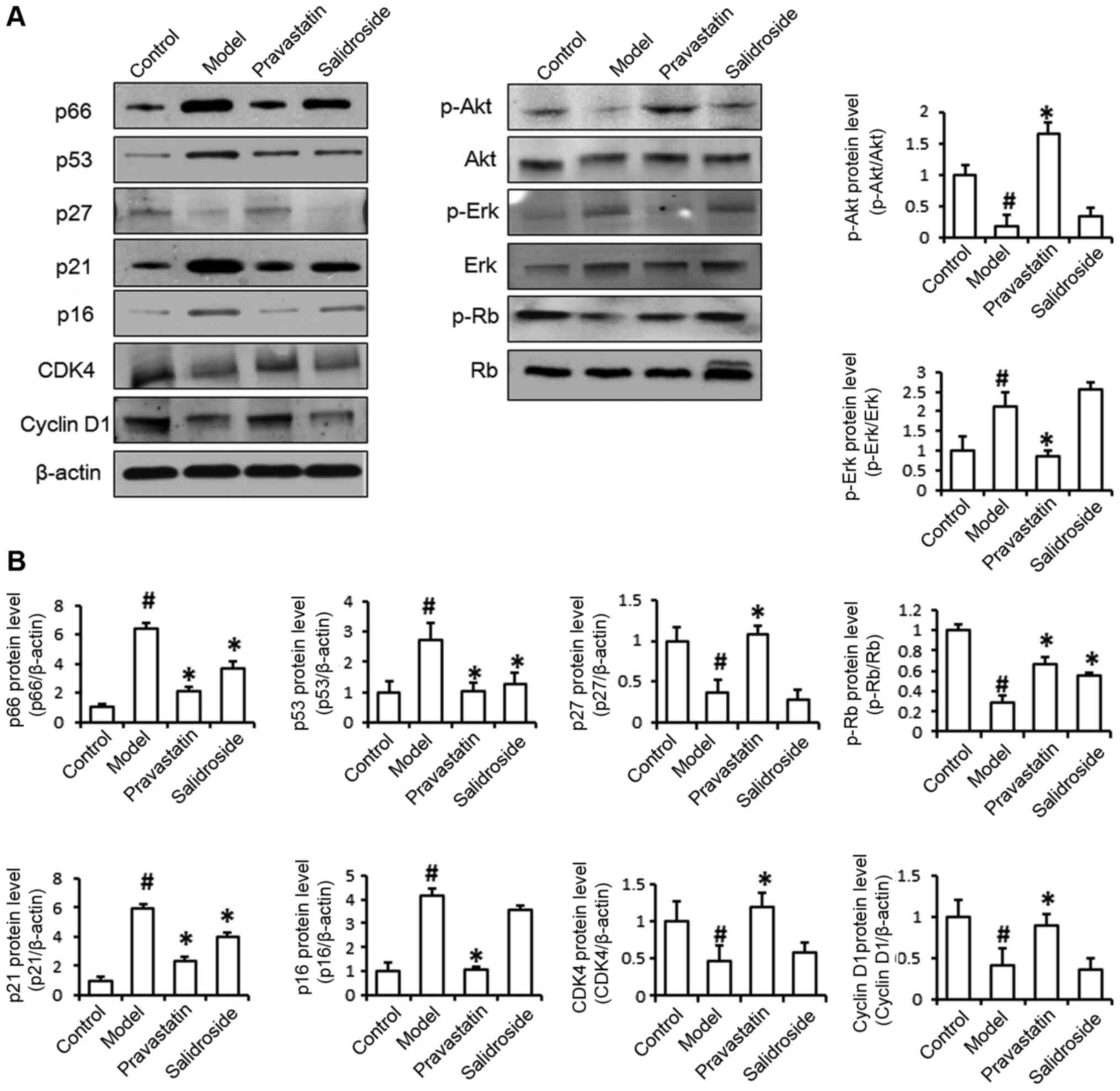 | Figure 3.Salidroside treatment represses
senescence-related protein expression. (A) The levels of p66, p53,
p27, p21, p16, CDK-4, Cyclin D1, p-Akt, Akt, p-Erk, Erk, p-Rb and
Rb protein expression were detected by western blotting. It is
showed that protein levels of p66, p53 and p21 were decreased in
ox-LDL (model) and salidroside-treated cells compared with
ox-LDL-treated cells, protein levels of p27, p16, CDK4, Cyclin-D1,
p-Akt and p-Erk have no differences between two groups. (B) The
histogram of the analysis of the protein bands. Pravastatin was
used as a positive control. Data represent the mean ± standard
deviation from 3 independent experiments. #P<0.05 vs.
control and *P<0.05 vs. model. Ox-LDL, oxidized low-density
lipoprotein; p53, cellular tumor antigen 53; p21, cyclin-dependent
kinase inhibitor 1; p16, cyclin-dependent kinase inhibitor 2A;
CDK-4, cyclin-dependent kinase-4; Akt, protein kinase B; p,
phosphorylated; Erk, extracellular signal-related kinase; Rb,
retinoblastoma. |
Salidroside prevents cell cycle arrest
via Rb phosphorylation in senescent endothelial cells
According to the result that salidroside could
phosphorylate Rb protein, we examined the effects of salidroside on
cell cycle progression in ox-LDL-treated EA.hy926 cells to more
fully elucidate the mechanisms by which inhibiting aging-related
protein expression prevents endothelial cell senescence. In the
setting of ox-LDL treatment, the percentage of EA.hy926 cells in
G0/G1 phase was significantly enhanced, whereas the percentage of
cells in S phase was markedly reduced. However, the cell cycle
profile of the salidroside-treated group was characterized by an
increase in the percentage of cells in S phase and a reduced
fraction of cells in G0/G1 phase (Fig.
4A and B). Our cell proliferation assay results confirmed that
salidroside also promoted EA.hy926 cell proliferation (Fig. 4C). These results suggested that
salidroside-induced senescence-related protein downregulation was
involved in EA.hy926 cell cycle progression. Overall, these results
indicated that salidroside regulated the cell cycle to slow cell
senescence. Taken together, our findings indicate that
salidroside-mediated p66, p53 and p21 regulation and Rb
phosphorylation influence ox-LDL-induced endothelial cell
senescence.
Discussion
Endothelial senescence is characterized by reduced
endothelial function accompanied by vascular functional impairment
and AS plaque formation. Approaches designed to retard the aging
process are expected to have therapeutic value with respect to AS
prevention and treatment. Salidroside is a major active ingredient
from the medicinal plant Rhodiola rosea L. and is commonly
used as an antioxidant (21),
anti-inflammatory (22) and an
anti-lipid agent (23). Although
salidroside has been shown to be useful in treating various types
of diseases, its effects on vascular endothelial cell
senescence-related AS development are still unclear. In the present
study, we demonstrated that salidroside was capable of protecting
vascular endothelial cells from lipid deposition and senescence
caused by exogenous ox-LDL-induced damage. Then, we explored the
possible molecular mechanisms underlying the anti-aging effects of
salidroside on senescence-related molecule expression and found
that salidroside promoted cell cycle progression from G0/G1 phase
to S phase via Rb phosphorylation.
Endothelial cell senescence in atherosclerotic
lesions is related to the expression of various aging-related
genes, which contribute to decreases in vasodilation and increases
in lipid infiltration. Our results demonstrate that salidroside
decreases cholesterol deposition in EA.hy926 cells and facilitates
migration capacity recovery and indicate that salidroside very
likely prevents cell senescence. Impaired endothelial function is
the hallmark of endothelial senescence, which is mediated primarily
by the aging-related proteins, which are produced by aging cells,
play important roles in endothelial senescence and are involved in
multiple processes that cause endothelial dysfunction, including
oxidative stress and energy metabolism (24,25).
In the present study, salidroside treatment
decreased the protein levels of p66, p53 and p21 protein expression
in ox-LDL-treated EA.hy926 cells in an in vitro AS model,
but the mRNA levels of p53 did not show differences between two
groups, it's mean that the posttranslational modifications might
decide the p53 protein level. In addition, changes in the numbers
of SA-β-gal-positive EA.hy926 cells coincided with changes in the
levels of the abovementioned aging-related proteins. These results
are in accordance with those of previous studies showing the
inhibitory effects of salidroside on adipogenesis (26) and cell senescence (27). The molecular mechanisms underlying
cellular senescence are generally very sophisticated. According to
previous reports, most researchers believe that the p53-p21 and
p16-pRb pathways are the main regulators of senescence (28). These senescence-related pathways
can be activated by telomere shortening, DNA damage or oxidative
stress (29). The signaling
pathways activated by these stresses facilitate increases in p53
and Rb protein expression, the combined levels of which determine
whether cells enter senescence (30). However, as various endogenous and
exogenous stimuli cause specific types of cell damage, the
senescence pathway that is activated may vary depending on the
cell-type. In our study, both the p53-p21 pathway and the p16-pRb
pathway were activated in ox-LDL-treated EA.hy926 cells. We
subsequently assessed cell cycle progression via PI staining and
confirmed that ox-LDL-treated cells arrested in G0/G1 phase. These
results indicate that Rb phosphorylation inhibits cell senescence
induced by the aging-related p53-p21 and p16-pRb pathways. The
mechanisms underlying pRb pathway-mediated cell growth are complex
(31). In senescent cells, pRb
dephosphorylation blocks mitogenic signaling and leaves the cells
in G1 phase (32); however, the
results of studies regarding Rb function are controversial.
Additional studies regarding the molecular mechanisms underlying
cell senescence are needed.
Previous studies have shown that salidroside has
anti-fatigue, anti-aging, immune regulation, free radical
scavenging and other pharmacological effects. Inour study, we found
that salidroside ameliorates ox-LDL-induced lipid deposition,
growth arrest and cell senescence in EA.hy926 cells. Its possible
mechanism is that salidroside inhibits the protein expression of
p53, p21 and p16, promotes phosphorylation of Rb protein, promotes
cell entry into S phase, prevent pre-aging of cells. Non-aging
cells can prevent excessive intracellular lipid deposition by
accelerating cholesterol transport. Therefore, we concluded that
salidroside prevented the development of AS by inhibiting cell
senescence.
Acknowledgements
We would like to thank Wengong Wang (Peking
University Health Science Center) for his helpful comments on the
manuscript. The present study was supported by grants from Natural
Science Foundation of China (program no. 81373706, 81503626) and
from the Shanghai Health Bureau Youth Fund (program no.
201540254).
References
|
1
|
Hashimoto J and Ito S: Aortic stiffness
determines diastolic blood flow reversal in the descending thoracic
aorta: Potential implication for retrograde embolic stroke in
hypertension. Hypertension. 62:542–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li H, Horke S and Förstermann U: Vascular
oxidative stress, nitric oxide and atherosclerosis.
Atherosclerosis. 237:208–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tousoulis D, Oikonomou E, Economou EK,
Crea F and Kaski JC: Inflammatory cytokines in atherosclerosis:
Current therapeutic approaches. Eur Heart J. 37:1723–1732. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah P, Bajaj S, Virk H, Bikkina M and
Shamoon F: Rapid progression of coronary atherosclerosis: A review.
Thrombosis. 2015:6349832015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camici GG, Savarese G, Akhmedov A and
Luscher TF: Molecular mechanism of endothelial and vascular aging:
Implications for cardiovascular disease. Eur Heart J. 36:3392–3403.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Camici GG and Lüscher TF:
Cardiovascular determinants of life span. Pflugers Arch.
459:315–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Migliaccio E, Giorgio M, Mele S, Pelicci
G, Reboldi P, Pandolfi PP, Lanfrancone L and Pelicci PG: The p66shc
adaptor protein controls oxidative stress response and life span in
mammals. Nature. 402:309–313. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Cosentino F, Camici GG, Akhmedov A,
Vanhoutte PM, Tanner FC and Lüscher TF: Oxidized low-density
lipoprotein activates p66Shc via lectin-like oxidized low-density
lipoprotein receptor-1, protein kinase C-beta and c-Jun N-terminal
kinase kinase in human endothelial cells. Arterioscler Thromb Vasc
Biol. 31:2090–2097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin-Padura I, de Nigris F, Migliaccio
E, Mansueto G, Minardi S, Rienzo M, Lerman LO, Stendardo M, Giorgio
M, De Rosa G, et al: p66Shc deletion confers vascular protection in
advanced atherosclerosis in hypercholesterolemic apolipoprotein E
knockout mice. Endothelium. 15:276–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou R, Han L, Li G and Tong T: Senescence
delay and repression of p16INK4a by Lsh via recruitment of histone
deacetylases in human diploid fibroblasts. Nucleic Acids Res.
37:5183–5196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skowronska-Krawczyk D, Zhao L, Zhu J,
Weinreb RN, Cao G, Luo J, Flagg K, Patel S, Wen C, Krupa M, et al:
P16INK4a upregulation mediated by SIX6 defines retinal ganglion
cell pathogenesis in glaucoma. Mol Cell. 59:931–940. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Romanov VS, Pospelov VA and Pospelova TV:
Cyclin-dependent kinase inhibitor p21 (Waf1): Contemporary view on
its role in senescence and oncogenesis. Biochemistry (Mosc).
77:575–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shih CT, Chang YF, Chen YT, Ma CP, Chen
HW, Yang CC, Lu JC, Tsai YS, Chen HC and Tan BC: The
PPARgamma-SETD8 axis constitutes an epigenetic, p53-independent
checkpoint on p21-mediated cellular senescence. Aging Cell.
16:797–813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang M, Luo L, Yao L, Wang C, Jiang K, Liu
X, Xu M, Shen N, Guo S, Sun C and Yang Y: Salidroside improves
glucose homeostasis in obese mice by repressing inflammation in
white adipose tissues and improving leptin sensitivity in
hypothalamus. Sci Rep. 6:253992016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Wang Y, Li H, Xiong R, Zhao Z,
Chu X, Li Q, Sun S and Chen S: Neuroprotective effects of
salidroside through PI3K/Akt pathway activation in Alzheimer's
disease models. Drug Des Devel Ther. 10:1335–1343. 2016.PubMed/NCBI
|
|
16
|
Yang ZR, Wang HF, Zuo TC, Guan LL and Dai
N: Salidroside alleviates oxidative stress in the liver with
non-alcoholic steatohepatitis in rats. BMC Pharmacol Toxicol.
17:162016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi Z, Qi S, Ling L, Lv J and Feng Z:
Salidroside attenuates inflammatory response via suppressing
JAK2-STAT3 pathway activation and preventing STAT3 transfer into
nucleus. Int Immunopharmacol. 35:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xing SS, Yang XY, Zheng T, Li WJ, Wu D,
Chi JY, Bian F, Bai XL, Wu GJ, Zhang YZ, et al: Salidroside
improves endothelial function and alleviates atherosclerosis by
activating a mitochondria-related AMPK/PI3 K/Akt/eNOS pathway.
Vascul Pharmacol. 72:141–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao X, Jin L, Shen N, Xu B, Zhang W, Zhu
H and Luo Z: Salidroside inhibits endogenous hydrogen peroxide
induced cytotoxicity of endothelial cells. Biol Pharm Bull.
36:1773–1778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dou F, Huang L, Yu P, Zhu H, Wang X, Zou
J, Lu P and Xu XM: Temporospatial expression and cellular
localization of oligodendrocyte myelin glycoprotein (OMgp) after
traumatic spinal cord injury in adult rats. J Neurotrauma.
26:2299–2311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calcabrini C, De Bellis R, Mancini U,
Cucchiarini L, Potenza L, De Sanctis R, Patrone V, Scesa C and
Dachà M: Rhodiola rosea ability to enrich cellular
antioxidant defences of cultured human keratinocytes. Arch Dermatol
Res. 302:191–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pooja, Bawa AS and Khanum F:
Anti-inflammatory activity of Rhodiola rosea- ‘a
second-generation adaptogen’. Phytother Res. 23:1099–1102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Rong X, Li W, Yang Y, Yamahara J
and Li Y: Rhodiola crenulata root ameliorates derangements
of glucose and lipid metabolism in a rat model of the metabolic
syndrome and type 2 diabetes. J Ethnopharmacol. 142:782–788. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bianchi G, Di Giulio C, Rapino C, Rapino
M, Antonucci A and Cataldi A: p53 and p66 proteins compete for
hypoxia-inducible factor 1 alpha stabilization in young and old rat
hearts exposed to intermittent hypoxia. Gerontology. 52:17–23.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hooten Noren N, Martin-Montalvo A, Dluzen
DF, Zhang Y, Bernier M, Zonderman AB, Becker KG, Gorospe M, de Cabo
R and Evans MK: Metformin-mediated increase in DICER1 regulates
microRNA expression and cellular senescence. Aging Cell.
15:572–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pomari E, Stefanon B and Colitti M:
Effects of two different Rhodiola rosea extracts on primary
human visceral adipocytes. Molecules. 20:8409–8428. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG,
Li RG, Song DQ, Li YY, Li DD and Wang Z: Salidroside protects human
fibroblast cells from premature senescence induced by H(2)O(2)
partly through modulating oxidative status. Mech Ageing Dev.
131:723–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beauséjour CM, Krtolica A, Galimi F,
Narita M, Lowe SW, Yaswen P and Campisi J: Reversal of human
cellular senescence: Roles of the p53 and p16 pathways. EMBO J.
22:4212–4222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin N, Raguz S, Dharmalingam G and Gil
J: Co-regulation of senescence-associated genes by oncogenic
homeobox proteins and polycomb repressive complexes. Cell Cycle.
12:2194–2199. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ben-Porath I and Weinberg RA: The signals
and pathways activating cellular senescence. Int J Biochem Cell
Biol. 37:961–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malumbres M and Barbacid M: RAS oncogenes:
The first 30 years. Nat Rev Cancer. 3:459–465. 2003. View Article : Google Scholar : PubMed/NCBI
|


















