Introduction
Depression, is a mental health disorder
characterized by pervasive and persistent low mood, frequently
occurring alongside self-abasement and loss of interest in social
contact, which can adversely affect an individuals normal life
(1–3). Globally, >300,000,000 individuals
of all ages suffer from depression (4). Depression is a major contributor to
the overall global burden of diseases. However, owing to its
unknown pathogenesis and complex pathogenic factors, the
therapeutic efficacy of the classical antidepressants used in
clinical practice to treat depression is not ideal. Therefore,
novel antidepressants with the desired effect and superior levels
of toleration are required.
Gastrodia elata (G. elata), known as ‘Tian
Ma’ in Chinese, is a saprophytic perennial herb, which is widely
used in traditional Chinese medicine and cuisine. Medically, it has
been used to treat a variety of nervous and cerebrovascular
diseases, including headache, dizziness, tetanus and epilepsy
(5). In neurological
investigations, gastrodin, one of major active components of G.
elata, was reported to have a protective effect against
1-methyl-4-phenylpyridinium-induced cytotoxicity in human
dopaminergic SH-SY5Y cells (6).
The polysaccharides of G. elata (GEP), as the major active
components in G. elata (7),
have been demonstrated to exert several pharmacological effects,
including reducing hypertension and improving serum lipid levels
(8), and exhibiting anti-dengue
virus bioactivities (9). In
addition, the extraction rate of GEP reached ~8.58% in our previous
experiment, and the antidepressant-like activity of a G.
elata ethanol extract in mice was observed (10). Although current understanding of
how polysaccharides isolated from G. elata may exert a
pharmacological neuroprotective effect, investigations on the
neuroprotective effects and underlying mechanism of GEP have been
limited.
PC12 cells, a cell line with typical neuron
characteristics and which generates a high level of glucocorticoid
receptors, have been used as a useful model to simulate the state
of glucocorticoid damage on hippocampal neurons when treated with
high concentrations of corticosterone (CORT) (11). In addition, CORT is the major
rodent glucocorticoid, and exposure to continued high CORT
concentrations can cause lymphocyte, cortex and hippocampal nerve
cell damage (12), which can be
reversed by antidepressants. There is also sufficient evidence to
suggest that a drug possessing the ability to reverse CORT-induced
neurotoxicity may have a possible therapeutic potential in
preventing or treating major depression (13).
In previous years, several experimental and clinical
data have provided support for the hypothesis that dysregulation of
the hypothalamus-pituitary-adrenal (HPA) axis is involved in the
pathogenesis of depression (14,15).
It is usually acknowledged that the HPA axis is activated in
response to stress, which results in the increased concentrations
of glucocorticoids in the circulating blood (16).
Multiple molecular mechanisms are involved in
CORT-induced cell apoptosis, including the mitochondrial apoptotic
pathway (17), extracellular
signal-regulated kinase (ERK)1/2 pathway (18), phosphoinositide 3-kinase (PI3K)/Akt
signaling pathway (19,20) and endoplasmic reticulum (ER)
stress-mediated pathway (21).
Additionally, on the basis of our previous study, the incubation of
PC12 cells with CORT significantly upregulated [Ca2+]
concentrations (22), which were
bound up with the apoptotic signals generated at the ER (23).
Oxidative stress is a hallmark of various
neuropathological disorders, and the underlying mechanism in
several neurodegenerative diseases and brain injuries, including
Parkinson's disease (24),
Alzheimer's disease (25,26), autism (27), chronic fatigue syndrome (28) and depression (29). Oxidative stress can cause base
damage, which is predominantly indirect, and can cause strand
breaks in DNA (30). Persistent
oxidative stress leads to reactive oxygen species (ROS) and
reactive nitrogen species (RNS) formation. ROS and RNS exacerbate
oxidative stress by attacking organelles, including mitochondria
(31).
ER is a multifunctional organelle and is a requisite
in all cells, which can perform protein folding and calcium
storage. As cells maintain homeostasis, when cellular homeostasis
has been disrupted, a series of signaling pathways are activated to
rebalance the cellular biochemical processes. Among all signaling
pathways, unfolded proteins cause an unfolded protein response
(UPR) as a stress response in the ER, which is also known as ER
stress (32).
In the present study, the neuroprotective effect of
GEP against CORT-induced apoptosis was evaluated in PC12 cells,
which possess typical neuron characteristics with high expression
levels of glucocorticoid receptors, and discussed whether the
neuroprotective effects of GEP were via inhibiting the oxidative
stress and ER stress-mediated pathway.
Materials and methods
Plant materials
G. elata was collected from Anqing (Anhui,
China). The plants were identified by Professor Hong Zhang of
Renmin Hospital of Wuhan University (Wuhan, China). A voucher
specimen (no. 1406906) was deposited in the herbarium of the
Institute of Botany, Chinese Academy of Sciences (Beijing, China).
The plant material was dried in the open air and retained for
use.
Chemicals and reagents
The following were used in the present study: Rat
PC12 adrenal gland tumor cell line (Cell Resource Center, Shanghai
Institutes for Biological Sciences, Shanghai, China), Cell Counting
Kit-8 (CCK-8; Dojindo Corporation, Kumamoto Japan); DMEM (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA; cat. no. SH30022.01);
penicillin and streptomycin (Gibco; Thermo Fisher Scientific, Inc;
cat. no. 15140-122); fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc; cat. no. 10099-141); CORT (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany; cat. no. C2505-500MG); LDH assay kit
(Nanjing Jiancheng Biological Engineering Research Center, Nanjing,
China); Hoechst 33258 (Beyotime Institute of Biotechnology, Haimen,
China; cat. no. C0003-2); DCFH-DA (Sigma-Aldrich; Merck Millipore;
cat. no. D6883/50 mg); Antibodies against GRP78, glucose-regulated
protein, 78 kDa (GRP78; Abcam, Cambridge, UK; cat. no. ab108613),
X-box binding protein 1 (XBP1; Abcam; cat. no. ab37152), growth
arrest- and DNA damage-inducible gene 153 (GADD153; BIOSS, Beijing,
China; cat. no. bs-8875R), GAPDH (Abcam; cat. no. ab37168), caspase
12 (BIOSS; cat. no. bs1105R) and caspase 9 (ProteinTech Group,
Inc., Chicago, IL, USA; cat. no. 10380-1-ap); and horseradish
peroxidase (HRP)-goat anti rabbit immunoglobulin G (KPL, Inc.
Gaithersburg, MD, USA; cat. no. 074-1506). All other reagents used
were of analytical grade.
Isolation and purification of GEP
GEP was prepared as follows: 100 g dry G.
elata tuber powder was extracted for 1 h three times with 95%
ethanol at 70°C. The extract was filtered using filter paper and
the filtrate discarded. The filtered residue was immersed in water
at a ratio of 1:30, and extraction was performed for 1 h three
times in a water bath at 60°C. The filtrate was then collected and
concentrated to 200 ml as the final volume, following which it was
precipitated with 600 ml of 95% ethanol (concentrated solution: 95%
ethanol; 1:3). The precipitate was dissolved in 100 ml of water and
deproteinized 7–8 times with Sevag reagent (chloroform: n-butanol;
5:1) until no protein was detected using an ultraviolet
spectrophotometer. Following dialysis (molecular weight cut-off 1.0
kDa) against water for 24 h and lyophilization, crude
polysaccharide was obtained. The yield of GEP was 8.58% (w/w). The
purity of the crude polysaccharide was 97%.
The crude polysaccharide was dissolved in distilled
water at a concentration of 10 mg/ml and then fractionated using a
Sephadex G-75 column (3.5×75 cm). Elution was performed with 0.15 M
NaCl at a flow rate of 1 ml/min. The polysaccharide elution profile
was determined using the phenol-sulfuric acid method, and the
fractions (test tube nos. 40–53) containing polysaccharide were
lyophilized to obtain the purified polysaccharide, referred to as
GEP (33,34).
Cell culture and treatment
The PC12 cells were maintained in DMEM supplemented
with penicillin (100 U/ml), streptomycin (100 µg/ml) and 10% FBS in
a humidified atmosphere of 95% air and 5% CO2 at
37°C.
For all experiments, cells in the exponential phase
of growth were used. CORT was dissolved in dimethyl sulphoxide,
which had a final concentration of <0.1% (v/v). All
manipulations were run in three duplicates for each treatment group
in the experiment.
To examine the neuroprotective effect of GEP, the
appropriate concentration of CORT was selected on the basis of
Fig. 1B. A dose of 200 µmol/l CORT
was selected to induce PC12 cell apoptosis. The PC12 cells were
divided into the following groups: Control group; CORT (200 µmol/l)
group; and CORT (200 µmol/l)+GEP (250, 500 and 1,000 µg/ml) groups.
For the CORT+GEP groups, GEP was added to the PC12 cells for 30 min
prior to treatment with CORT. In all experiments, with the
exception of the control group, CORT was applied 48 h following
treatment with GEP.
Measurement of cell viability
The PC12 cells were seeded in a 96-well culture
plate at 1×105 cells/ml. The cell viability was
quantified using a CCK-8 kit according to the manufacturer's
protocol. Briefly, 10 µl of CCK-8 solution (5 mg/ml) was added to
each well of a 96-well plate and incubated for 1.5 h at 37°C in the
dark. The optical density value was measured at an absorption
wavelength of 450 nm on a microplate reader. Cell viability is
expressed as a percentage of the untreated control group.
Measurement of LDH release
The cytotoxicity was determined by the release of
LDH using a diagnostic kit according to the manufacturer's
protocol. Briefly, following treatment, 2 ml of the culture
supernatant in a 6-well plate was collected from each well and
centrifuged at 1,000 × g for 5 min at 4°C; following which another
2 ml culture was added into each well, and the cells crushed by
ultrasound to free the LDH in the cells. The absorbance of each of
the samples was measured at 490 nm with a microplate reader, and
background absorbance from the culture medium, which was not used
for any cell cultures, was subtracted from all absorbance
measurements. Each experiment was performed three times. LDH
release was defined as the ratio of LDH in the media to total LDH
(LDH in the media and LDH in the cell) according to the following
equation: LDH release (%) = (LDH activity in the media/total LDH
activity) × 100%.
Measurement of intracellular ROS
levels
ROS levels were measured using the DCFH-DA method.
DCFH-DA is a non-fluorescent compound. It can be enzymatically
converted to a highly fluorescent compound, DCF, in the presence of
ROS. In brief, following treatment, the cells were washed with
phosphate-buffered saline (PBS) and incubated with DCFH-DA at a
final concentration of 10 µmol/l for 30 min at 37°C in the dark.
The fluorescence intensity was measured in a microplate reader at
an excitation wavelength of 485 nm and an emission wavelength of
535 nm, following washing of the cells three times with PBS to
remove extracellular DCFH-DA and adjustment of the cell number in
the different groups consistently. The level of intracellular ROS
was calculated as a percentage of that in the untreated
control.
Assessment with Hoechst 33258
staining
Hoechst 33258 staining, which distinguishes
apoptotic cells from normal cells based on nuclear chromatin
condensation and fragmentation, was used for the qualitative and
quantitative analyses of apoptotic cells in the present study.
Following treatment, the cells on slides were fixed in
stain-fixative overnight at 4°C. The slides were then washed twice
with PBS to remove the extra stain-fixative, following which the
slides were incubated with 5 µg/ml Hoechst 33342 for 10 min, and
then washed twice with PBS. Finally, the staining was visualized
and images were captured using fluorescence microscopy. All the
procedures were performed in the dark. The apoptotic nuclei were
counted in at least 400 cells from three non-overlapping fields in
each group, and expressed as a percentage of the total number of
nuclei counted: Rate of apoptosis (%) = apoptotic cells/total cells
× 100%.
ER morphology staining
The morphological staining of the ER in the PC12
cells was determined using the cationic probe,
3,3-dihexyloxacarbocyanine iodide, which can selectively accumulate
together in the ER and present with green fluorescence (35,36).
The PC12 cells were cultured in a 6-well plate and, at the end of
treatment, the cells were washed with PBS three times, prior to
being loaded with a carbocyanine staining solution of DiOC6
(3) at 37°C for 15 min. Images
were captured using a fluorescence microscope with excitation and
emission wavelengths of 488 and 505 nm, respectively.
Western blot analysis
The PC12 cells were cultured in 6-well plates and
treatments were performed as described above, following which the
cells were harvested, washed once with PBS, and then lysed with
cell lysis buffer containing 1% phenylmethylsulfonyl fluoride for
30 min at 4°C, followed by centrifugation at 2,000 × g for 5 min at
4°C. The concentration of protein was determined using a
bicinchoninic acid protein assay. Equal quantities of protein (40
µg) were separated by electrophoresis with 15% sodium dodecyl
sulfate polyacrylamide gels, followed by transfer onto
nitrocellulose membranes. These membranes were incubated with 5%
(w/v) non-fat milk powder in Tris-buffered saline containing 0.1%
(v/v) Triton X-100 (TBST) for 1 h to block nonspecific binding
sites. The membranes were then incubated overnight at 4°C with the
one of the following primary antibodies: Rabbit anti-GRP78
(1:1,000), rabbit anti-XBP-1 (1:1,000), rabbit anti-GADD153
(1:1,000), rabbit anti-caspase 9 (1:1,000) or rabbit anti-caspase
12 (1:1,000). Following washing three times with TBST, the
membranes were incubated for 30 min at room temperature in the dark
with the HRP-goat anti rabbit secondary antibodies (1:10,000).
Following rewashing four times with TBST, the membranes were
developed using enhanced chemiluminescence. Each independent
experiment was performed three times. Western blot analysis was
performed using AlphaEaseFC V.4 Software (ProteinSimple, San Jose,
USA). Values were normalized to corresponding levels of GADPH and
expressed as a percentage of the control value. The results are
presented as the mean ± standard deviation.
Statistical analysis
Data were analyzed using SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA). Differences between groups were
compared using one-way analysis of variance and the least
significant difference test to determine whether the results were
statistically significant. P≤0.05 was considered to indicate a
statistically significant difference. All results are expressed as
the mean ± standard deviation.
Results
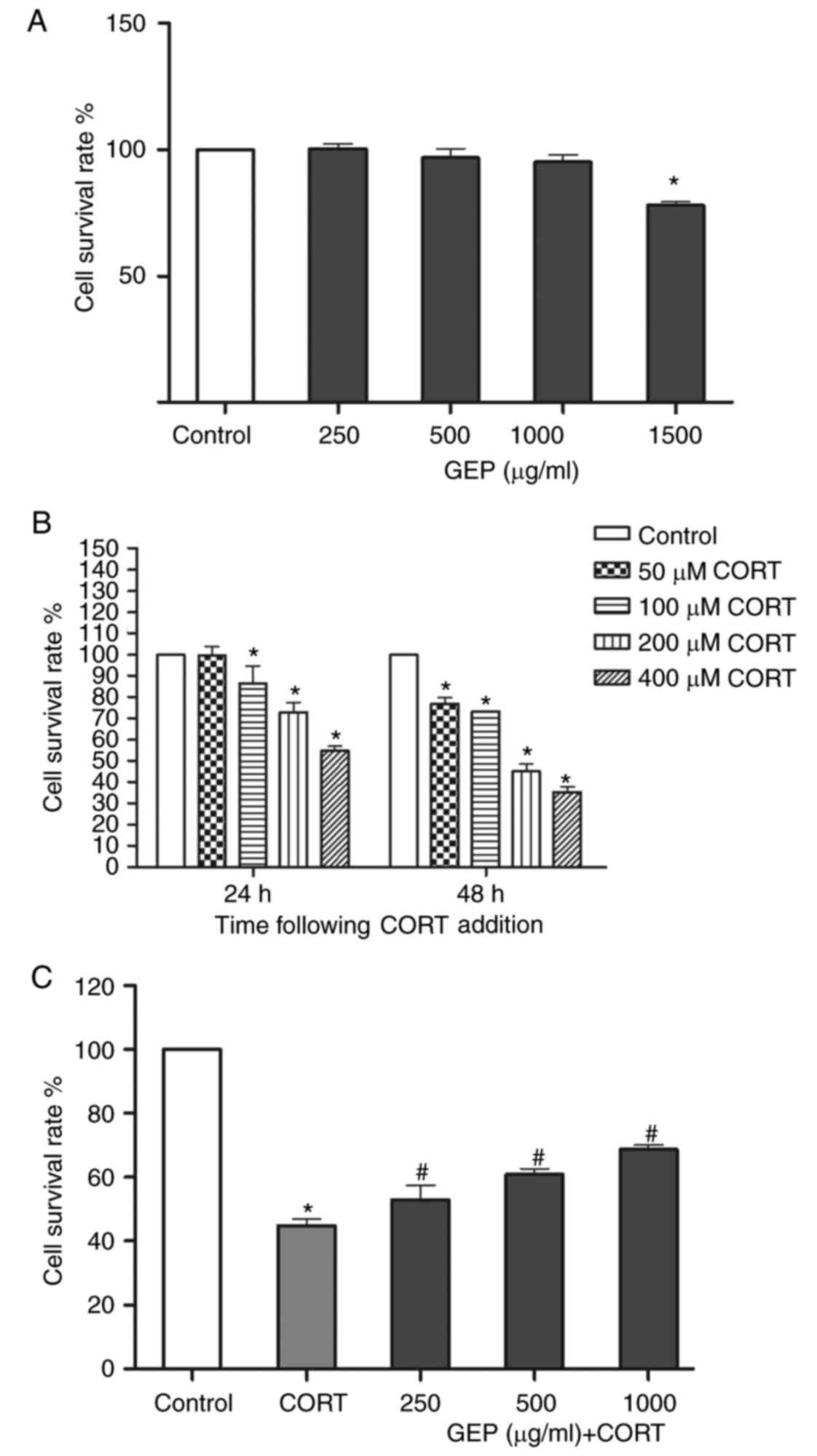
Effect of GEP on PC12 cell viability
determined using a CCK-8 assay
The viability of the PC12 cells following exposure
to GEP and CORT was determined using a CCK-8 assay. As shown in
Fig. 1A, no significant changes
were observed in the viability of cells treated with individual GEP
at concentrations of 250, 500 and 1,000 µg/ml, whereas 1,500 µg/ml
led to visible cell damage. Therefore, 250–1,000 µg/ml of GEP was
selected for the subsequent experiments.
CORT gradually reduced cell viability as the
concentration and treatment time increased (Fig. 1B). When treated with 200 µM CORT
for 48 h, cell viability decreased to 44.95±3.64%, therefore this
concentration was used in the subsequent experiments.
Effect of GEP on CORT-induced PC12
cell viability determined using a CCK-8 assay
The viability of PC12 cells following exposure to
CORT was determined using a CCK-8 assay. As shown in Fig. 1C, stimulation with 200 µM CORT
alone resulted in a significant decrease in cell viability,
compared with that in the control group. However, pretreatment with
GEP at various concentrations (250, 500 and 1,000 µg/ml) in the
presence of CORT led to a significant increase in cell survival
rates to 53.05±7.76, 60.83±3.53 and 68.73±2.24%, respectively.
These results demonstrated that GEP alleviate the toxic effect of
CORT on PC12 cells.
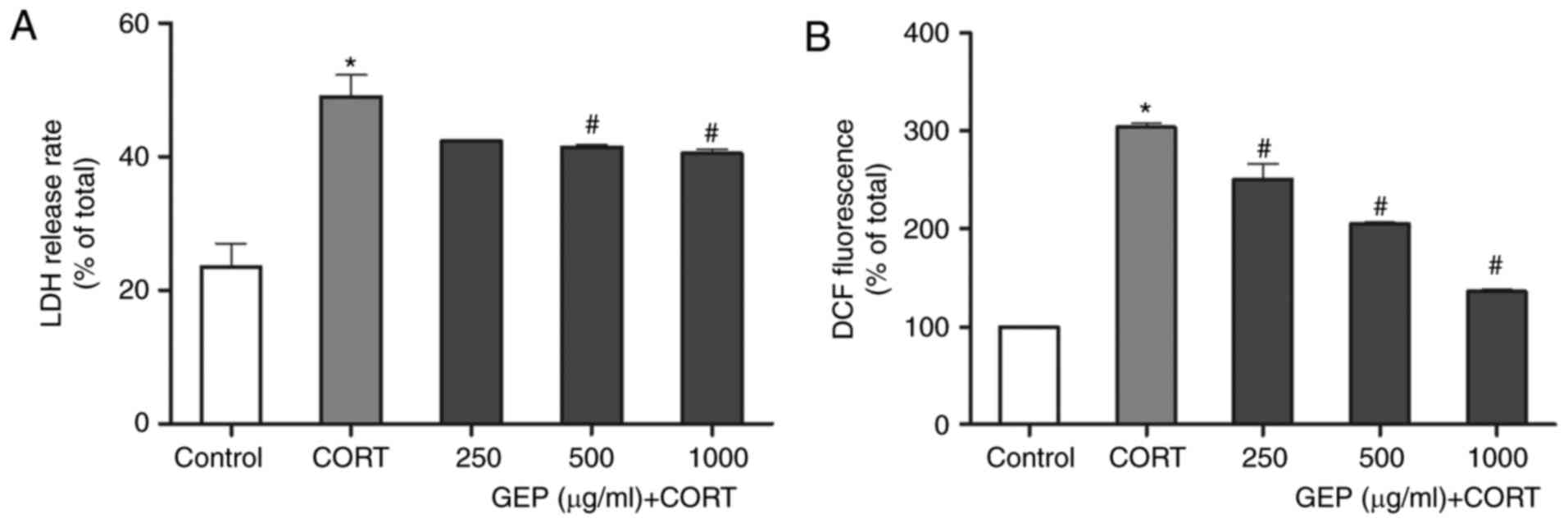
Effect of GEP on CORT-induced LDH
leakage in PC12 cells
An LDH assay was performed to investigate damage to
cell membranes following CORT treatment. As shown in Fig. 2A, compared with control group, an
increase in the level of LDH release was observed in the
CORT-treated group (P<0.05). By contrast, pretreatment with GEP
alleviated this effect, and the percentages of LDH release in the
groups treated with 250, 500 and 1,000 µg/ml GEP were 42.33±0.04,
41.37±0.66 and 40.46±1.16% respectively. The 250 µg/ml pretreatment
concentration reduced the release but without statistical
significance. These data further indicated that GEP had a
protective effect towards CORT-damaged PC12 cells. However, this
method measures necrosis, which can occur in vitro
sequentially to the induction of apoptosis or as a primary event
(37). The results of the present
study showed that GEP alleviated the increase in LDH levels with
minimal difference between each dose. According to literature, the
release of the cytosolic LDH enzyme is used to evaluate membrane
integrity, thereby indicating cell death by necrosis (38). Therefore, it was suggested that the
protective effect of GEP on PC12 cell membrane integrity was
limited.
Measurement of intracellular ROS
levels
As shown in Fig.
2B, the intracellular level of ROS in the CORT-treated group
markedly increased to 304.30±6.72% of the control, which suggested
that CORT induced oxidative stress. When the cells were pretreated
with different concentrations of GEP (250, 500 and 1,000 µg/ml) in
the presence of 200 µM CORT for 48 h, the level reduced
significantly to 250.82±26.83, 205.60±2.41 and 136.13±3.37%,
respectively.
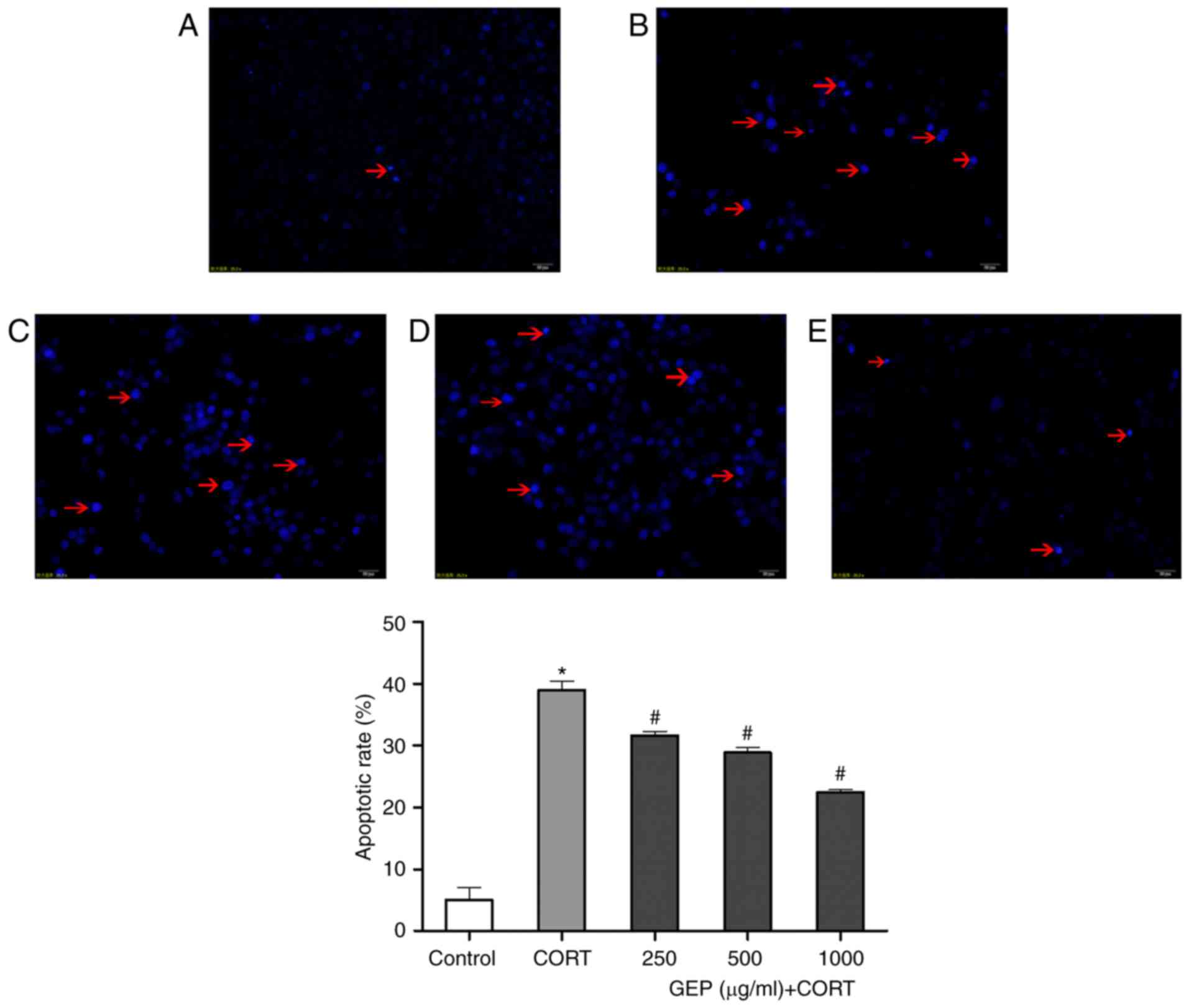
Effect of GEP on CORT-induced
apoptosis in PC12 cells, determined using Hoechst 33258
staining
Hoechst 33258 is DNA-specific, and stains the
condensed chromatin in apoptotic cells. Early apoptotic cells show
an increased uptake of the vital DNA dye Hoechst 33342, compared
with live cells due to changes in membrane permeability (39). As shown in Fig. 3A-E, normal PC12 cells had a uniform
soft fluorescence, were light blue in color, and had round nuclei
with sharp edges. Treatment with CORT for 48 h led to cells
exhibiting typical characteristics of apoptosis, including small,
bright nuclei with irregular margins and apparent fluorescent
debris as indicated by the red arrows. However, with GEP
pretreatment, the cell nuclei appeared similar to those in the
control, suggesting that GEP reduced the apoptotic effect of
CORT.
Compared with the control group, the percentage of
apoptotic nuclei in the CORT-treated cells was significantly
increased, with apoptosis increased to 38.98±2.52% (P<0.05).
However, pretreatment with GEP (250, 500 and 1,000 µg/ml) reduced
the apoptotic rates to 31.60±1.14, 28.90±1.38 and 22.51±0.67%,
respectively (P<0.05; Fig.
3).
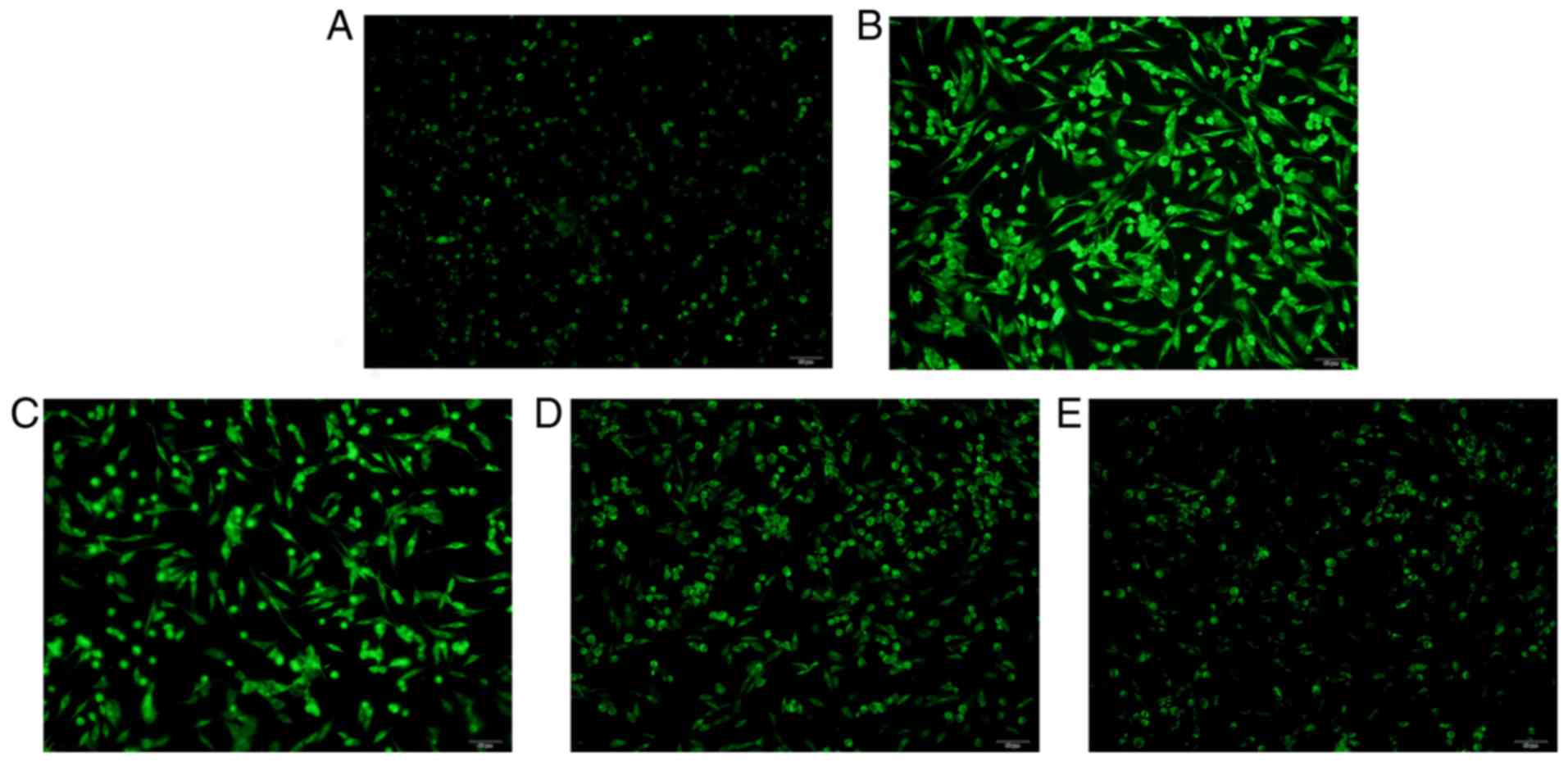
Effect of GEP on CORT-induced ER
stress in PC12 cells
CORT treatment resulted in the reinforced assembling
of 3,3-dihexyloxacarbocyanine iodide to the electronegative ER
membrane to cause a significant increase in green fluorescence,
compared with that in the control group (Fig. 4A-E). GEP pretreatment reduced the
green fluorescence comparatively. The results revealed that GEP
markedly alleviated the ER stress induced by CORT.
Effect of GEP on ER stress-associated
proteins
In order to examine whether the CORT-induced
apoptosis of PC12 cells is associated with ER stress, the present
study analyzed the expression levels of GRP78, XBP-1, GADD153,
caspase 9 and caspase 12, which are biomarkers of ER activation,
using western blot analysis. As shown in Fig. 5A-E, the expression levels of GRP78,
XBP-1, GADD153, caspase 9 and caspase 12 were significantly
increased in the PC12 cells following incubation of the cells with
200 µM CORT. However, the upregulation of these biomarkers was
attenuated by GEP pretreatment in a concentration-dependent
manner.
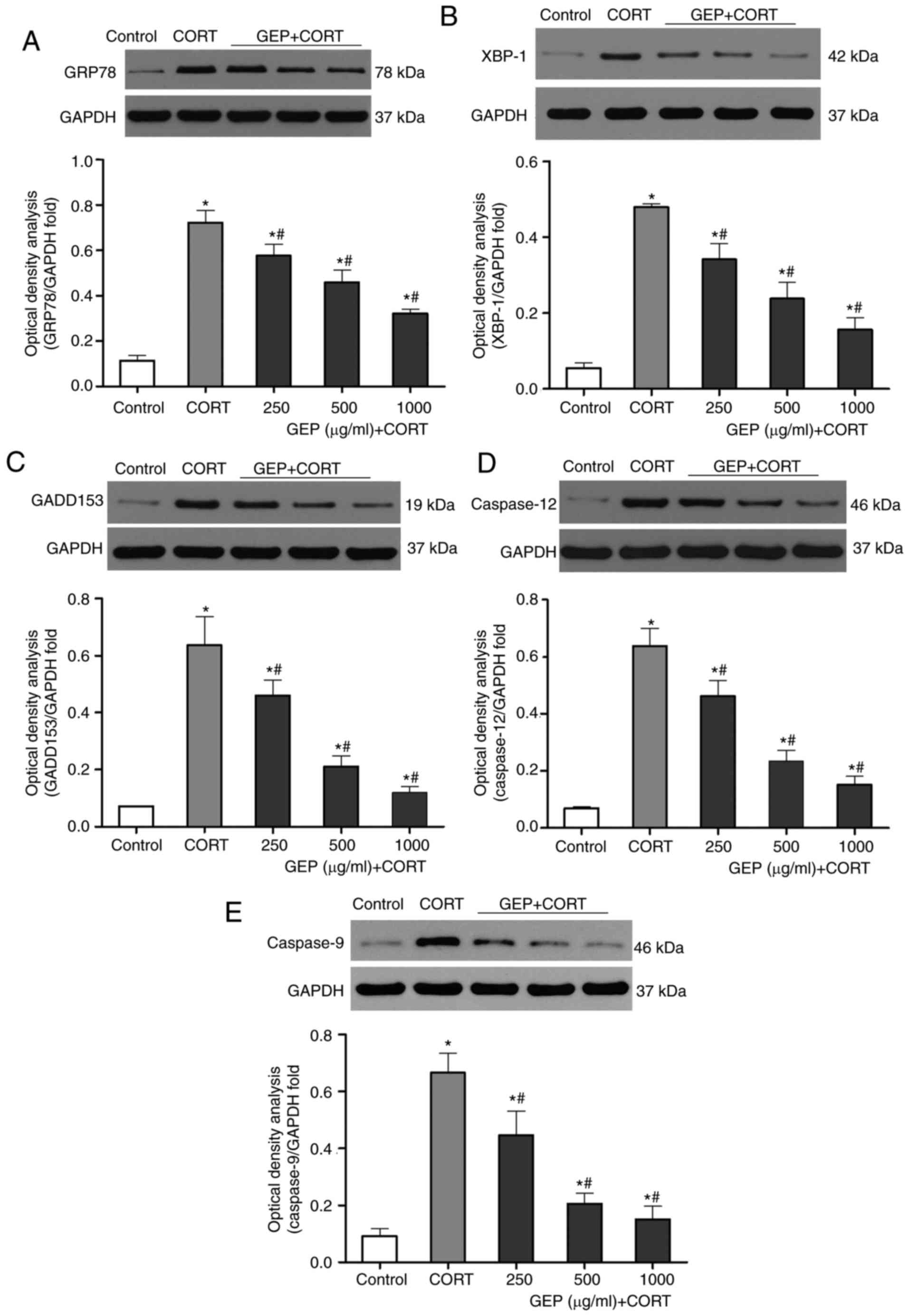 | Figure 5.Effects of GEP on expression levels
of GRP78, XBP-1, GADD153, caspase 12 and caspase 9 in CORT-treated
PC12 cells. Expression levels were assessed using western blot
analysis, with data presented as the mean ± standard deviation
(n=3). *P<0.05, compared with the control group;
#P<0.05, compared with the CORT group. The groups
comprised a control group, and groups of cells treated with 200 µM
CORT, 200 µM CORT+250 µg/ml GEP, 200 µM CORT+500 µg/ml GEP, and 200
µM CORT+1,000 µg/ml GEP. Expression levels of (A) GRP78, (B) XBP-1,
(C) GADD153, (D) caspase 12, and (E) caspase 9 were determined. All
levels were relative to GAPDH. CORT, corticosterone; GEP,
polysaccharides from Gastrodia elata; GRP78,
glucose-regulated protein, 78 kDa; XBP-1, X-box binding protein 1;
GADD153, growth arrest-and DNA damage-inducible gene 153. |
Discussion
The present study demonstrated that GEP exerted a
protective effect against CORT-induced apoptosis in PC12 cells,
which was confirmed using a CCK-8 assay and LDH detection, and from
evidence that the PC12 cells underwent conformational changes
through morphological observation and Hoechst 33258 staining.
Therefore, GEP may offer potential to as a novel therapeutic agent
in the treatment of depression.
In the present study, PC12 cells were used to
establish a model of depression. The PC12 cell line has typical
neuron characteristics and generates a high level of glucocorticoid
receptors. Several other cell lines were also suitable for use; for
example, SH-SY5Y, C6 and BV2 cell lines. The use of in vivo
models of depression is also required in the future.
Based on previous studies, the association between
ROS generation and cellular antioxidant systems determines whether
cells are ultimately damaged, despite ROS being found in all living
cells (40). A number of previous
studies suggested that glucocorticoid treatment had a negative
effect on the survival of hippocampal cultures during oxidative
stress, which was caused by generating superfluous ROS (41). Mitochondrial ROS induce the
activation of a large number of mitochondrial apoptotic proteins,
leading to cell apoptosis and death (42). In the nervous system, apoptosis can
be activated in certain physiological and pathological conditions,
and apoptotic cells have been found in the hippocampus of patients
with depression (43). Therefore,
apoptosis in hippocampal neurons may be one of the pathogenetic
factors involved in clinical depression. The results of the present
study indicated that the PC12 cells treated with CORT generated
excessive ROS combined with an increased apoptotic rate. However,
GEP significantly reversed this effect by lowering the level of
ROS.
ER stress has been demonstrated to be a significant
potential connection between high levels of ROS and cell apoptosis.
On the basis of our previous study, the incubation of PC12 cells
with CORT was found to upregulate [Ca2+] concentrations
significantly (22), which was
bound up with apoptotic signals generated at the ER (23). It was also found that the
upregulated concentration of [Ca2+] was reduced by
treatment with GEP (22). In
addition, alterations of intra-ER Ca2+ led to the
production of ER stress-induced ROS. In the present study, ER
morphological staining revealed that treatment with CORT (200 µM)
caused a significant increase in green fluorescence, compared with
that in the control group, which indicated that CORT likely induced
the ER stress in the PC12 cells. It is known that DiOC6 (3) can be a useful fluorescent dye for
staining the ER. It is a positively charged molecule, which
permeates through the plasma membrane. At low concentrations, it
accumulates in mitochondria due to their large negative membrane
potential. At higher concentrations, the dye stains other
membranes, including the ER. In the present study on ER stress,
DiOC6 (3) was selected to
determine whether ER stress was activated as a qualitative trail.
Considering that DiOC5 (3) is not
a fluorochrome aimed at the ER, this may be a limitation in the
present study and therefore, it was validated using western blot
analysis. GEP pretreatment reduced green fluorescence
comparatively, which indicated that ER stress may be involved in
the neuroprotective effect of GEP against CORT-induced apoptosis.
Therefore, the expression of ER stress-related proteins were
measured to elucidate the underlying mechanism.
The UPR is now a well-characterized signaling
pathway. In the canonical model of the UPR, UPR signaling is
initiated by three major sensing molecules: PKR-like ER kinase
(PERK), inositol-requiring 1α (IRE1α), and activating transcription
factor-6α (ATF6α) (44). The
activation of IRE1α and ATF6α can lead to the induction of
chaperones, including GRP78, which increase protein-folding
capacity; the activation of PERK leads to a reduction in protein
production (45). In the course of
this transfer, the activated IRE1 catalyzes the splicing of an
unconventional intron from ubiquitously expressed XBP-1-unspliced,
into the XBP-1-spliced isoform (46). The XBP-1-spliced isoform is a
transcription factor, which increases the protein folding capacity
of the ER and turnover of misfolded proteins by inducing
ER-resident chaperones (45).
Therefore, XBP-1-spliced is a key constituent of the UPR, which
indicates that it is also a key constituent of ER stress. The
continuous overproduction of XBP-1-spliced induces apoptosis
(47).
C/EBP homologous protein (CHOP), also known as
GADD153, is a transcription factor encoded by the DDIT3 gene
(48), which has been confirmed as
one of the highest inducible genes during ER stress by microarray
studies. Accumulated evidence suggests that the overexpression of
CHOP, which is also a downstream target of XBP-1, can lead to
enhanced oxidant injury and apoptosis (49). An increased CHOP level is a
significant mediator of ER stress-mediated cell death (50). In the present study, significantly
increased levels of XBP-1 and GADD153 were found in the
CORT-induced injury PC12 cells, which, as mentioned above, are
major apoptotic factors. Combined with the results of ROS
measurement and ER morphological staining, the results indicated
that CORT induced PC12 cell apoptosis by activating ER stress.
As mentioned above, in the canonical model, the
intraluminal domains of these initiators, namely the amino termini
of IRE1 and PERK, and the carboxyl terminus of ATF6, are bound by
the chaperone GRP78 in the absence of stress and are rendered
inactive (50,51). Following the activation of ER
stress, it has been shown that the expression level of GRP78 is
significantly enhanced (53,53).
GRP78 is an abundant ER chaperone, which is crucial for ER
function, acting as a master modulator of the UPR. In the present
study, in addition to the significant PC12 cell apoptosis induced
by CORT, the expression level of GRP78 was increased. However,
pretreatment of the cells with GEP significantly reduced the
expression of these proteins in a concentration-dependent manner.
These results indicated that the neuroprotective effect of GEP
against CORT-induced apoptosis was through inhibition of the ER
stress apoptotic pathway.
In addition to these ER chaperone proteins, caspase
12 possesses the capability to activate caspase 9, subsequently
initiating ER stress-induced apoptosis (54,55).
In the present study, CORT activated caspases via excessive ER
stress cleaving procaspase into active caspase 9, which in turn
activated downstream caspase-cleavage, leading to apoptosis
(56). However, GEP pretreatment
downregulated the expression of caspase 12, together with caspase
9.
It is known that GEP is a type of polysaccharide,
which is a complex molecule with a high molecular weight. The
concentration of GEP used in the present study was as high as 1,000
µg/ml, which was higher than any physiological concentration. This
may be a limitation of the present study, however, three
concentrations (250, 500 and 1,000 µg/ml) of GEP were used to show
the neuroprotective effect of GEP. For future clinical application,
further investigation is required.
Taken together, the above findings revealed that the
neuroprotective effect of GEP occurred through inhibiting the
oxidative stress and ER stress-mediated apoptotic pathways.
In conclusion, the results obtained from the in
vitro model of depression showed that GEP, which partly
reversed the pathological changes induced by CORT, was beneficial
in the cytoprotection of neurons, as it may contribute to the
attenuation of intracellular ROS and downregulation of the protein
expression levels of GRP78, XBP-1, GADD153, caspase 12 and caspase
9 via inhibition of the ER stress-mediated pathway. The results of
the present study suggested that the neuroprotective effect of GEP
may be one of the available mechanisms underlying its
antidepressant-like effects. These results indicate that further
investigation of GEP is warranted, which may be a potential
candidate in developing antidepressants.
Acknowledgements
This study was financially supported by the
Provincial Natural Science Foundation (grant no. 2010CDB06201).
References
|
1
|
Clark DB, Bukstein O and Cornelius J:
Alcohol use disorders in adolescents: Epidemiology, diagnosis,
psychosocial interventions, and pharmacological treatment. Paediatr
Drugs. 4:493–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dennis CL and Dowswell T: Interventions
(other than pharmacological, psychosocial or psychological) for
treating antenatal depression. Cochrane Database Syst Rev:
CD006795. 2013. View Article : Google Scholar
|
|
3
|
Zhang K, Zhu Y, Zhu Y, Wu S, Liu H, Zhang
W, Xu C, Zhang H, Hayashi T and Tian M: Molecular, Functional, and
structural imaging of major depressive disorder. Neurosci Bull.
32:273–285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
World Health Organization (WHO):
Depression fact sheet. WHO; Geneva: 2017, http://www.who.int/mediacentre/factsheets/fs369/en/Updated
February 2017.
|
|
5
|
Jiangsu New Medical College: Chinese
Medicine Dictionary. Shanghai Scientific and Technological Press;
Shanghai: 1979, (In Chinese).
|
|
6
|
An H, Kim IS, Koppula S, Kim BW, Park PJ,
Lim BO, Choi WS, Lee KH and Choi DK: Protective effects of
Gastrodia elata Blume on MPP+−induced
cytotoxicity in human dopaminergic SH-SY5Y cells. J Ethnopharmacol.
130:290–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang G, Hu Y, Liu L, Cai J, Peng C and Li
Q: Gastrodin protects against MPP+-induced oxidative stress by up
regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway
in human dopaminergic cells. Neurochem Int. 75:79–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee OH, Kim KI, Han CK, Kim YC and Hong
HD: Effects of acidic polysaccharides from Gastrodia rhizome
on systolic blood pressure and serum lipid concentrations in
spontaneously hypertensive rats fed a high-fat diet. Int J Mol Sci.
13:698–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu H, Tang W, Tong X, Ding K and Zuo J:
Structure elucidation and sulfated derivatives preparation of two
alpha-D-glucans from Gastrodia elata Bl. and their
anti-dengue virus bioactivities. Carbohyd Res. 342:2230–2236. 2007.
View Article : Google Scholar
|
|
10
|
Zhou BH, Li XJ, Liu M, Wu Z and Ming Hu X:
Antidepressent-like activity of the Gastrodia elata ethanol
extract in mice. Fitotorapia. 77:592–594. 2007. View Article : Google Scholar
|
|
11
|
Li ZY, Guo Z, Liu YM, Liu XM, Chang Q,
Liao YH and Pan RL: Neuroprotective effects of total saikosaponins
of Bupleurum yinchowense on corticosterone-induced apoptosis in
PC12 cells. J Ethnopharmacol. 148:794–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao QQ, Huang Z, Ip SP, Xian YF and Che
CT: Protective effects of piperine against corticosterone-induced
neurotoxicity in PC12 cells. Cell Mol Neurobiol. 32:531–537. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou YZ, Li X, Gong WX, Tian JS, Gao XX,
Gao L, Zhang X, Du GH and Qin XM: Protective effect of
isoliquiritin against corticosterone-induced neurotoxicity in PC12
cells. Food Funct. 8:1235–1244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aihara M, Ida I, Yuuki N, Oshima A, Kumano
H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H and Mikuni
M: HPA axis dysfunction in unmedicated major depressive disorder
and its normalization by pharmacotherapy correlates with alteration
of neural activity in prefrontal cortex and limbic/paralimbic
regions. Psychiatry Res. 155:245–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murray F, Smith DW and Hutson PH: Chronic
low dose corticosterone exposure decreased hippocampal cell
proliferation, volume and induced anxiety and depression like
behaviours in mice. Eur J Pharmacol. 583:115–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson SA, Fournier NM and Kalynchuk LE:
Effect of different doses of corticosterone on depression-like
behavior and HPA axis responses to a novel stressor. Behav Brain
Res. 168:280–288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang Y, Li Z, Liu Y, Liu X, Chang Q, Liao
Y and Pan R: Neuroprotective effect of water extract of Panax
ginseng on corticosterone-induced apoptosis in PC12 cells and its
underlying molecule mechanisms. J Ethnopharmacol. 159:102–112.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Zhou J, Qian J, Cheng X, Wu H, Li L,
Qian C, Su J, Wu D, Burns L, et al: Target genes involved in
corticosterone-induced PC12 cell viability and neurite disorders: A
potential molecular mechanism of major depressive disorder.
Psychiat Res. 235:206–208. 2016. View Article : Google Scholar
|
|
19
|
Zhang H, Liu B, Wu J, Xu C, Tao J, Duan X,
Cao Y and Dong J: Icariin inhibits corticosterone-induced apoptosis
in hypothalamic neurons via the P13-K/Akt signaling pathway. Mol
Med Rep. 6:967–972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Z, Yang R, Fu X, Wang YQ and Wu GC:
Astrocyte-conditioned medium protecting hippocampal neurons in
primary cultures against corticosterone-induced damages via
PI3-K/Akt signal pathway. Brain Res. 1114:1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Shen S, Li Z, Jiang Y, Si J, Chang
Q, Liu X and Pan R: Cajaninstilbene acid protects
corticosterone-induced injury in PC12 cells by inhibiting oxidative
and endoplasmic reticulum stress-mediated apoptosis. Neurochem Int.
78:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou B, Liang Y, Shen H, et al: Protective
effects of polysaccharides of Gastrodia elata blume against
corticosterone-induced apoptosis in PC12 cells. J Chinese Med
Material. 630–633. 2013.(In Chinese).
|
|
23
|
van Vliet AR and Agostinis P: When under
pressure, get closer: PERKing up membrane contact sites during ER
stress. Biochem Soc Trans. 44:499–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dias V, Junn E and Mouradian MM: The role
of oxidative stress in parkinson's disease. J Parkinson Dis.
3:461–491. 2013.
|
|
25
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pohanka M: Alzheimer's disease and
oxidative stress: A review. Curr Med Chem. 21:356–364. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
James SJ, Cutler P, Melnyk S, Jernigan S,
Janak L, Gaylor DW and Neubrander JA: Metabolic biomarkers of
increased oxidative stress and impaired methylation capacity in
children with autism. Am J Clin Nutr. 80:1611–1617. 2004.PubMed/NCBI
|
|
28
|
Kennedy G, Spence VA, McLaren M, Hill A,
Underwood C and Belch JJ: Oxidative stress levels are raised in
chronic fatigue syndrome and are associated with clinical symptoms.
Free Radical Bio Med. 39:584–589. 2005. View Article : Google Scholar
|
|
29
|
Jiménez-Fernández S, Gurpegui M,
Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M and Correll CU:
Oxidative stress and antioxidant parameters in patients with major
depressive disorder compared to healthy controls before and after
antidepressant treatment: Results from a meta-analysis. J Clin
Psychiat. 76:1658–1667. 2015. View Article : Google Scholar
|
|
30
|
Parellada M, Moreno C, Mac-Dowell K, Leza
JC, Giraldez M, Bailón C, Castro C, Miranda-Azpiazu P, Fraguas D
and Arango C: Plasma antioxidant capacity is reduced in Asperger
syndrome. J Psychiatr Res. 46:394–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De la Monte SM and Tong M: Brain metabolic
dysfunction at the core of Alzheimer's disease. Biochem Pharmacol.
88:548–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng TB, He JS, Niu SM, Zhao L, Pi ZF, Shao
W and Liu F: A gallic acid derivative and polysaccharides with
antioxidative activity from rose (Rosa rugosa) flowers. J Pharm
Pharmacol. 56:537–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tuvaanjav S, Shuqin H, Komata M, Ma C,
Kanamoto T, Nakashima H and Yoshida T: Isolation and antiviral
activity of water-soluble Cynomorium songaricum Rupr.
polysaccharides. J Asian Nat Prod Res. 18:159–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Banjerdpongchai R, Kongtawelert P,
Khantamat O, Srisomsap C, Chokchaichamnankit D, Subhasitanont P and
Svasti J: Mitochondrial and endoplasmic reticulum stress pathways
cooperate in zearalenone-induced apoptosis of human leukemic cells.
J Hematol Oncol. 3:502010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Banjerdpongchai R, Punyati P, Nakrob A,
Pompimon W and Kongtawelert P: 4′-Hydroxycinnamaldehyde from
Alpinia galanga (Linn.) induces human leukemic cell apoptosis via
mitochondrial and endoplasmic reticulum stress pathways. Asian Pac
J Cancer P. 12:593–598. 2011.
|
|
37
|
Sardão VA, Oliveira PJ, Holy J, Oliveira
CR and Wallace KB: Morphological alterations induced by doxorubicin
on H9c2 myoblasts: Nuclear, mitochondrial, and cytoskeletal
targets. Cell Biol Toxicol. 25:227–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bernuzzi F, Recalcati S, Alberghini A and
Cairo G: Reactive oxygen species-independent apoptosis in
doxorubicin-treated H9c2 cardiomyocytes: Role for heme oxygenase-1
down-modulation. Chem Biol Interact. 177:12–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Atale N, Gupta S, Yadav UC and Rani V:
Cell-death assessment by fluorescent and nonfluorescent cytosolic
and nuclear staining techniques. J Microsc. 255:7–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McIntosh LJ and Sapolsky RM:
Glucocorticoids increase the accumulation of reactive oxygen
species and enhance adriamycin-induced toxicity in neuronal
culture. Exp Neurol. 141:201–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McIntosh LJ, Cortopassi KM and Sapolsky
RM: Glucocorticoids may alter antioxidant enzyme capacity in the
brain: Kainic acid studies. Brain Res. 791:215–222. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One. 9:e890212014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lucassen PJ, Müller MB, Holsboer F, Bauer
J, Holtrop A, Wouda J, Hoogendijk WJ, De Kloet ER and Swaab DF:
Hippocampal apoptosis in major depression is a minor event and
absent from subareas at risk for glucocorticoid overexposure. Am J
Pathol. 158:453–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: Coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shapiro DJ, Livezey M, Yu L, Zheng X and
Andruska N: Anticipatory UPR activation: A protective pathway and
target in cancer. Trends Endocrinol Metab. 27:731–741. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kaufman RJ: Stress signaling from the
lumen of the endoplasmic reticulum: Coordination of gene
transcriptional and translational controls. Gene Dev. 13:1211–1233.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Allagnat F, Christulia F, Ortis F, Pirot
P, Lortz S, Lenzen S, Eizirik DL and Cardozo AK: Sustained
production of spliced X-box binding protein 1 (XBP1) induces
pancreatic beta cell dysfunction and apoptosis. Diabetologia.
53:1120–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ramji DP and Foka P:
CCAAT/enhancer-binding proteins: Structure, function and
regulation. Biochem J. 365:561–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang M, Xu A, Wu X, Zhang Y, Guo Y, Guo
F, Pan Z and Kong L: Japanese encephalitis virus induces apoptosis
by the IRE1/JNK pathway of ER stress response in BHK-21 cells. Arch
Virol. 161:699–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shen J, Chen X, Hendershot L and Prywes R:
ER stress regulation of ATF6 localization by dissociation of
BiP/GRP78 binding and unmasking of Golgi localization signals. Dev
Cell. 3:99–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Y, Bai C, Lu D, Wu X, Gao L and
Zhang W: Endoplasmic reticulum stress and autophagy participate in
apoptosis induced by bortezomib in cervical cancer cells.
Biotechnol Lett. 38:357–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chandrika BB, Maney SK, Lekshmi SU and
Retnabhai ST: Endoplasmic reticulum targeted Bcl2 confers long term
cell survival through phosphorylation of heat shock protein 27. Int
J Biochem Cell Biol. 42:1984–1992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang Y, Lv H, Liao M, Xu X, Huang S, Tan
H, Peng T, Zhang Y and Li H: GRP78 counteracts cell death and
protein aggregation caused by mutant huntingtin proteins. Neurosci
Lett. 516:182–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent activation
of caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|