Introduction
C-C chemokine receptor type 5 (CCR5) is a
co-receptor for human immunodeficiency virus (HIV)-1 present on the
surface of target cells, including cluster of differentiation
(CD)4+ T lymphocytes (1). A small fraction of Europeans (1%) are
homozygous for a 32-bp deletion within the coding region of both
CCR5 alleles (CCR5Δ32/Δ32 genotype), which produces a
polymorphic form of CCR5 that is not present on the cell surface,
and thus confers strong protection against HIV-1 infection
(2). CCR5 antagonists can block
HIV-1 entry into target cells; at present, one small molecule CCR5
antagonist has been approved for clinical use (3).
In 2007, an HIV-1-infected patient with acute
myeloid leukemia received transplantation of bone marrow stem cells
from a donor with the CCR5Δ32/Δ32 genotype, and the viral
load in this patient has since been undetectable (4,5).
Therefore, replacement of host CD4+ T lymphocytes with
engineered CCR5Δ32/Δ32 genotype cells is believed to
represent a method by which HIV-1 infection may be cured. Various
gene-targeting techniques could be used to produce genetically
engineered cells, including zinc finger nucleases (ZFNs) (6,7),
transcription activator-like effector nucleases (TALENs) (8–10)
and the RNA-guided CRISPR/Cas9 nuclease system (11,12),
which can be used to induce random mutations (deletion and/or
insertions) or insert a specific gene at specific loci.
Various techniques have been used to disrupt
CCR5 in hematopoietic stem and progenitor cells (HSPCs),
CD4+ T lymphocytes and induced pluripotent stem cells
(iPSCs) (7,13–17).
Disruption of CCR5 by ZFNs can efficiently inhibit HIV-1
infection of CD4+ T cells (7). In addition, ZFN modification of
CCR5 in primary human CD4+ T cells protects cells
from infection with CCR5- and CXCR4-trophic HIV-1 strains (6). TALENs recognize only one nucleotide,
instead of the three required for ZFNs (9), and can target sites in the
CCR5 loci with less cytotoxicity than ZFNs (8). This technique has been reported to
protect CCR5-expressing T cells from R5-tropic HIV (10). In addition, Wang et al
(11) recently silenced
CCR5 via Cas9 and CCR5-specific single-guide RNA in
CEM cells, whereas Hou et al (12) extended this to CXCR4 in
primary CD4+ T cells.
Although bi-allelic disruption of the CCR5
gene can prevent infection of target cells, including
CD4+ T lymphocytes, concerns have been raised suggesting
that cells with non-functional CCR5 may lose some important
immune functions (18); however,
individuals with the CCR5Δ32/Δ32 genotype do not experience
any discernable deleterious clinical effects (19,20).
Recently, Ye et al (21)
homozygously reproduced the naturally existing CCR5Δ32
mutation in iPSCs by combining the TALENs or CRISPR/Cas9 technique
with the PiggyBac technique, as a ‘TTAA’ tetranucleotide sequence
happens to be located close to the to-be-deleted 32 bp region. The
established CCR5Δ32/Δ32 iPSC clones maintained pluripotency
and resistance to HIV-1 infection, further indicating that the
CCR5Δ32/Δ32 genotype is safe for cells.
Site-specific, size-controlled and homozygous DNA
deletion remains a major challenge in mammalian genome engineering.
The present study established an efficient method to homozygously
reproduce the natural CCRΔ32 mutation in CD4+ U87
cells using a TALENs-mediated homologous recombination technique.
Engineered CD4+ U87 cells with the CCR5Δ32/Δ32
genotype exhibited significant resistance to HIV-1 infection.
Materials and methods
Cell culture
CD4+ U87 cells were acquired from
American Type Culture Collection (Manassas, VA, USA).
CD4+ U87 cells were originally derived from glioma cells
expressing CCR5 and CXCR4, and were stably
transfected with a CD4 receptor gene to mimic CD4+ T
lymphocytes, and a puromycin gene resistance for selection.
CD4+ U87 cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 1% penicillin and streptomycin, 1%,
amphotericin B, 1% sodium pyruvate, 1% L-glutamine and 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
CO2 incubator at 37°C.
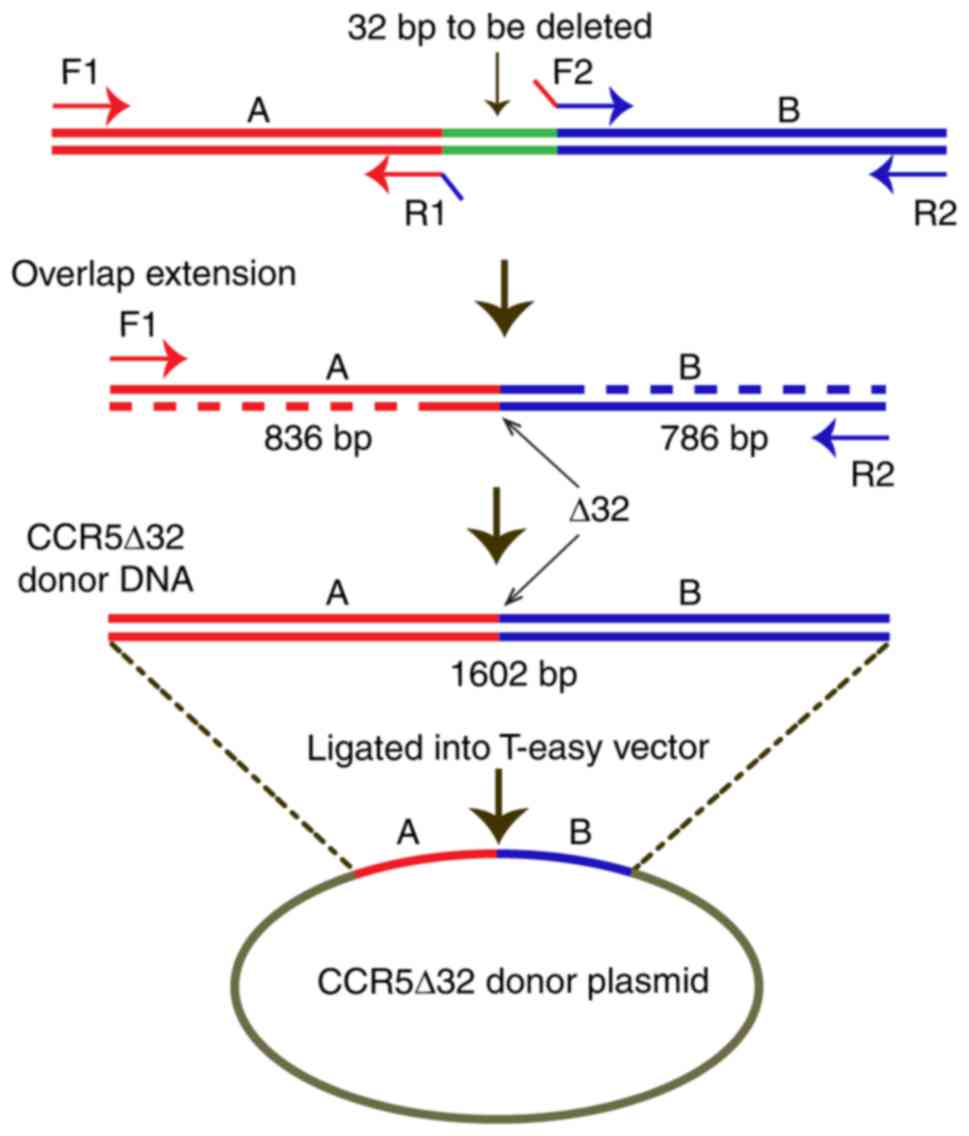
Construction of CCR5Δ32 donor DNA
plasmids
CCR5Δ32 DNA plasmids were constructed by
overlap extension PCR. To mimic the naturally occurring
CCR5Δ32, mutation, the 32 bp DNA fragment (3,299–3,330 bp)
was deleted from the wild-type CCR5 (Gene ID:1234,
https://www.ncbi.nlm.nih.gov/gene/1234). Two sets of
primers, F1 (5′-CACAAGATTTTATTTGGTGAGA-3′) and R1
(5′-CTATCTTTAATGTATGGAAAATGAGAGCTG-3′), and F2
(5′-TTTCCATACATTAAAGATAGTCATCTTGGG-3′) and R2
(5′-ATACATAAGGAACTTTCGGAGT-3′), were designed for both sides of the
32 bp DNA fragment, as indicated in Fig. 1. The two homologous arms, 836 and
786 bp in lengths, were separately amplified by PCR with the
primers F1/R1 and F2/R2, respectively, and were then used as DNA
templates for the next round of PCR with the primers F1 and R2. The
products (1,602 bp in length) were confirmed to contain the correct
CCR5Δ32 sequence by gene sequencing (data not shown), and
were finally ligated into EcoRI/BamHI digested T-easy
vectors (Promega Corporation, Madison, WI, USA).
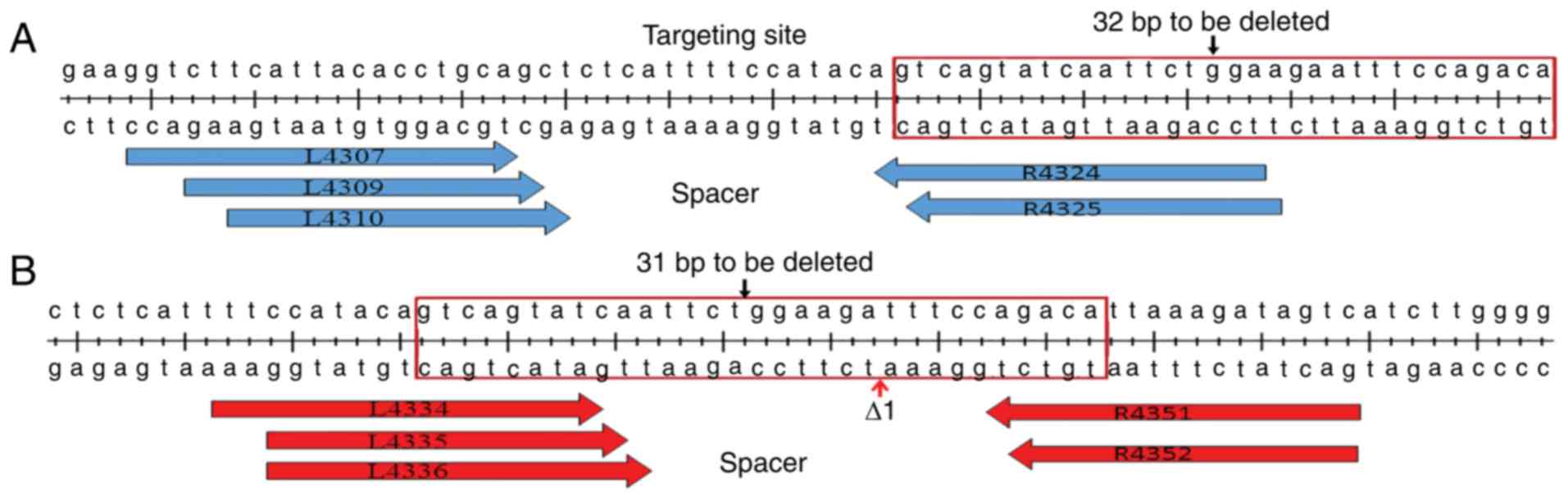
Designation and selection of
TALENs
CCR5-TALENs were designed with the right arm mostly
overlapping the to-be-deleted 32 bp region and the left arm 14–18
bp upstream (Fig. 2A). It is
critical to ensure arms are mostly within the specific regions to
prevent the donor DNA and the mutated CCR5Δ32 from further
targeting by CCR5-TALENs. In addition, the CCR5Δ1-TALENs were
designed with both arms overlapping the to-be-deleted 31 bp region
(Fig. 2B). The TALENs plasmids
were constructed by one-step ligation using the Fast TALE™ TALEN
Assembly kit (Sidansai Biotechnology Co., Ltd., Shanghai, China).
Altogether, six pairs of TALENs plasmids were designed and
constructed to target both CCR5 and CCR5Δ1. After a
preliminary transfection, two pairs of TALENs plasmids, L4309/R4324
for CCR5, and L4336/R4352 for CCR5Δ1, with the
highest targeting efficiencies were selected for use in the
subsequent experiments. The plasmids were designated CCR5-TALENs
and CCR5Δ1-TALENs accordingly.
Transfection
CD4+ U87 cells (~1×106) were
mixed with 8 µg paired CCR5-TALENs plasmids (each 4 µg) and 2 µg
CCR5Δ32 donor DNA in a cuvette (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with 100 µl Opti-minimal essential medium
(Gibco; Thermo Fisher Scientific, Inc.). Electroporation was
conducted at 150 V using a transfection system (NEPA21; Nepa Gene
Co., Ltd., Chiba, Japan). Transfected CD4+ U87 cells
were transferred into one well of a 6-well plate and cultured at
37°C for 3 days, after which the cells underwent a second and third
round of transfection using the same conditions. After three rounds
of transfection, DNA was extracted using the Blood Genomic DNA
Extraction Mini kit (Tiangen Biotech Co., Ltd., Beijing, China) for
T7 endonuclease 1 (T7E1) enzyme analysis, and ≥50 transfected cells
were cultured individually. A single clone with the CCR5Δ32
mutation was screened by monoculture and sequencing. In order to
generate homozygous CCR5Δ32 mutations, transfection of the
CD4+ U87 cells with CCR5Δ32/Δ1 was performed
under the same conditions using the CCR5Δ1-TALENs plasmids for a
further two rounds in place of the CCR5-TALENs plasmids, and no
donor DNA was added.
T7E1 enzyme analyses
The genomic region, encompassing the TALENs
targeting site, was amplified by polymerase chain reaction (PCR),
and PCR products were denatured and annealed. Some heteroduplex DNA
was formed as a result of random mutations or homologous
recombination. The annealed DNA was then digested with 5 units of
T7E1 enzyme (Beijing Viewsolid Biotech Co., Ltd., Beijing, China)
at 37°C for 15 min. The heteroduplex DNA fragments were cut at the
mismatch point and 1% agarose gel electrophoresis was performed to
separate the DNA fragments. The TALENs targeting rate was
calculated according to the DNA band intensities measured by
grayscale technique (Photoshop CS6; Adobe Systems, Inc., San Jose,
CA, USA).
Nested PCR
In order to avoid contamination of undegenerated
CCR5Δ32 donor DNA, two pairs of primers were designed to
amplify the targeting sites. The first round of PCR was performed
with primers F3 (5′-TTCATCATCCTCCTGACAATCG-3′) and R3a
(5′-CTCAAGAATCAGCAATTCTC-3′), and product length was 1,048 bp.
Since R3a was located 18 bp downstream from the primer R2 used to
amplify donor DNA, there was no chance for R3a to anneal to donor
DNA when contaminated. The products from the first round of PCR
were purified by gel extraction, and the second round of PCR was
performed with primers F3 and R3b (5′-TGGTCCAACCTGTTAGAGCTAC-3′) to
amplify the targeting sites; product length, 479 bp. The PCR
product was ligated into T-easy vectors and then transformed into
DH5a competent cells, and successfully transformed clones were gene
sequenced using Sanger sequencing.
Determination of the p24 antigen
Cells were challenged with BaL-HIV-1 obtained from
State Key Laboratory for Infectious Disease Prevention and Control
(Beijing, China), a CCR5-trophic virus isolate, at a multiplicity
of infection of 0.06, for 4 h at 37°C with 8 µg/ml Polybrene. The
challenged cells were rinsed three times to remove the free virus
and were cultured as above for 12 days. The culture supernatants
were collected every 48 h and replaced with fresh medium. p24
content in the culture supernatants was assessed in triplicate by
ELISA (632200; Clontech Laboratories, Inc., Mountainview, CA, USA)
according to the manufacturer's protocol (22,23).
This experiment was performed in a P-3 laboratory situated in the
Chinese Center for Disease Control and Prevention (Beijing, China)
strictly according to the guidelines.
Results
TALENs-mediated homozygous CCR5Δ32
mutation
CCR5-TALENs and CCR5Δ1-TALENs plasmids were used to
induce homozygous CCR5Δ32 mutation (Fig. 3). CD4+ U87 cells were
initially transfected with CCR5-TALENs plasmids and the
CCR5Δ32 donor DNA fragments carried in T-easy vectors. After
three rounds of transfection without any antibiotic selection, the
CCR5 gene in ≤50% of CD4+ U87 cells was targeted
by T7E1 enzyme analysis (Fig. 3A).
Two of the 29 (6.9%) single-cell cultured clones were revealed to
carry bi-allelic mutations, and one of these contained a 1 bp
deletion (Δ1) on one allele and a 32 bp deletion (Δ32) on the other
(representing 1.7% of the transfected alleles). The latter was
confirmed to carry the natural CCR5Δ32 mutation by gene
sequencing (Fig. 3C). The
bi-allelic mutated CD4+ U87 cells with the
CCR5Δ32/Δ1 genotype underwent a further two rounds of
transfection with CCR5Δ1-TALENs, without any donor DNA. It was
assumed that the mutated CCR5Δ32 alleles themselves could be
used as donor DNA for the predicted homologous recombination.
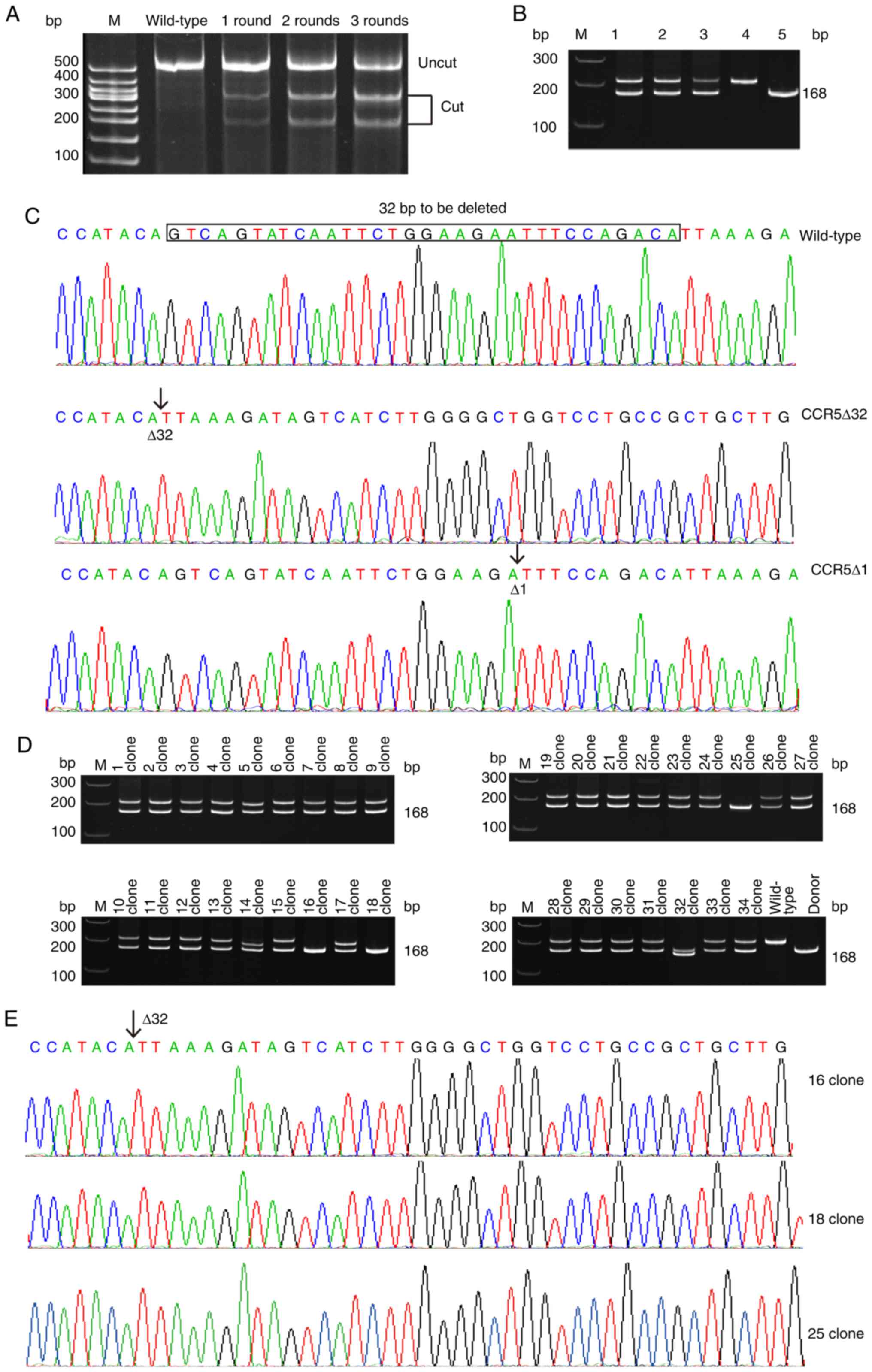 | Figure 3.TALENs-mediated homozygous
recombination. (A) T7E1 enzyme analysis of transfected
CD4+ U87 cells after each transfection with CCR5-TALENs.
The targeting efficiencies after first, second and third rounds of
transfection were 14.80, 38.20 and 50.04%, respectively. M, 100 bp
marker; wild-type, wild-type cells; lanes 1–3, transfected cells
after the first, second and third rounds of transfections. (B) PCR
of CCR5Δ32/Δ1 genotype cells. M, 100 bp marker; lane 1,
prior to transfection; lane 2, after CCR5-TALENs transfection; lane
3, after CCR5Δ1-TALENs transfection; the CCR5Δ32 band (168
bp) was 2.87-times brighter than the CCR5Δ1 band; lane 4,
wild-type cells (negative control); lane 5, CCR5Δ32 donor
DNA (positive control). (C) Sequencing of wild-type CD4+
U87 cells and those with CCR5Δ32/Δ1 mutations. (D) PCR of 34
single-cell cultured clones of CD4+ U87 cells with
CCR5Δ32/Δ1 mutations post-transfection with CCR5Δ1-TALENs. The
CCR5Δ32 band was observed in all clones; however, as a
single band, it was only seen in clones 16, 18 and 25
post-transfection with CCR5Δ1-TALENs. M, 100 bp marker; lanes 1–34,
single-cell cultured cells post-transfection with CCR5Δ1-TALENs;
wild-type, wild-type cells (negative control); donor,
CCR5Δ32 donor DNA (positive control). (E) Gene sequencing of
clones 16, 18 and 25. CCR5, C-C chemokine receptor type 5; CD4,
cluster of differentiation 4; PCR, polymerase chain reaction; T7E1,
T7 endonuclease 1; TALENs, transcription activator-like effector
nucleases. |
As expected, PCR revealed that the genomic DNA of
cells with the CCR5Δ32/Δ1 genotype contained similar levels
of CCR5Δ32 and CCR5Δ1 nucleic acids. However, after
two rounds of transfection, the CCR5Δ32 alleles were
gradually enriched and became the major alleles with increasing
homologous recombination. The intensity of the CCR5Δ32 band
was eventually close to 3-fold (2.87-fold, as determined by gray
scale measurement) that of the CCR5Δ1 band (Fig. 3B).
At this point, a total of 50 single cells were
randomly selected for single cell culture, and DNA was separately
extracted from 34 successfully cultured clones. Subsequent PCR
analysis using primers F4 (5′-CTCCCAGGAATCATCTTTACC-3′) and R4
(5′-TCATTTCGACACCGAAGCAG-3′), with a short product length of 200
bp, indicated that clones 16, 18 and 25 were homozygous for the
CCR5Δ32 mutation, whereas in clones 5, 13, 14, 17 and 32
CCR5Δ1 appeared to be randomly mutated (Fig. 3D). Subsequent gene sequencing
confirmed that clones 16, 18 and 25 (representing 8.8% of the
transfected alleles, and 37.5% of the targeted alleles) were
homozygous for the CCR5Δ32 mutation (Fig. 3E).
Off-targeting analysis of CCR5-TALENs
and CCR5Δ1-TALENs
A Basic Local Alignment Search Tool (BLAST) search
of the National Center for Biotechnology Information (NCBI)
database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed
CCR2 to have the most similar genomic DNA sequence to
CCR5; therefore, the off-target effects of the two TALENs on
this gene were investigated. An analysis investigating the homology
between the potential targeting region of CCR2 (4,312–4,711
bp) and that of CCR5 (4,023–4,422 bp), which was the
targeting region of the CCR5-TALENs and the CCR5Δ1-TALENs, revealed
a score of 73%, analyses of all other potential targeting regions
revealed scores <50%. The present study amplified the potential
targeting region of CCR2 in CD4+ U87 cells with
CCR5Δ32/Δ32 mutations by PCR, followed by T7E1 analysis and
gene sequencing (data not shown). The present study confirmed that
no off-target CCR2 mutations were generated.
HIV-1 challenge test
Wild-type CD4+ U87 cells and the
CCR5Δ32/Δ32 genotype clones were challenged with BaL-HIV-1
for 4 h to assess resistance to infection. The level of p24 in the
culture supernatants was assessed every 48 h for the following 12
days by ELISA. In wild-type cultures, p24 was detected after 48 h
and peaked after 8 days. Conversely, p24 was not detected in the
supernatants of CCR5Δ32/Δ32 genotype cells at any time point
(Fig. 4).
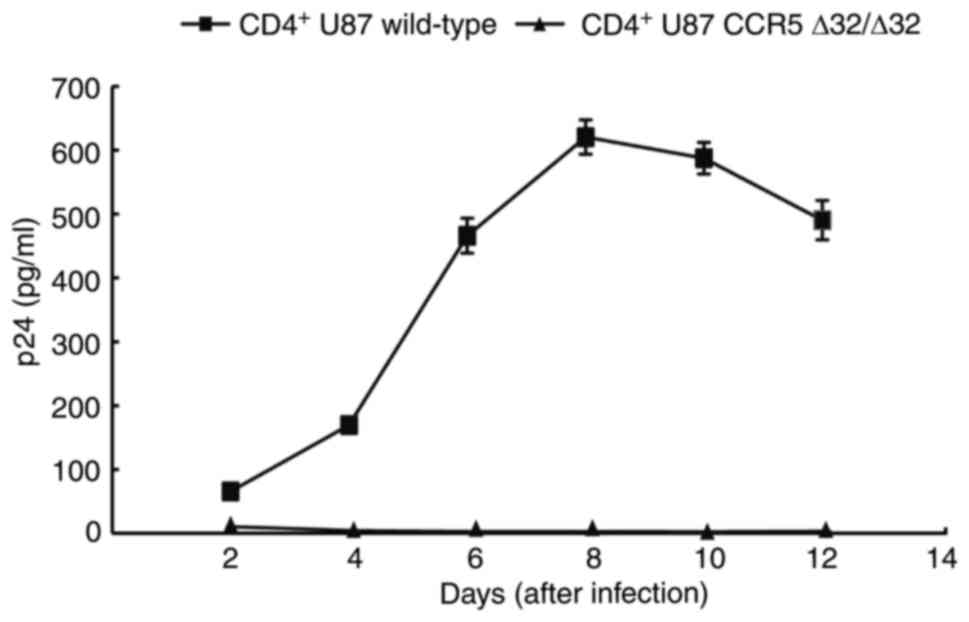 | Figure 4.HIV-1 challenge. The mean
concentration of p24 in the supernatant at 2, 4, 6, 8, 10 and 12
days after challenge was 58.47±2.35, 162.23±4.78, 458.78±27.34,
613.35±26.78, 580.35±24.73 and 483.34±30.85 in wild-type
CD+ U87 cells, whereas almost no p24 was detected in
CCR5Δ32/Δ32 genotype cultures. CCR5, C-C chemokine receptor
type 5; CD4, cluster of differentiation 4; HIV-1, human
immunodeficiency virus-1. |
Discussion
The present study successfully reproduced the
naturally existing CCR5Δ32/Δ32 genotype in CD4+
U87 cells by combining TALENs with a traditional homologous
recombination technique. This study reported a novel technique
capable of producing site-specific, size-controlled and homozygous
DNA deletion within the mammalian genome. Introduction of the
CCR5Δ32/Δ32 mutation, rather than entirely knocking out
CCR5, may ease concerns regarding the potentially
unfavorable clinical effects of CCR5 knockout (18). TALENs-mediated homologous
recombination achieved a significantly higher frequency of
recombination, without application of selection, compared with
traditional homologous recombination techniques without off-target
DNA integration into the genome (24).
In order to ensure that the donor CCR5Δ32
genes are saved from further targeting by TALENs, the binding
domain of one arm of the TALENs must be located mostly within the
to-be-deleted 32 bp region of CCR5 (Fig. 2A and B). Once CCR5 is
randomly mutated with insertions or deletions, including
CCR5Δ1, new TALENs may be needed to target the randomly
mutated CCR5, even though the insertions or deletions did
not occur within the binding domains of the original TALENs arms.
After repeated transfection with CCR5-TALENs, gene sequencing
indicated that the CCR5Δ32/Δ1 genotype remained unchanged.
These results suggested that a new pair of TALENs may be required
even though mutation did not occur within the binding domains of
CCR5-TALENs. Six pairs of CCR5Δ1-TALENs were therefore designed;
one of which was revealed to work well. These results indicated
that these TALENs are not only specific for the binding domains,
but may also be specific for the 3-dimensional structures
surrounding the binding domains. This property increases safety of
these TALENs, and suggests that off-target effects will be rare. In
the present study, the originally designed CCR5-TALENs were no
longer functional when a 1 bp deletion had occurred 3 bp downstream
of the right arm of the CCR5-TALENs (Fig. 2).
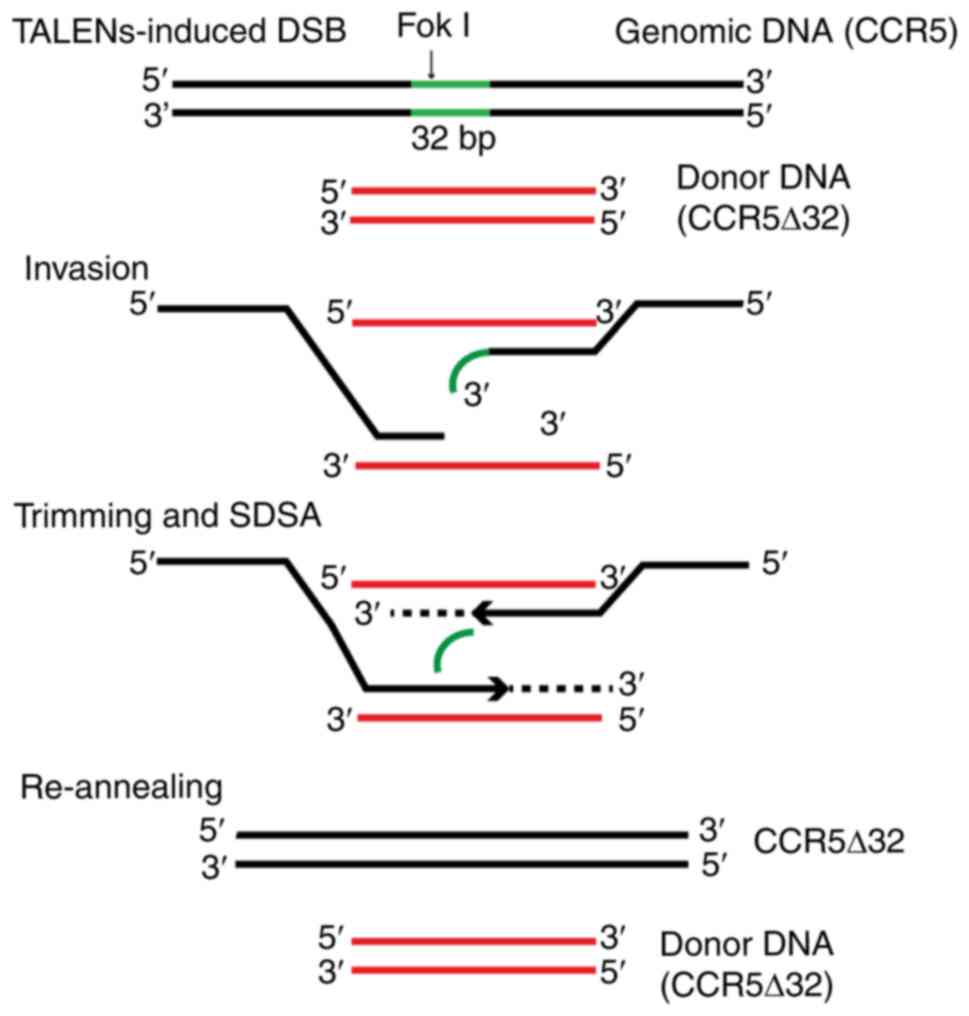
To the best of our knowledge, homozygous deletion of
a predetermined length of genomic DNA using ZFNs, TALENs or
CRISPR/Cas9 has not been previously reported. The mechanism
involved, therefore, requires discussion. Theoretically,
homology-directed repair occurs in a manner most consistent with
the synthesis-dependent strand annealing (SDSA) model of double
strand break (DSB) repair (25,26).
However, since constructed CCR5Δ32 donor DNA templates were
used, when DSB occurred, one out of two or both 5′ to 3′ broken
ends of each DSB, bearing the to-be-deleted sequence, will not find
the homologous sequence on the donor DNA templates to anneal with;
therefore, SDSA would not be able to start. However, the present
study suggested that this non-homologous end could be trimmed off
by some unidentified DNA polymerase. Therefore, the DSB repair
process with the predetermined 32 bp deletion used in this study
may involve the five steps as illustrated in Fig. 5: i) DSB, double strand DNA was
broken apart in the targeting region by FokI connected with
TALENs; ii) invasion, both 5′ to 3′ ends invaded into the opened
double strands of donor DNA and annealed with the corresponding
homologous sequences, at least one bearing a non-homologous
‘floating’ tail; iii) trimming, the non-homologous tail is excised
by an unidentified DNA polymerase; iv) SDSA, SDSA starts with donor
DNA sequences as templates; and v) re-annealing, the two newly
synthesized DNA single strands leave the donor DNA templates,
re-anneal, and may continue SDSA with each other as templates, if
necessary, until the DSB is completely repaired.
The homologous recombination frequency after the
first three rounds of transfection was 1.7% when the TALENs
targeting rate was ≤50.4%. However, after the last two rounds of
transfections it reached 8.8%, when the TALENs targeting rate was
only 23.5% (Fig. 3). However,
dividing these two homologous recombination frequencies by the
TALENs targeting rate, indicates that the homologous recombination
frequency after the first three rounds of transfection was 3.4%,
and after the last two rounds of transfection was 37.5%. To better
determine the frequencies of TALENs targeting and homologous
recombination, three types of frequency were discussed: i)
Frequency of targeting (T7E1 analysis), which reflects how many
cells with mutated CCR5 were generated; ii) frequency of homologous
recombination, which reflects the number of clones containing the
CCR5Δ32 allele (formula: F2=the number of
CCR5Δ32 alleles/2× the number of sequenced clones); iii)
frequency of homologous recombination in targeted CCR5,
which reflects the accuracy of DNA autonomous repair (formula:
F3=the number of CCR5Δ32 alleles/the number of
targeted alleles including CCR5Δ32 alleles). This homologous
recombination difference may be attributed to homologous
recombination, as longer homologous arms will have higher
homologous recombination frequencies. The total length of the
homologous arms for the first three rounds of transfection was only
1,602 bp, whereas for the last two rounds of transfections the
homologous arms were the whole chromatids. This significant
difference may explain the 10-fold difference in homologous
recombination frequency between the last two rounds and first three
rounds of transfection. The homologous recombination frequencies
could be further improved if the homologous arm length was
increased. Notably, since 3 of the 8 targeted mutations edited by
CCR5Δ1-TALENs that occurred during DSB repair processes in the last
two rounds of transfection were caused by homologous recombination,
the DSB induced by TALENs may have a tendency to be repaired,
restoring the ‘original’ sequences.
A major disadvantage of the present study is
choosing the CD4+−U87 cell line to generate homozygous
CCR5Δ32 mutations instead of directly using T lymphocytes. T
lymphocytes could be used directly once the technique is ready for
clinical application. The present study chose the
CD4+−U87 cell line to preliminarily establish the
technique simply because these cells grow fast and are easy to
manipulate. Furthermore, they mimic T lymphocytes very well in
terms of expressing CD4, CCR5 and CXCR4, which is
required for the HIV challenge test following gene editing. Since
this modified TALENs technique has been well established in editing
the U87 cell line, our further studies aim to optimize the
technique, and make it easier to alter the CCR5 gene in T
lymphocyte cells.
To the best of our knowledge, off-target occurrence
should be avoided when using genome editing for therapeutic
applications. To minimize off-target modification, the following
strategies were employed in the present study: i) Potential
off-target sites prediction, after obtaining homozygous
CCR5Δ32 mutations in three clones, potential off-target
sites were analyzed in the targeting regions of CCR5-TALENs and
CCR5Δ1-TALENs. Homology (73%) was detected between CCR5
(4,023–4,422 bp) and CCR2 (4,312–4,711 bp). The CCR2
gene was revealed to possess the highest homology to the targeting
region of CCR5, whereas other genes exhibited lower homology
(≤50%) when aligned with CCR5 (4,023–4,422 bp), as
determined using a BLAST search in NCBI. ii) Detecting off-target
modification by T7E1 analysis and gene sequencing; since the
CCR2 gene exhibited the highest homology to the targeting
region of CCR5, a primer was designed to specifically
amplify CCR2 (4,312–4,711 bp) with corresponding template
DNA extracted from wild-type cells, CCR5Δ32/Δ1 cells, and
clones 16, 18 and 25. Amplified 400 bp CCR2 fragments were analyzed
by T7E1 analysis, and the results demonstrated that none were
cleaved by T7E1, indicating that no potential off-target effects
occurred in the CCR2 homologous region (data not shown). In
addition, 400 bp PCR products were ligated into T-easy vector and
sequenced (50 successfully transformed clones for each PCR product
were sent for gene sequencing) and the sequencing results confirmed
that no potential off-target effects occurred in the CCR2
homologous region (data not shown). Since no off-target effects
were detected in the CCR2 gene, it is very unlikely that
other off-target effects will occur in genes with low homology to
CCR5. Furthermore, TALENs, instead of CRISPR/Cas9, were used
in the present study to edit the target gene due to the following
reasons: i) TALENs exhibit an efficient editing efficacy, although
it is lower compared with CRISPR/Cas9 (21); ii) notably, TALENs exhibit much
lower off-target modification than CRISPR/Cas9 (27).
In conclusion, to the best of our knowledge, the
present study reproduced the CCR5Δ32/Δ32 genotype without
selection for the first time in CD4+ U87 cells. This
mutation conferred resistance against HIV-1 infection. Our future
studies aim to adapt this technique in HSPCs or CD4+ T
lymphocytes, producing clinically useful cells for therapeutic use
in HIV-positive patients.
Acknowledgements
The present study was supported by grants from the
Ministry of Science and Technology of China (grant no.
2013ZX10001-004-002-005), the Science and Technology Department of
Hubei Province (grant no. 2012FFA037), the Hubei Provincial
Department of Education (grant nos. B2013111 and B2015484) and the
Science and Technology Department of Shiyan City Government (grant
no. 069S, 2013).
Glossary
Abbreviations
Abbreviations:
|
TALENs
|
transcription activator-like effector
nucleases
|
|
CCR5
|
C-C chemokine receptor type 5
|
|
ZFNs
|
zinc finger nucleases
|
|
HSPCs
|
hematopoietic stem and progenitor
cells
|
|
SDSA
|
synthesis-dependent strand
annealing
|
|
T7E1
|
T7 endonuclease 1
|
References
|
1
|
Deng H, Liu R, Ellmeier W, Choe S, Unutmaz
D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al:
Identification of a major co-receptor for primary isolates of
HIV-1. Nature. 381:661–666. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu R, Paxton WA, Choe S, Ceradini D,
Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR,
et al: Homozygous defect in HIV-1 coreceptor accounts for
resistance of some multiply-exposed individuals to HIV-1 infection.
Cell. 86:367–377. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fätkenheuer G, Pozniak AL, Johnson MA,
Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD,
Rockstroh JK, Dezube BJ, et al: Efficacy of short-term monotherapy
with maraviroc, a new CCR5 antagonist, in patients infected with
HIV-1. Nat Med. 11:1170–1172. 2015. View
Article : Google Scholar
|
|
4
|
Hütter G, Nowak D, Mossner M, Ganepola S,
Müßig A, Allers K, Schneider T, Hofmann J, Kücherer C, Blau O, et
al: Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell
transplantation. N Engl J Med. 360:692–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allers K, Hütter G, Hofmann J,
Loddenkemper C, Rieger K, Thiel E and Schneider T: Evidence for the
cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation.
Blood. 117:2791–2799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DiGiusto DL, Krishnan A, Li L, Li H, Li S,
Rao A, Mi S, Yam P, Stinson S, Kalos M, et al: RNA-based gene
therapy for HIV with lentiviral vector-modified CD34(+) cells in
patients undergoing transplantation for AIDS-related lymphoma. Sci
Transl Med. 2:362010. View Article : Google Scholar
|
|
7
|
Tebas P, Stein D, Tang WW, Frank I, Wang
SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, et al: Gene
editing of CCR5 in autologous CD4 T cells of persons infected with
HIV. N Engl J Med. 370:901–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mussolino C, Morbitzer R, Lütge F,
Dannemann N, Lahaye T and Cathomen T: A novel TALE nuclease
scaffold enables high genome editing activity in combination with
low toxicity. Nucleic Acids Res. 39:9283–9293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holkers M, Maggio I, Liu J, Janssen JM,
Miselli F, Mussolino C, Recchia A, Cathomen T and Gonçalves MA:
Differential integrity of TALE nuclease genes following adenoviral
and lentiviral vector gene transfer into human cells. Nucleic Acids
Res. 41:e632014. View Article : Google Scholar
|
|
10
|
Mock U, Machowicz R, Hauber I, Horn S,
Abramowski P, Berdien B, Hauber J and Fehse B: mRNA transfection of
a novel TAL effector nuclease (TALEN) facilitates efficient
knockout of HIV co-receptor CCR5. Nucleic Acids Res. 43:5560–5571.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Ye C, Liu J, Zhang D, Kimata JT
and Zhou P: CCR5 gene disruption via lentiviral vectors expressing
Cas9 and single guided RNA renders cells resistant to HIV-1
infection. PLoS One. 9:e1159872014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou P, Chen S, Wang S, Yu X, Chen Y, Jiang
M, Zhuang K, Ho W, Hou W, Huang J and Guo D: Genome editing of
CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection.
Sci Rep. 5:155772015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perez EE, Wang J, Miller JC, Jouvenot Y,
Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, et al:
Establishment of HIV-1 resistance in CD4+ T cells by genome
editing using zinc-finger nucleases. Nat Biotechnol. 26:808–816.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Krymskaya L, Wang J, Henley J, Rao
A, Cao LF, Tran CA, Torres-Coronado M, Gardner A, Gonzalez N, et
al: Genomic editing of the HIV-1 coreceptor CCR5 in adult
hematopoietic stem and progenitor cells using zinc finger
nucleases. Mol Ther. 21:1259–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holt N, Wang J, Kim K, Friedman G, Wang X,
Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC and Cannon PM:
Human hematopoietic stem/progenitor cells modified by zinc-finger
nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol.
28:839–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maier DA, Brennan AL, Jiang S,
Binder-Scholl GK, Lee G, Plesa G, Zheng Z, Cotte J, Carpenito C,
Wood T, et al: Efficient clinical scale gene modification via zinc
finger nuclease-targeted disruption of the HIV co-receptor CCR5.
Hum Gene Ther. 24:245–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mandal PK, Ferreira LM, Collins R,
Meissner TB, Boutwell CL, Friesen M, Vrbanac V, Garrison BS,
Stortchevoi A, Bryder D, et al: Efficient ablation of genes in
human hematopoietic stem and effector cells using CRISPR/Cas9. Cell
Stem Cell. 15:643–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carrington M, Kissner T, Gerrard B, Ivanov
S, O'Brien SJ and Dean M: Novel alleles of the chemokine-receptor
gene CCR5. Am J Hum Genet. 61:1261–1267. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berger EA, Murphy PM and Farber JM:
Chemokine receptors as HIV-1 coreceptors: roles in viral entry,
tropism, and disease. Annu Rev Immunol. 17:657–700. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinson JJ, Chapman NH, Rees DC, Liu YT
and Clegg JB: Global distribution of the CCR5 gene 32-basepair
deletion. Nat Genet. 16:100–103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye L, Wang J, Beyer AI, Teque F, Cradick
TJ, Qi Z, Chang JC, Bao G, Muench MO, Yu J, et al: Seamless
modification of wild-type induced pluripotent stem cells to the
natural CCR5Δ32 mutation confers resistance to HIV infection. Proc
Natl Acad of Sci USA. 111:9591–9596. 2014. View Article : Google Scholar
|
|
22
|
Goudsmit J, Lange JM, Paul DA and Dawson
GJ: Antigenemia and antibody titers to core and envelope antigens
in AIDS, AIDS-related complex and subclinical human
immunodeficiency virus infection. J Infect Dis. 155:558–560. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilen CB, Wang J, Tilton JC, Miller JC,
Kim KA, Rebar EJ, Sherrill-Mix SA, Patro SC, Secreto AJ, Jordan AP,
et al: Engineering HIV-resistant human CD4+ T cells with
CXCR4-specific zinc-finger nucleases. PLoS Pathog. 7:e10020202011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song H, Chung SK and Xu Y: Modeling
disease in human ESCs using an efficient BAC-based homologous
recombination system. Cell Stem Cell. 6:80–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nassif N, Penney J, Pal S, Engels W and
Gloor G: Efficient copying of nonhomologous sequences from ectopic
sites via P-element-induced gap repair. Mol Cell Biol.
14:1613–1625. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Symington LS: Role of RAD52 epistasis
group genes in homologous recombination and double-strand break
repair. Microbiol Mol Biol Rev. 66:630–670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu Y, Foden JA, Khayter C, Maeder ML,
Sander JD, Reyon D and Joung JK: High-frequency off-target
mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat
Biotechnol. 31:822–826. 2013. View Article : Google Scholar : PubMed/NCBI
|