Introduction
Diacylglycerol kinases (DGKs) are endogenous lipid
regulation enzymes, which are involved in multiple cellular
signaling pathways by regulating the levels of intracellular
secondary messengers diacylglycerol (DAG) and phosphatidyl acid
(PA) (1,2). Currently, 10 DGK isoforms have been
documented in mammals, and are grouped into five categories
according to their structure and number of specific domains. DGKθ
is the sole member of group V (3).
Compared with other DGK members, which contain two cysteine-rich
domains, DGKθ has three, in addition to an N-terminal
proline/glycine-rich domain, a pleckstrin homology domain and a
Ras-associating domain (4). DGKθ
was initially found to be expressed in mouse brains (4), and was subsequently reported to be
the most abundant isoform in hepatocytes (5).
There is evidence that abnormal enzyme activity of
DGKθ may be associated with insulin resistance. Hepatic DAG
accumulation can activate protein kinase Cε (PKCε) in the liver,
which is associated with hepatic insulin resistance (6,7).
DGKθ has been identified as the major isoform mediating DAG
accumulation (5,8,9). In
addition, DGKδ, which has a similar substructure to DGKθ (4), has been shown to be directly linked
to insulin resistance in the skeletal muscle of patients with type
2 diabetes (10).
Nonalcoholic fatty liver disease (NAFLD) is an
independent risk factor for type 2 diabetes and cardiovascular
diseases (11). The prevalence of
NAFLD is ~30% in the general population, and up to three times
higher in those with type 2 diabetes. Studies have suggested that
abnormality of the DAG-PKCε signaling pathway can link NAFLD with
hepatic insulin resistance (11).
Therefore, it is likely that DGKθ is the key signaling molecule in
this pathway and involved in the pathogenesis of NAFLD.
In the present study, CRISPR/Cas9 genome editing
technology was used to establish a DGKθ-knockout hepatic cell line.
It was found that this cell line had markedly increased
intracellular lipid content. The gene expression levels of key
proteins in the pathways involved in lipid metabolism were
evaluated. These proteins included fatty acid synthase (FAS),
peroxisome proliferator-activated receptor-γ (PPARγ), sterol
regulatory element-binding protein-1c (SREBP-1c), carnitine
palmitoyltransferase1a (CPT1a) and long-chain
L-3-hydroxyacyl-coenzyme A dehydrogenase α (HADHα). Key proteins in
pathways involved in insulin resistance, including PKCε and insulin
receptor substrate 1 (IRS-1), and in gluconeogenesis, including
mechanistic target of rapamycin (mTOR) and Akt, were also assessed.
This cell line may offer potential for investigating NAFLD and its
associated hepatic insulin resistance.
Materials and methods
Cell culture
The human liver cancer cell line HepG2 was purchased
from American Type Culture Collection (Manassas, VA, USA) and
cultured in high-glucose Dulbecco's modified Eagle medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C, 5%
CO2 (v/v). A total of ~1×105 HepG2 cells were
treated with DGKθ inhibitor R59949 at 10 µM, or DGKθ agonist GW4064
(both from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 1 µM
for 24 h at 37°C.
Plasmid construction
The targeting regions for four pairs of single-guide
RNA (sgRNA) located in exon 6, exon 7 or exon 8 of human DGKθ were
selected using the CRISPR Design website (http://crispr.mit.edu). The sgRNAs were synthesized at
the Beijing Genomics Institute (Beijing, China). The sequences of
the oligonucleotides are shown in Table I. According to a previously
described method (12), the human
U6 promoter and sgRNA backbone were sequentially cloned into pUC19
(Clontech Laboratories, Inc., Mountain view, CA, USA), the obtained
plasmid was called the pUC19/U6-BsaI-sgRNA backbone vector. The
synthesized oligos were annealed, and ligated into the BsaI sites
of the pUC19/U6-BsaI-sgRNA backbone vector under the control of the
U6 promoter. The resultant plasmids were referred to as
pUC19/U6-DGKθ sgRNA1, pUC19/U6-DGKθ sgRNA2, pUC19/U6-DGKθ sgRNA3
and pUC19/U6-DGKθ sgRNA4.
 | Table I.Primer sequences used for sgRNA
synthesis and the detection of sgRNA biological activity. |
Table I.
Primer sequences used for sgRNA
synthesis and the detection of sgRNA biological activity.
| Primer | Sequence (5′-3′) |
|---|
| hDGKθ sgRNA1
Forward |
ACCGCCCTGCAGGAGGCCGCACTGCGG |
| hDGKθ sgRNA1
Reverse |
AAACCCGCAGTGCGGCCTCCTGCAGGG |
| hDGKθ sgRNA2
Forward |
ACCGGAGGGGGGCGACGGCGCCGACGG |
| hDGKθ sgRNA2
Reverse |
AAACCCGTCGGCGCCGTCGCCCCCCTC |
| hDGKθ sgRNA3
Forward |
ACCGACACAGGCAACTCCGGAGTCCGG |
| hDGKθ sgRNA3
Reverse |
AAACCCGGACTCCGGAGTTGCCTGTGT |
| hDGKθ sgRNA4
Forward |
ACCGAAGCCAGTTCCGCCTCGTCACGG |
| hDGKθ sgRNA4
Reverse |
AAACCCGTGACGAGGCGGAACTGGCTT |
| hDGKθ sgRNA detection
Forward |
GCTTCAGCAAGACGCAGAG |
| hDGKθ sgRNA detection
Reverse |
CAGGTCCAAACCCAAAAGGT |
To construct the donor vector, an up homologous arm,
909 bp in length and located upstream of the targeting sites, was
amplified through nest polymerase chain reaction (PCR) using two
pairs of primers (Table II) based
on a template of human genomic DNA. PCR was conducted in a 50 µl
reaction volume, consisting of 5 µl 10X PrimeSTAR buffer, 100 ng
genomic DNA template, 0.2 µM each primer, 10 mM dNTPS and 1 unit
PrimeSTAR HS DNA Polymerase (all from Clontech Laboratories, Inc.)
according to the following conditions: 29 cycles of 94°C for 30
sec; 98°C for 10 sec, 58°C for 15 sec and 72°C for 1 min and a 10
min extension step at 72°C. Similarly, a down homologous arm 975 bp
in length was obtained using two pairs of primers (Table II). Subsequently, the donor vector
pAd5/DGKθ-up/down-arm was constructed by sequentially inserting the
up and down homologous arms into the backbone vector according to
the previously described method (12). This vector also contained an
eGFP-T2A-Neomycin expression cassette between the up and down
homologous arms for positive selection, and a PGK-TK-T2A-mCherry
expression cassette located at the 3′-terminal of the down
homologous arm for negative selection (Fig. 1D).
 | Table II.Primer sequences used for donor
construction and RT-qPCR amplification. |
Table II.
Primer sequences used for donor
construction and RT-qPCR amplification.
| Primer | Sequence (5′-3′) |
|---|
| hDGKθ up arm nest
Forward |
GGCGAGAGTCAGGAGTGAAG |
| hDGKθ up arm nest
Reverse |
GGAGAAGGGCCTGAGCTG |
| hDGKθ up arm SalI
Forward |
GTCGACAGAGTTGCGCAGGTGAAGAG |
| hDGKθ up arm ClaI
Reverse |
AATCGATACCAGGTCCAGGGAAAGACC |
| hDGKθ down arm nest
Forward |
CGTACCCTGTGCCTGCTC |
| hDGKθ down arm nest
Reverse |
GTGACATCTCACCCCAAAGG |
| hDGKθ down arm SalI
Forward |
GTCGACAGGCACGGTGAGTAGACAGC |
| hDGKθ down arm BamHI
Reverse |
GGATCCCAGAGCCTCTTGGAGGAAGA |
| hDGKθ knock-in
detection nest Forward |
TGGTGATTCCACACTGGCTTG |
| hDGKθ knock-in
detection nest Reverse |
ATGCCAGATGAAAACAGCGAG |
| hDGKθ knock-in
detection Forward |
CACCAGGATCACGTGAGTGTA |
| hDGKθ knock-in
detection Reverse |
CAGGTCCAAACCCAAAAGGT |
| PKCε RT-qPCR
Forward |
GACGAGTTCGTCACCGATGT |
| PKCε RT-qPCR
Reverse |
CTTTAGGGGCTTCACCCGAC |
| INSR RT-qPCR
Forward |
GTACCCCGGAGAGGTGTGTC |
| INSR RT-qPCR
Reverse |
CCCGGAAGAGCAGCAAGTAA |
| IRS1 RT-qPCR
Forward |
CTGGGGGTTTGGAGAATGGT |
| IRS1 RT-qPCR
Reverse |
GTCTTCATTCTGCTGTGATGTCC |
| FAS RT-qPCR
Forward |
CAGAGCAGCCATGGAGGAG |
| FAS RT-qPCR
Reverse |
TTGATGCCTCCGTCCACGAT |
| PPAR-γ RT-qPCR
Forward |
ACCCAGAAAGCGATTCCTTCA |
| PPAR-γ RT-qPCR
Reverse |
TCCACTTTGATTGCACTTTGGT |
| SREBP1c RT-qPCR
Forward |
CTCCGGCCACAAGGTACACA |
| SREBP1c RT-qPCR
Reverse |
GAGGCCCTAAGGGTTGACACAG |
| CPT1a RT-qPCR
Forward |
GGAATGAAATTCCCACTGTCTGTC |
| CPT1a RT-qPCR
Reverse |
CAGTTCAGCCATCGCTGTTGTA |
| HADHα RT-qPCR
Forward |
GCCATCAATGGATCCTGCCT |
| HADHα RT-qPCR
Reverse |
CAGGCACACCCACCATTTTG |
| AKT1 RT-qPCR
Forward |
GGCAAGGTGATCCTGGTGAA |
| AKT1 RT-qPCR
Reverse |
ACAGGTGGAAGAACAGCTCG |
| mTOR RT-qPCR
Forward |
AAGCCGCGCGAACCTC |
| mTOR RT-qPCR
Reverse |
TGGCATCTGAGCTGGAAACC |
| hDGKθ RT-qPCR
Forward |
ATCCGGCAGATGTCTGTGC |
| hDGKθ RT-qPCR
Reverse |
ATGTGACTCACGGACACCAC |
T7E1 assay
Genomic DNA was extracted using the TIANamp Blood
DNA kit (Tiangen Biotech, Inc., Beijing, China). The target site
was amplified by nest PCR, the product of which was then purified
using an AxyPrep DNA Gel Extraction kit (Axygen Biotechnology,
Hangzhou, China). The purified product was then denatured and
re-annealed, and digested with T7E1 (New England Biolabs, Ipswich,
MA, USA). The digested product was then separated by 1.2% agarose
gel electrophoresis. The gel was stained in running buffer
containing 0.5 µg/ml ethidium bromide at room temperature for 15–30
min, then the images were captured under FR-98A Gel Imaging System
(Shanghai Furi Science & Technology Co., Ltd., Shanghai,
China).
Establishment of the DGKθ
gene-knockout liver cancer cell line
To construct the human DGKθ gene-knockout Liver
cancer cell line, pUC19/CMV-Cas9-U6-sgRNAX (X represents the sgRNA
with the highest activity) was generated according to a previously
described method (12). A total of
~1.5×105 HepG2 cells were then co-transfected with 4 µg
of pUC19/CMV-Cas9-U6-sgRNAX and 8 µg of pAd5/DGKθ-up/down-arm at
37°C for 48 h at an efficiency of ~20% using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) followed by
screening in DMEM containing G418 (1 mg/ml) and GCV (1 mg/ml). A
single cell clone was obtained through limited dilution following
positive and negative selection, which was then confirmed by PCR
that was performed in a 50 µl reaction volume, consisting of 5 µl
10X PrimeSTAR, 100 ng genomic DNA template, 0.2 µM each primer, 10
mM dNTPs, and 1 unit PrimeSTAR HS DNA Polymerase (all from Clontech
Laboratories, Inc.) according to the following conditions: 30
cycles of 94°C for 30 sec; 98°C for 10 sec, 58°C for 15 sec and
72°C for 90 min, followed by a 10 min extension step at 72°C, then
the PCR products were sent to Bejing Genomics Institute Genomics
Co., Ltd. (Shenzhen, China) for sequencing.
MTT assay
A total of 1×103 wild-type (WT) HepG2
cells or DGKθ gene-knockout HepG2 cells were cultured in 96-well
plates. MTT solution (20 µl; American Type Culture Collection) was
added to each well at 37°C at 24, 48, 72 and 96 h, respectively.
Following incubation with MTT for 4 h, 200 µl DMSO was added to
each well for 35 min at 37°C. The absorbance in each well was then
measured at 570 nm on a microplate reader (Thermo Fisher
Scientific, Inc.). Each group contained six replicates and the
experiment was repeated three times.
Oil Red O staining and determination
of optical density (OD) values
The WT HepG2 cells and DGKθ-knockout HepG2 cells
grown in 24-well plates were harvested. The cells were stained with
Oil Red O (Sigma-Aldrich; Merck KGaA), and quantification of Oil
Red O-based steatosis was performed, as previously described
(13). The cell nuclei were
stained with hematoxylin for 15 sec and washed with saturated
Li2CO3 solution. Images were captured using a
Leica DFC 420 C microscope (Leica Microsystems GmbH). The
experiments were performed in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA (1 µg), purified with an RNeasy Mini kit
(Qiagen, Inc., Valencia, CA, USA) was used to synthesize cDNA,
followed by amplification of the target gene that was carried out
in a 25 µl reaction volume, consisting of 150 ng cDNA, 0.2 µM each
primer, 12.5 µl 2X SYBR buffer (Takara Biotechnology Co., Ltd.,
Dalian, China) containing 10 mM dNTPs and 1 unit DNA Taq polymerase
according to the following conditions: 39 cycles of 95°C for 30
sec; 95°C for 5 sec and 60°C for 30 sec. The sequences of the
primers used are listed in Table
II. All tests were performed in triplicate and the data were
normalized to GAPDH and quantified using the 2−ΔΔCq
method (14). ΔCq was calculated
by subtracting the Cq value of GAPDH from the Cq value of the
target gene. The fold change was generated using the formula
2-ΔΔCq.
Western blot analysis
The proteins were extracted from the cells using
extraction buffer as previously described (13) and quantified using Pierce
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.), which were then applied (80 µg/lane) to a gel
for 10% SDS-PAGE and subsequently electrotransferred onto
methanol-pretreated polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with PBS
buffer containing 3% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) and 0.5% v/v Tween-20 for 1 h at room temperature. The
membranes were then incubated with primary antibodies targeting FAS
(1:500; cat no. ab82419), CPT1a (1:300; cat no. ab128568), PPARγ
(1:500; cat no. ab66343), mTOR (1:500; cat no. ab25880),
phosphorylated (p-)mTOR (1:300; cat no. ab109268), PKCε (1:500; cat
no. ab63638), p-PKCε (S729; 1:500; cat no. ab63387), IRS1 (1:500;
cat no. ab52167) and p-IRS1 (Y632; 1:300; cat no. ab109543) from
Abcam (Cambridge, UK), and primary antibodies targeting DGKθ
(1:500; cat no. 17885-1-AP), SREBP-1c (1:500; cat no. 14088-1-AP),
HADHα (1:500; cat no. 10758-1-AP), AKT (1:500; cat no. 10176-2-AP)
and p-AKT (1:300; cat no. 66,444-1-Ig) from ProteinTech Group, Inc.
(Chicago, IL, USA) at 37°C for 1 h. Finally, the membranes were
incubated with secondary antibodies, horse radish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) polyclonal
antibody (1:10,000; cat no. ZB-2301; Beijing Zhongshan Jinqiao
Biological Technology Ltd., Beijing, China) or HRP-conjugated goat
anti-mouse IgG polyclonal antibody (1:10,000; cat no. ZB-2305;
Beijing Zhongshan Jinqiao Biological Technology Ltd., Beijing,
China) at 37°C for 1 h and visualized on a Tanon 5500
Chemiluminescence Imaging system (Tanon Science and Technology Co.,
Ltd., Shanghai, China), and the protein levels were visualized
using a Supersignal West Pico chemiluminescent detection system
(Tanon Science and Technology Co., Ltd.), according to the
manufacturer's protocol. Protein levels were determined using
ImageCal software (version 4.0; Tanon Science and Technology Co.,
Ltd.).
PA and DAG assay
The WT HepG2 cells and DGKθ-knockout HepG2 cells
were grown on 60 mm plates for 48 h and lysed with RIPA buffer
containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.1%
SDS and 1 mM phenylmethanesulfonyl fluoride. The total lipids in
the lysates were harvested by centrifugation at 10,000 × g for 10
min at 4°C. The PA content was quantified using a Total PA kit
(HZbscience, Shanghai, China) according to the manufacturer's
protocol. The quantity of DAG in each sample was determined using a
Human DAG ELISA kit (Cusabio Biotech Co., Ltd., Barksdale, DE,
USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences between the means of each group were analyzed
using one-way analysis of variance with Dunnett's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference. The statistical analyses were
performed using Prism 6.0 software (GraphPad Software, Inc., La
Jolla, CA, USA) and SPSS Statistics 20 software (IBM SPSS, Armonk,
NY, USA).
Results
Generation of a DGKθ-knockout liver
cancer cell line using the Cas9/sgRNA technique
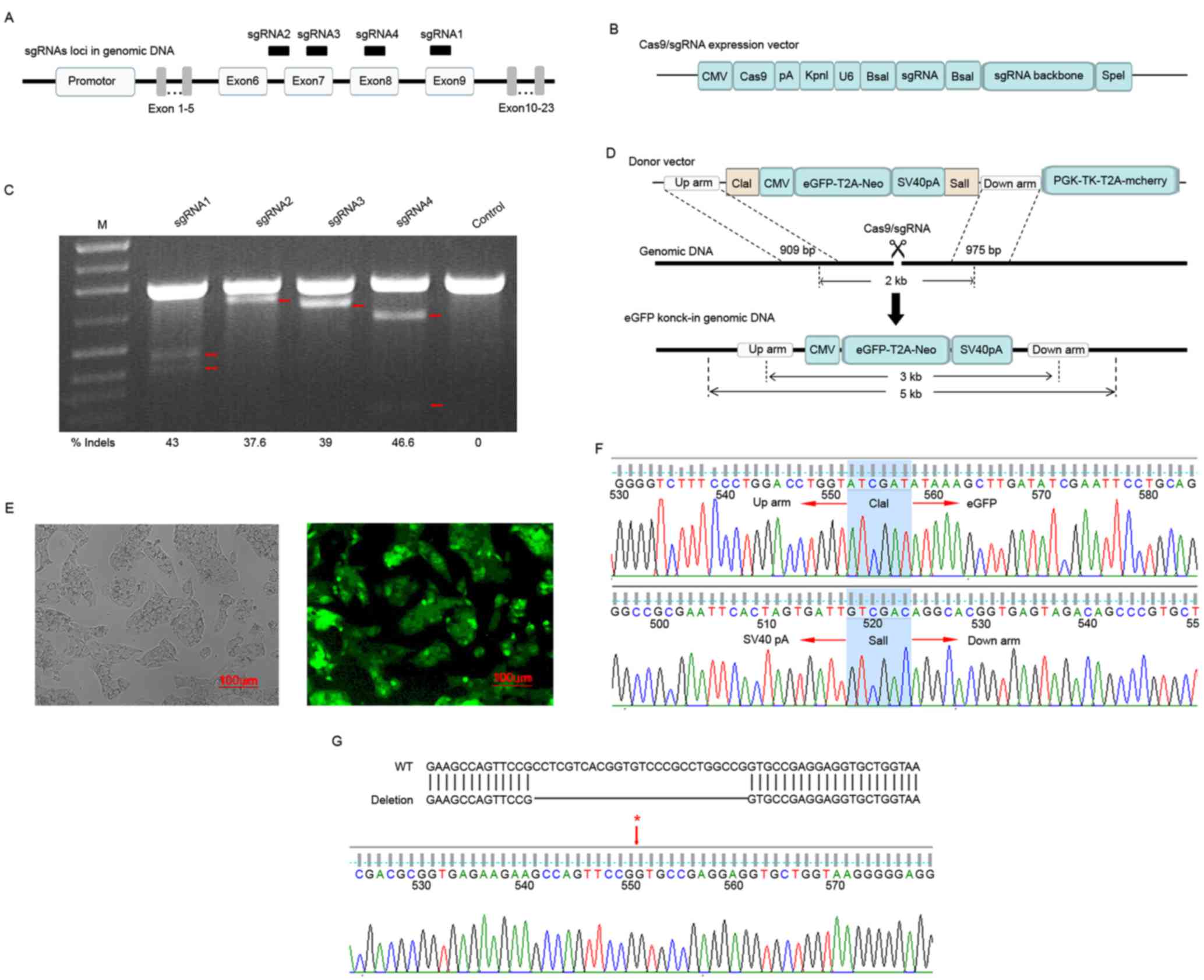
In the present study, the DGKθ gene-knockout liver
cancer cell line HepG2 was established using the Cas9/sgRNA
technique, as follows. Firstly, the pUC19/CMV-Cas9-U6-sgRNA4 vector
was constructed according to the previously described method
(12). The vector carried sgRNA4,
which had the highest cleavage activity among the four pairs of
human DGKθ-targeting sgRNAs (Fig.
1A-C). Secondly, the donor vector pAd5/DGKθ-up/down-arm, which
contained up- and down-homologous arms for homologous
recombination, a neomycin-T2A-eGFP expression cassette for positive
selection and a TK expression cassette for negative selection
(Fig. 1D), was generated according
to the previously described method (12). The Liver cancer cell line was then
transfected with pUC19/CMV-Cas9-U6-sgRNA4 and pAd5/DGKθ-up/down-arm
donor vector followed by screening with G418. As the donor vector
contained an eGFP expression cassette, the cells with homologous
recombination exhibited green fluorescence (Fig. 1E). Finally, the DGKθ gene-knockout
liver cancer cell line HepG2 carrying a targeted integration in one
allele (Fig. 1F) and a 26 bp
deletion in the other allele was confirmed by sequencing (Fig. 1G).
Characterization of the DGKθ-knockout
liver cancer cell line
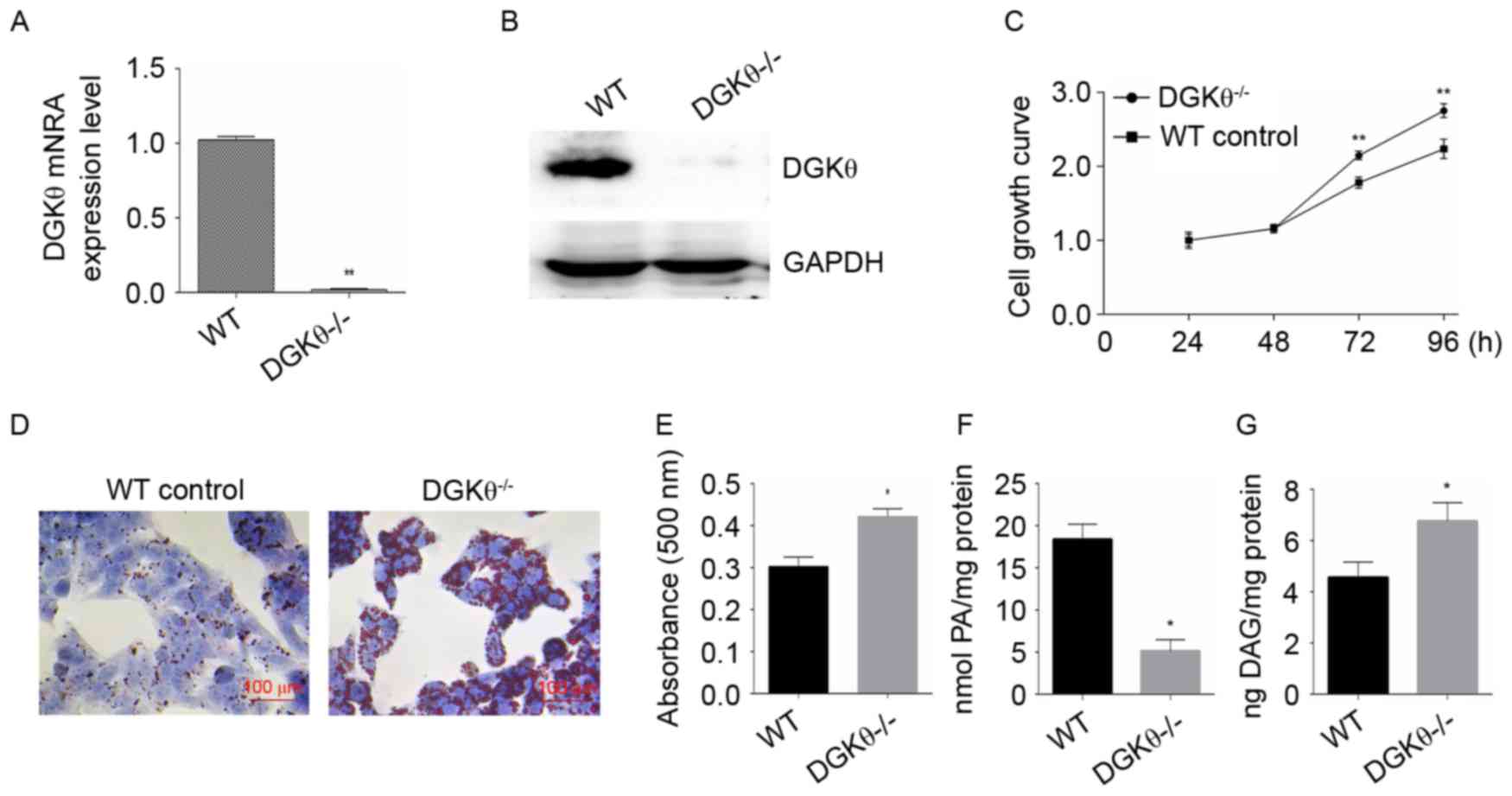
RT-qPCR and western blot analyses were performed to
confirm the knockout of the DGKθ gene in the liver cancer cell line
HepG2 (Fig. 2A and B). The effect
of knockout of the DGKθ gene on the growth of liver cancer cells
was then investigated using an MTT assay. The knockout of the DGKθ
gene promoted the growth of the liver cancer cells (Fig. 2C). Oil Red O staining showed that
the DGKθ gene-knockout HepG2 cells had 32% higher intracellular
lipid content (Fig. 2D), compared
with the WT HepG2 cells (Fig. 2E).
As expected, the content of intracellular PA was significantly
decreased (Fig. 2F), whereas the
content of DAG was increased (Fig.
2G) in the DGKθ gene-knockout HepG2 cells.
Subsequently, the present study examined the
expression levels of genes associated with lipid synthesis (FAS,
PPARγ and SREBP-1c) and genes associated with lipolysis (CPT1a and
HADHα) in the DGKθ-knockout HepG2 cells at the mRNA and protein
levels. The results indicated that DGKθ gene-knockout increased the
expression levels of FAS, PPARγ and SREBP-1c, and suppressed the
expression of CPT1a (Fig. 3A-C),
compared, with the levels in the WT liver cancer cell line HepG2.
These changes were observed at the mRNA and protein levels. Similar
results were found in WT HepG2 cells treated with the DGKθ
inhibitor, R59949. However, the DGKθ agonist, GW4064, had an
opposite effect at the mRNA level for FAS only, compared with the
knockout of DGKθ and treatment with DGKθ inhibitor. No effects on
HADHα were observed in any of the treatment groups (Fig. 3A-C).
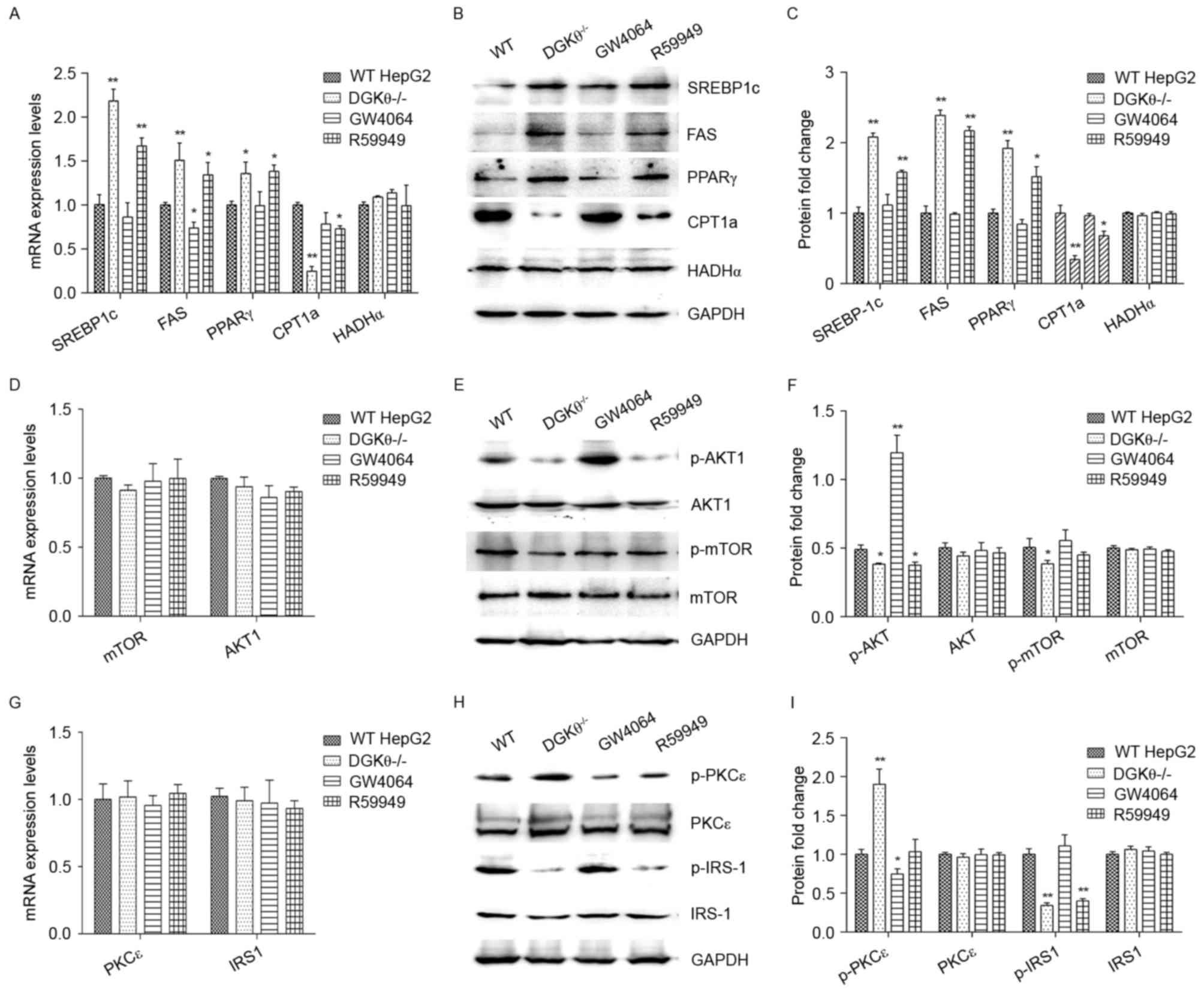 | Figure 3.Effects of the knockout of DGKθ on the
expression levels of signaling proteins involved in lipid
metabolism, insulin resistance and gluconeogenesis pathways. (A)
Expression levels of proteins involved in lipid metabolism pathway
signaling, SREBP-1c, FAS, PPARγ, CPT1a and HADHα, were analyzed
using RT-qPCR and (B) western blot analyses with (C)
semi-quantification. (D) Expression levels of the proteins involved
in the gluconeogenesis pathway signaling, mTOR and AKT, were
detected by RT-qPCR and (E) western blot analyses with (F)
semi-quantification. (G) The expression levels of the insulin
resistance pathway signaling proteins PKCε and IRS-1 were measured
by RT-qPCR and (H) western blot analyses with (I)
semi-quantification. For western blot analysis, 80 µg of proteins
were loaded in each lane. Protein expression values were normalized
to GAPDH and data are presented as the mean ± standard error of the
mean of three independent experiments, each performed in
triplicate. *P<0.05 and **P<0.01, vs. control. DGKθ,
diacylglycerol kinase θ; WT, wild-type control; DGKθ-/-, DGKθ
gene-knockout; GW4064, WT Liver cancer cells treated with 1 µM
GW4064; R59949, WT Liver cancer cells treated with 10 µM R59949;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; SREBP1c, sterol regulatory element-binding protein-1c;
FAS, fatty acid synthase, PPAR-γ, peroxisome proliferator-activated
receptor-γ; CPT1a, carnitine palmitoyltransferase1A; HADHα,
long-chain L-3-hydroxyacyl-coenzyme A dehydrogenase α; mTOR,
mechanistic target of rapamycin; PKCε, protein kinase Cε; IRS1,
insulin receptor substrate 1; p-, phosphorylated. |
The expression levels of the signaling proteins
involved in the glucose metabolism pathway, mTOR and Akt, were also
analyzed. The results showed that DGKθ-knockout affected neither
the mRNA nor the protein levels of mTOR and Akt (Fig. 3D-F). The effect of DGKθ-knockout on
the levels of protein phosphorylation were then determined. No
significant changes were observed in the total protein levels of
mTOR and Akt, however, the phosphorylation levels of these proteins
were significantly decreased in the DGKθ-knockout group (Fig. 3E and F). Treatment with the DGKθ
inhibitor R59949 decreased the level of p-Akt, whereas the DGKθ
agonist GW4064 significantly increased the level of p-Akt. Neither
R59949 nor GW4064 treatment affected the level of p-mTOR.
Finally, the present study examined whether the
DGKθ-knockout affected the expression levels of insulin resistance
mediators, PKCε and IRS-1. The results showed no significant change
in the expression levels of PKCε and IRS-1 by DGKθ knockout at the
mRNA or protein levels. However, the level of p-PKCε (serine 729)
was significantly increased, and the level of p-IRS-1 at tyrosine
632 (a stimulatory site for insulin signaling) was significantly
decreased in the DGKθ-knockout group (Fig. 3G-I). In addition, the DGKθ
inhibitor R59949 decreased the level of p-IRS-1, and the DGKθ
agonist GW4064 significantly decreased the level of p-PKCε.
Discussion
In the present study, a DGKθ gene-knockout liver
cancer cell line HepG2 was produced using CRISPR/Cas9 technology,
which exhibited a marked increase in the accumulation of
intracellular lipids. This cell line was then evaluated for the
expression of genes associated with lipid and glucose metabolism,
confirming that the established cell line offers potential for
investigating NAFLD and its associated hepatic insulin
resistance.
CRISPR/Cas9 is a next-generation targeted genome
editing technology. Compared with ZFN technology or TALEN
technology, it is easier to manipulate (15–17).
In the present study, an efficient CRISPR/Cas9 system designed.
Four pairs of sgRNA targeting the human DGKθ gene were first
obtained with an indel frequency up to 46.6%. The donor vector,
which carried the positive and negative selection markers, improved
the selection efficiency. The DGKθ gene-knockout Liver cancer cell
line was successfully generated by integrating the exogenous
fragment into one allele, and deleting a 26-bp base on the other
allele.
The results of the present study showed that the
DGKθ-knockout liver cancer cell line HepG2 exhibited increased
expression of all three of the lipid synthesis-related genes
examined (FAS, PPARγ and SREBP-1c) and decreased the expression of
the lipolysis-related gene, CPT1a. This may be the cause of the
increased intracellular lipid content of this cell line. Of note,
Cai et al reported that DGKθ gene-knockdown using short
hairpin RNA led to a decrease in the expression of SREBP-1c;
however, this was performed in human adrenocortical cells (18), which may have a lipid metabolism
pathway differing from that of human hepatocytes. The
overexpression of FAS has been shown to promote not only
lipogenesis but also the growth of breast cancer cells (19). This may explain why the
DGθ-knockout Liver cancer cells exhibited an increased growth rate.
The increased FAS and decreased CPT1a of the DGKθ-knockout liver
cancer cells may also be caused by the increased activity of
SREBP-1c in this cell line. SREBP-1c has been reported to activate
the transcription of FAS (19) and
downregulate lipolytic enzyme genes (20).
In a previous study, DGKθ was shown to modulate
cellular DAG and PA, which further modulated DAG-sensitive proteins
associated with hepatic insulin resistance, including PKCε
(6), and PA-sensitive proteins,
including mTOR and Akt, which are associated with glucose
production (21–24). As expected, the results of the
present study showed that the DGKθ-knockout HepG2 cells expressed
an increased level of p-PKCε, possibly due to increased
intracellular DAG, and a decreased level of p-IRS-1. These changes
have been reported to be mediate insulin resistance (6,25).
Consistent with a previous study on DGKθ silencing (24,26),
the DGKθ-knockout HepG2 cells in the present study expressed lower
levels of p-mTOR and p-AKT, which may have been caused by decreased
PA. Based on the results from the present study, the roles of DGKθ
in lipid accumulation, insulin resistance and glucose production
are summarized in Fig. 4.
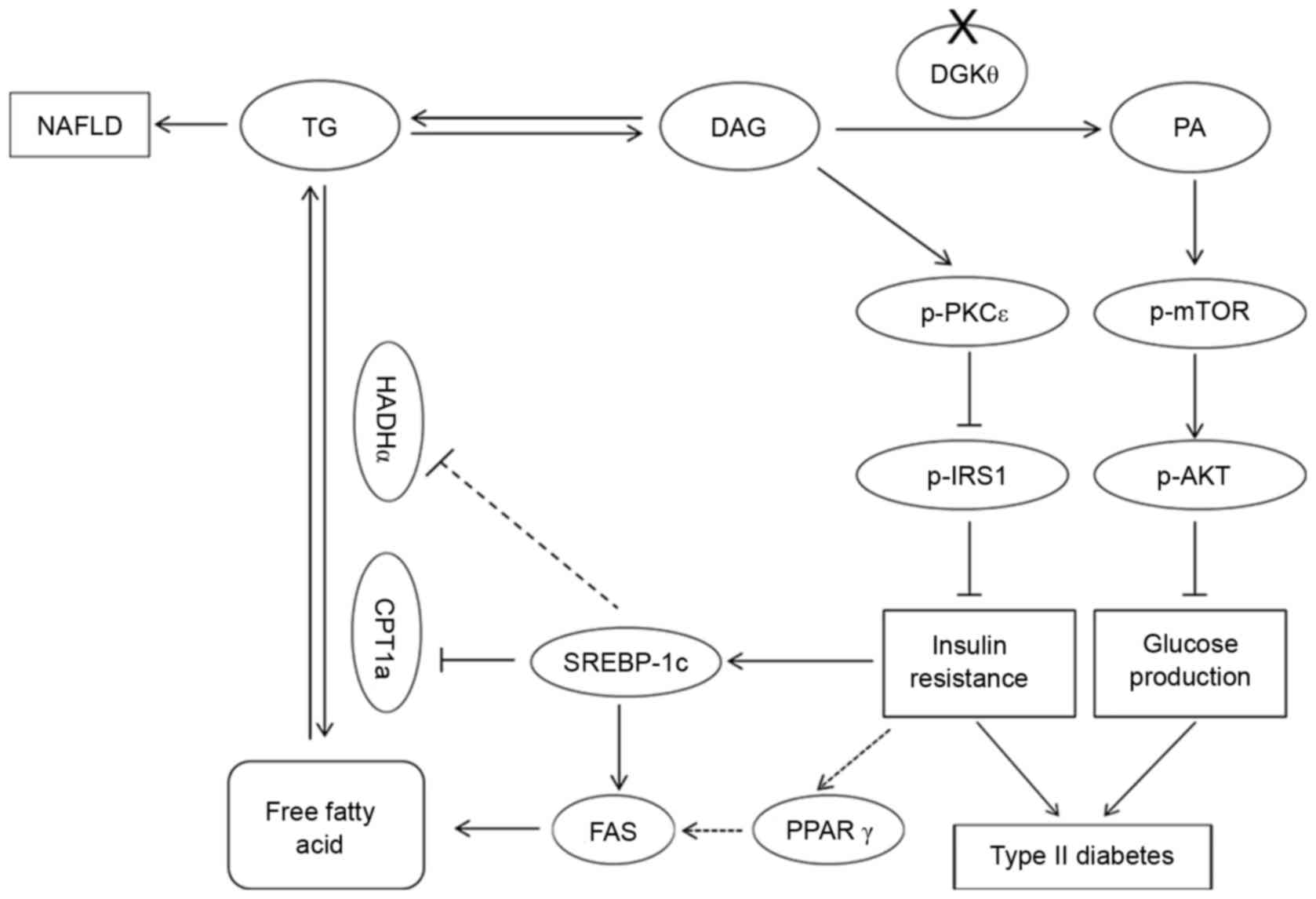 | Figure 4.Illustration demonstrating the roles
of DGKθ in lipid accumulation, insulin resistance and glucose
production. DGKθ gene knockout leads to a decrease in the level of
PA, which causes type 2 diabetes by increasing the levels of p-mTOR
and AKT, and an increase in the level of DAG, which causes insulin
resistance, type 2 diabetes and NAFLD. Solid lines indicate
confirmed regulatory associations, while dotted lines indicate
undetermined hypotheses. DGKθ, diacylglycerol kinase; p-,
phosphorylated; NAFLD, nonalcoholic fatty liver disease; PA,
phosphatidyl acid; DAG, diacylglycerol; mTOR, mechanistic target of
rapamycin; PKCε, protein kinase Cε; IRS1, insulin receptor
substrate 1; PPAR-γ, peroxisome proliferator-activated receptor-γ;
SREBP1c, sterol regulatory element-binding protein-1c; FAS, fatty
acid synthase; CPT1a, carnitine palmitoyltransferase1A; HADHα,
long-chain L-3-hydroxyacyl-coenzyme A dehydrogenase α; TG,
triglyceride. |
In conclusion, the present study successfully
generated a DGKθ-knockout Liver cancer cell line using the
CRISPR/Cas9 technique. This cell line provides a valuable tool for
investigating the pathogenesis of, and developing treatments for,
NAFLD and type 2 diabetes.
Acknowledgements
This study was supported by the Fundamental Research
Funds for Innovation Founds of Graduate Programs, Shaanxi Normal
University (grant no. 2015CXS024), research grants to Dr Haibin Xia
and Dr Kai Cai from the National Natural Science Foundation of
China (grant nos. 81272543, 81471772 and 31470058) and the Natural
Science Foundation of Shaanxi Province, China (grant nos. 2014JZ005
and 2015JQ8302).
References
|
1
|
Cai J, Abramovici H, Gee SH and Topham MK:
Diacylglycerol kinases as sources of phosphatidic acid. Biochim
Biophys Acta. 1791:942–948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griner EM and Kazanietz MG: Protein kinase
C and other diacylglycerol effectors in cancer. Nat Rev Cancer.
7:281–294. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mérida I, Avila-Flores A and Merino E:
Diacylglycerol kinases: At the hub of cell signaling. Biochem J.
409:1–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Houssa B, Schaap D, van der Wal J, Goto K,
Kondo H, Yamakawa A, Shibata M, Takenawa T and van Blitterswijk WJ:
Cloning of a novel human diacylglycerol kinase (DGKtheta)
containing three cysteine-rich domains, a proline-rich region, and
a pleckstrin homology domain with an overlapping Ras-associating
domain. J Biol Chem. 272:10422–10428. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su AI, Wiltshire T, Batalov S, Lapp H,
Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al:
A gene atlas of the mouse and human protein-encoding
transcriptomes. Proc Natl Acad Sci USA. 101:pp. 6062–6067. 2004;
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cantley JL, Yoshimura T, Camporez JP,
Zhang D, Jornayvaz FR, Kumashiro N, Guebre-Egziabher F, Jurczak MJ,
Kahn M, Guigni BA, et al: CGI-58 knockout sequesters
diacylglycerols in lipid droplets/ER-preventing
diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad
Sci USA. 110:pp. 1869–1874. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S,
Befroy D, Romanelli AJ and Shulman GI: Mechanism of hepatic insulin
resistance in non-alcoholic fatty liver disease. J Biol Chem.
279:32345–32353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baldanzi G, Alchera E, Imarisio C,
Gaggianesi M, Dal Ponte C, Nitti M, Domenicotti C, van Blitterswijk
WJ, Albano E, Graziani A and Carini R: Negative regulation of
diacylglycerol kinase theta mediates adenosine-dependent hepatocyte
preconditioning. Cell Death Differ. 17:1059–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai K and Sewer MB: cAMP-stimulated
transcription of DGKθ requires steroidogenic factor 1 and sterol
regulatory element binding protein 1. J Lipid Res. 54:2121–2132.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chibalin AV, Leng Y, Vieira E, Krook A,
Björnholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T, et
al: Downregulation of diacylglycerol kinase delta contributes to
hyperglycemia-induced insulin resistance. Cell. 132:375–386. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birkenfeld AL and Shulman GI: Nonalcoholic
fatty liver disease, hepatic insulin resistance, and type 2
diabetes. Hepatology. 59:713–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao D, Zhang W, Li Y, Liu K, Zhao J, Sun
X, Shan L, Mao Q and Xia H: A novel luciferase knock-in reporter
system for studying transcriptional regulation of the human Sox2
gene. J Biotechnol. 219:110–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JY, Kim Y, Im JA and Lee H: Oligonol
suppresses lipid accumulation and improves insulin resistance in a
palmitate-induced in HepG2 hepatocytes as a cellular steatosis
model. BMC Complement Altern Med. 15:1852015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riordan SM, Heruth DP, Zhang LQ and Ye SQ:
Application of CRISPR/Cas9 for biomedical discoveries. Cell Biosci.
5:332015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wang Y, Wu X, Wang J, Wang Y, Qiu
Z, Chang T, Huang H, Lin RJ and Yee JK: Unbiased detection of
off-target cleavage by CRISPR-Cas9 and TALENs using
integrase-defective lentiviral vectors. Nat Biotechnol. 33:175–178.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shalem O, Sanjana NE, Hartenian E, Shi X,
Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG and
Zhang F: Genome-scale CRISPR-Cas9 knockout screening in human
cells. Science. 343:84–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai K, Lucki NC and Sewer MB: Silencing
diacylglycerol kinase-theta expression reduces steroid hormone
biosynthesis and cholesterol metabolism in human adrenocortical
cells. Biochim Biophys Acta. 1841:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Li H, Zhang Y, Li L, Fang R, Li Y,
Liu Q, Zhang W, Qiu L, Liu F, et al: Oncoprotein HBXIP modulates
abnormal lipid metabolism and growth of breast cancer cells by
activating the LXRs/SREBP-1c/FAS signaling cascade. Cancer Res.
76:4696–4707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferré P and Foufelle F: SREBP-1c
transcription factor and lipid homeostasis: Clinical perspective.
Horm Res. 68:72–82. 2007.PubMed/NCBI
|
|
21
|
Lu M, Wan M, Leavens KF, Chu Q, Monks BR,
Fernandez S, Ahima RS, Ueki K, Kahn CR and Birnbaum MJ: Insulin
regulates liver metabolism in vivo in the absence of hepatic
Akt and Foxo1. Nat Med. 18:388–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacinto E, Facchinetti V, Liu D, Soto N,
Wei SY, Jung SY, Huang Q, Qin J and Su B: SIN1/MIP1 maintains
rictor-mTOR complex integrity and regulates Akt phosphorylation and
substrate specificity. Cell. 127:125–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toschi A, Lee E, Xu L, Garcia A, Gadir N
and Foster DA: Regulation of mTORC1 and mTORC2 complex assembly by
phosphatidic acid: Competition with rapamycin. Mol Cell Biol.
29:1411–1420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai K and Sewer MB: Diacylglycerol kinase
θ couples farnesoid X receptor-dependent bile acid signalling to
Akt activation and glucose homoeostasis in hepatocytes. Biochem J.
454:267–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumashiro N, Erion DM, Zhang D, Kahn M,
Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, et al:
Cellular mechanism of insulin resistance in nonalcoholic fatty
liver disease. Proc Natl Acad Sci USA. 108:pp. 16381–16385. 2011;
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gual P, Le Marchand-Brustel Y and Tanti
JF: Positive and negative regulation of insulin signaling through
IRS-1 phosphorylation. Biochimie. 87:99–109. 2005. View Article : Google Scholar : PubMed/NCBI
|