Introduction
It is known that viruses trigger typically strong
immunological responses to stop viral dissemination and resolve
infection. However, certain viruses may cause persistent infections
in the central nervous system (CNS) without an immunological
reaction or symptoms of acute inflammation, including lymphocytic
choriomeningitis virus and rabies (1,2). The
mechanisms underlying persistent viruses, and the latent
associations between brain function impairment and neurological or
neuropsychiatric diseases, remain to be completely elucidated.
Borna disease virus (BDV), a single-stranded,
non-cytolytic RNA virus with a non-segmented genome of ~8.9 kb with
six open reading frames (3,4), is
a highly neurotropic virus and causes persistent infections in the
CNS of the infected individuals for their entire life. BDV was
first reported as the causative agent of Borna disease, causing
nonpurulent encephalomyelitis in horses in Germany at the end of
the 19th century (5), and natural
BDV has been observed to infect a series of warm-blooded species
ranging from birds to non-human primates (6,7). In
neonatal rats, BDV infection leads to severe neurodegeneration in
the cortex and hippocampus, and generates neurodevelopmental
abnormalities and complex behavioral changes. BDV infection has
been demonstrated to be associated with human psychiatric diseases,
including schizophrenia, mental disorders including learning
difficulties, affective disorders and autism (8–10),
and certain BDV-associated antibodies, antigens and RNAs have been
identified in patients with neurological diseases, including
Parkinson's disease, chronic fatigue syndrome, Guillain-Barre
syndrome, viral encephalitis and multiple sclerosis (11). Increasing evidence has indicated
that BDV infection may induce neuronal loss, gliocyte impairment
and dysfunction in the development of neural stem/progenitor cells
(12–14). However, it may be noted that
differences in the genetic background of the host, viral strains
and even viral proteins may exert different effects on apoptotic
activity (15,16). Epidemiological studies have been
performed to address the latent association between BDV infection
and human neurological diseases (17,18),
although the controversy of the possible association remains under
debate and certain studies have reported an absence of these
associations (19).
Hu-H1 and Strain V are two different BDV strains.
Hu-H1 is a natural strain which was recovered in 1994 from the
blood cells of a female patient with bipolar I disorder in Germany
(4). The lab-derived Strain V is a
non-natural strain, which was originated from the brain of a horse
with fatal Borna disease in 1927 (20). Compared with Strain V, Hu-H1
exhibits few meaningful point mutations at the molecular level and
a differing pathogenicity at the host level, including the
induction behavioral changes in rabbits without fatal disease
(21).
In previous studies, it was observed that BDV
strains Hu-H1 and Strain V infected oligodendrocyte (OL) cells and
rat neurons, leading to different alterations in cell
proliferation, apoptosis and metabonomics (21,22).
The present study sought to further investigate the pathomechanisms
involved in SH-SY5Y cell dysfunction induced by various viral
strains. The present study revealed that two BDV strains may serve
a divergent antiproliferative and apoptotic role in SH-SY5Y cell
and provided an insight for future examination of the strain
differences and underlying pathomechanisms.
Materials and methods
Viral strains and cell culture
The virus strains and human OL cell lines were
provided by Professor Hanns Ludwig and Professor Liv Bod of the
Free University of Berlin (Berlin, Germany), and the viral solution
was obtained by freezing and thawing the persistently BDV-infected
human OL cells (OL cell lysates). The SH-SY5Y cell line (cat. no.
SCSP-5014; Stem Cell Bank, Chinese Academy of Sciences, Shanghai,
China) was cultured in Dulbecco's modified Eagle's medium (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) containing 5% fetal
bovine serum (cat. no. 10099158; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 1% penicillin and 1% streptomycin, and was
in a 5% CO2 incubator at 37°C.
Immunofluorescent staining
A total of 1×105 cells/well were seeded
in six-well plates, and 200 µl volumes of the BDV strains were
separately added to each well to form the infected cells following
adherence overnight. A total of 2 h subsequent to infection, the
medium was replaced with 3 ml new culture medium and culture was
continued for 3 days. Following removal of the medium, the cells
were fixed with 4% paraformaldehyde solution for 30 min at room
temperature. As previously described (21), immunofluorescence staining was
performed for BDV nucleoprotein detection. The primary antibody was
a gift from Prof. Xie Peng of Chongqing University. The primary
antibody rabbit anti-p40 (1:100) was incubated at 4°C overnight.
Following rinsing three times with phosphate buffer, cells were
incubated with fluorescein isothiocyanate (FITC)-conjugated
anti-rabbit immunoglobulin G (1:200; cat. no. A0562, Beyotime
Institute of Biotechnology, Haimen, China) in dark for 2 h at 37°C.
Standard immunofluorescence staining and analysis was performed as
described previously (21).
Cell proliferation and apoptosis
analysis
A Cell Counting Kit-8 (CCK8) assay kit (Beyotime
Institute of Biotechnology) was used for cell proliferation
analysis. Cells were cultured in 96-well plates overnight at a
density of 1,000 cells/well and were infected with BDV strains
(multiplicity of infection, 4) as previously described (21). At 0–7 days post-infection, 10 µl
CCK8 solution was added to each well for 2 h and the optical
density was measured in triplicate using an ELISA reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at λ=450 nm.
For the apoptosis analysis, cells were harvested
following infection for 1, 3 and 5 days by trypsinization without
EDTA, washed twice with cold phosphate buffer and stained with an
annexin-FITC/propidium iodide (PI) apoptosis detection kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), according to the
manufacturer's protocol. Apoptotic cells were assessed by flow
cytometry and the results were recorded by matched Cell Quest
software (version 5.1; BD Biosciences, San Jose, CA, USA). The
experiment was repeated three times independently.
Cell cycle analysis
At 2 h post-infection with the BDV strains, cells
were replaced with new medium without serum for 24 h to induce a G1
arrest, and were harvested at 0, 6, 15 and 24 h following the
release of G1 arrest by adding 5% fetal bovine serum to the medium.
All cells were fixed overnight using 70% ethanol at 4°C, followed
by incubation with 100 U/ml RNase A and 50 µg/ml PI for 30 min at
37°C subsequent to washing twice with cold phosphate buffer.
Analyses were performed on a flow cytometer by measurement of the
percentage of cells in different phases of the cell cycle.
Western blot analysis
Western blotting was performed as previously
described (19). Cell lysates were
prepared from the cell sample using radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology) combined with a
protease inhibitor. Protein concentrations were determined using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Proteins from each sample (40 µg) were separated by 10%
SDS-PAGE, and transferred to polyvinylidene fluoride membranes
(Merck KGaA, Darmstadt, Germany). Following blocking by 5% fetal
bovine serum for 12 h at 4°C, the membranes were probed with the
primary antibodies rabbit monoclonal anti-apoptosis regulator BAX
(Bax; Sigma-Aldrich; Merck KGaA; cat. no. SAB5500012) and
anti-apoptosis regulator Bcl-2 (Bcl-2; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), followed by incubation with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody for 1 h at 25°C (1:1,000; cat. no. AB501-01A; NovoProtein
Biotech Co., Ltd., Shanghai, China). A chemiluminescence detection
kit (Beyotime Institute of Biotechnology) was employed to detect
the labeled bands. β-actin (1:1,000; Sangon Biotech Co., Ltd.,
Shanghai, China) served as an internal reference and the relative
protein expression of target proteins was determined. Image Pro
Plus 6.0 software was used for densitometric analysis (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
GraphPad Prism version 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for statistical analysis. All results
are presented as the mean ± standard deviation of three independent
experiments. The statistical analysis was performed using one-way
analysis of variance with Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
P40 protein assay in BDV-Infected
SH-SY5Y
As a specific biomarker of BDV infection, BDV
nucleoprotein P40 was testified in the Strain V and Hu-H1 cells by
immunofluorescent staining to evaluate the permissivity of SH-SY5Y
to BDV infection. On day 3 post-infection, the two BDV
strain-infected groups exhibited fluorescent green focal points,
stained positive for P40, while the observation of the control
cells revealed no fluorescent expression (Fig. 1). The results suggested that Strain
V and Hu-H1 successfully infected SH-SY5Y cells directly, as P40 is
a specific marker of BDV infection.
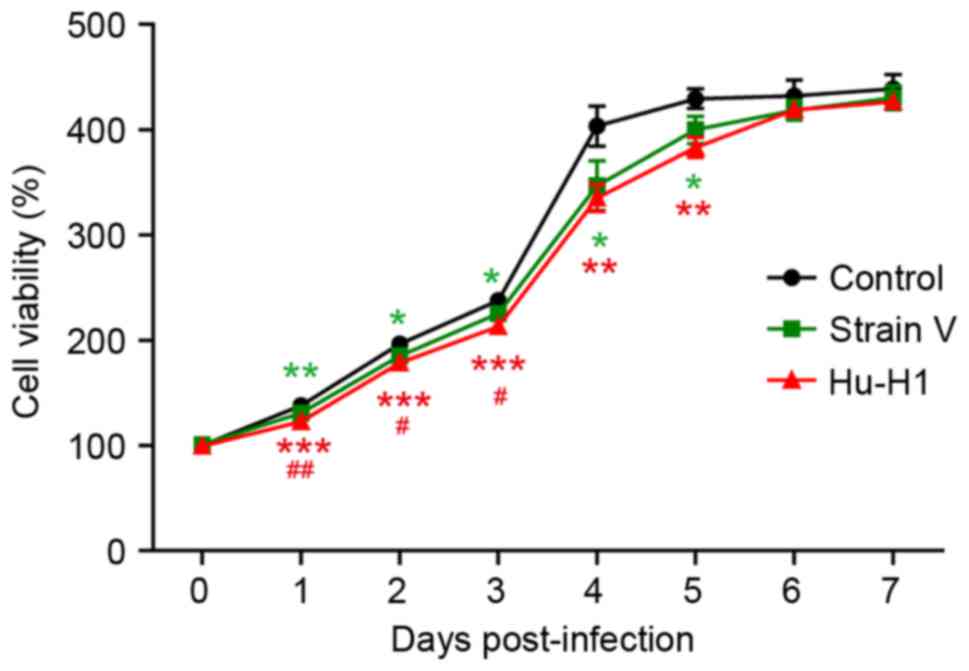
BDV strain infection decreases SH-SY5Y
cell viability
The CCK8 assay revealed a gradual increase in
SH-SY5Y cell proliferation between days 1 and 7 in the three groups
(Table I; Fig. 2). The control group exhibited
significantly increased cell viability compared with the BDV
strains groups (P<0.05), and the Hu-H1-infected cells exhibited
a significantly decreased viability compared with the Strain
V-infected cells (P<0.01).
 | Table I.Cell viability (%) of control, Strain
V and Hu-H1 cells (mean ± standard deviation; n=3). |
Table I.
Cell viability (%) of control, Strain
V and Hu-H1 cells (mean ± standard deviation; n=3).
|
| Group | P-value |
|---|
|
|
|
|
|---|
| Day | Control | Strain V | Hu-H1 | Control vs. Strain
V | Control vs.
Hu-H1 | Strain V vs.
Hu-H1 |
|---|
| 0 |
100.00±1.12 |
100.63±2.42 |
99.48±1.15 | ns | ns | ns |
| 1 |
137.92±0.96 |
130.68±2.75 |
122.63±0.45 | P<0.01 | P<0.001 | P<0.01 |
| 2 |
196.42±4.59 |
185.22±3.93 |
178.51±2.32 | P<0.05 | P<0.001 | P<0.05 |
| 3 |
237.75±3.97 |
225.40±4.42 |
212.97±1.57 | P<0.05 | P<0.001 | P<0.05 |
| 4 |
403.40±19.02 |
346.78±23.64 |
334.97±12.76 | P<0.05 | P<0.01 | ns |
| 5 |
429.43±9.41 |
399.82±12.82 |
383.18±9.49 | P<0.05 | P<0.01 | ns |
| 6 |
431.93±15.22 |
418.43±9.45 |
418.78±2.70 | ns | ns | ns |
| 7 |
438.91±13.56 |
430.32±11.31 |
426.57±3.05 | ns | ns | ns |
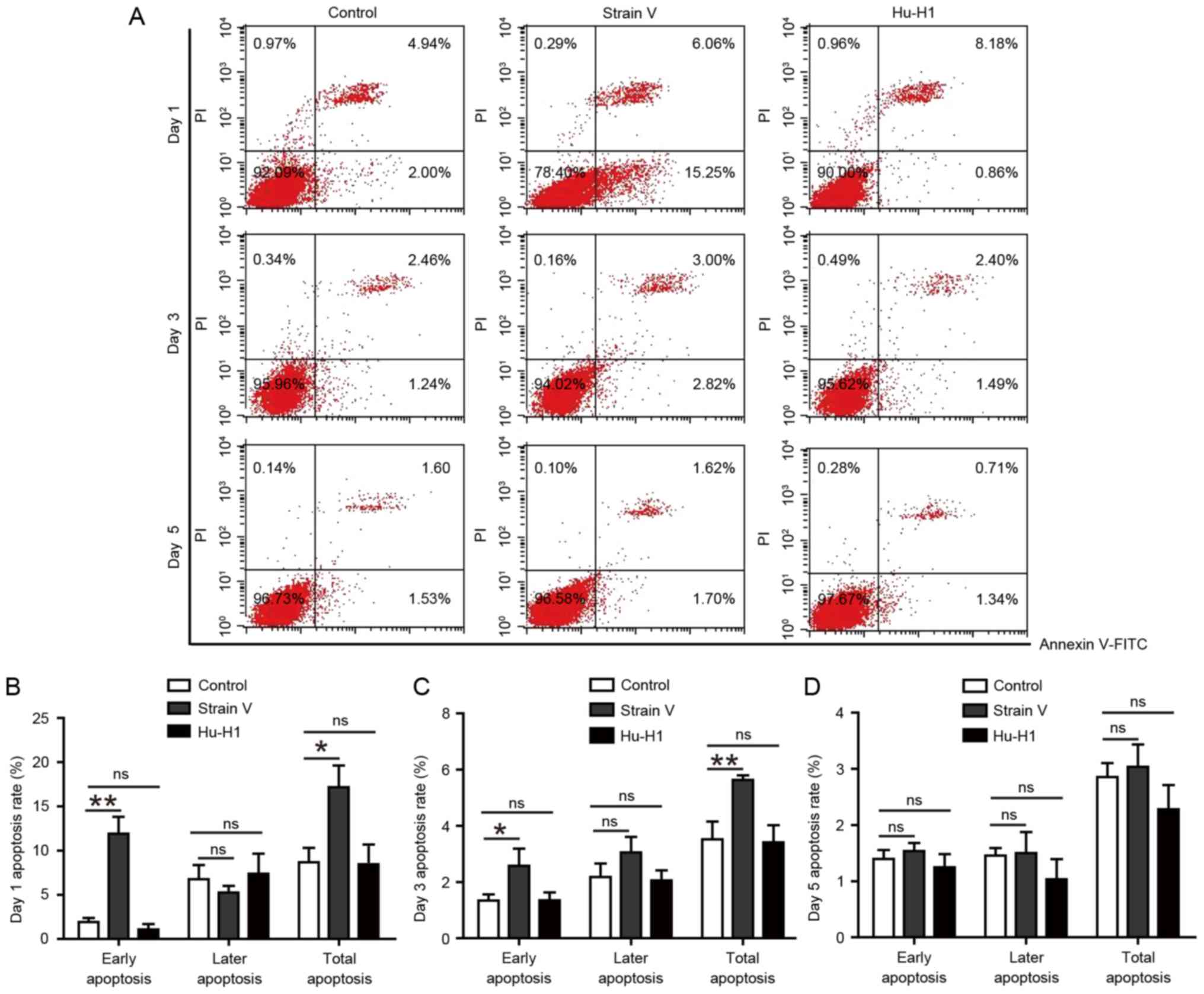
Strain V, although not the Hu-H1
strain, promotes apoptosis in SH-SY5Y cells
According to the protocol of annexin V-FITC/PI
labeling, cellular apoptosis was determined by flow cytometry on
days 1, 3 and 5 post-infection (Fig.
3A). There was no significant difference between the control
and Hu-H1group (P>0.05). The Strain V group exhibited an
increased apoptotic percentage compared with the control and
Hu-H1groups on days 1 and 3 post-infection (P<0.05). However, on
day 5 post-infection there was no statistically significant
difference among the three groups (P>0.05). Therefore, only
Strain V exerted an apoptotic effect at the initial stage of the
assay (Fig. 3B-D). It may be
suggested that Strain V induced cellular apoptosis upon initial
infection, while the Hu-H1 strain exerted no effect on cellular
apoptosis.
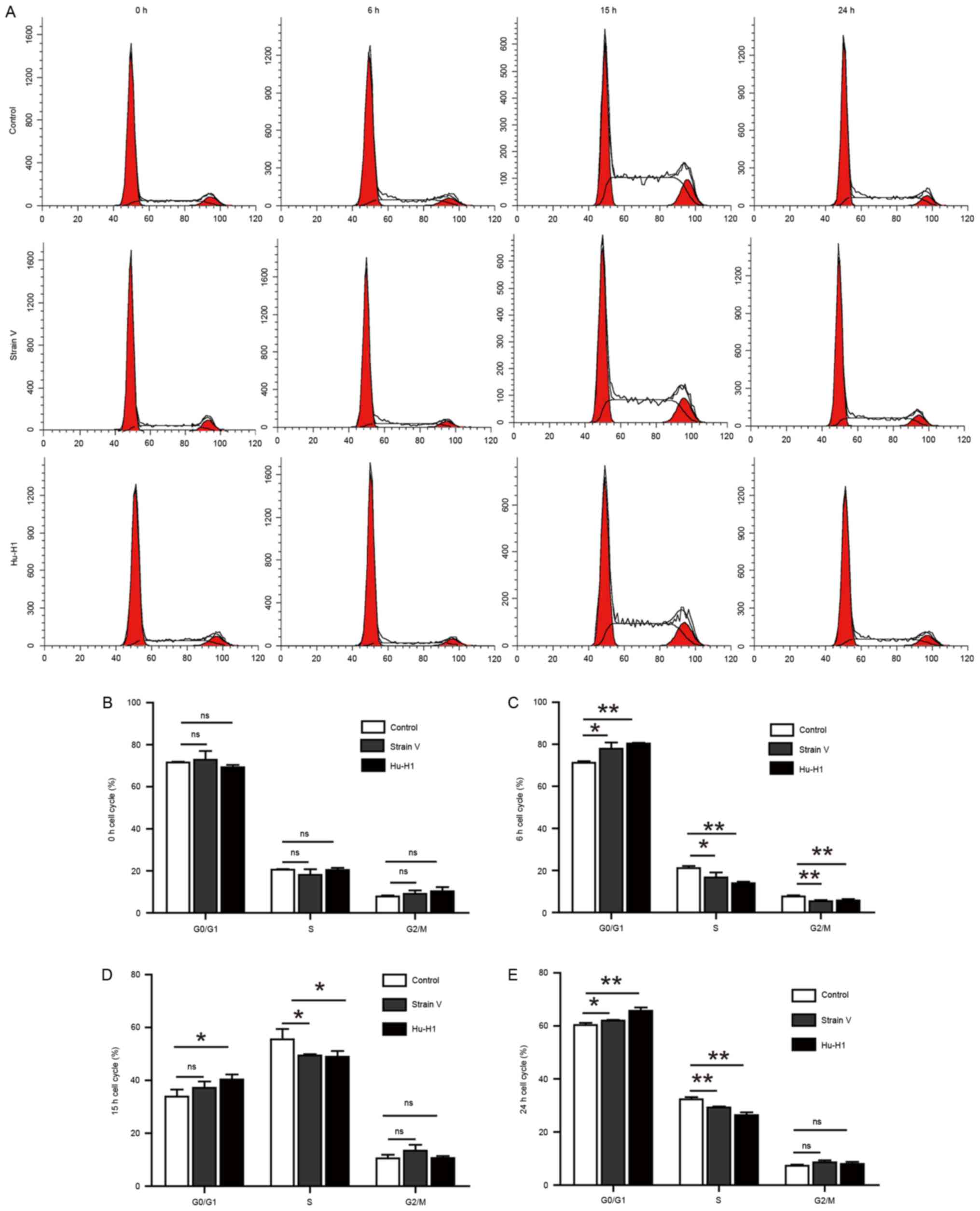
BDV strain infection induces SH-SY5Y
cell cycle arrest to different degrees
Following fixing with 70% ethanol overnight, the
harvested cells were stained with PI and analyzed by flow cytometry
to assay cell cycle distribution. It was observed that there was a
larger percentage of cells in the G0/G1 phases and fewer cells in
the S or G2/M phases when the cell cycle was arrested (Fig. 4A). At 0 h post-serum release, there
was no significant difference among the three groups (P>0.05;
Fig. 4B). Between 6 and 24 h
post-serum release, the two BDV strains exhibited distinct cell
cycle arrest compared with the control group (Fig. 4C-E). In addition, the Hu-H1 strain
exerted a more significantly inhibitory effect compared with Strain
V.
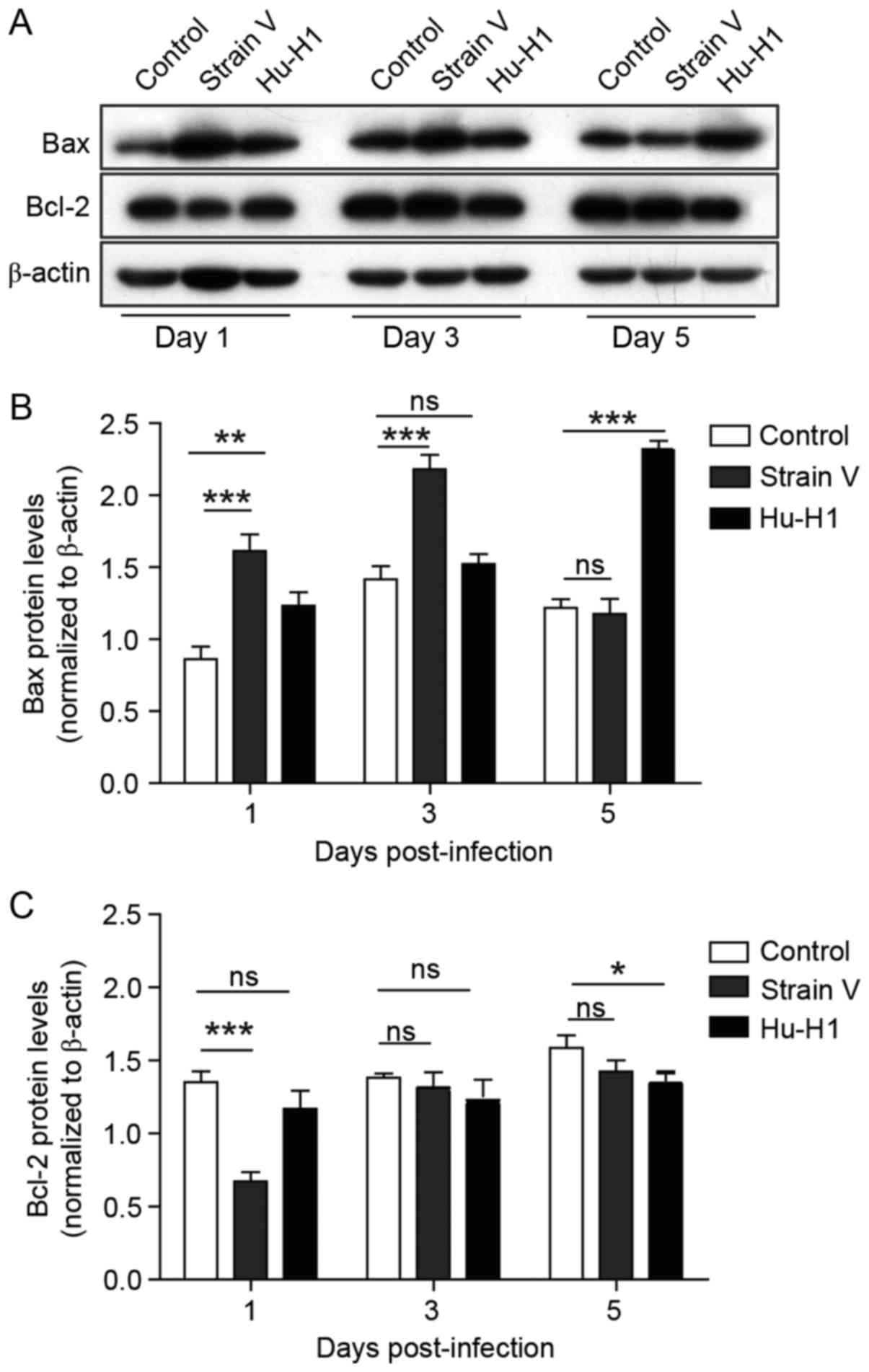
Different effects of BDV strain
infection on apoptotic pathway protein expression
In order to investigate whether proteins in the
apoptotic pathways were differently regulated, the relative
expression of the apoptosis-associated proteins Bax and Bcl-2 were
measured via western blotting (Fig.
5A). The western blot analysis results demonstrated that the
Bax protein in BDV strain cells was upregulated compared with the
control (P<0.01) on days 1–5 post-infection, particularly in
Strain V cells (Fig. 5B). By
contrast, Bcl-2, the anti-apoptotic protein, exhibited decreased
levels in Strain V cells compared with corresponding controls
(P<0.01), and there was no significant difference between Hu-H1
strains and controls until 5 days post-infection (P>0.05)
(Fig. 5C).
Discussion
As a neurotropic virus, which causes CNS dysfunction
in a wide range of warm-blooded species, BDV may lead to persistent
infection and biomolecular alterations in neurological cells
(17,23). Based on the cellular phenotype of
SH-SY5Y cells, the present study revealed different proliferative
and apoptotic effects that distinguished among the non-infected
control, BDV Strain V- and Hu-H1-infected cells for the first time,
to the best of our knowledge. The results of the present study
suggested that the biological functions of natural BDV Hu-H1 and
non-natural BDV Strain V were different due to properties of the
hosts, and provided evidence for biological strain differences. The
present study may provide a basis for biological characterization
and an insight into taxonomical issues at the species level.
The results of the present study indicated that BDV
strains were able to inhibit SH-SY5Y cellular proliferation and
obstruct the cell cycle, and that human Hu-H1 exerted stronger
inhibitory effects compared with Strain V. A previous study
demonstrated that Strain V was able to promote OL cell
proliferation while Hu-H1 inhibited OL cell proliferation (21), and Planz et al (24) reported that BDV nucleoprotein He/80
interacted with the cyclin-dependent kinase 1-cyclin B1 complex and
prolonged the G2 cell cycle phase. These previous results implied
that the virus may directly or indirectly regulate certain cell
cycle regulatory proteins, cytokines or growth factors during the
proliferation and cell cycle of SH-SY5Y cells to execute its
inhibitory effects. The Hu-H1 strain has been demonstrated to
undergo a number of species crossings in animals and tissue culture
cells (25), thus may be
exhibiting divergent influences on different human cells. In
addition, although the molecules binding to the viral proteins may
be the same in different cell lines, virus strains may affect
different signaling or metabolic pathways, as revealed in a
metabonomic study of the two BDV strains in rat cortical neurons
(21); elucidating the differences
in specific mechanisms requires further investigation.
The BDV non-natural Strain V exerted significant,
although temporary, effects on the apoptosis of SH-SY5Y cells,
while the Hu-H1 strain exerted almost no effect. In order to
examine the underlying mechanisms of apoptosis, the relative
expression of Bax and Bcl-2 protein was detected by western blot
analysis. The results of the present study demonstrated that Bax
and Bcl-2 proteins were involved in the SH-SY5Y cellular apoptosis
induced by BDV initial infection, and the Hu-H1 strain also
promoted apoptosis in the infected OL cells, while laboratory
strain V was demonstrated to inhibit cell apoptosis. In addition,
Wu et al (16) reported
that granule cell neurons of the dentate gyrus were not affected in
Sprague-Dawley rats with BDV infection. Therefore, different
strains may cause unpredictable apoptotic effects in different
hosts. The different inherent properties of BDV strains may explain
why they may cause acute nonpurulent encephalomyelitis in animals
and chronic asymptomatic infection in humans.
Based on the genomic and protein sequences (26), the taxonomy within the family of
the Bornaviridae is intuitive and comprehensible. However, even
with high conservation of BDV sequence, strains may cause different
effects between hosts. Therefore, the host phenotype alone is
inadequate to explain the mechanisms of action of BDV strains.
Studies into the acetylation and metabonomics of BDV infection
(20,27) have provided a novel perspective for
understanding the pathogenic mechanisms of BDV infection. However,
further analysis of biological functions and systematic
interpretations are required to better define BDV strains in future
studies.
References
|
1
|
De la Torre JC, Mallory M, Brot M, Gold L,
Koob G, Oldstone MB and Masliah E: Viral persistence in neurons
alters synaptic plasticity and cognitive functions without
destruction of brain cells. Virology. 220:508–515. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dietzschold B, Li J, Faber M and Schnell
M: Concepts in the pathogenesis of rabies. Future Virol. 3:481–490.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cubitt B, Oldstone C and de la Torre JC:
Sequence and genome organization of Borna disease virus. J Virol.
68:1382–1396. 1994.PubMed/NCBI
|
|
4
|
Briese T, Schneemann A, Lewis AJ, Park YS,
Kim S, Ludwig H and Lipkin WI: Genomic organization of Borna
disease virus. Proc Natl Acad Sci USA. 91:4362–4366. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dürrwald R and Ludwig H: Borna disease
virus (BDV), a (zoonotic?) worldwide pathogen. A review of the
history of the disease and the virus infection with comprehensive
bibliography. Zentralbl Veterinarmed B. 44:147–184. 1997.PubMed/NCBI
|
|
6
|
Bode L, Dürrwald R and Ludwig H: Borna
virus infections in cattle associated with fatal neurological
disease. Vet Rec. 135:283–284. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hagiwara K, Tsuge Y, Asakawa M, Kabaya H,
Okamoto M, Miyasho T, Taniyama H, Ishihara C, de la Torre JC and
Ikuta K: Borna disease virus RNA detected in Japanese macaques
(Macaca fuscata). Primates. 49:57–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hornig M, Solbrig M, Horscroft N,
Weissenböck H and Lipkin WI: Borna disease virus infection of adult
and neonatal rats: Models for neuropsychiatric disease. Curr Top
Microbiol Immunol. 253:157–177. 2001.PubMed/NCBI
|
|
9
|
Pletnikov MV, Moran TH and Carbone KM:
Borna disease virus infection of the neonatal rat: Developmental
brain injury model of autism spectrum disorders. Front Biosci.
7:d593–d607. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams BL, Hornig M, Yaddanapudi K and
Lipkin WI: Hippocampal poly(ADP-Ribose) polymerase 1 and caspase 3
activation in neonatal bornavirus infection. J Virol. 82:1748–1758.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Xu MM, Zeng L, Liu S, Liu X, Wang
X, Li D, Huang RZ, Zhao LB, Zhan QL, et al: Evidence for Borna
disease virus infection in neuropsychiatric patients in three
western China provinces. Eur J Clin Microbiol Infect Dis.
33:621–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das S and Basu A: Viral infection and
neural stem/progenitor cell's fate: Implications in brain
development and neurological disorders. Neurochem Int. 59:357–366.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brnic D, Stevanovic V, Cochet M, Agier C,
Richardson J, Montero-Menei CN, Milhavet O, Eloit M and Coulpier M:
Borna disease virus infects human neural progenitor cells and
impairs neurogenesis. J Virol. 86:2512–2522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scordel C, Huttin A, Cochet-Bernoin M,
Szelechowski M, Poulet A, Richardson J, Benchoua A, Gonzalez-Dunia
D, Eloit M and Coulpier M: Borna disease virus phosphoprotein
impairs the developmental program controlling neurogenesis and
reduces human GABAergic neurogenesis. PLoS Pathog. 11:e10048592015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poenisch M, Burger N, Staeheli P, Bauer G
and Schneider U: Protein X of Borna disease virus inhibits
apoptosis and promotes viral persistence in the central nervous
systems of newborn-infected rats. J Virol. 83:4297–4307. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YJ, Schulz H, Lin CC, Saar K, Patone G,
Fischer H, Hübner N, Heimrich B and Schwemmle M: Borna disease
virus-induced neuronal degeneration dependent on host genetic
background and prevented by soluble factors. Proc Natl Acad Sci
USA. 110:1899–1904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Wang Z, Zhu D, Xu M, Chen X, Peng D,
Iwata Y and Xie P: Detection and analysis of Borna disease virus in
Chinese patients with neurological disorders. Eur J Neurol.
16:399–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Zhang L, Lei Y, Liu X, Zhou X, Liu
Y, Wang M, Yang L, Zhang L, Fan S and Xie P: Meta-analysis of
infectious agents and depression. Sci Rep. 4:45302014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hornig M, Briese T, Licinio J, Khabbaz RF,
Altshuler LL, Potkin SG, Schwemmle M, Siemetzki U, Mintz J,
Honkavuori K, et al: Absence of evidence for bornavirus infection
in schizophrenia, bipolar disorder and major depressive disorder.
Mol Psychiatr. 17:486–493. 2012. View Article : Google Scholar
|
|
20
|
Zwick W: Neuere Untersuchungen über die
seuchenhafte Gehirn-u. Rückenmarksentzündung (Borna'sche Krankheit)
der Pferde. Dtsch. Z. Nervenheilk. 110:316–322. 1929. View Article : Google Scholar
|
|
21
|
Liu S, Bode L, Zhang L, He P, Huang R, Sun
L, Chen S, Zhang H, Guo Y, Zhou J, et al: GC-MS-Based metabonomic
profiling displayed differing effects of Borna Disease Virus
natural strain Hu-H1 and laboratory Strain V infection in rat
cortical neurons. Int J Mol Sci. 16:19347–19368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li D, Lei Y, Deng J, Zhou C, Zhang Y, Li
W, Huang H, Cheng S, Zhang H, Zhang L, et al: Human but not
laboratory Borna Disease Virus inhibits proliferation and induces
apoptosis in human oligodendrocytes in vitro. PLoS One.
8:e666232013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang R, Gao H, Zhang L, Jia J, Liu X,
Zheng P, Ma L, Li W, Deng J, Wang X, et al: Borna disease virus
infection perturbs energy metabolites and amino acids in cultured
human oligodendroglia cells. PLoS One. 7:e446652012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Planz O, Pleschka S, Oesterle K,
Berberich-Siebelt F, Ehrhardt C, Stitz L and Ludwig S: Borna
disease virus nucleoprotein interacts with the CDC2-cyclin B1
complex. J Virol. 77:11186–11192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ludwig H, Furuya K, Bode L, Klein N,
Dürrwald R and Lee DS: Biology and neurobiology of Borna disease
viruses (BDV), defined by antibodies, neutralizability and their
pathogenic potential. Arch Virol Suppl. 7:111–133. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuhn JH, Dürrwald R, Bào Y, Briese T,
Carbone K, Clawson AN, deRisi JL, Garten W, Jahrling PB,
Kolodziejek J, et al: Taxonomic reorganization of the family
Bornaviridae. Arch Virol. 160:621–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Liu S, Bode L, Liu C, Zhang L, Wang
X, Li D, Lei Y, Peng X, Cheng Z and Xie P: Persistent human Borna
disease virus infection modifies the acetylome of human
oligodendroglia cells towards higher energy and transporter levels.
Virology. 485:58–78. 2015. View Article : Google Scholar : PubMed/NCBI
|