Introduction
Atherosclerosis has been one of the most threatening
types of diseases in the world, causing severe damage to health
(1,2). The migration of vascular smooth
muscle cells (VSMCs), the most common pathological change in
atherosclerosis, occurs in response to arterial injury and exerts a
vital role in the development of atherosclerotic plaques (3,4).
Directional migration of VSMCs from the media to the intima through
the basement membrane and collagenous matrix, and their subsequent
proliferation are the key processes for self-remodeling of vessels
(5). However, the molecular
mechanism of abnormal VSMC phenotypic modulation in atherosclerosis
is not fully understood.
Micro (mi)RNAs are a class of highly conserved small
noncoding RNAs that regulate target genes by binding to the
3′-untranslated region (3′-UTR) of messenger RNA transcripts to
repress their translation or mediate their degradation (6,7).
Certain critical roles of miRNAs in VSMC phenotypic change have
previously been reported (8–11).
Among the various miRNAs, miR-92a is a component of the miR-17-92
cluster, which is highly expressed in young endothelial cells in
comparison with senescent endothelial cells, which exhibit
increased oxidative stress and apoptosis (12). However, whether a miRNA-dependent
mechanism contributes to hydrogen peroxide
(H2O2)-induced changes in VSMCs remains
unknown.
As reported previously, matrix metalloproteinases
(MMPs) are major mediators of the vulnerability of atherosclerotic
plaques. The balance between MMPs and tissue inhibitor of
metalloproteinases (TIMPs) is important for the regulation of VSMC
migration (13–16). It is well established that MMP9,
inhibited by TIMP3, is a major molecular mediator that degrades the
extracellular matrix (ECM), and detection of MMP9 supports the role
of MMPs in vulnerable atherosclerotic plaques (17). High expression levels of MMP9 in
atherosclerotic plaques contribute to plaque hemorrhage and rupture
in specific MMP knockout mice and patients with acute coronary
syndrome (18). However, the
molecular mechanisms for MMP9/TIMP3 regulation in VSMCs remain
elusive.
In order to investigate the possible effect of
miR-92a and its underlying mechanisms on the migration of VSMCs in
atherosclerosis, the present study utilized a cell model of
H2O2-induced VSMCs obtained from rat thoracic
aortas for experimental purposes.
Materials and methods
Experimental animals
A total of 20 male Sprague-Dawley (SD) rats
(Specific-pathogen-free degree, aged 4–5 weeks, weight 100–150 g)
were purchased from Tongji University Laboratory Animal Center
(Shanghai, China), and housed in a temperature-controlled feeding
facility (22°C) with a 12-h light/dark cycle and free access to
water and rodent diet. This study conformed to the Guide for Care
and Use of Laboratory Animals and procedures were approved by the
Animal Care and Use Committee of Tongji University School of
Medicine (Shanghai, China).
Primary culture, identification and
transfection of VSMCs
Primary VSMCs were isolated from SD rat thoracic
aortas, as described previously (19). In brief, animals were sacrificed by
intraperitoneal injection of 2% pentobarbital sodium (50 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and perfused with
saline for 5 min. The thoracic aortas were aseptically harvested
with removal of the intima, longitudinally cut into ~1
mm2 fragments, explanted with the lumen side down on
cell culture dishes (Corning Life Sciences, Beijing, China)
containing Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and maintained
in a 37°C and humidified 5% CO2 incubator for 7 days,
followed by discarding the tissue fragments and further collection
of sprouted VSMCs. The following experiments were performed on the
VSMCs between passages 4 and 7 in DMEM with 2.5% FBS.
VSMCs were identified by their morphology and
immunofluorescence detection of marker proteins, such as
alpha-smooth muscle actin (α-SMA) using rabbit anti-α-SMA
polyclonal antibody (ab5694; 1:100; Abcam, Cambridge, UK), and
their purity was ensured by multiple fluorescent stainings with
α-SMA antibody and nucleic acid dye, DAPI (2 mg/ml; Beyotime
Institute of Biotechnology, Haimen, China) using a laser scanning
confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany). To
determine the effect of H2O2 on miR-92a
expression in VSMCs, VSMCs were treated with 100 µM
H2O2 (Sangon Biotech Co., Ltd., Shanghai,
China) at 37°C for 24 h.
For the transfection experiments, double-stranded
miR-92a mimics or miRNA negative control (miR-NC; 100 nM; Shanghai
GenePharma Co., Ltd., Shanghai, China), and sirtuin 1 (SIRT1) siRNA
or NC siRNA (100 nM; Shanghai GenePharma Co., Ltd.) were
respectively transfected into VSMCs using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocols. Afterwards, VSMCs were cultured
for 48 h and then used for subsequent assays.
Transwell migration assay
Cell migration assays were detected using a
Transwell chamber with 8-µm filter inserts (Corning Life Sciences,
Beijing, China) without Matrigel. Following transfection and
H2O2 treatment, ~2×104 VSMCs in
100 µl DMEM medium with 1% bovine serum albumin (BSA; Sangon
Biotech Co., Ltd., Shanghai, China) were seeded into the upper
chamber. The lower chamber was filled with 600 µl DMEM medium
containing 10% FBS. After 24 h, VSMCs on the upper chamber were
gently scraped away, and the cells that had migrated through the
filter into the lower chamber were fixed with 4% paraformaldehyde
(Sangon Biotech Co., Ltd.) for 15 min at room temperature and
stained with DAPI (2 mg/ml). Then, the cells from 9 independent,
randomly selected visual fields were counted under a laser scanning
confocal microscope (magnification, ×200; Leica Microsystems GmbH)
for quantitative analysis. Experiments were performed in
triplicate.
Protein extraction and western blot
analysis
Cellular protein was extracted with ice-cold
radioimmunoprecipitation assay lysis buffer containing a mixture of
proteasome inhibitors (Beyotime Institute of Biotechnology) for
western blot analysis. The BCA method (BCA Protein Assay kit;
Beyotime Institute of Biotechnology) was used to measure the
protein concentrations of the supernatant by a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA) according to the
manufacturer's protocols. Equal amounts (30 µg) of protein were
then separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) at 80 V for 0.5 h and subsequently 120 V
for 1.5 h at room temperature, and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA) at 100 V for
1.5 h at 4°C. The membranes were then blocked with 3% BSA in
phosphate-buffered saline (PBS; Sangon Biotech Co., Ltd.) for 30
min at room temperature and incubated with specific primary
antibodies, including rabbit anti-MMP9 polyclonal antibody
(ab38898; 1:1,000; Abcam), rabbit anti-TIMP3 monoclonal antibody
(5673; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
and mouse anti-GAPDH monoclonal antibody (97166; 1:1,000; Cell
Signaling Technology, Inc.), overnight at 4°C. Tris-buffered saline
and Tween 20 (Sangon Biotech Co., Ltd.) was used to wash the
membranes (3 times for 10 min) and the membranes were incubated
with secondary antibodies, including goat anti-rabbit
immunoglobulin G (IgG)-horseradish peroxidase (HRP) (L3012-2;
1:10,000; Signalway Antibody LLC, College Park, MD, USA), or goat
anti-mouse IgG-HRP (L3032-2; 1:10,000; Signalway Antibody LLC), for
1 h at room temperature. The standard chemical luminescence method
(BeyoECL Plus kit; Beyotime Institute of Biotechnology) was used to
visualize the antigen by exposing the membranes to Kodak X-Omat AR
film (Seebio Biotech, Inc., Shanghai, China) in accordance with the
manufacturer's protocols. The resultant films were scanned on a gel
imaging and analysis system and analyzed by Quantity One software
version 4.4 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Relative protein expression of MMP9 and TIMP3 was normalized by the
internal control, GAPDH, in the same sample.
MTT assay
MTT assay was used to evaluate the effect of miR-92a
mimics on VSMC viability. Briefly, following transfection and
H2O2 treatment, VSMCs were cultured in DMEM
medium in 96-well plates at a density of 6,000 per well at 37°C
under 5% CO2. After 24 h of maintenance, 20 µl MTT (5
mg/ml in PBS; Beyotime Institute of Biotechnology) was added to
each plate and incubated for 4 h at 37°C. Subsequently, the
supernatant was removed and 150 µl DMSO (Sangon Biotech Co., Ltd.)
was added to each well. Following 10 min of incubation at room
temperature, absorbance of formazan dissolved in DMSO was
immediately assessed at a wavelength of 490 nm using a microplate
reader (BioTek Instruments, Inc.). Experiments were performed in
quintuplicate.
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was isolated from VSMCs using TRIzol
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols and its concentration was determined
with NanoDrop Spectrophotometer (Thermo Fisher Scientific, Inc.).
Reverse transcription of miRNA or ordinary RNA was performed using
miRcute miRNA First-Strand cDNA Synthesis kit (Tiangen Biotech Co.,
Ltd., Beijing, China) or PrimeScript RT Reagent kit with gDNA
Eraser (Perfect Real Time; Takara Biotechnology Co., Ltd., Dalian,
China) according to the suggested protocol, respectively. Then,
miR-92a expression level was measured with miRcute miRNA qPCR
Detection kit (Tiangen Biotech Co., Ltd.) under the conditions:
94°C for 2 min; 40 cycles (94°C for 20 sec and 60°C for 34 sec),
while the expression level of SIRT1 mRNA was evaluated with SYBR
Green PCR Master Mix Kit (Takara Biotechnology Co., Ltd.) under the
conditions: 95°C for 6 min; 40 cycles (95°C for 15 sec and 60°C for
34 sec), using 7900 HT Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). U6 snRNA and GAPDH mRNA served as
endogenous controls. The relative expression level was computed
according to the 2−ΔΔCq analysis method (20). Experiments were performed in
triplicate and the following primers were used: Forward,
5′-ACAGGCCGGGACAAGTGCAATA-3′ and reverse,
5′-GCTGTCAACGATACGCTACGTAACG-3′ for miR-92a; forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′ for
U6; forward, 5′-CCAGATCCTCAAGCCATGT-3′ and reverse,
5′-TTGGATTCCTGCAACCTG-3′ for SIRT1; and forward,
5′-TGGGCTACACTGAGCACCAG-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAAT-3′
for GAPDH.
Luciferase reporter assay
Verification of miR-92a targeting SIRT1 was
conducted by luciferase reporter assay in HEK-293T cells, as
described previously (21).
Briefly, wild-type 3′-UTR sequence of SIRT1 (WT-UTR) predicted to
interact with miR-92a, was synthesized by GenePharma Co., Ltd and
inserted into the dual-luciferase reporter vector (Promega
Corporation, Madison, WI, USA), yielding the WT-UTR plasmid.
Mutations (Mut-UTR) of potential miR-92a binding sites in WT-UTR
were subjected to the Site-Directed Mutagenesis kit (Stratagene;
Agilent Technologies, Inc., Santa Clara, CA, USA), generating the
Mut-UTR plasmid. All plasmids were confirmed with DNA sequencing.
HEK-293T cells were cultured in 12-well plates at 37°C under 5%
CO2 and cotransfected with the plasmids carrying 3′-UTR
variants, and miR-92a mimics or miR-NC for 48 h, using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Then, luciferase values of cell lysates were
determined with a Dual-Luciferase Reporter Assay System (Promega
Corporation) according to the protocol. All data were normalized by
Renilla luciferase activity. Experiments were performed in
triplicate.
Statistical analysis
All experiments were repeated 3 times. All
statistical analyses were performed using SPSS version 20.0 (IBM
Corp., Armonk, NY, USA). Data were presented as means ± standard
deviation. Student's t-test was used for comparisons between two
different groups. One-way analysis of variance, followed by Tukey's
post hoc test was performed to compare between multiple
experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
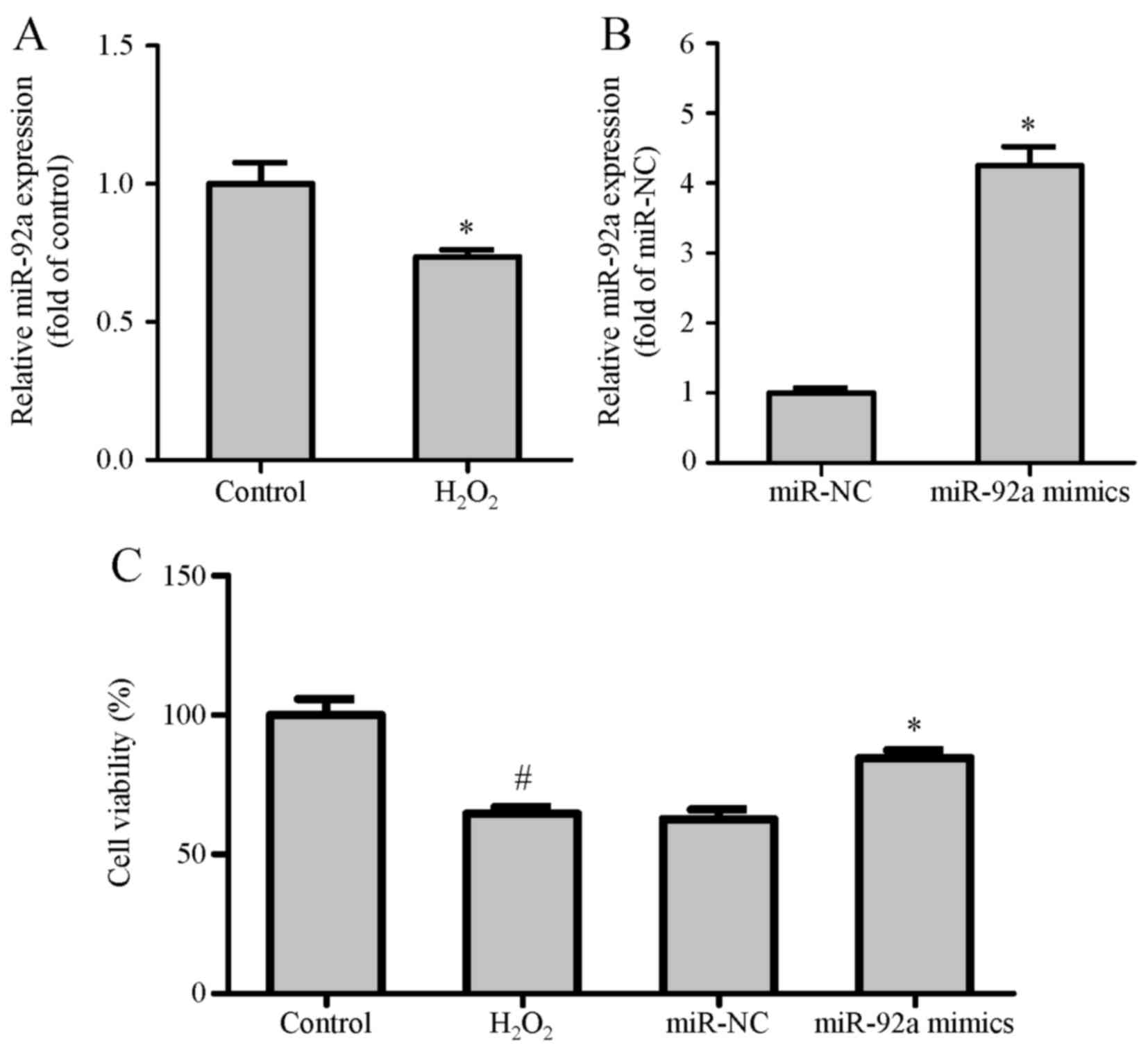
Regulation of miR-92a expression level
in VSMCs
In the present study, the expression level of
miR-92a was decreased in VSMCs with H2O2
treatment for 24 h (Fig. 1A). This
result intimated that miR-92a exerted a potential protective effect
in VSMCs. Following transfection and H2O2
treatment, the expression of miR-92a in VSMCs was detected by qPCR
(Fig. 1B). The outcomes revealed
that the miR-92a expression level in the miR-92a mimic group was
significantly higher than that in the miRNA negative control
(miR-NC) group.
miR-92a overexpression inhibits the
migration of VSMCs induced by H2O2
treatment
To investigate the potential effect of miR-92a on
VSMC viability, cell viability was detected via MTT assay. It was
found that H2O2 remarkably attenuated the
viability of normal VSMCs. In contrast, cell viability in miR-92a
mimic-transfected VSMCs was identified to be improved compared with
miR-NC-transfected VSMCs following H2O2
treatment for 24 h (Fig. 1C).
In addition, cell migration assays were performed to
evaluate whether miR-92a overexpression inhibited
H2O2-induced VSMC migration. The Transwell
migration assay demonstrated that the number of VSMCs in the
H2O2 group that migrated through the membrane
was greater compared with the control group. Notably, the miR-92a
mimic group markedly decreased the number of VSMCs that migrated
through the membrane when compared with the
H2O2 group or the miR-NC group (Fig. 2A-E). These results illustrate that
upregulated miR-92a expression by mimics suppresses
H2O2-induced VSMC migration in the absence of
adverse effects on cell viability.
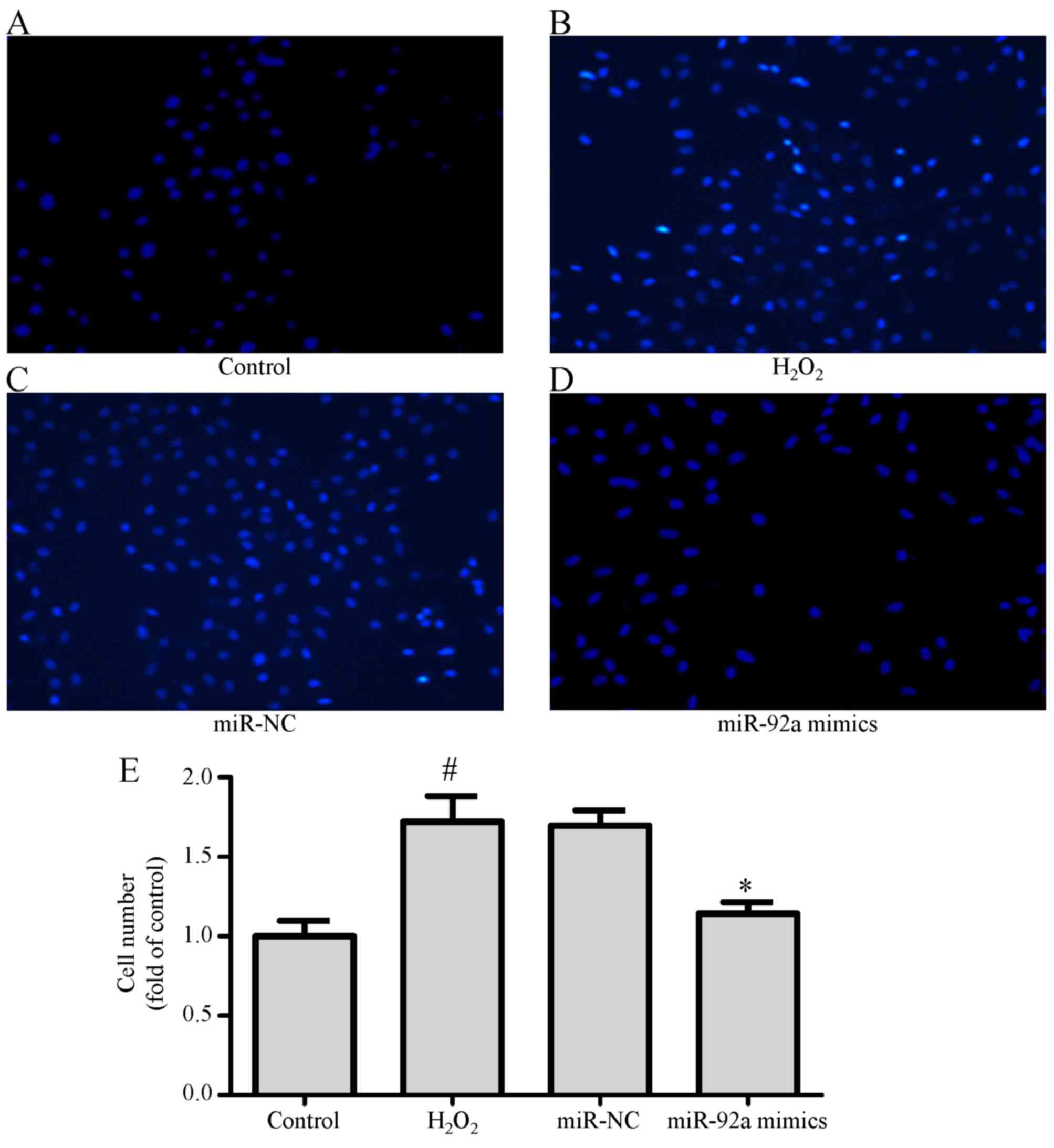 | Figure 2.miR-92a overexpression inhibits
H2O2-induced VSMC migration in vitro.
Following transfection and H2O2 treatment,
VSMCs were divided into four groups as follows: (A) Control, (B)
H2O2, (C) miR-NC, (D) miR-92a mimics for
Transwell migration assay, and (E) quantitative analysis of VSMCs
vertically migrating into the lower chamber, based on DAPI staining
and counting under confocal microscopy (magnification, ×200).
Values are presented as means ± SD; n=3; *P<0.05 vs. miR-NC
group and #P<0.05 vs. control group. miR, microRNA;
H2O2, hydrogen peroxide; NC, negative
control; VSMC, vascular smooth muscle cell; SD, standard
deviation. |
miR-92a overexpression regulates the
expression levels of MMP9 and TIMP3
miR-92a may target MMP9 and TIMP3, and influence
their expression in certain types of cancer cells (22). However, the mechanism between
miR-92a and MMP9/TIMP3 in H2O2-induced VSMCs
remains unclear. MMP9, inhibited by TIMP3, is involved in the
progression of ECM degradation and VSMC migration. In the current
study, the expression levels of MMP9 and TIMP3 in VSMCs following
the above-mentioned transfection and H2O2
treatment were evaluated by western blot assay. The results
demonstrated that H2O2 clearly caused the
upregulation of MMP9 and the downregulation of TIMP3 in VSMCs.
Nevertheless, transfection of miR-92a mimics markedly decreased the
expression level of MMP9, but clearly increased the expression
level of TIMP3 in comparison with transfection of miR-NC in
H2O2-treated VSMCs (Fig. 3A-C). These data suggest that the
expression levels of MMP9 and TIMP3 are modulated by miR-92a in
VSMCs.
 | Figure 3.miR-92a overexpression regulates the
expression levels of MMP9 and TIMP3. VSMCs were transfected with
miR-92a mimics or miR-NC, induced by H2O2,
and further processed for (A) western blot analysis of MMP9 and
TIMP3 protein expression levels, and densitometric analysis of (B)
MMP9 and (C) TIMP3 protein expression levels normalized to GAPDH,
in each group. Values are presented as means ± SD; n=3; *P<0.05
vs. miR-NC group and #P<0.05 vs. control group. miR,
microRNA; H2O2, hydrogen peroxide; NC,
negative control; MMP9, matrix metalloproteinase 9; TIMP3, tissue
inhibitor of metalloproteinase 3; VSMC, vascular smooth muscle
cell; SD, standard deviation. |
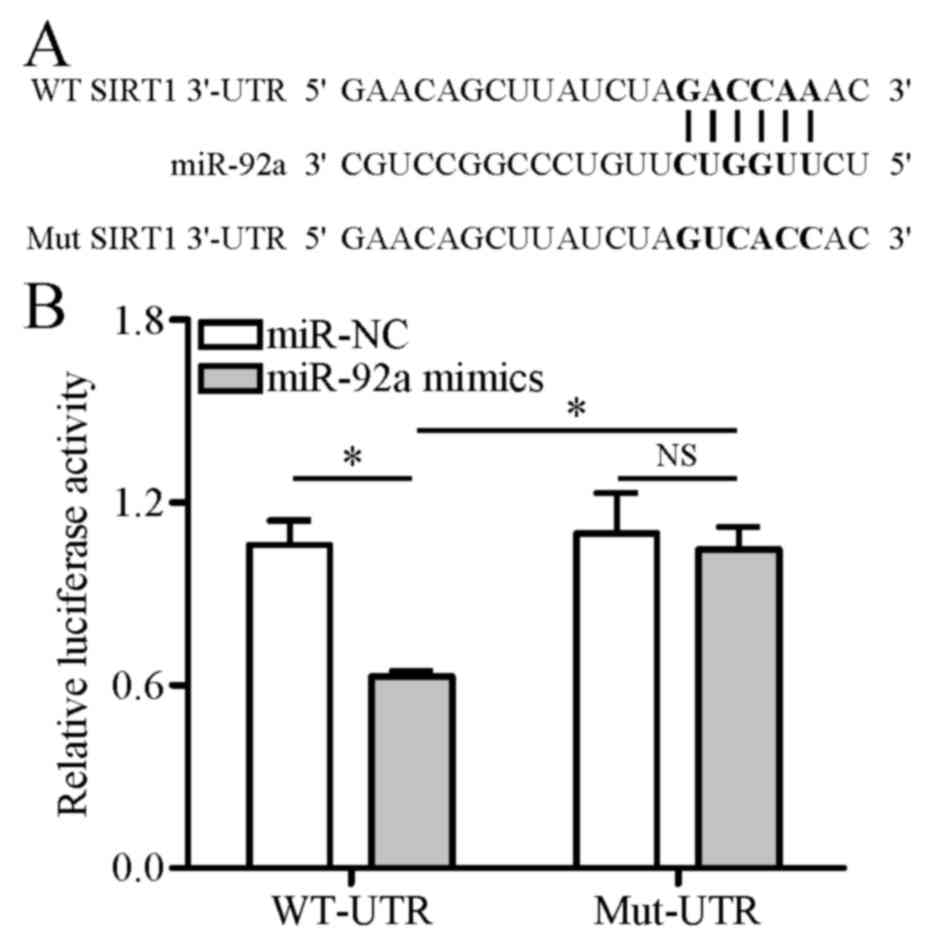
SIRT1 is a direct target of
miR-92a
To verify whether miR-92a directly targets the SIRT1
gene, dual-luciferase reporter vectors harboring a wild-type or
mutant 3′-UTR region of SIRT1 were constructed and transfected into
HEK-293T cells. Dual-luciferase reporter assay demonstrated that
miR-92a mimics significantly weakened the luciferase activity of
the reporter vector containing a wild-type SIRT1 3′-UTR sequence
(WT-UTR) compared with miR-NC. However, this inhibitive effect of
miR-92a mimics was eliminated when the reporter vector contained a
mutant SIRT1 3′-UTR sequence (Mut-UTR), which was unable to
interact with miR-92a (Fig. 4A and
B). These results indicate that miR-92a directly binds to
SIRT1.
Silencing SIRT1 regulates the
expression levels of MMP9 and TIMP3 and inhibits the migration of
VSMCs induced by H2O2 treatment
The above-mentioned findings suggested that miR-92a
may regulate the expression levels of MMP9 and TIMP3 to inhibit
VSMC migration by targeting SIRT1. To ascertain whether this
influence can be indicated by silencing SIRT1, SIRT1 siRNA was
transfected into VSMCs before H2O2 treatment.
The transfection efficiency of SIRT1 siRNA and its influence on the
expression levels of MMP9 and TIMP3 were demonstrated by qPCR and
western blot analysis, respectively. The data revealed that the
overexpression of SIRT1 mRNA resulting from
H2O2 stimulation was markedly reduced in the
SIRT1-siRNA group compared with the NC-siRNA group (Fig. 5A). As presented in Fig. 5B-D, SIRT1 siRNA decreased the
expression level of MMP9, and increased the expression level of
TIMP3 in H2O2-treated VSMCs. Notably, the
Transwell migration assay demonstrated that the quantity of VSMCs
in the SIRT1-siRNA group that migrated via the membrane was lower
than that in the H2O2 group or the NC-siRNA
group (Fig. 6A-E). Collectively,
these outcomes demonstrated that miR-92a regulates the expression
levels of MMP9 and TIMP3 via SIRT1 signaling, and further
interferes with H2O2-induced VSMC
migration.
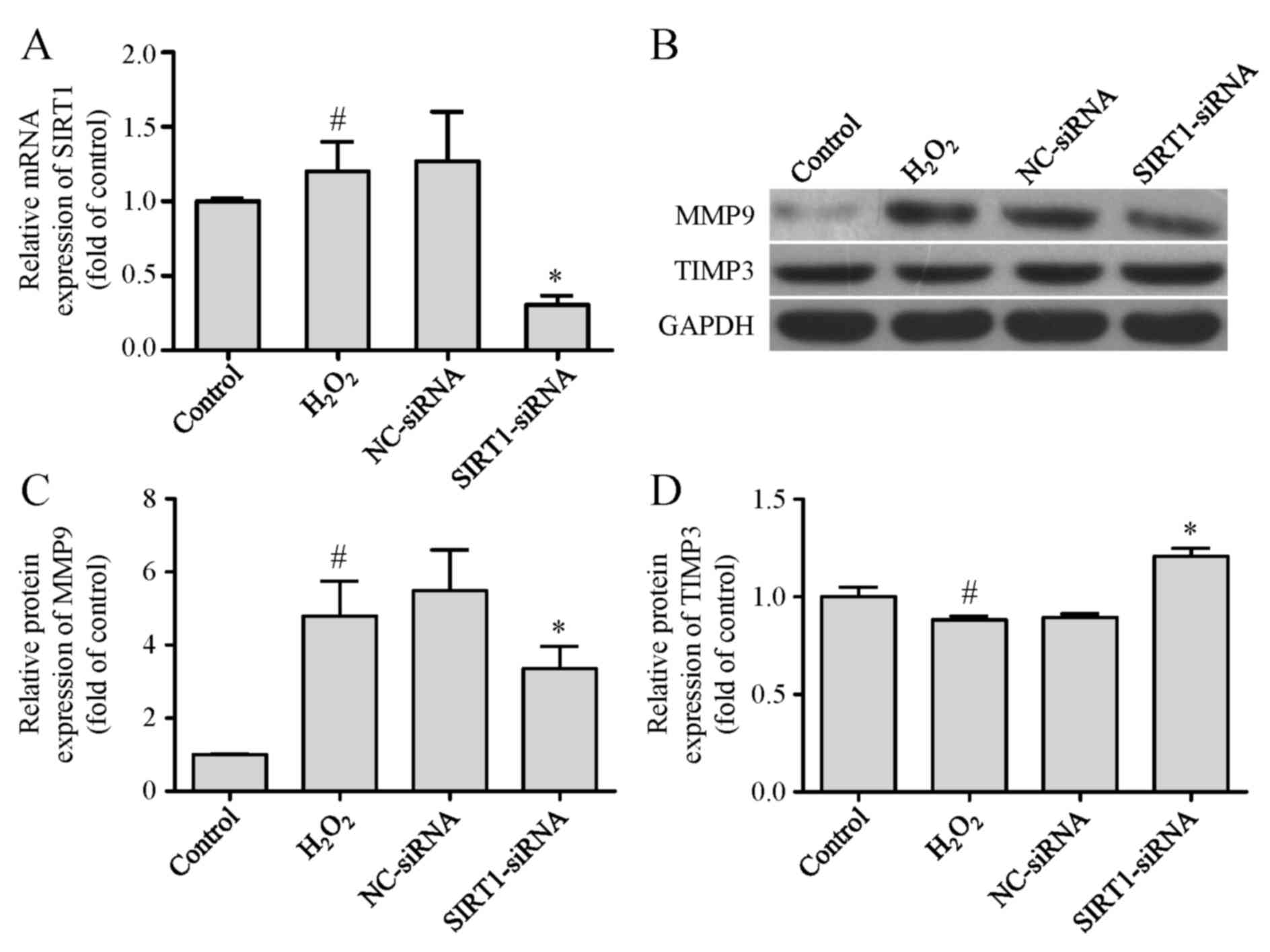 | Figure 5.Silencing SIRT1 regulates the
expression levels of MMP9 and TIMP3. VSMCs were transfected with
SIRT1-siRNA or NC-siRNA, induced by H2O2, and
further processed for (A) qPCR analysis of SIRT1 mRNA expression
level normalized to GAPDH, (B) western blot analysis of MMP9 and
TIMP3 protein expression levels, and densitometric analysis of (C)
MMP9 and (D) TIMP3 protein expression levels normalized to GAPDH,
in each group. Values are presented as means ± SD; n=3; *P<0.05
vs. NC-siRNA group and #P<0.05 vs. control group.
SIRT1, sirtuin 1; H2O2, hydrogen peroxide;
NC, negative control; MMP9, matrix metalloproteinase 9; TIMP3,
tissue inhibitor of metalloproteinase 3; VSMC, vascular smooth
muscle cell; qPCR, quantitative polymerase chain reaction; SD,
standard deviation. |
 | Figure 6.Silencing SIRT1 inhibits
H2O2-induced VSMC migration in vitro.
Following transfection and H2O2 treatment,
VSMCs were divided into four groups as follows: (A) Control, (B)
H2O2, (C) NC-siRNA, (D) SIRT1-siRNA for
Transwell migration assay, and (E) quantitative analysis of VSMCs
vertically migrating into the lower chamber, based on DAPI staining
and counting under confocal microscopy (magnification, ×200).
Values are presented as means ± SD; n=3; *P<0.05 vs. NC-siRNA
group and #P<0.05 vs. control group. SIRT1, sirtuin
1; H2O2, hydrogen peroxide; NC, negative
control; VSMC, vascular smooth muscle cell; SD, standard
deviation. |
Discussion
In the current study, miR-92a was demonstrated to be
capable of suppressing migration in
H2O2-treated VSMCs. To fully investigate the
role of miR-92a and the underlying mechanisms in injured VSMCs, a
model of H2O2-treated VSMCs was established,
and the downregulation of miR-92a was demonstrated by this model,
which was consistent with the study by Zhang et al (10). The migration of VSMCs is known to
be one of the essential prerequisites for atherogenesis following
arterial injury (4). In the
present study, the overexpression of miR-92a by mimics was
confirmed to inhibit VSMC migration and regulate MMP9 and TIMP3
expression levels via SIRT1 signaling.
miRNAs are engaged in a variety of biological
processes and multiple pathogenic events, including cell migration,
proliferation, inflammation, oxidation, and apoptosis (23,24).
As reported previously, miR-92a overexpression inhibits
H2O2-induced VSMC apoptosis by directly
targeting the mitogen-activated protein kinase kinase 4-c-Jun
N-terminal kinase 1 signaling pathway (10). In the current study, miR-92a
overexpression suppressed cell migration by inhibiting the ratio of
MMP9/TIMP3 in H2O2-induced VSMCs. Of note,
VSMC migration is known to be an indispensable element in the
incidence and progression of atherosclerotic lesions (25). Additionally, MMPs and TIMPs
together maintain the balance of the ECM, and contribute to the
regulation of VSMC migration (14,26).
A previous study identified that the significant decrease of TIMP3
expression levels in human carotid atherosclerotic plaques
associated with type 2 diabetes mellitus, exhibit a high
correlation with MMP9 overactivity in these subjects (27). Therefore, the present data
suggested that miR-92a overexpression is considered to be an
important potential target for therapeutic intervention of
atherosclerosis.
To further explain the association by which miR-92a
action affects VSMC migration, the expression level of SIRT1 mRNA,
potentially interacting with miR-92a, was measured in
H2O2-induced VSMCs. In the present study,
H2O2 decreased mRNA expression levels of
miR-92a but increased that of SIRT1 in VSMCs, suggesting that VSMC
migration was related to the expression changes of SIRT1 targeted
by miR-92a. SIRT1, a member of the sirtuin family (SIRT1-7) or
silent information regulator 2 proteins, performs various
biological functions in the development of cardiovascular diseases
(28). Cardellini et al
(27) indicated that SIRT1
signaling was involved in regulating MMP9/TIMP3 activity of
diabetic atherosclerosis. Furthermore, it has been proposed that
SIRT1 is a downstream target of miRNAs in vascular injury (29–31).
In the current study, it was identified that SIRT1 siRNA played a
similar role with miR-92a mimics in the regulation of MMP9 and
TIMP3. Specifically, SIRT1 siRNA attenuated the expression level
and secretion of MMP9, and promoted TIMP3 expression by knockdown
of SIRT1 gene in H2O2-induced VSMCs.
Moreover, SIRT1 was successfully demonstrated as a target gene of
miR-92a in a dual-luciferase reporter assay and silencing SIRT1 did
not result in a migration-promoting effect in vitro, which
was also similar to miR-92a overexpression. Taken together, the
results indicate that miR-92a decreases the expression level of
MMP9 and increases the expression level of TIMP3 via SIRT1
signaling, which accounts for the inhibition of VSMC migration.
In conclusion, miR-92a overexpression modulates MMP9
and TIMP3 expression levels and prevents
H2O2-induced VSMC migration by repressing
SIRT1, implying that miR-92a may exert an atheroprotective role in
atherogenesis. Therefore, upregulation of miR-92a expression may
present a novel therapeutic strategy in protecting against the
dysfunction of VSMCs in atherosclerosis.
Acknowledgements
The authors would like to thank Mr. Jun Tian (The
Second Military Medical University, Shanghai, China) for data
analysis in the current research, and Miss Zhen Guo (Pfeiffer
University, Misenheimer, NC, USA) for language modification of the
manuscript.
References
|
1
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verweij SL, van der Valk FM and Stroes ES:
Novel directions in inflammation as a therapeutic target in
atherosclerosis. Curr Opin Lipidol. 26:580–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stringa E, Knäuper V, Murphy G and
Gavrilovic J: Collagen degradation and platelet-derived growth
factor stimulate the migration of vascular smooth muscle cells. J
Cell Sci. 113:(Pt 11). 2055–2064. 2000.PubMed/NCBI
|
|
4
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
5
|
Zhang J, Zou F, Tang J, Zhang Q, Gong Y,
Wang Q, Shen Y, Xiong L, Breyer RM, Lazarus M, et al:
Cyclooxygenase-2-derived prostaglandin E2 promotes
injury-induced vascular neointimal hyperplasia through the
E-prostanoid 3 receptor. Circ Res. 113:104–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albinsson S and Sessa WC: Can microRNAs
control vascular smooth muscle phenotypic modulation and the
response to injury? Physiol Genomics. 43:529–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robinson HC and Baker AH: How do microRNAs
affect vascular smooth muscle cell biology? Curr Opin Lipidol.
23:405–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ning B, Gao L, Liu RH, Liu Y, Zhang NS and
Chen ZY: microRNAs in spinal cord injury: Potential roles and
therapeutic implications. Int J Biol Sci. 10:997–1006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chio CC, Lin JW, Cheng HA, Chiu WT, Wang
YH, Wang JJ, Hsing CH and Chen RM: MicroRNA-210 targets
antiapoptotic Bcl-2 expression and mediates hypoxia-induced
apoptosis of neuroblastoma cells. Arch Toxicol. 87:459–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Zhou M, Wang Y, Huang W, Qin G,
Weintraub NL and Tang Y: miR-92a inhibits vascular smooth muscle
cell apoptosis: Role of the MKK4-JNK pathway. Apoptosis.
19:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv H, Zhang Z, Wang Y, Li C, Gong W and
Wang X: MicroRNA-92a promotes colorectal cancer cell growth and
migration by inhibiting KLF4. Oncol Res. 23:283–290. 2016.
View Article : Google Scholar
|
|
12
|
Rippe C, Blimline M, Magerko KA, Lawson
BR, LaRocca TJ, Donato AJ and Seals DR: MicroRNA changes in human
arterial endothelial cells with senescence: Relation to apoptosis,
eNOS and inflammation. Exp Gerontol. 47:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lenglet S, Thomas A, Chaurand P, Galan K,
Mach F and Montecucco F: Molecular imaging of matrix
metalloproteinases in atherosclerotic plaques. Thromb Haemost.
107:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao B, Luo X, Shi H and Ma D: Tissue
factor pathway inhibitor-2 is downregulated by ox-LDL and inhibits
ox-LDL induced vascular smooth muscle cells proliferation and
migration. Thromb Res. 128:179–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vigetti D, Moretto P, Viola M, Genasetti
A, Rizzi M, Karousou E, Pallotti F, De Luca G and Passi A: Matrix
metalloproteinase 2 and tissue inhibitors of metalloproteinases
regulate human aortic smooth muscle cell migration during in vitro
aging. FASEB J. 20:1118–1130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
2013:9283152013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halade GV, Jin YF and Lindsey ML: Matrix
metalloproteinase (MMP)-9: A proximal biomarker for cardiac
remodeling and a distal biomarker for inflammation. Pharmacol Ther.
139:32–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hobeika MJ, Thompson RW, Muhs BE, Brooks
PC and Gagne PJ: Matrix metalloproteinases in peripheral vascular
disease. J Vasc Surg. 45:849–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha JM, Yun SJ, Jin SY, Lee HS, Kim SJ,
Shin HK and Bae SS: Regulation of vascular smooth muscle phenotype
by cross-regulation of krüppel-like factors. Korean J Physiol
Pharmacol. 21:37–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu J, Wang L, Yang H, Ding D, Zhang L,
Wang J, Chen Q, Zou Q, Jin Y and Liu X: Rab14 suppression mediated
by MiR-320a Inhibits cell proliferation, migration and invasion in
breast cancer. J Cancer. 7:2317–2326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jo DH and Kim JH, Cho CS, Cho YL, Jun HO,
Yu YS, Min JK and Kim JH: STAT3 inhibition suppresses proliferation
of retinoblastoma through down-regulation of positive feedback loop
of STAT3/miR-17-92 clusters. Oncotarget. 5:11513–11525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McManus DD and Freedman JE: MicroRNAs in
platelet function and cardiovascular disease. Nat Rev Cardiol.
12:711–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Y, Zhang MJ, Li BH, Chen L, Pi Y, Yin
YW, Long CY, Wang X, Sun MJ, Chen X, et al: PPARγ inhibits VSMC
proliferation and migration via attenuating oxidative stress
through upregulating UCP2. PLoS One. 11:e01547202016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi YF, Chi JF, Tang WL, Xu FK, Liu LB, Ji
Z, Lv HT and Guo HY: Effects of rosuvastatin on the production and
activation of matrix metalloproteinase-2 and migration of cultured
rat vascular smooth muscle cells induced by homocysteine. J
Zhejiang Univ Sci B. 14:696–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cardellini M, Menghini R, Martelli E,
Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A,
Ippoliti A, et al: TIMP3 is reduced in atherosclerotic plaques from
subjects with type 2 diabetes and increased by SirT1. Diabetes.
58:2396–2401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma L and Li Y: SIRT1: Role in
cardiovascular biology. Clin Chim Acta. 440:8–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Volkmann I, Kumarswamy R, Pfaff N, Fiedler
J, Dangwal S, Holzmann A, Batkai S, Geffers R, Lother A, Hein L and
Thum T: MicroRNA-mediated epigenetic silencing of sirtuin1
contributes to impaired angiogenic responses. Circ Res.
113:997–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Menghini R, Casagrande V, Cardellini M,
Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A,
Novelli G, Melino G, et al: MicroRNA 217 modulates endothelial cell
senescence via silent information regulator 1. Circulation.
120:1524–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumarswamy R, Volkmann I, Beermann J, Napp
LC, Jabs O, Bhayadia R, Melk A, Ucar A, Chowdhury K, Lorenzen JM,
et al: Vascular importance of the miR-212/132 cluster. Eur Heart J.
35:3224–3231. 2014. View Article : Google Scholar : PubMed/NCBI
|