Introduction
Pulmonary fibrosis is a fatal disorder, considered
as the outcome of many chronic pulmonary diseases (1). Fibrotic alveolar epithelial cells can
develop for various reasons mainly due to overexposure to
irradiation, smoke inhalation and disease of respiratory system.
The early pathological lesion is demonstrated in a form of an acute
alveolus inflammation, involving inflammatory cells, immune
effector cells and cytokines, such as transforming growth factor-β1
(TGF-β1), tumor necrosis factor-α (TNF-α) and nuclear factor-κB
(NF-κB). Pulmonary fibrosis is most prevalent among 50–70year-olds,
and the estimated annual incidence among men and women is 10 and 7
per 100,000 individuals, respectively (2,3). The
death rate of pulmonary fibrosis is gradually increasing and the
risk is positively correlated with age (4). Traditionally, treatment of the
disease relies on corticosteroids, but the response rate is not
satisfactory and the drug is rarely used as a prophylaxis (5). Developing a therapeutic strategy
against the disease is an urgent matter for patient who developed
the symptoms of lung fibrosis.
Ulinastatin (UTI), usually used as a urinary trypsin
inhibitor, is a 67 kDa glycoprotein normally purified from healthy
human urine (6). UTI is a
Kunitz-type protease inhibitor containing two active functional
domains and no overlapping regions with an effective role against a
broad range of enzymes (7).
Previous studies demonstrated that UTI is able to inhibit numerous
inflammatory proteases including trypsin, chymotrypsin, neutrophil
elastase and plasmin (8). Since
the number of proteases increases from the beginning of infection
and inflammation, it is rational to use UTI as an effective
anti-inflammatory molecule (9).
Clinically, UTI has been widely used as a drug for the treatment of
severe inflammatory responses, such as burn, sepsis and acute
pancreatitis (10). No adverse
toxicological effects of ulinastatin were observed during
preliminary treatment. UTI was reported to be able to decrease the
inflammatory reaction and mitigate the lung damage caused by smoke
and lipopolysaccharide in rats subjected to the treatment of
pulmonary disorder (11).
Nonetheless, the mechanisms underlying the therapeutic process
remain unclear, and the dosage-dependent effect has not been
thoroughly investigated. Thus, the present study aimed to unravel
the signaling pathway mediating the therapeutic effect of UTI in
pulmonary fibrosis on a molecular level using Sprague-Dawley rats.
In addition, correlation between UTI dosage and treatment efficacy
was analyzed.
Materials and methods
Animals and reagents
A total of 90 male Sprague-Dawley rats used for all
experiments were 9 weeks old and weighed 290–320 g (Super-B&K
Laboratory Animal Corp, Shanghai, China). The materials used for
this study included bleomycin (BLM; Tai He Pharmaceutical Co. Ltd.,
Tianjin, China) and UTI (Techpool Bio-Pharma Co. Ltd., Guangzhou,
China). Pentobarbital sodium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved in physiological saline and the
concentration was adjusted to 10 mg/ml. Enzyme-linked immunosorbent
assay (ELISA) kits specific to TGF-β1 (Abcam, cat. no. ab119558,
USA), TNF-α (Abcam, cat. no. ab46070, USA) and NF-κB (Abcam, cat.
no. ab28856, USA), and a western blotting kit (Amersham; GE
Healthcare Lice Sciences, Little Chalfont, UK) were used in this
study.
Rat model of pulmonary fibrosis
Rats were randomly allocated into one of 3 groups:
Negative control (n=30), model (n=30) and ulinastatin treatment
(n=30). All rats were bred and maintained in accordance with the
‘Care and Use of Laboratory Animals’ guidelines published by the
National Institute of Health of China (12). For the model group, 250 µl BLM (4
g/l) was injected into lungs through the trachea, to achieve the
dosage of 5 mg/kg body weight. Once BLM was injected, the animals
were rotated immediately to ensure an equal distribution of the
chemical in lungs. A total of 250 µl physiological saline solution
was introduced into the lungs of rats as a control. In the UTI
treatment group, rats were given different doses of UTI (high and
low) by intraperitoneal injection one day following BLM
administration. A total of 15 rats were treated with a high dose of
UTI (100,000 U/kg body weight/day) and 15 rats were treated with a
low dose of UTI (20,000 U/kg body weight/day). A total of 5 rats
were treated with sodium pentobarbital sedative overdose at 7, 14
and 28 days in all three groups. A piece of lung tissue was
collected from dead rats and stored at −70°C for further
analysis.
The present study received ethical approval from the
Animal Ethics Committee of the Shandong University (Jinan,
China).
Histopathological examination
Paraffin-embedded sections of pulmonary tissues were
stained with hematoxylin-eosin (H&E) and Masson's trichrome
stain for morphological studies (13). A piece of the left lung was fixed
in 10% formaldehyde solution for 12 h. Blocks of the lung tissue
were dehydrated and embedded in paraffin, cut into 5 µm slices,
incubated at 60°C overnight, dewaxed, then stained with H&E or
Masson's trichrome for 5 and 10 min, respectively, at room
temperature. Finally, the general pathological changes of the lung
tissue and collagen fibrils were observed and captured using an
optical microscope.
Alveolitis and pulmonary fibrosis of the
experimental rats were classified according to the Szapiel's method
(14). The alveolitis grades were:
0, no alveolitis; 1, mild alveolitis characterized by alveolar
interval widening due to cell infiltration, with a lesion area
<20% of the lung; 2, moderate alveolitis characterized by a
lesion area of 20–50% of the lung; 3, diffuse alveolar inflammation
characterized by a lesion area >50% of the lung.
The pulmonary interstitial fibrosis grades were: 0,
no pulmonary fibrosis; 1, mild pulmonary fibrosis characterized by
an involvement area <20% of the lung; 2, moderate lung
interstitial fibrosis characterized by a disordered alveolar
structure and an involvement area 20–50% of the lung; 3, severe
pulmonary fibrosis characterized by an integrated alveolar, a
disordered physical lung structure and an involvement area >50%
of the lung.
Western blot analysis
Lung samples from three mice per group were randomly
selected from each group and protein expression levels were
determined by western blot analysis. For tissue lysate preparation,
frozen lung tissues were homogenized at 4°C with 100 ~200 µl
radioimmunoprecipitation assay (RIPA) lysis buffer (cat. no.
P0013B, Beyotime Institute of Biotechnology, Haimen, China)
supplemented with 1% protease and phosphatase inhibitor cocktail
(Beyotime Institute of Biotechnology, cat. no. P1005), followed by
centrifuged at 10,000 × g for 10 min at 4°C. Total tissue lysate
was collected as a supernatant. Equal amounts of protein lysates
(30 µg/lane) were resolved on a SDS-PAGE gel (12%), and the
separated bands were transferred onto a 0.22-µm nitrocellulose
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using a
transfer tank (Bio-Rad Laboratories, Inc.). Subsequently, the
membrane was blocked at room temperature for 2 h in PBS containing
0.1% Tween-20 (Sigma-Aldrich; Merck KGaA) and 5% skimmed milk. The
blocked negative control was then incubated overnight at 4°C with
rabbit polyclonal primary antibodies (anti-TGF-β1, anti-TNF-α and
anti-NF-κB, Santa Cruz Biotechnology, Inc.). An antibody specific
toglyceraldehyde-3-phosphate dehydrogenase, a house keeping
protein, served as an internal reference. After the incubation, the
membranes were washed in a dilution buffer containing
1XTris-buffered saline (TBS, Beyotime Institute of Biotechnology,
cat. no. P0233) and 0.1% Tween-20 (Beyotime Institute of
Biotechnology, cat. no. ST825) and 5% BSA (cat. no. A8020, Beijing
Solarbio Science and Technology, Co., Ltd., Beijing, China) and
incubated for 1 h with horseradish peroxidase (HRP)-coupled
developing antibody (HRP-conjugated anti-rabbit, diluted 1:8,000,
Santa Cruz Biotechnology, Inc.). After the final wash with the
dilution buffer (3 times, 10 min each) the blots were
immunodetected with enhanced chemiluminescence (Cell Signaling
Technology, Inc., Danvers, MA, USA). The grayscale value for each
band representing the concentration of the corresponding protein
was measured using a molecular imager (Bio-Rad Laboratories,
Inc.).
ELISA analysis
The frozen lung tissue was thawed and homogenized at
4°C, followed by centrifugation at 10,000 × g at 4°C for 20 min.
Subsequently, 20 µl supernatant was pipetted and transferred into
an Eppendorf tube for future use. The calibrator diluent with
different concentrations of standard TGF-β1, TNF-α and NF-κB, and
triplicate samples were added to individual polystyrene plate
wells. Tissue was incubated for 2 h at 37°C. Between each step of
the procedure, the plates were washed three times using TBS (pH
7.4). PBS was used as a negative control. A total of 100 µl
antigen-specific biotin conjugate (Cell Signaling Technology, cat.
no. L27A9) was added to each well of the plate, and then the plate
was incubated at room temperature for 40 min, followed by an
addition of 100 µl streptavidin-HRP conjugate. The plate was
incubated at room temperature for 30 min, 100 µl 3, 3, 5,
5-Tetramethylbenzidine (TMB) substrate solution was added to each
well, and then the plate was incubated at room temperature for 30
min in the dark. Finally, 100 µl stop solution was added to each
well, and the plate was measured at a wavelength of 450 nm using a
680-microplate reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed using
Statistical Package for the Social Sciences 13.0 software (SPSS,
Inc., Chicago, IL, USA). Data are presented as the mean ± standard
deviation from three separate experiments. Comparison of multiple
groups was performed using analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Histopathological characteristics of
the lung tissues
The comparison between lung tissues from different
groups was presented in Fig. 1.
The control group demonstrated a thin interstitial matrix and
regular alveolar lumen (Fig. 1A).
7 days following the experiment, a mild alveolitis with localized
fibrosis became evident, indicating that BLM caused rat lung injury
(Fig. 1B). Symptoms such as
hemorrhage, widened alveolar septa and infiltration of numerous
macrophages occurred following more than 14 days of the experiment
and represented a severe alveolitis and pulmonary fibrosis
(Fig. 1C and D). When the injured
rats were administered UTI treatment, typical inflammatory
symptoms, such as hemorrhage, alveolar exudates and neutrophil
accumulation were evidently alleviated compared with the injury
model group (Fig. 1E and F)
indicating that UTI is therapeutic to pulmonary fibrosis and
alveolitis. As demonstrated by the results from the histopathology
grading (Table I), UTI treatment
mitigated alveolitis and lung fibrosis in all three modeling
periods, with recovery rates >40%. UTI cured >65%
interstitial fibrosis rats induced by 7 days of the experimental
injury, demonstrating therapeutic potential of UTI in the treatment
of pulmonary disorders.
 | Table I.Histopathological grade system for the
assessment of pulmonary alveolitis and interstitial fibrosis. |
Table I.
Histopathological grade system for the
assessment of pulmonary alveolitis and interstitial fibrosis.
|
|
|
| Grade |
|---|
|
|
|
|
|
|---|
| Parameter | Group | n | 7 d | 14 d | 28 d |
|---|
| Pulmonary | Control | 10 |
0±0 |
0±0 |
0±0 |
| Alveolitis | Modeling | 10 |
2.4±0.5 |
2.6±0.4 |
2.0±0.4 |
|
| Ulinastatin | 10 |
1.3±0.2 |
1.2±0.3 |
1.0±0.2 |
| Interstitial | Control | 10 |
0±0 |
0±0 |
0±0 |
| Fibrosis | Modeling | 10 |
0.9±0.3 |
2.3±0.2a |
2.7±0.4a |
|
| Ulinastatin | 10 |
0.3±0.1 |
1.2±0.3a |
1.2±0.5a |
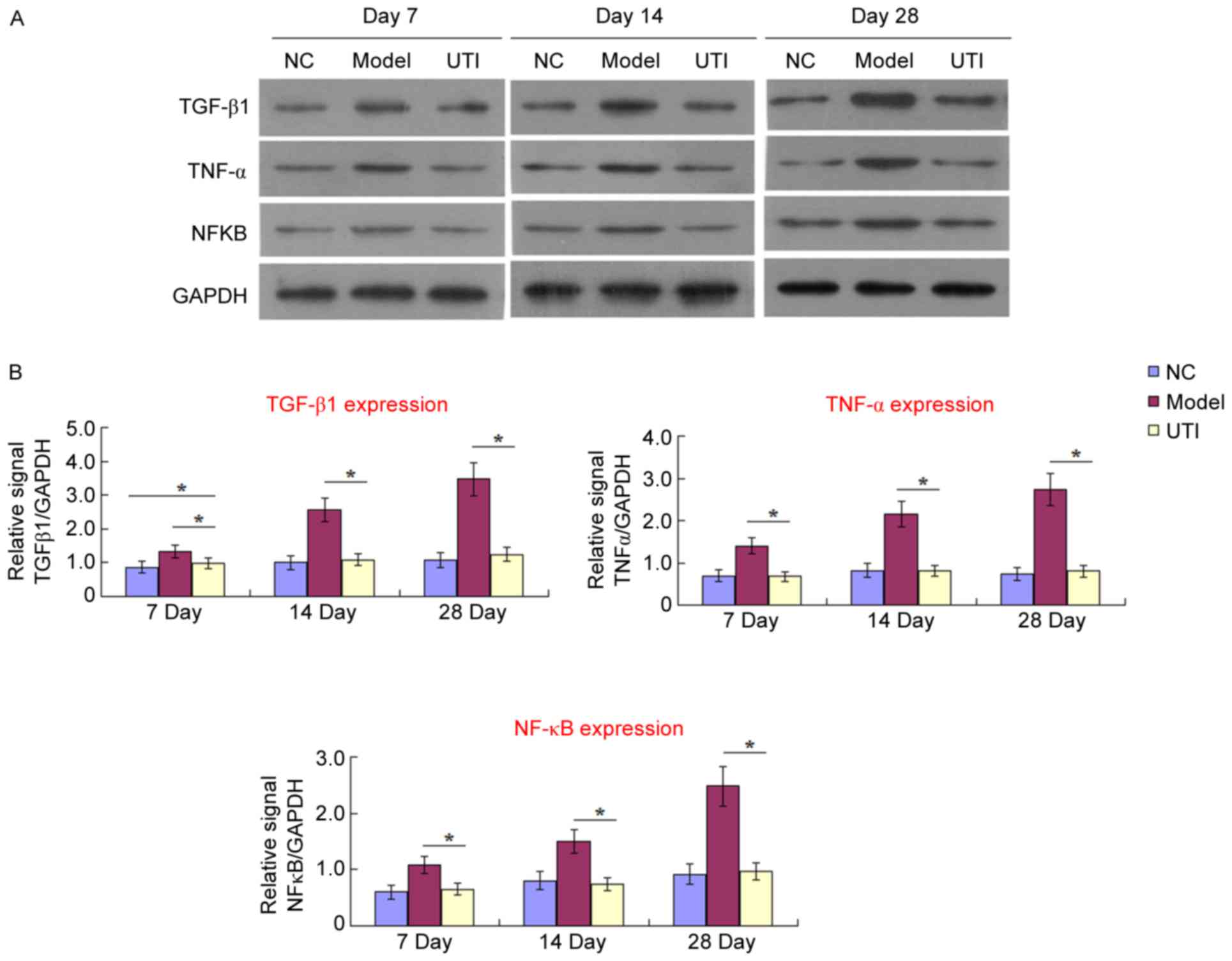
Expression of TGF-β1, TNF-α and NF-κB
in lungs of the experimental rats
Expression levels of TGF-β1, TNF-α and NF-κB were
investigated using western blotting to determine specific
functional pathways of UTI activity on lung fibrosis. As presented
in Fig. 2, expression of all three
inflammation-associated cytokines increased in the model group from
day 7 to 28, compared with the control group. This increase
indicated that the inflammatory response was activated by BLM and
contributed to histopathological injuries (Fig. 1). When UTI treatment was applied,
the expression of these cytokines was significantly reduced. The
expression of TNF-α and NF-κB was restored to the normal level,
indicating that the likely mechanism of UTI action on pulmonary
fibrosis is downregulation of inflammatory regulators contributing
mostly to the migration, proliferation, and differentiation of
resident mesenchymal cells (15).
UTI-mediated mitigation of pulmonary
fibrosis demonstrated by ELISA
To quantify the number of cytokines expressed during
alveolitis and fibrotic formation, ELISA using HRP/TMB system
chromogenic agent was used. Fig. 3
summarizes the changes in expression levels of TGF-β1, TNF-α and
NF-κB. UTI significantly inhibited these cytokines in the treatment
groups, compared with the negative control group. Additionally,
expression levels of TGF-β1, TNF-α and NF-κB in groups with both
high and low dose UTI treatment were significantly reduced 4 weeks
post injury compared with the modeling, untreated group.
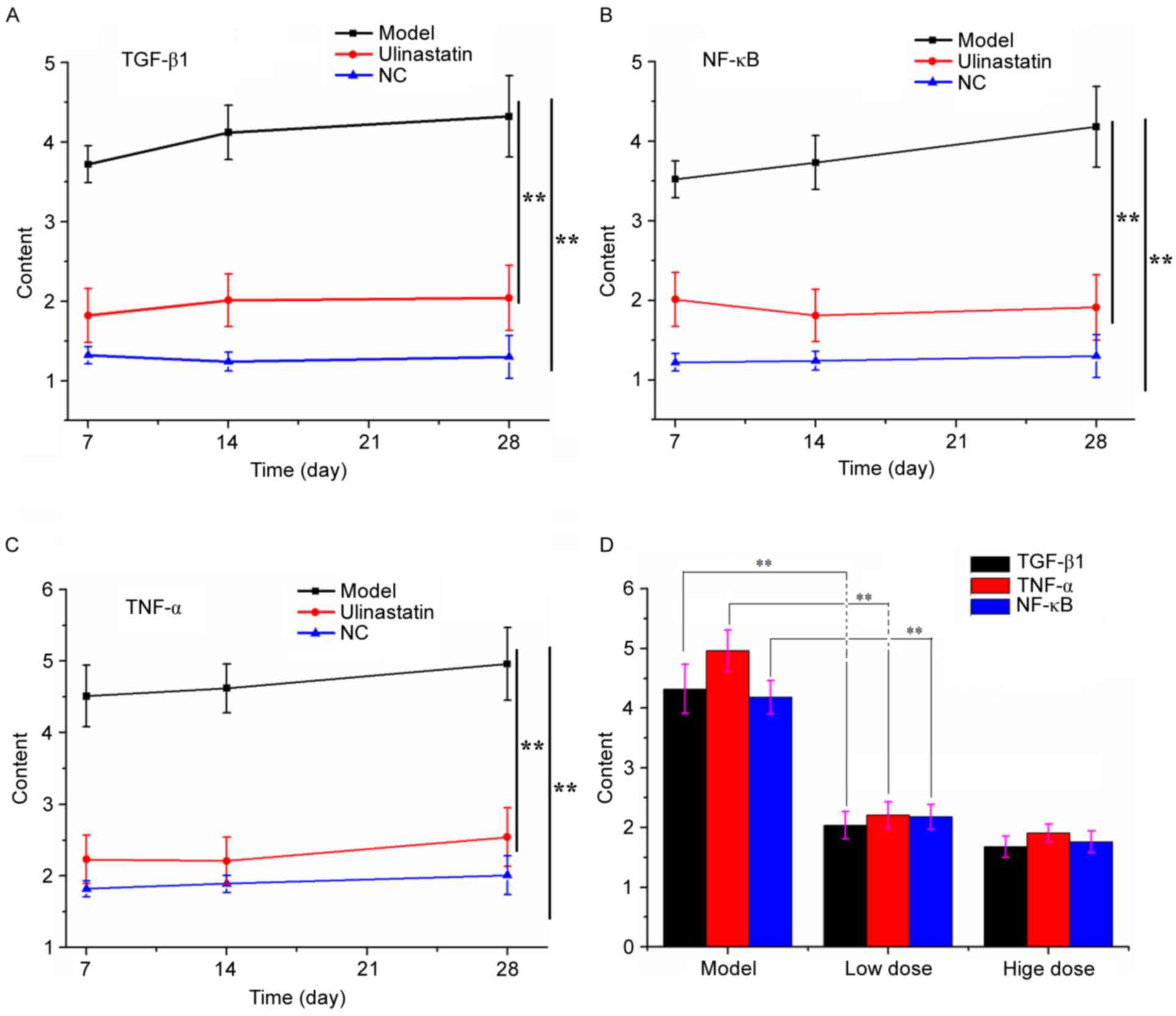 | Figure 3.Changes in the expression of TGF-β1,
NF-κB and TNF-α throughout the experiment. Expression of (A)
TGF-β1, (B) NF-κB and (C) TNF-α in the negative control, model and
ulinastatin treatment group at 7, 14 and 28 days. **P<0.01 (D)
Comparison of TGF-β1, NF-κB and TNF-α between the modeling group,
low dose UTI treatment group (20,000 U/kg body weight/day) and high
dose treatment group (100,000 U/kg body weight/day). Data are
presented as the mean ± standard deviation. **P<0.01 vs. the
control group. UTI, ulinastatin; NC, negative control; TGF-β1,
transforming growth factor-β1; NF-κB, nuclear factor-κB; TNF-α,
tumor necrosis factor-α. |
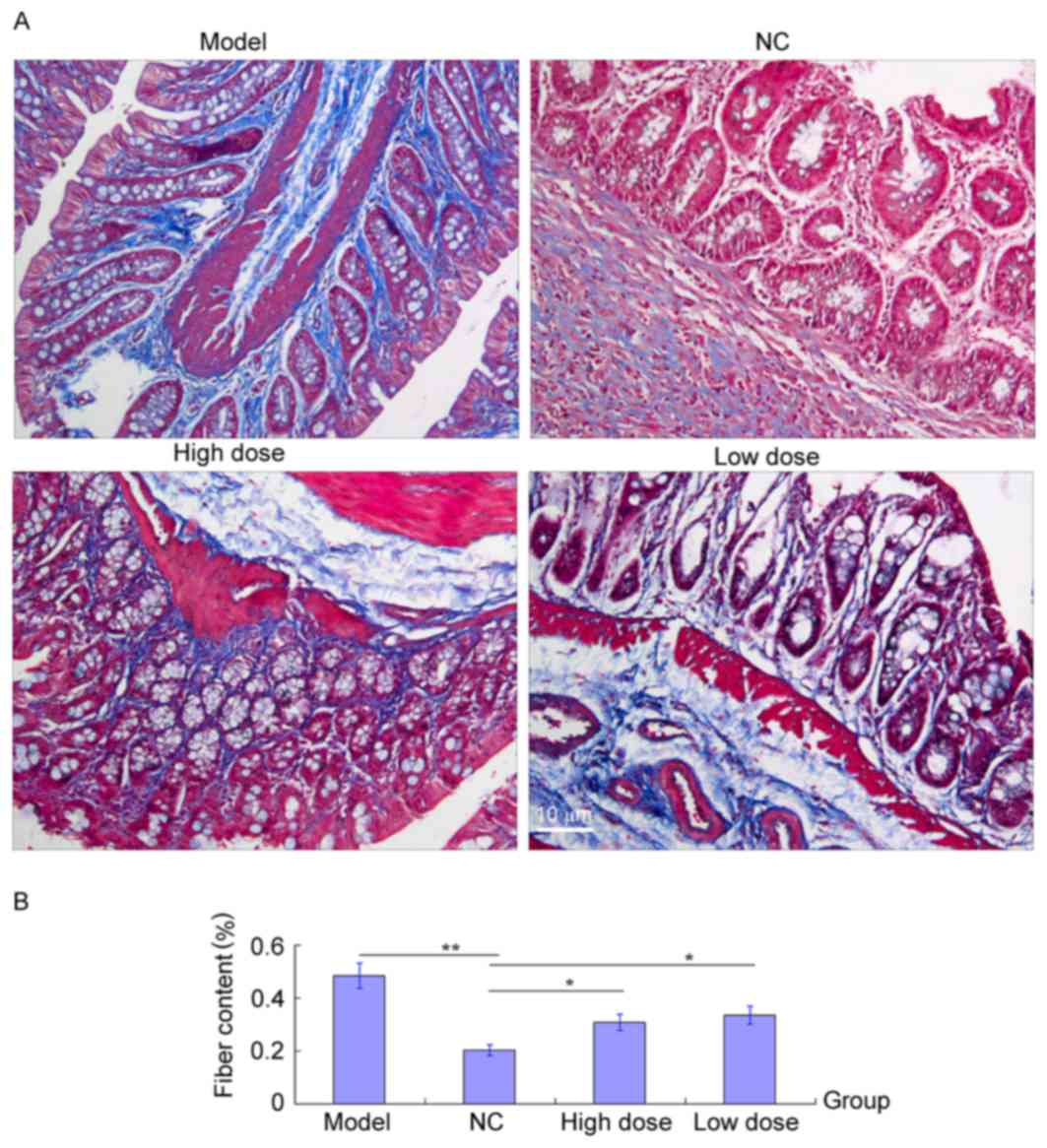
Masson staining to measure healing of
the fibrotic lung tissues
Masson trichrome staining was used to evaluate the
degree of pulmonary fibrosis by calculating the proportion of the
blue-fibrosis stain to the whole area (16). As presented in Fig. 4A, fibrous tissue in a normal range
(<20%) was observed in the lung of rats from the
control/untreated group. BLM modeling resulted in the formation of
interstitial collagens depositions leading to dysplastic fibrosis
of the lung (area >50%). When UTI treatment was applied, both in
a low and high dose, the area of fibrotic tissue was significantly
reduced compared with the BLM modeling group, indicating that
progression of pulmonary fibrosis was inhibited by UTI, which could
have also contributed to the replacement of fibrotic collagen and
the dissipation of inflammatory cells. The fibrosis area in UTI
group was larger than that of the control group (Fig. 4B) possibly due to a short course of
treatment. Longer treatment with UTI and a refined protocol may
cure pulmonary fibrosis.
Discussion
Pulmonary fibrosis is a progressive, irreversible,
and usually lethal lung disease. Its incidence and prevalence
increase markedly with age, and the median survival is 3 years
following diagnosis (17).
Research into the etiology of this devastating lung injury revealed
that the cause of pulmonary fibrosis is heterogeneous but probably
arising from the interplay between genetic and environmental
factors (18). Although the
disease received tremendous attention, the exact mechanisms of
pathogenesis is still unclear, leading to limited availability of
treatment options for patient with both idiopathic and induced
pulmonary fibrosis. Traditionally, glucocorticoids,
immunosuppressors and cytotoxic agents are used to treat the lung
disorder; however, positive responses are recorded in 10–30% of the
treated patients, and long-term use of these drugs may cause
undesirable side effects (19).
Therefore, developing a novel therapeutic strategy with low
cytotoxicity and high efficacy is needed.
The present study investigated the effect of UTI
treatment on BLM-induced pulmonary fibrosis in rats. UTI treatment
significantly mitigated pulmonary injury as demonstrated by the
histological reduction of inflammatory exudates and collagen
deposition compared with the model group with chronic fibrosing
alveolitis. UTI treatment resulted in a more efficient lung gas
exchange, less pulmonary microvascular leakage, and decreased
tissue injury and fibrosis formation. Similar results are also
reported by other research teams focusing on the anti-inflammatory
effects of UTI (20–22). It was also demonstrated that high
dose UTI (100,000 U/kg body weight/day) more effectively prevented
BLM-induced pulmonary injury during the acute inflammation phase,
than the traditional therapeutic strategy. Rats from the modeling
group suffered more pronounced inflammation (several died following
28 days of modeling) than those in the treatment groups, suggesting
that UTI elicited a protective effect. In addition, acute
inflammatory symptoms in the control group were more severe
compared with both low and high dose UTI treatment group,
suggesting that pretreatment with high dose UTI might have more
protective effect than after-treatment with either a low or high
dose.
UTI is an anti-inflammatory agent used for treatment
of many inflammation disorders such as pancreatitis, arthritis,
nephritis and associated disorders (23–25).
Previous studies demonstrated that UTI can inhibit the accumulation
of pro-inflammatory cytokines (26–28),
and the present study investigated the expression of cytokines in
response to UTI administration. The results of the present study
demonstrated that UTI can markedly reduce the expression levels of
TGF-β1, a key mediator of fibrosis formation. Fibroblasts,
myoblasts and macrophages have been proposed as TGF-β1 effectors in
the progression of lung fibrosis (29,30).
Therefore, reducing the production of TGF-β1 should inhibit the
abnormal growth of fibroblasts and myoblasts, which are responsible
for excessive collagen deposition and alveolar membrane collapse,
resulting in the amelioration of fibrosis in lungs. TNF-α (also
known as cachectin), secreted by macrophages, is able to induce the
differentiation and proliferation of fibroblasts, leading to the
formation of fasciculate collagen fibers in extracellular matrix
(31). The way UTI acts on TNF-α
signaling pathway is similar to the effect it has on TGF-β1.
Inflammatory components were not demonstrated to be involved in
TNF-α and TGF-β1 signaling pathways, and this may explain the low
efficacy of glucocorticoids in the treatment of chronic fibrosing
alveolitis. In addition, since UTI is not cytotoxic, it may be used
to prevent lung fibrosis in high genetic or environmental risk
patients.
NF-κB is a transcription factor that regulates genes
responsible for both innate and adaptive immune responses. It was
reported that NF-κB directly or indirectly controls the expression
of several cytokines, such as TGF-β1 and TNF-α which were
investigated in the present study (32). Normally, NF-κB is in a relatively
inactivate state and promotes hardly any gene expression. Upon
exogenous stimulation and phosphorylation, it can promote
expression of certain genes. Previous studies demonstrated that
during inflammatory response NF-κB initiates mRNA synthesis of
TGF-β1 and TNF-α, leading to elevated expression of these cytokines
in serum and plasma (33),
suggesting that NF-κB regulates the expression of TGF-β1 and TNF-α
in inflammatory cells. In the present study, pulmonary fibrosis was
positively associated with the enhanced activity of NF-κB, and
production of TGF-β1 and TNF-α. Lung injury was attenuated with the
downregulation of NF-κB, TGF-β1 and TNF-α upon UTI application, as
demonstrated by ELISA. Since NF-κB regulates the expression of
TGF-β1 and TNF-α, one possible mechanism of UTI action on lung
fibrosis is that UTI inhibits NF-κB, causing decreased expression
of TGF-β1 and TNF-α and mitigation of the inflammatory reaction and
renewal of fibrotic tissues. As described in the present study, the
activity of NF-κB, and expression of TGF-β1 and TNF-α decreased
simultaneously upon UTI administration, indicating that UTI
simultaneously acts on three factors and downregulates them in the
pulmonary cells exhibiting inflammatory symptoms.
In conclusion, the present study demonstrated that
UTI can significantly ameliorate the symptoms of pulmonary injury
and the subsequent development of pulmonary fibrosis in a rat
model. The functional mechanism of UTI is likely a simultaneous
downregulation of NF-κB, TGF-β1 and TNF-α along with their
associated signaling pathways. High dose UTI treatment (100,000
U/kg body weight/day) may in the future demonstrate a therapeutic
effect for lung fibrosis in high risk people exposed to radiation,
smoke and silica particles. Based on the data presented in this
study, UTI represents a promising therapeutic strategy for
pulmonary fibrosis and inflammatory disorders. Evaluation of
long-term side-effects and dosage-related cytotoxicity of UTI needs
to be performed before initiation of clinical applications.
Acknowledgements
The present study was supported by The Natural
Science Foundation of Shandong Province (grant. no. ZR2011HM063)
and Research Foundation of Tian Pu (grant. no. 01201006).
References
|
1
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar :
|
|
2
|
Raghu G, Weycker D, Edelsberg J, Bradford
WZ and Oster G: Incidence and prevalence of idiopathic pulmonary
fibrosis. Am J Respir Crit Care Med. 174:810–816. 2006. View Article : Google Scholar
|
|
3
|
Gribbin J, Hubbard RB, Le Jeune I, Smith
CJ, West J and Tata LJ: Incidence and mortality of idiopathic
pulmonary fi brosis and sarcoidosis in the UK. Thorax. 61:980–985.
2006. View Article : Google Scholar :
|
|
4
|
American Thoracic Society; European
Respiratory Society: American Thoracic Society/European Respiratory
Society International Multidisciplinary Consensus Classification of
the Idiopathic Interstitial Pneumon Society (ERS) was adopted by
the ATS board of directors, June 2001 and by the ERS executive
committee, June 2001. Am J Respir Crit Care Med. 165:277–304.
2002.
|
|
5
|
Henderson WR Jr, Chi EY, Ye X, Nguyen C,
Tien YT, Zhou B, Borok Z, Knight DA and Kahn M: Inhibition of
Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses
pulmonary fibrosis. Proc Natl Acad Sci USA. 107:14309–14314. 2010.
View Article : Google Scholar :
|
|
6
|
Kanai T, Ishiwata T, Kobayashi T, Sato H,
Takizawa M, Kawamura Y, Tsujimoto H, Nakatani K, Ishibashi N,
Nishiyama M, et al: Ulinastatin, a urinary trypsin inhibitor, for
the initial treatment of patients with Kawasaki disease: A
retrospective study. Circulation. 124:2822–2828. 2011. View Article : Google Scholar
|
|
7
|
Sato H, Kajikawa S, Kuroda S, Horisawa Y,
Nakamura N, Kaga N, Kakinuma C, Kato K, Morishita H, Niwa H and
Miyazaki J: Impaired fertility in female mice lacking urinary
trypsin inhibitor. Biochem Biophys Res Commun. 281:1154–1160. 2001.
View Article : Google Scholar
|
|
8
|
Umeadi C, Kandeel F and Al-Abdullah IH:
Ulinastatin is a novel protease inhibitor and neutral protease
activator. Transplant Proc. 40:387–389. 2008. View Article : Google Scholar
|
|
9
|
Pugia MJ and Lott JA: Pathophysiology and
diagnostic value of urinary trypsin inhibitors. Clin Chem Lab Med.
43:1–16. 2005. View Article : Google Scholar
|
|
10
|
Wang G, Wen J, Wilbur RR, Wen P, Zhou SF
and Xiao X: The effect of somatostatin, ulinastatin and Salvia
miltiorrhiza on severe acute pancreatitis treatment. Am J Med Sci.
346:371–376. 2013. View Article : Google Scholar
|
|
11
|
Wei W, Ma B, Li HY, Jia Y, Lv K, Wang G,
Zhang J, Zhu S, Tang H, Sheng Z and Xia Z: Biphasic effects of
selective inhibition of transforming growth factor beta1 activin
receptor-like kinase on LPS-induced lung injury. Shock. 33:218–224.
2010. View Article : Google Scholar
|
|
12
|
Wang J: Care and Use of Laboratory
Animals. 8th edition. Shanghai Science and Technology Press; 2012,
View Article : Google Scholar
|
|
13
|
O'connor WN and Valle S: A combination
Verhoeffs elastic and Masson's trichrome stain for routine
histology. Stain Technol. 57:207–210. 1982. View Article : Google Scholar
|
|
14
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic rat. Am Rev Respir Dis.
120:893–899. 1979.
|
|
15
|
Hakenjos L, Bamberg M and Rodemann HP:
TGF-beta1-mediated alterations of rat lung fibroblast
differentiation resulting in the radiation-induced fibrotic
phenotype. Int J Radiat Biol. 76:503–509. 2000. View Article : Google Scholar
|
|
16
|
Moore BB and Hogaboam CM: Murine models of
pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol.
294:L152–L160. 2008. View Article : Google Scholar
|
|
17
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar
|
|
18
|
American Thoracic Society. Idiopathic
pulmonary fibrosis: Idiopathic pulmonary fibrosis: Diagnosis and
treatment. International consensus statement. American Thoracic
Society (ATS), and the European Respiratory Society (ERS). Am J
Resp Crit Care Med. 161:646–664. 2000. View Article : Google Scholar
|
|
19
|
Tobin RW, Pope CE II, Pellegrini CA, Emond
MJ, Sillery J and Raghu G: Increased prevalence of gastroesophageal
reflux in patients with idiopathic pulmonary fibrosis. Am J Resp
Crit Care Med. 158:1804–1808. 1998. View Article : Google Scholar
|
|
20
|
Wang X, Xue Q, Yan F, Li L, Liu J, Li S
and Hu S: Ulinastatin as a neuroprotective and anti-inflammatory
agent in infant piglets model undergoing surgery on hypothermic
low-flow cardiopulmonary bypass. Pediatr Anesth. 23:209–216. 2013.
View Article : Google Scholar
|
|
21
|
Shin IW, Jang IS, Lee SM, Park KE, Ok SH,
Sohn JT, Lee HK and Chung YK: Myocardial protective effect by
ulinastatin via an anti-inflammatory response after regional
ischemia/reperfusion injury in an in vivo rat heart model. Korean J
Anesthesiol. 61:499–505. 2011. View Article : Google Scholar :
|
|
22
|
Park KH, Lee KH, Kim H and Hwang SO: The
anti-inflammatory effects of ulinastatin in trauma patients with
hemorrhagic shock. J Korean Med Sci. 25:128–134. 2010. View Article : Google Scholar
|
|
23
|
Gao CJ, Huan JN, Li W and Tang JJ:
Protective effects of ulinastatin on pancreatic and renal damage in
rats following early scald injury. Burns. 35:547–552. 2009.
View Article : Google Scholar
|
|
24
|
Larsen C, Ostergaard J, Larsen SW, Jensen
H, Jacobsen S, Lindegaard C and Andersen PH: Intra-articular depot
formulation principles: role in the management of postoperative
pain and arthritic disorders. J Pharm Sci. 97:4622–4654. 2008.
View Article : Google Scholar
|
|
25
|
Ning XH, Ge XF, Cui Y and An HX:
Ulinastatin inhibits unilateral ureteral obstruction-induced renal
interstitial fibrosis in rats via transforming growth factor β
(TGF-β)/Smad signalling pathways. Int Immunopharmacol. 15:406–413.
2013. View Article : Google Scholar
|
|
26
|
Inoue K, Takano H, Yanagisawa R, Sakurai
M, Shimada A, Yoshino S, Sato H and Yoshikawa T: Protective role of
urinary trypsin inhibitor in acute lung injury induced by
lipopolysaccharide. Exp Biol Med (Maywood). 230:281–287. 2005.
View Article : Google Scholar
|
|
27
|
Tanaka R, Fujita M, Tsuruta R, Fujimoto K,
Aki HS, Kumagai K, Aoki T, Kobayashi A, Izumi T, Kasaoka S, et al:
Urinary trypsin inhibitor suppresses excessive generation of
superoxide anion radical, systemic inflammation, oxidative stress,
and endothelial injury in endotoxemic rats. Inflamm Res.
59:597–606. 2010. View Article : Google Scholar
|
|
28
|
Molor-Erdene P, Okajima K, Isobe H, Uchiba
M, Harada N and Okabe H: Urinary trypsin inhibitor reduces
LPS-induced hypotension by suppressing tumor necrosis factor-alpha
production through inhibition of Egr-1 expression. Am J Physiol
Heart Circ Physiol. 288:H1265–H1271. 2005. View Article : Google Scholar
|
|
29
|
Park KJ, Oh YT, Kil WJ, Park W, Kang SH
and Chun M: Bronchoalveolar lavage findings of radiation induced
lung damage in rats. J Radiat Res. 50:177–182. 2009. View Article : Google Scholar
|
|
30
|
Anscher MS, Kong FM, Andrews K, Clough R,
Marks LB, Bentel G and Jirtle RL: Plasma transforming growth factor
beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol
Biol Phys. 41:1029–1035. 1998. View Article : Google Scholar
|
|
31
|
Chen G and Goeddel DV: TNF-R1 signaling: A
beautiful pathway. Science. 296:1634–1635. 2002. View Article : Google Scholar
|
|
32
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Ann Rev Immunol. 16:225–260. 1998. View Article : Google Scholar
|
|
33
|
Cox RA, Burke AS, Jacob S, Oliveras G,
Murakami K, Shimoda K, Enkhbaatar P, Traber LD, Herndon DN, Traber
DL and Hawkins HK: Activated nuclear factor kappa B and airway
inflammation after smoke inhalation and burn injury in sheep. J
Burn Care Res. 30:489–498. 2009. View Article : Google Scholar
|