Introduction
Colorectal cancer (CRC) is one of the most common
cancers worldwide, with over 1 million new cases diagnosed
annually, along with 600,000 CRC-associated deaths (1). The 5-year survival rate for patients
with early stage CRC is ~80% (2).
Current guidelines for the treatment of CRC are based on the stages
of tumor progression. Surgery still remains the primary treatment
option for CRC, in conjunction with radiation therapy or
chemotherapy.
The chemotherapeutic drug 5-fluorouracil (5-FU) is
fundamental for the treatment of CRC. Patients with advanced stages
of CRC are often have treated with a combination of various
chemotherapeutic agents, including 5-FU, capecitabine, irinotecan,
oxaliplatin, bevacizumab cetuximab, and panitumumab (3,4).
However, ~40–50% of patients with advanced stage CRC may relapse or
succumb due to the reduced efficacy of chemotherapy as a result of
drug resistance. The mechanisms of cancer cell drug resistance
involve complex systems, including increased drug efflux, reduced
drug uptake, altered metabolism of drugs, altered expression of
drug targets, reduced affinity of drug targets, activation of
detoxification system, enhanced repair of drug-induced defects and
resistance to apoptosis (5–7).
Therefore, discovery of novel and more effective therapeutic agents
which may reverse cancer cell drug resistance and increase clinical
efficacy of CRC treatment is urgently required.
Genetic abnormalities of the
phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT) pathway
are a common aspect of various cancers (8,9). In
addition, previous studies have revealed that the PI3K/AKT
signaling pathway is involved in the development of cancer cell
drug-resistance. PI3K is a lipid kinase that generates the
secondary messenger lipid phosphatidylinositol (3–5)-triphosphate (PIP3), which in turn
recruits and activates various proteins including AKT, a
serine/threonine kinase (10,11).
Phosphorylation of AKT (p-AKT) mediates the activation of various
downstream target genes involved in the regulation of cell
proliferation, survival, angiogenesis, metastasis and drug
resistance (12). The PI3K/AKT
pathway may be involved in the regulation of a number of cellular
processes, including cell death and survival, protein synthesis and
metabolism (13). The activation
of the PI3K/AKT signaling pathway and dysregulation of apoptosis
are the major factors involved in the chemo-resistance of cancer
cells by conferring acquired resistance to various chemotherapeutic
drugs (14). Therefore, AKT is a
novel target for the development of therapeutic drugs which may
improve the outcomes of cancer chemotherapy.
Traditional Chinese Medicine (TCM) has been used for
the treatment of various illnesses and diseases for thousands of
years. Previous studies reported beneficial effects and reduced
side effects of TCM in increasing the efficacy of cancer treatment,
especially in combination with standard chemotherapeutic drugs
(15–17). Therefore, the use of TCM promising
when treating cancer cell drug-resistance and may allow for
improved treatment of cancer. Hedyotis diffusa Willd (HDW)
is a major component of TCM with potent anti-cancer effects on
various cancers, such as ovarian, hepatocellular and cervical
cancer (18–21). Previous studies have also
demonstrated that HDW may inhibit CRC cell proliferation (22–26).
These studies primarily focused on the effect of EEHDW on regular
CRC cells. Recently, the present study reported that ethanol
extract of HDW (EEHDW) may reduce 5-FU resistance in CRC HCT-8/5-FU
cells by regulating the expression of permeability-glycoprotein and
ATP binding cassette subfamily G member 2 (ABCG2) (27). However, the underlying mechanism of
EEHDW overcoming drug resistance in human CRC HCT-8/5-FU cells
remains to be fully elucidated. The present study aimed to
investigate the inhibitory mechanism of EEHDW in terms of
proliferation and apoptosis of CRC cells via regulation of the
PI3K/AKT pathway.
Materials and methods
Materials and reagents
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin-streptomycin, trypsin-EDTA, TRIzol reagent, and
bicinchoninic acid (BCA) protein assay kit, RIPA cell lysis buffer
(pierce, 89,900) were obtained from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). 5-FU, DMSO (cat. no. D5879), and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
cat. no. M-2128) were obtained from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). Annexin V/propidium iodide (PI) apoptosis
assay kit and DAPI were purchased from Nanjing KeyGen Biotech Co.,
Ltd. (Nanjing, China). PrimeScript™ RT Reagent kit was purchased
from Takara Bio, Inc. (Otsu, Japan). Goldview Nucleic Acid Gel
stain (cat. no. G8142) was purchased from Beijing Solarbio Life
Science and Technology, Ltd., (Beijing, China). Primary antibodies
for β-actin (cat. no. 4967), B cell leukemia/lymphoma (Bcl-2; cat.
no. 4223), Bcl-2 associated X (Bax; cat. no. 5023), cyclin D1 (cat.
no. 2978), cyclin dependent kinase 4 (CDK4; cat. no. 2906), p21
(cat. no. 2947), PI3K (cat. no. 4257), p-AKT (Ser473; cat. no.
4060), AKT (cat. no. 2938) and phosphatase and tensin homolog
(PTEN; cat. no. 9559), and horseradish peroxidase (HRP)-conjugated
secondary antibodies (cat. no. 7074) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
EEHDW preparation
HDW was purchased from a commercial supplier (Guo Yi
Tang Chinese Herbal Medicine Store, Fujian, China) and the EEHDW
was obtained as previously described (22). Stock solutions of EEHDW were
prepared by dissolving 500 mg EEHDW powder in 1 ml DMSO to a final
concentration of 500 mg/ml and stored at −20°C. Working solutions
of EEHDW were made by diluting the stock solution in RPMI-1640
culture medium. The final concentration of DMSO in the medium was
<0.5%.
Cell culture
Human colon carcinoma HCT-8/5-FU cell line was
obtained from Nanjing KeyGen Biotech Co., Ltd. HCT-8/5-FU cells
were cultured RPMI-1640 media supplemented with 10% FBS and 100
U/ml penicillin and 100 g/ml streptomycin in a humidified 37°C
incubator supplemented with 5% CO2.
Evaluation of cell viability of
HCT-8/5-FU by MTT assay
Cell viability was determined using the MTT and
colorimetric assay. Briefly, HCT-8/5-FU cells were seeded into
96-well plates at a density of 1.0×104 cells/well. After
24 h, the cells were treated with different doses of EEHDW (0.5–2.0
mg/ml) for different periods of time (24 and 48 h). Treatment with
0.1% DMSO was included as the vehicle control. Following treatment,
100 µl MTT (0.5 mg/ml) were added to each well and cells were
incubated for an additional 4 h at 37°C. Subsequently, the MTT
formazan precipitate was dissolved in 100 µl DMSO and the
absorbance was measured at 570 nm using an ELISA plate reader
(Model EXL800, BioTek Instruments, Inc., Winooski, VT, USA).
Colony formation
HCT-8/5-FU cells from exponentially growing cultures
were seeded into 12-well culture plates at a density of
1.0×105 cells/well and were treated with different
concentrations of EEHDW for 24 h. Cells were then harvested and
seeded into 6-well plates at a final density of 1.0×103
cells/well in 2 ml fresh RPMI-1640 medium. Following incubation for
8 days, colonies were fixed in MeOH-HAc (3:1, v/v) for 10 min in
room temperature, stained with 0.1% crystal violet (Beyotime
Biotech Co., Ltd. (Shanghai, China) for 10 min at room temperature
and counted. Cell colony formation was calculated by showing
survival of the control cells as 100%.
Detection of apoptosis by flow
cytometry analysis with Annexin V/PI staining and DAPI staining
assay
HCT-8/5-FU cells at a density of 2.0×105
cells/well were seeded into 6-well plates and treated with
different concentrations of EEHDW for 24 h. Subsequently, apoptosis
of HCT-8/5-FU cells was determined by flow cytometry analysis using
a fluorescence activated cell sorting (FACS) caliber (BD
Biosciences, Franklin Lakes, NJ, USA) and Annexin V-fluorescein
isothiocyanate/PI kit, according to the manufacturer's protocol.
Annexin V-negative/PI-negative cells indicated viable cells,
whereas Annexin V-positive/PI-negative and Annexin
V-positive/PI-positive cells indicated cells undergoing early and
late stage apoptosis, respectively.
HCT-8/5-FU cell morphology and apoptosis was
monitored following staining with DAPI. HCT-8/5-FU cells were
seeded on 12-mm diameter round, glass cover slips in 24-well plates
and treated with EEHDW (0.5, 1.0, 2.0 mg/ml) for 24 h. The cover
slips were then washed with PBS, fixed with 4% paraformaldehyde for
10 min and stained with DAPI (4 µg/ml) for 10 min at room
temperature, then observed under fluorescence microscopy. Cells
with clear condensed nuclei were identified as apoptotic cells.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
HCT-8/5-FU cells were seeded into 6-well plates at a
density of 4×105 cells/well and treated with different
concentrations of EEHDW for 24 h. RNA was extracted from HCT-8/5-FU
cells using TRIzol reagent (Takara Bio, Inc.). cDNA was obtained
using reverse transcription with PrimeScript RT reagent kit,
according to the manufacturer's protocol. PCR was performed to
determine the mRNA expression levels of Bax, Bcl-2, cyclin D1, CDK4
and p21. GAPDH was used as an internal control. The primers used
for amplification of Bax, Bcl-2, cyclin D1, CDK4 and p21
transcripts are as follows: GAPDH forward (F)
5′-GTCATCCATGACAACTTTGG-3′ and reverse (R)
5′-GAGCTTGACAAAGTGGTCGT-3′; Bcl-2 F 5′-CAGCTGCACCTGACGCCCTT-3′ and
R 5′-GCCTCCGTTATCCTGGATCC-3′; Bax F 5′-TGCTTCAGGGTTTCATCCAGG-3′ and
R 5′-TGGCAAAGTAGAAAAGGGCGA-3′; CDK4 F 5′-CATGTAGACCAGGACCTAAGC-3′
and R 5′-AACTGGCGCATCAGATCCTAG-3′; cyclin Dl F
5′-TGGATGCTGGAGGTCTGCGAGGAA-3′ and R
5′-GGCTTCGATCTGCTCCTGGCAGGC−3′; p21 F
5′-GAGCGATGGAACTTCGACTTTGTC-3′ and R
5′-GGCGTTTGGAGTGGTAGAAATCTG−3′. The PCR was repeated 3 independent
times. A BIO-RAD S1000 Thermal Cycler (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to perform the experiment under the
following conditions: 95°C for 30 sec, annealing at the 60°C for 30
sec and extension at 72°C for 30 sec for 30 cycles. Samples were
analyzed by 1.5% agarose gel electrophoresis. The DNA bands were
examined using a gel documentation system (Gel Doc 2000; Bio-Rad
Laboratories, Inc.). PCR results were calculated based on 3
independent experiments.
Western blot analysis
Western blot analysis was used to observe HCT-8/5-FU
cell apoptosis induced by EEHDW treatment and expression of
PI3K/AKT signaling pathways. HCT-8/5-FU cells were seeded into 25
cm2 flasks at a density of 1.0×106
cells/flask in 5 ml RPMI-1640 media and treated with different
concentrations of EEHDW for 24 h. Cells were lysed using RIPA cell
lysis buffer containing protease inhibitors, and the resulting
protein concentration in each sample was determined by BCA assay.
Equal quantities of protein (50 µg) were then separated on 10%
SDS-PAGE gel and transferred onto PVDF membranes. The membranes
were blocked for 2 h with 5% non-fat dry milk at room temperature,
then incubated with β-actin, Bax, Bcl-2, Cyclin D1, CDK4, p21,
PI3K, p-AKT, AKT and PTEN primary antibodies (1:1,000) overnight at
4°C. Following washing and subsequent incubation with
HRP-conjugated secondary antibodies (1:2,000) for 2 h at room
temperature, protein bands of interest were detected using enhanced
chemiluminescence by SuperSignal West Pico Chemiluminescent
Substrate. Image Lab™ software version 3.0 (Bio-Rad Laboratories,
Inc.) was used for densitometric analysis and quantification of
western blots.
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA)
by using Student's t-test or one-way AVOVA analysis. LSD and
Dunnet's were used as post-hoc tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
EEHDW reduces HCT-8/5-FU cell
viability
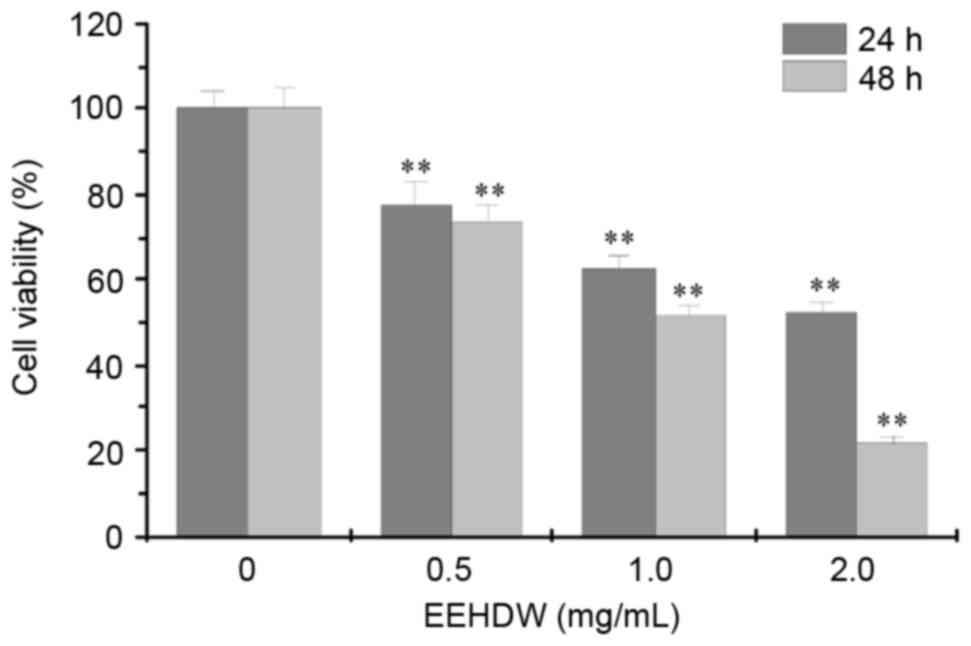
HCT-8/5-FU cell viability was measured using MTT
assay following exposure to different concentrations of EEHDW for
24 h and 48 h. As presented in Fig.
1, treatment with 0.5–2.0 mg/ml of EEHDW for 24 and 48 h
significantly reduced cell viability of HCT-8/5-FU cells by
22.22–47.58% and 26.67–78.27%, respectively compared with untreated
control cells (P<0.01; Fig. 1).
This suggests that EEHDW inhibited HCT-8/5-FU cell viability in a
concentration- and time-dependent manner.
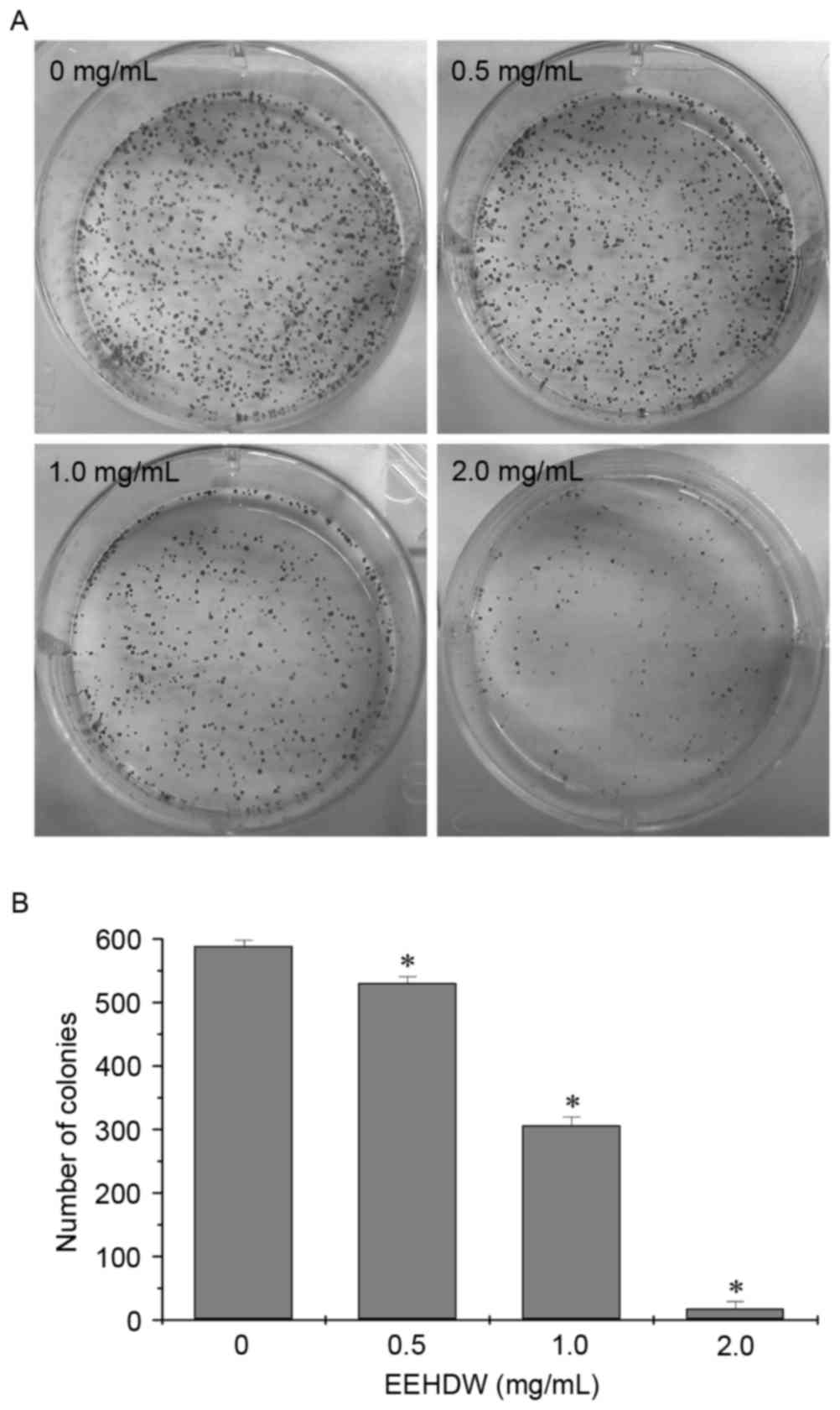
EEHDW inhibits the colony formation
ability of HCT-8/5-FU cells
In order to fully evaluate the effect of EEHDW on
HCT-8/5-FU cells, the number of colonies formed by HCT-8/5-FU cells
following EEHDW treatment was examined using a colony formation
assay. As presented in Fig. 2,
treatment with 0.5, 1.0 and 2.0 mg/ml EEHDW for 24 h significantly
reduced the cell survival rate of HCT-8/5-FU cells when compared
with untreated control cells in a dose-dependent manner
(P<0.05).
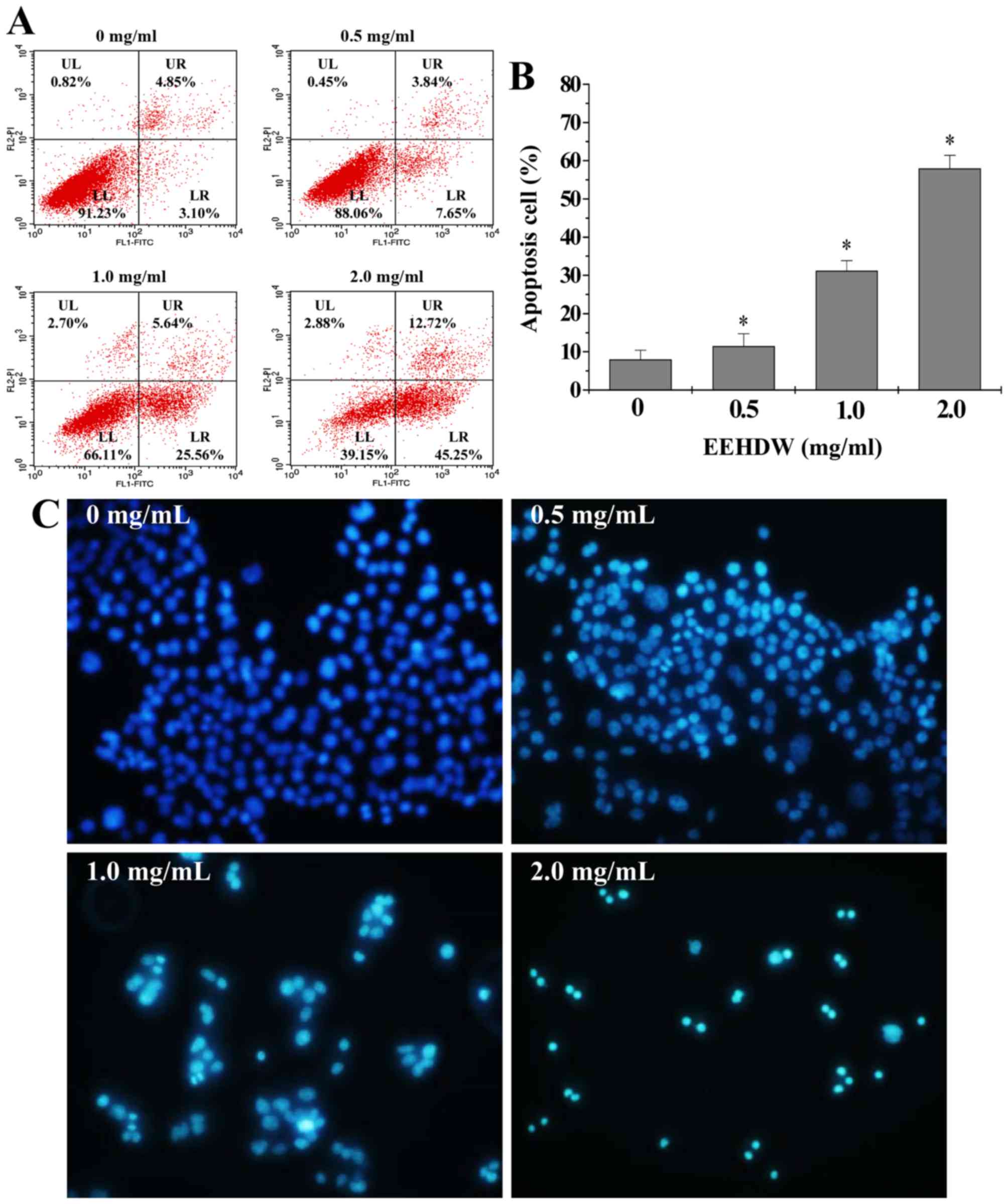
EEHDW induces apoptosis in HCT-8/5-FU
cells
Due to the innate resistance of the cancer cells to
apoptosis, clinical treatment for various cancers such as CRC is
frequently insufficient. The present study examined the possibility
that EEHDW may overcome drug-resistance and induce apoptosis of
HCT-8/5-FU cells. Annexin-V/PI staining and FACS analysis
demonstrated that EEHDW treatment significantly increased the
percentage of HCT-8/5-FU cells undergoing early-stage (lower right
quadrant) and late-stage apoptosis (upper right quadrant) in a
dose-dependent manner when compared with the untreated control
cells (Fig. 3A and B; P<0.05).
The cellular and nuclear morphology of HCT-8/5-FU cells was
examined using DAPI staining. As presented in Fig. 3C, EEHDW treated cells showed more
intense staining and apparent DNA condensation when compared with
untreated control cells, suggesting that EEHDW treatment promoted
HCT-8/5-FU cell apoptosis.
EEHDW affects the expression of cyclin
D1, CDK4, p21, Bcl-2 and Bax in HCT-8/5-FU cells
The proliferation of the majority of animal cells is
primarily regulated in the G1/S transition, one of the two main
checkpoints in the cell cycle which is mediated by the
pro-proliferative cyclin D1 and CDK4. Apoptosis is tightly
regulated by Bcl-2 family proteins, including anti-apoptotic
members such as Bcl-2 and pro-apoptotic members such as Bax
(28–30). In order to investigate the
underlying mechanisms of EEHDW and how it overcomes the drug
resistance of cancer cells, the present study examined the mRNA and
protein expression levels of cyclin D1, CDK4, p21, Bax and Bcl-2
following EEHDW treatment in HCT-8/5-FU cells. As presented in
Fig. 4, EEHDW treatment
significantly reduced cyclin D1, CDK4 and Bcl-2 mRNA and protein
expression; however, p21 and Bax mRNA and protein expression levels
in HCT-8/5-FU cells were increased when compared with untreated
control cells (P<0.05). This suggests that EEHDW likely
modulates the drug resistance of CRC HCT-8/5-FU cells by regulating
the expression of cyclin D1, CDK4, p21, Bcl-2 and Bax.
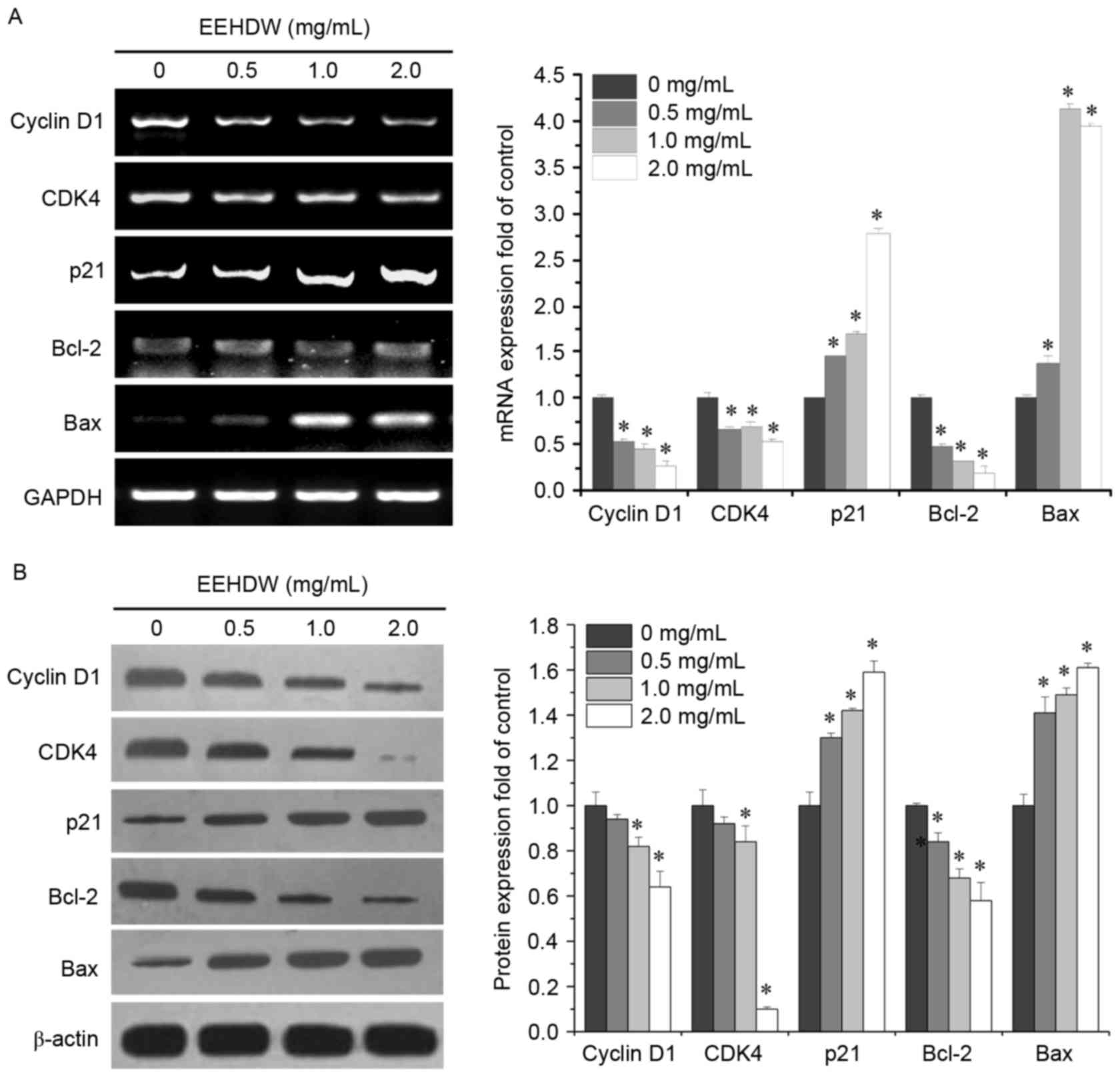 | Figure 4.Effect of EEHDW on the mRNA and
protein expression levels of cyclin D1, CDK4, p21, Bcl-2 and Bax in
HCT-8/5-FU cells. HCT-8/5-FU cells were treated with the indicated
concentration of EEHDW for 24 h. The mRNA (A) expression levels of
cyclin D1, CDK4, p21, Bcl-2, and Bax were subsequently determined
by using reverse transcription-polymerase chain reaction.
Densitometric analysis was conducted to indicated the mRNA
expression fold change. The data were normalized to the mean mRNA
expression of untreated control. The protein (B) expression levels
of cyclin D1, CDK4, p21, Bcl-2, and Bax were subsequently
determined by western blot analysis. Densitometric analysis was
conducted to indicated the protein expression fold-change. Data
were normalized to the mean protein expression of untreated control
(fold of control). *P<0.05 vs. untreated control cells. CDK4,
cyclin dependent kinase 4; Bcl-2, B cell leukemia/lymphoma 2; Bax,
Bcl2 associated X, apoptosis regulator; EEHDW, ethanol extract of
Hedyotis diffusa Willd; 5-FU, 5-fluorouracil. |
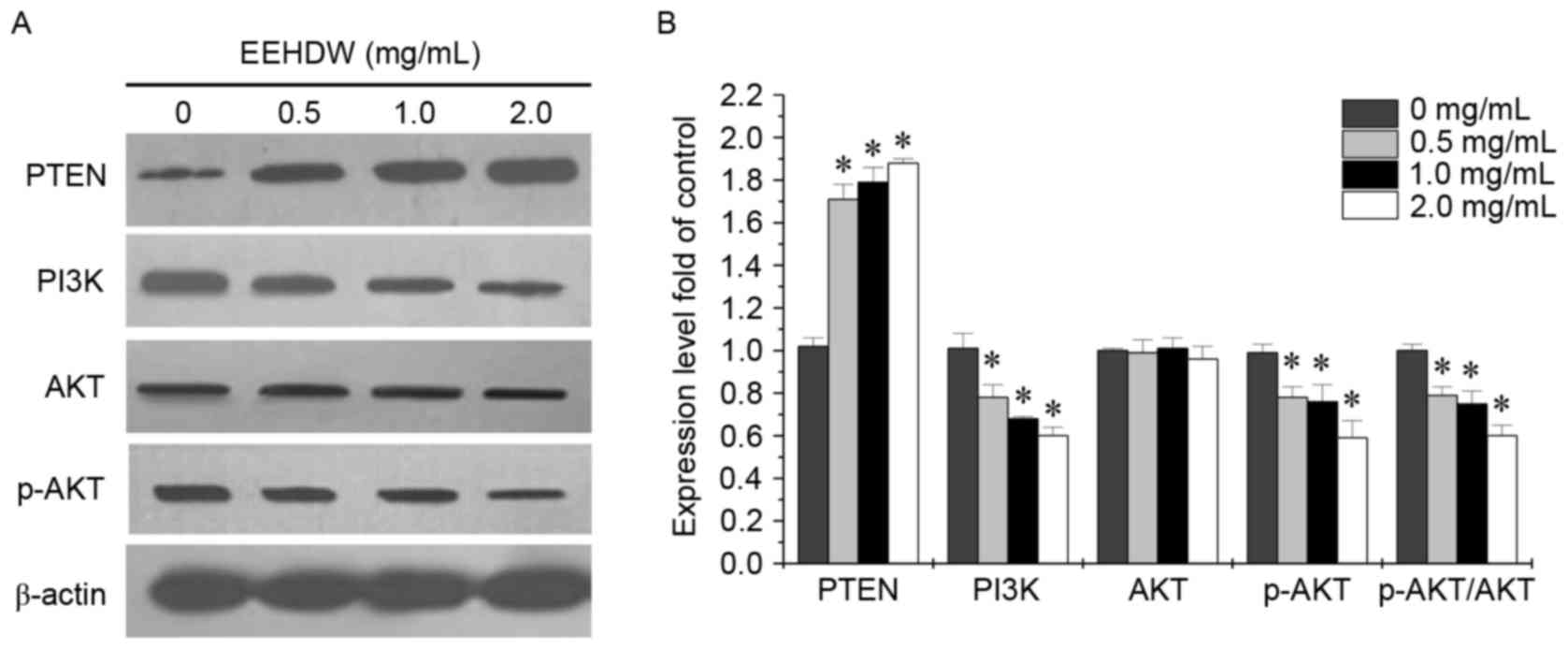
EEHDW inhibits the activation of the
PI3K/AKT pathway in HCT-8/5-FU cells
The PI3K/AKT signaling pathway has a crucial role in
numerous cellular systems and its activation is closely associated
with the prevention of cellular apoptosis. AKT is activated by
phospholipid binding and activation loop phosphorylation at Thr308
by pyruvate dehydrogenase kinase 1 and by phosphorylation within
the carboxy terminus at Ser473. In order to determine the mechanism
behind EEHDW's ability to overcome drug resistance of CRC, the
present study examined the expression of key proteins involved in
the PI3K/AKT signaling pathway, including PI3K, p-AKT, AKT and PTEN
following EEHDW treatment. As presented in Fig. 5, EEHDW treatment significantly
reduced PI3K and p-AKT expression and the ratio of p-AKT to total
AKT; however, led to increased PTEN expression in HCT-8/5-FU cells,
in a dose-dependent manner when compared with untreated control
cells (P<0.05). This suggests that EEHDW may also modulate the
drug-resistance of CRC HCT-8/5-FU cells via regulation of the
PI3K/AKT signaling pathway.
Discussion
EEHDW is a major component commonly used in
traditional Chinese medicine for the clinical treatment of CRC. Our
previous study determined that Hedyotis diffusa Willd
inhibits the growth of CRC, possibly via the inhibition of tumor
angiogenesis by regulating the Hedgehog signaling pathway (25). It also induced the cell apoptosis
via the IL-6-inducible STAT3 pathway (26). However, TCM including HDW have
multiple components. There are various compounds in EEHDW such as
ursolic and oleanolic acid, kaempferol, luteolin and they may
regulate multiple signaling pathways (31). Our previous study revealed that
EEHDW significantly reduced the viability of CRC HCT-8/5-FU cells
by inhibiting cell proliferation and inducing cell apoptosis
(27). A previous study
demonstrated that EEHDW may restore the sensitivity of multi-drug
resistant cancer cells to various chemotherapeutic agents (32).
However, the mechanisms of drug-resistance in cancer
cells involve complex systems, including increased drug efflux,
reduced drug uptake and resistance to apoptosis (5–7). Our
previous study demonstrated that EEHDW is effective for increasing
5-FU accumulation in HCT-8/5-FU cells and that EEHDW may reverse
5-FU resistance by inhibiting the expression of an ABC transporter
protein, ABCG2 (27). In addition,
resistance to apoptosis has been implicated as a major factor
involved in chemo-resistance of cancer cells. The present study
aimed to elucidate the mechanisms of EEHDW in inhibiting
proliferation and inducing apoptosis in CRC cells via regulation of
the PI3K/AKT pathway.
The current study demonstrated that the growth and
viability of CRC HCT-8/5-FU cells was significantly inhibited by
EEHDW treatment, through inhibition of cell proliferation and
induction of cellular apoptosis. One of the hallmarks of cancerous
cells is their ability to resist the intrinsic process of cellular
apoptosis (33). Eliminating or
reducing the ability of cancer cells to resist apoptosis has become
a key target for anti-cancer therapy (34). Therefore, determining the
underlying mechanisms involved in cell apoptotic pathways has
become a crucial step in the clinical treatment of cancer (35). Additionally, cancer cells have the
ability to upregulate the expression of anti-apoptotic regulators
such as Bcl-2, whilst downregulating pro-apoptotic factors such as
Bax (36,37). The present study demonstrated that
EEHDW may induce apoptosis of HCT-8/5-FU cells and restore its
sensitivity to chemotherapy. In addition, the present study
determined that EEHDW treatment significantly reduced the
expression of Bcl-2, cyclin D1 and CDK4, whilst significantly
increasing the expression of Bax and p21, demonstrating that EEHDW
treatment may suppress proliferation and induce apoptosis of
HCT-8/5-FU cells.
The PI3K/AKT signaling pathway is involved in the
promotion of cell proliferation and apoptosis (10,38).
Previous studies have revealed that inhibition of the PI3K/AKT
pathway may restore the sensitivity of various cancer cells to
chemotherapeutic drugs (38–40).
In particular, p-AKT activation leads to the increased
transcription of downstream target genes such as Bax, Bcl-2, CDK4,
cyclin D1, ATP binding cassette subfamily B member 1 and ABCG2
(16,17,41),
which are associated with the regulation of various cellular
processes including cell cycle, proliferation and apoptosis.
Therefore, the PI3K/AKT signaling pathway and its downstream genes
are promising targets for the therapeutic treatment of cancer
(42). The present study detected
a significant increase in protein expression of PTEN and reduction
in the protein expression of PI3K or p-AKT following EEHDW
treatment in HCT-8/5-FU cells, demonstrating that EEHDW may
overcome the drug-resistance of cancer cells via inhibition of the
PI3K/AKT pathway. Therefore, the present study outlined the key
anti-drug resistance effect of EEHDW on CRC HCT-8/5-FU cells.
In conclusion, the current study demonstrated that
EEHDW treatment may inhibit proliferation and induce apoptosis of
5-FU resistant CRC cells via inhibition of p-AKT activation and
regulation of Bcl-2, Bax, cyclin D1, CDK4 and p21 expression.
Additionally, the present study demonstrated that regulation of the
PI3K/AKT pathway and its downstream target genes is a key mechanism
of EEHDW in overcoming 5-FU cancer drug resistance.
Acknowledgements
The present study was sponsored by the Research Fund
for the Doctoral Program of Higher Education of China (grant no.
20133519110003), Project Funding for the Training of Young and
Middle-aged Backbone Personnel of Fujian Provincial Health and
Family Planning Commission (grant no. 2016-ZQN-67) and the
Developmental Fund of Chen Keji Integrative Medicine (grant nos.
CKJ2014013 and CKJ2015007).
Glossary
Abbreviations
Abbreviations:
|
EEHDW
|
ethanol extract of Hedyotis
diffusa Wild
|
|
CRC
|
colorectal cancer
|
|
MTT
|
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
|
AKT
|
protein kinase B
|
|
5-FU
|
5-fluorouracil
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Cutsem E, Nordlinger B and Cervantes
A: ESMO Guidelines Working Group: Advanced colorectal cancer: ESMO
clinical practice guidelines for treatment. Ann Oncol. 21 Suppl
5:v93–v97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li W, Zhang H, Assaraf YG, Zhao K, Xu X,
Xie J, Yang DH and Chen ZS: Overcoming ABC transporter-mediated
multidrug resistance: Molecular mechanisms and novel therapeutic
drug strategies. Drug Resist Updat. 27:14–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mittal B, Tulsyan S, Kumar S, Mittal RD
and Agarwal G: Cytochrome P450 in cancer susceptibility and
treatment. Adv Clin Chem. 71:77–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan ST, Li ZL, He ZX, Qiu JX and Zhou SF:
Molecular mechanisms for tumour resistance to chemotherapy. Clin
Exp Pharmacol Physiol. 43:723–737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartholomeusz C and Gonzalez-Angulo AM:
Targeting the PI3K signaling pathway in cancer therapy. Expert Opin
Ther Targets. 16:121–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song L, Xiong H, Li J, Liao W, Wang L, Wu
J and Li M: Sphingosine kinase-1 enhances resistance to apoptosis
through activation of PI3K/AKT/NF-κB pathway in human non-small
cell lung cancer. Clin Cancer Res. 17:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Danielsen SA, Eide PW, Nesbakken A, Guren
T, Leithe E and Lothe RA: Portrait of the PI3K/AKT pathway in
colorectal cancer. Biochim Biophys Acta. 1855:104–121.
2015.PubMed/NCBI
|
|
14
|
Brotelle T and Bay JO: PI3K-AKT-mTOR
pathway: Description, therapeutic development, resistance,
predictive/prognostic biomarkers and therapeutic applications for
cancer. Bull Cancer. 103:18–29. 2016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu D, Lu Q and Hu X: Down-regulation of
P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by
honokiol. Cancer Lett. 243:274–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Xia Y, Wang H and Hou Z: Chinese
herbs of Shenghe Powder reverse multidrug resistance of gastric
carcinoma SGC-7901. Integr Cancer Ther. 6:400–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Angelini A, Di Ilio C, Castellani ML,
Conti P and Cuccurullo F: Modulation of multidrug resistance
p-glycoprotein activity by flavonoids and honokiol in human
doxorubicin- resistant sarcoma cells (MES-SA/DX-5): Implications
for natural sedatives as chemosensitizing agents in cancer therapy.
J Biol Regul Homeost Agents. 24:197–205. 2010.PubMed/NCBI
|
|
18
|
Zhang L, Zhang J, Qi B, Jiang G, Liu J,
Zhang P, Ma Y and Li W: The anti-tumor effect and bioactive
phytochemicals of Hedyotis diffusa willd on ovarian cancer
cells. J Ethnopharmacol. 192:132–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YL, Zhang J, Min D, Hongyan Z, Lin N
and Li QS: Anticancer effects of
1,3-dihydroxy-2-methylanthraquinone and the ethyl acetate fraction
of Hedyotis diffusa willd against HepG2 carcinoma cells
mediated via apoptosis. PLoS One. 11:e01515022016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang P, Zhang B, Gu J, Hao L, Hu F and
Han C: The study of the effect of Hedyotis diffusa on the
proliferation and the apoptosis of the cervical tumor in nude mouse
model. Cell Biochem Biophys. 72:783–789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuo YJ, Yang JS, Lu CC, Chiang SY, Lin JG
and Chung JG: Ethanol extract of Hedyotis diffusa willd
upregulates G0/G1 phase arrest and induces apoptosis in human
leukemia cells by modulating caspase cascade signaling and altering
associated genes expression was assayed by cDNA microarray. Environ
Toxicol. 30:1162–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis diffusa willd extract
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
23
|
Lin JM, Wei LH, Xu W, Hong Z, Liu X and
Peng J: Effect of Hedyotis diffusa willd extract on tumor
angiogenesis. Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
24
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis diffusa
willd inhibits colorectal cancer growth in vivo via inhibition of
STAT3 signaling pathway. Int J Mol Sci. 13:6117–6128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin J, Wei L, Shen A, Cai Q, Xu W, Li H,
Zhan Y, Hong Z and Peng J: Hedyotis diffusa willd extract
suppresses sonic hedgehog signaling leading to the inhibition of
colorectal cancer angiogenesis. Int J Oncol. 42:651–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin J, Li Q, Chen H, Lin H, Lai Z and Peng
J: Hedyotis diffusa willd. Extract suppresses proliferation
and induces apoptosis via IL-6-inducible STAT3 pathway inactivation
in human colorectal cancer cells. Oncol Lett. 9:1962–1970.
2015.PubMed/NCBI
|
|
27
|
Li Q, Wang X, Shen A, Zhang Y, Chen Y,
Sferra TJ, Lin J and Peng J: Hedyotis diffusa Willd
overcomes 5-fluorouracil resistance in human colorectal cancer
HCT-8/5-FU cells by downregulating the expression of P-glycoprotein
and ATP-binding casette subfamily G member 2. Exp Ther Med.
10:1845–1850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elledge SJ: Cell cycle checkpoints:
Preventing an identity crisis. Science. 274:1664–1672. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taulés M, Rius E, Talaya D, López-Girona
A, Bachs O and Agell N: Calmodulin is essential for
cyclin-dependent kinase 4 (Cdk4) activity and nuclear accumulation
of cyclin D1-Cdk4 during G1. J Biol Chem. 273:33279–33386. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Youle RJ and Strasser A: The Bcl-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis
diffusa willd suppresses proliferation of human HepG2 cells and
potentiates the anticancer efficacy of low-dose 5-fluorouracil by
inhibiting the CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu JJ, Lin M, Yu JY, Liu B and Bao JK:
Targeting apoptotic and autophagic pathways for cancer
therapeutics. Cancer Lett. 300:105–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Denicourt C and Dowdy SF: Medicine.
Targeting apoptotic pathways in cancer cells. Science.
305:1411–1413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuchiya T, Tsuno NH, Asakage M, Yamada J,
Yoneyama S, Okaji Y, Sasaki S, Kitayama J, Osada T, Takahashi K and
Nagawa H: Apoptosis induction by p38 MAPK inhibitor in human colon
cancer cells. Hepatogastroenterology. 55:930–935. 2008.PubMed/NCBI
|
|
38
|
Wu H, Hait WN and Yang JM: Small
interfering RNA-induced suppression of MDR1 (P-glycoprotein)
restores sensitivity to multidrug-resistant cancer cells. Cancer
Res. 63:1515–1519. 2003.PubMed/NCBI
|
|
39
|
Jiao M and Nan KJ: Activation of PI3
kinase/Akt/HIF-1α pathway contributes to hypoxia-induced
epithelial-mesenchymal transition and chemoresistance in
hepatocellular carcinoma. Int J Oncol. 40:461–468. 2012.PubMed/NCBI
|
|
40
|
Zhang HY, Zhang PN and Sun H: Aberration
of the PI3K/AKT/mTOR signaling in epithelial ovarian cancer and its
implication in cisplatin-based chemotherapy. Eur J Obstet Gynecol
Reprod Biol. 146:81–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lima RT, Martins LM, Guimarães JE, Sambade
C and Vasconcelos MH: Specific downregulation of Bcl-2 and xIAP by
RNAi enhances the effects of chemotherapeutic agents in MCF-7 human
breast cancer cells. Cancer Gene Ther. 11:309–316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar : PubMed/NCBI
|