Introduction
Atherosclerosis (AS) is an important factor leading
to cardiovascular and cerebrovascular diseases, which have a high
morbidity and mortality. The formation mechanisms of AS are
complicated and diverse (1,2),
while the structural and functional injury of endothelial cells
(ECs) is regarded as the initial step of AS and influences the
grade of AS (3,4). Vasomotor dysfunction is the primary
presentation of AS, therefore, the imbalance of factors that affect
vascular contraction and relaxation serve important roles in its
pathogenesis (5,6). Several types of cells, particularly
ECs, secrete factors involved in autoregulation, including nitric
oxide (NO) and endothelin (ET), thus regulating vasomotor function
(7–9).
The major functions of NO are to sustain vascular
tension, regulate blood pressure, inhibit the migration and
hyperplasia of vascular smooth muscle cells, and influence the
vasomotor function (10). The
formation of NO is regulated primarily by endothelial NO synthase
(eNOS) (11). ET, which is
associated with various human diseases, is comprised of three
isomers (ET-1, ET-2 and ET-3). At present, ET-1 is regarded as the
most potent endothelial-independent vasoconstrictor peptide
(12,13). The levels of NO and ET-1 that
regulate vascular endothelial function are important in the AS
process.
All-trans retinoic acid (ATRA), a natural derivative
of vitamin A, exerts extensive biological effects (14). As indicated in previous studies, it
inhibits the migration and hyperplasia of smooth muscle cells, in
addition to possessing anti-inflammatory and antifibrotic functions
(15–17). However, studies investigating the
effects of ATRA on the secretion of NO and ET-1 are rare. The
present study replicated an AS model to analyze the effects of ATRA
on AS rabbits, and compared the expression of ET-1, NO, eNOS and
phosphorylated (p)-eNOS (Ser-1177) levels in the plasma and
arterial tissue, in addition to determining the permeability of the
arterial wall, in order to investigate the potential mechanism of
ATRA on AS and to provide a novel basis for the treatment of AS in
the clinic.
Materials and methods
Duplication of AS model
A total of 24 male New Zealand pure breed white
rabbits (age, 10 weeks; weight 1.8±0.2 kg) were provided by Qingdao
Shandong Kangda group. Animals were housed at 22°C, 50% relative
humidity, 0.03% CO2 and 12 h light/dark cycle. The
rabbits were fed standard diet to acclimate for 1 week prior to
being randomly divided into three groups (n=8): Group A was the
control group, which was administered an ordinary diet; group B was
the high fat group; and group C was the ATRA (provided by the
Institute of Pharmacology, Anhui Medical University, Hefei, China)
intervention group. Groups B and C were administered high fat feed
(94% ordinary feed, 1% cholesterol and 5% lard), and all feed was
purchased from the Laboratory Animal Center of Anhui Medical
University (Hefei, China). The ATRA group received gavage (10
mg/kg/day) from week 2 (18). At
the end of week 12, the animals were sacrificed to obtain blood and
arterial specimens for fixation and analysis. All procedures were
approved by the Internal Animal Care and Use Committee of Anhui
Medical University.
Specimen preparation
For anesthesia, 3% pentobarbital sodium was
administered through the auricular vein and the carotid arterial
blood collected. After standing for 2 h, the blood was centrifuged
for 10 min at 1,006 × g, and the upper serum was removed and stored
at −80°C. The animals were dissected through the abdomen, the blood
was drained and the aorta was separated as soon as possible,
sections of which were embedded with optimal cutting temperature
compound (OCT) following the above procedures, other sections were
frozen with liquid nitrogen and stored at −80°C until use, prepared
for oil red O staining or were fixed with 4% paraformaldehyde at
room temperature for 24 h followed by embedding in paraffin and
sectioning at 4 µm.
Reagents
Triglyceride (TG; cat. no. 0949-2008) and total
cholesterol (TC; cat. no. 1568-2003) assay kits were provided by
Zhejiang Dong'ou Diagnostic Products Co., Ltd. (Wenzhou, China).
The plasma radioimmunoassay ET-1 kit (cat. no. D11PJA) was
purchased from the Beijing North Institute of Biological Technology
(Beijing, China). Low-density lipoprotein (LDL; cat. no. A113-2),
high-density lipoprotein (HDL; cat. no. A112-2) assay kits,
endothelial nitric oxide synthase assay kit (eNOS; cat. no. H195)
and a nitrate reductase NO kit (cat. no. A012) were obtained from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Sulfo-NHS-LC-biotin was purchased from Pierce (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Anti-β-actin (cat. no. BM0627)
antibody was obtained from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China), and ET-1 (cat. no. sc-517436), eNOS (cat. no.
sc-136977) and horseradish peroxidase-conjugated anti-mouse IgG
(cat. no. sc-2005) and anti-rabbit IgG (cat. no. sc-2004)
antibodies were provided by Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Anti-p-eNOS (Ser-1177; cat. no. 9571s) antibody was
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Stripping buffer was purchased from Beyotime Institute of
Biotechnology (Haimen, China).
Blood lipid detection
An enzyme coupling colorimetric method was utilized
to detect the serum TC and TG, while a precipitation method was
used to detect the HDL and LDL in model rabbits. All were performed
in strict accordance with the manufacturer's instructions for the
assay and ELISA kits.
Hematoxylin and eosin and oil red O
staining
The paraffin sections from each group were dewaxed
and hydrated in a graded series and stained at room temperature
with hematoxylin for 10 min and eosin for 5 min, which was followed
by mounting in neutral resin. Sections were observed under a light
microscope and images were captured. For oil red O staining, the
harvested whole specimen of arterial wall was placed in the stock
solution (oil red powder 0.5 g dissolved in 100 ml IPA), rinsed
with distilled water at a ratio of 6:4 respectively, placed in 40%
formaldehyde solution and observed under a light microscope to
capture images.
Western blot analysis
A total of 1 ml PBS was added to the frozen aorta,
which was cut it into pieces on ice. Subsequently, protein
extraction buffer (0.15 mol/l NaCl, 1.5 mmol/l MgCl2, 10
mmol/l KCl, 10 µg/ml aprotinin, 0.5 µg/ml leupeptin, 3 mmol/l
phenylmethylsulfonyl fluoride, 3 mmol/l dithiothreitol, 1 mmol/l
Na3VO4, 10 mmol/l hydroxymethyl aminomethane,
5 mmol/l ethylenediaminetetraacetic acid, pH 7.5) was added to
prepare the homogenate, followed by freeze-thawing three times at
−80°C and room temperature, and centrifugation for 15 min at 1,006
× g and 4°C. The supernatant was collected and the Lowry et
al (19) was applied to
quantitatively detect the protein concentration. The protein
samples (15 µl/lane) were subjected to 10% SDS/PAGE and
electrophoretically transferred onto a polyvinylidene fluoride
membrane. Non-specific binding was blocked by 5% skimmed milk at
room temperature for 2 h. Western blotting was performed using
β-actin (1:2,000 dilution), ET-1 (1:1,000 dilution), eNOS (1:2,000
dilution) and p-eNOS (Ser-1177; 1:400 dilution) primary antibodies.
All primary antibodies were incubated at 4°C overnight and the
secondary antibodies (1:8,000 dilution) were incubated with
membranes at room temperature for 1 h. Enhanced Chemiluminescence
reagent (Beyotime Institute of Biotechnology) was used for
visualization. All the values were normalized to the levels of
β-actin. ImageJ 2 software (National Institutes of Health,
Bethesda, MD, USA) was utilized in the quantitative analysis of
western blot images.
Detection of ET-1 and NO levels, and
eNOS activity in plasma
The whole blood (5 ml, 3.8% sodium citrate
anticoagulation) was centrifuged for 10 min at 1,006 × g, and the
upper plasma was removed and stored at −80°C and assayed within 2
weeks. ET-1 was measured by using an ET-1 radioimmunoassay kit.
Assay kits were used to detect the contents of NO and the activity
of eNOS. All the testing steps were performed in accordance with
the manufacturers' instructions.
Immunofluorescence for detecting
endothelial permeability
The permeability assay using the surface
biotinylation technique was performed for the aorta intima as
described by Zhu et al (20) with certain modifications. The
arterial tissue was filled with a freshly made Sulfo-NHS-LC-biotin
solution in Hanks Balance Salt Solution (HBS; 137 mM NaCl, 0.4 mM
MgSO4 x 7H2O, 5.3 mM KCl, 0.5 mM
MgCl2 × 6H2O, 1 mM CaCl2, 0.3 mM
Na2HPO4 x 7H2O, 0.45 mM
KH2PO4, 5.5 mM glucose) containing 1 mM
CaCl2 and 1 mM MgCl2) at 1 mg/ml for 30 min
at room temperature. The aortas were rinsed with PBS three times, 5
min each time embedded in OCT and cryosectioned. Frozen sections (6
µm) were incubated with 5% skimmed milk powder (pH 7.4) at 4°C
overnight. Subsequently, the milk was removed and 100 µl
Rhodamine600 Avidin D (XRITC-avidin, 1:200, cat. no. A-2005; Vector
Laboratories, Inc., Burlingame, CA, USA) was added, followed by
incubation at 4°C for 2 h. Rhodamine was then removed, and the
sections were rinsed with PBS for 10 min, PBS containing 0.05%
Tween-20 for 10 min and with pure PBS twice. The sections were
dried at room temperature, mounted and sealed. DAPI staining for 10
min at room temperature was used as a histological control. A
fluorescence microscope (Nikon E800; Nikon Corporation, Tokyo,
Japan) was used for observation and CCD-SPOT digital camera series
(Diagnostic Instruments, Inc., Sterling Heights, MI, USA) was used
to capture images. ImageJ 2 software (National Institutes of
Health) was used to quantitatively analyze the fluorescence
intensity of images.
Statistical analysis
Data are presented as the mean ± standard deviation,
and SPSS statistical software version 16.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Comparisons between
each group were performed using one-way analysis of variance and
the Student-Newman-Keuls method. P<0.05 was considered to
indicate a statistically significant difference.
Results
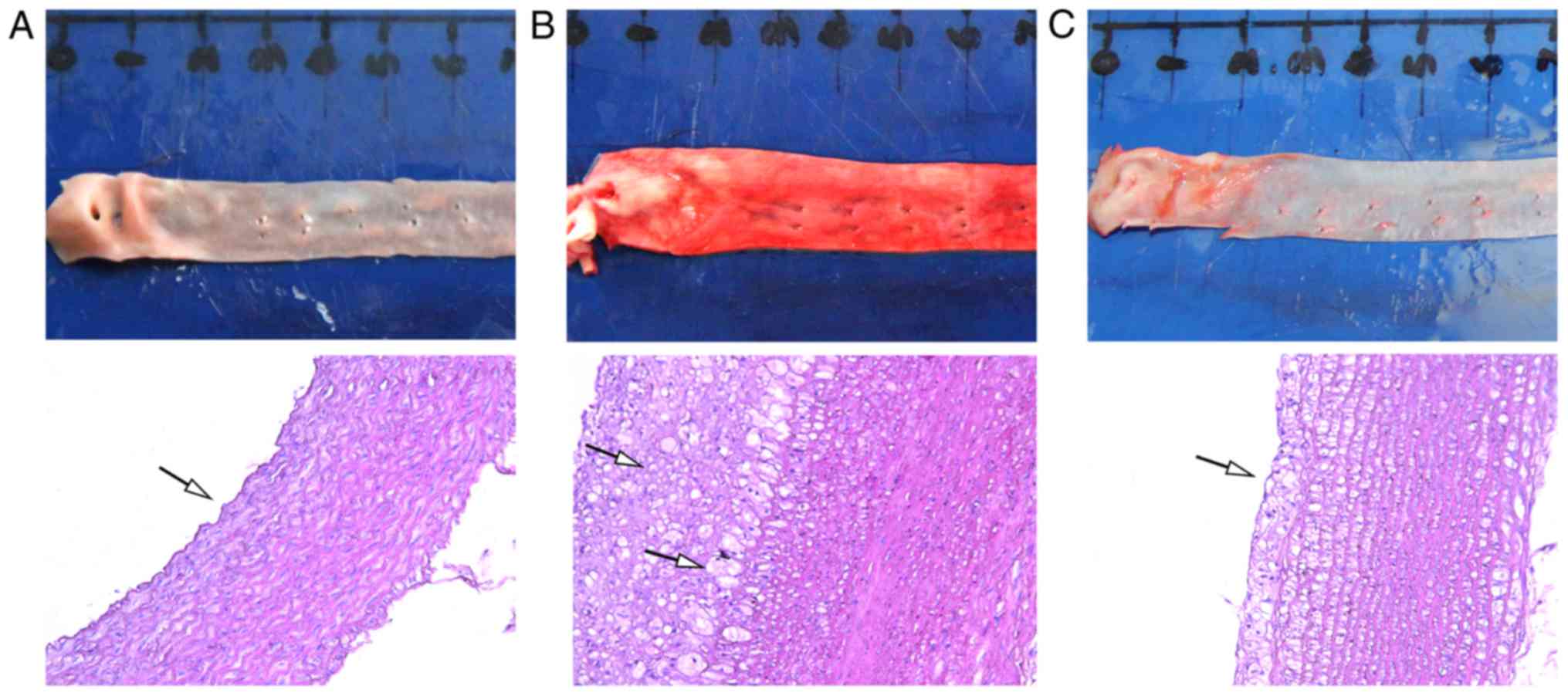
ATRA alleviates atherosclerotic plaque
formation in rabbits
Following hematoxylin and eosin and oil red O
staining, the artery intima of the control group was smooth and
continuous without intimal thickening, the smooth muscle cells
arranged in neat rows and the elastic fibers only slightly
disordered. Compared with the control group, the intima of the
artery wall in the high fat group was visibly thickened with
numerous elastic plate fractures and a large number of plaques;
inflammatory and foam cells were identified in the plaques. In the
ATRA group, a small amount of plaque formation was observed in the
arterial wall, and compared with the high fat group, the foam cells
and inflammatory cells were reduced (Fig. 1).
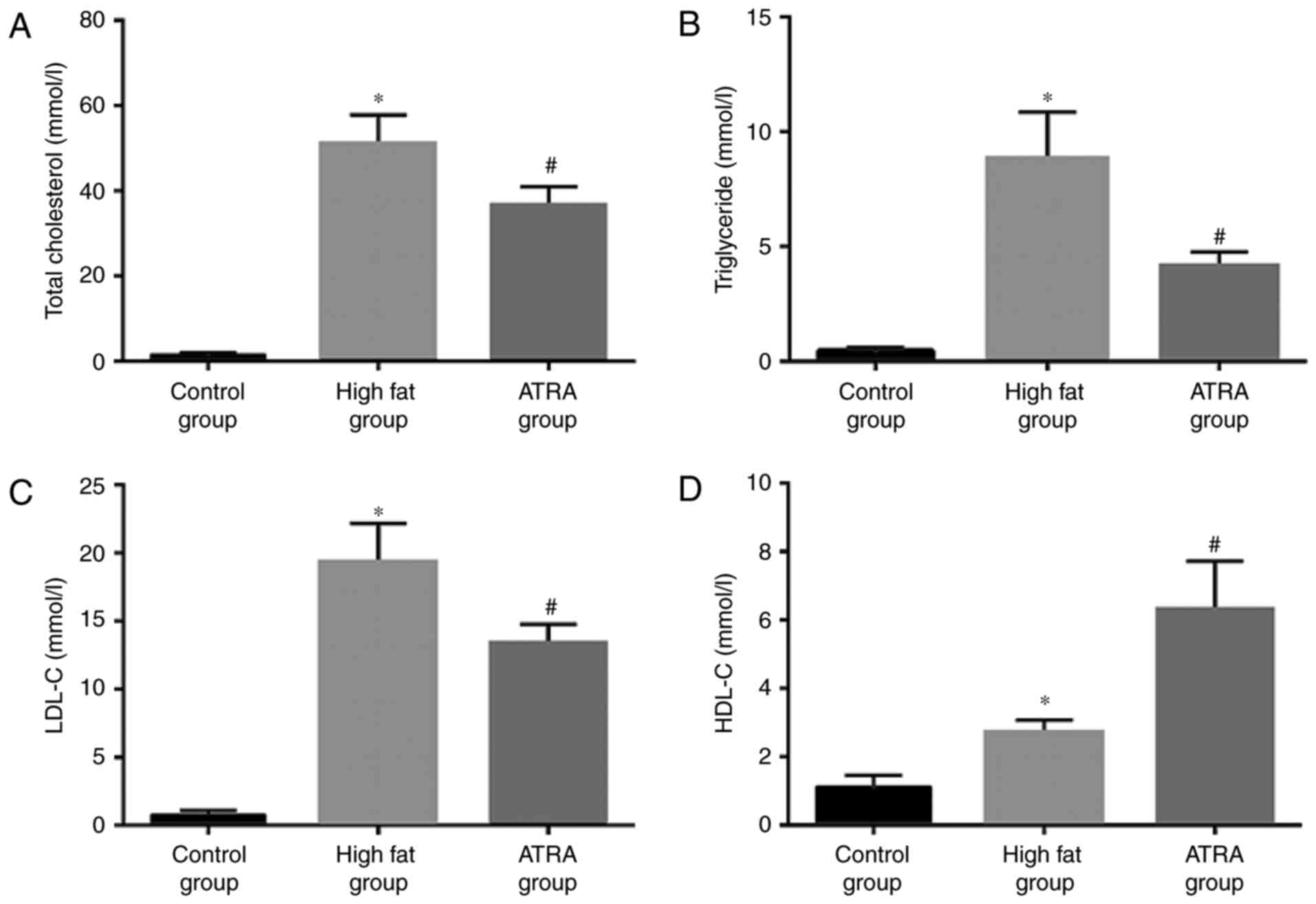
ATRA improves blood lipid levels in
atherosclerotic rabbits
Due to the close association between lipid levels
and AS, the serum levels of TC, TG, HDL and LDL in each group were
examined (Fig. 2). Compared with
the control group, the levels of TC, TG, LDL and HDL were
significantly higher in the high fat group (P<0.05). By
contrast, the levels of TC, TG and LDL in the ATRA group were lower
compared with the high fat group, while HDL levels were higher in
the ATRA group (P<0.05).
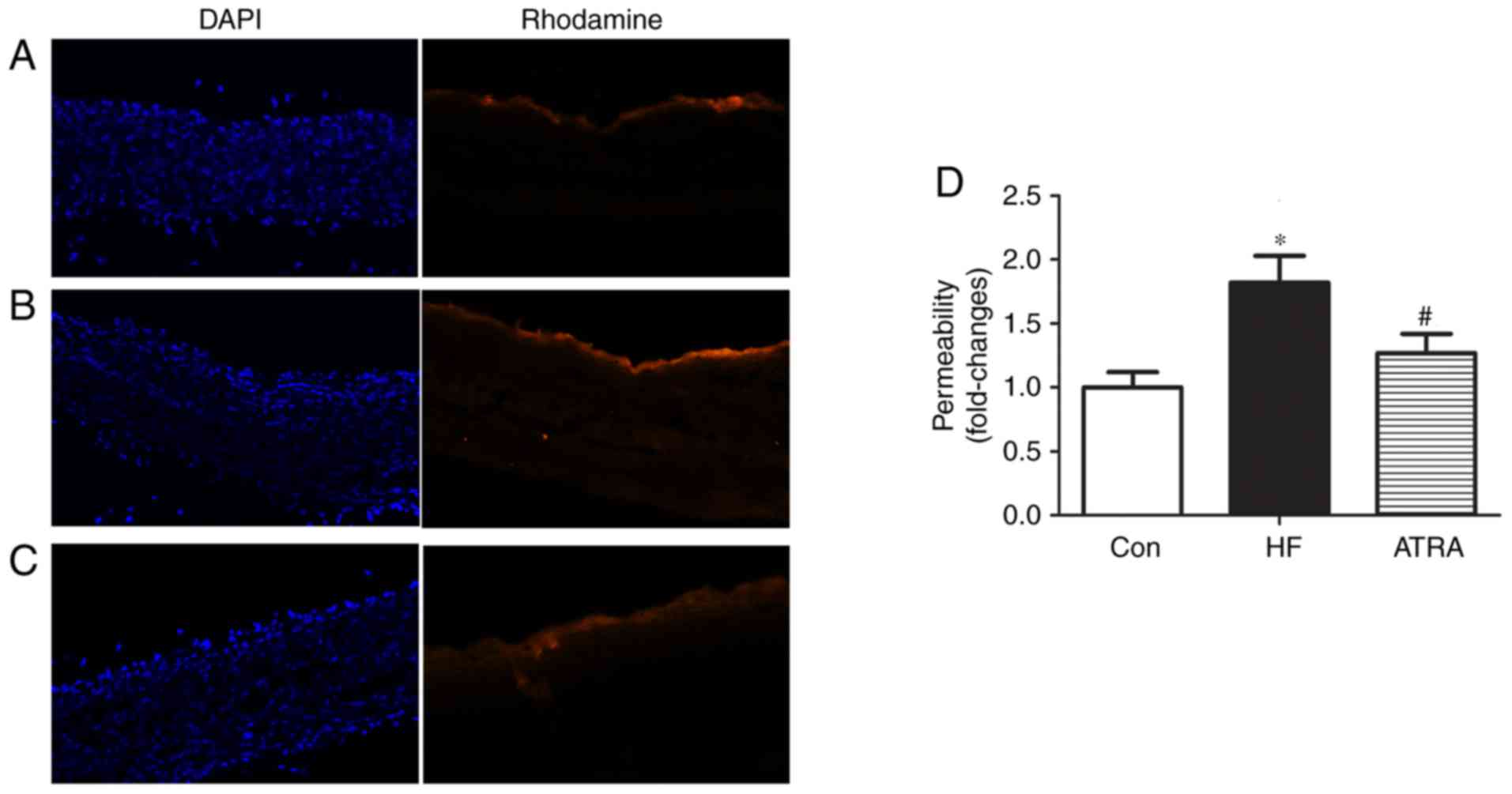
ATRA reduces artery endothelial
permeability of atherosclerotic rabbits
To determine the effect of ATRA treatment on
endothelial permeability, the transport of NHS-LC-biotin across the
aortic intima into the media was assessed. The NHS-LC-Biotin
concentration profiles were obtained as a function of the radial
distance through the aortic wall media layer. Only the endothelial
surface of the aorta intima was biotinylated in rabbits fed a
normal diet, indicating no paracellular leakage of the
NHS-LC-biotin (Fig. 3A), and
NHS-LC-biotin leakage into the aortic intima layers was increased
in the high fat group (Fig. 3B).
However, aortic endothelial permeability was clearly attenuated in
the ATRA group compared with the high fat group (Fig. 3C). The permeability in each group
was quantified (Fig. 3D).
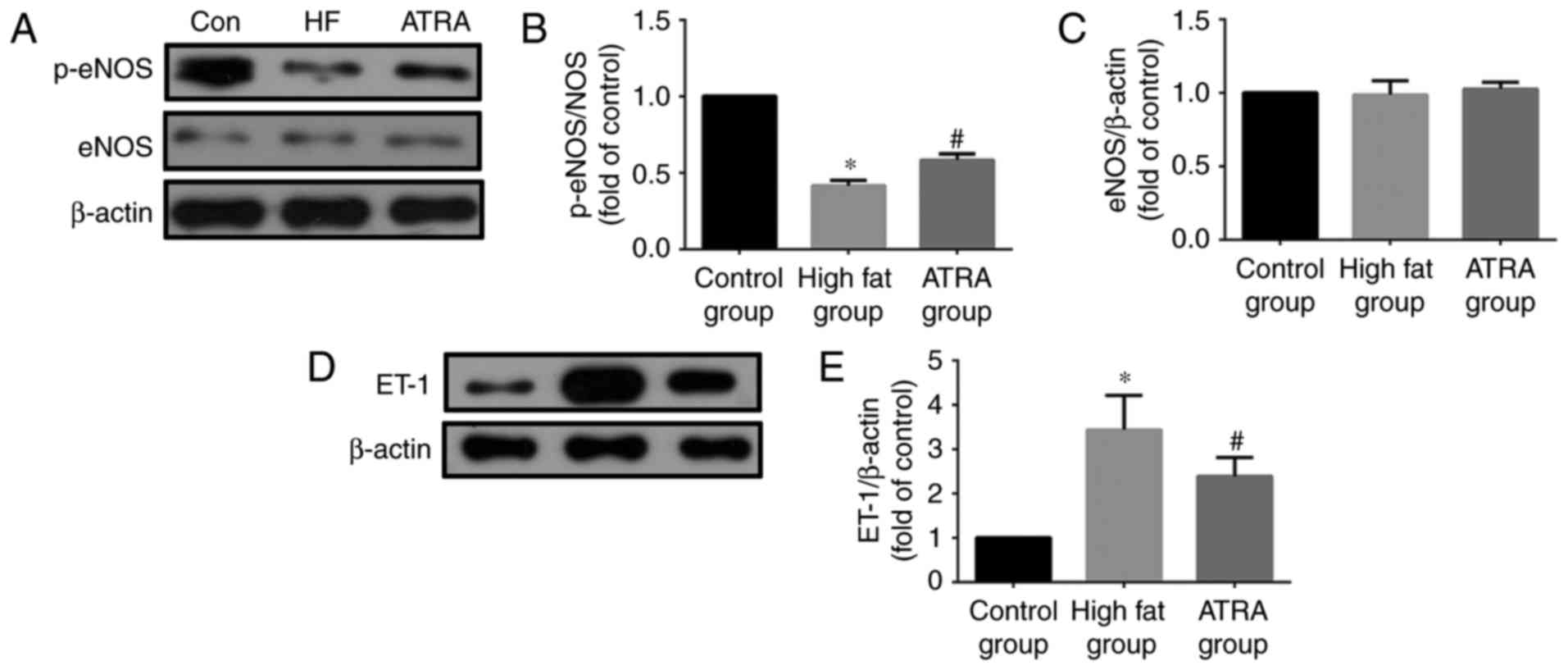
ATRA affects the expression of ET-1,
eNOS and p-eNOS in atherosclerotic rabbit aortas
Western blotting results demonstrated that the
expression of ET-1 in rabbit arterial tissue in the high fat group
was increased compared with the control group, however, its
expression was decreased in the ATRA group. ATRA significantly
increased the eNOS phosphorylation at Ser-1177 (p-eNOS); the
expression of p-eNOS in the high fat group was lower compared with
the control group and increased in the rabbits fed with ATRA. ATRA
did not affect the expression of eNOS in each group (Fig. 4).
Effect of ATRA on eNOS activity, and
ET-1 and NO levels, in the plasma of atherosclerotic rabbits
The results demonstrated that the content of NO and
eNOS activity in the high fat group was significantly lower
compared with the control group (P<0.05), while the level of NO
and eNOS activity were increased following ATRA treatment
(P<0.05; Fig. 5A and B). The
detection results of ET-1 were in contrast to the levels of NO, and
the expression of ET-1 in the ATRA group was significantly lower
compared with the high fat group (P<0.05; Fig. 5C).
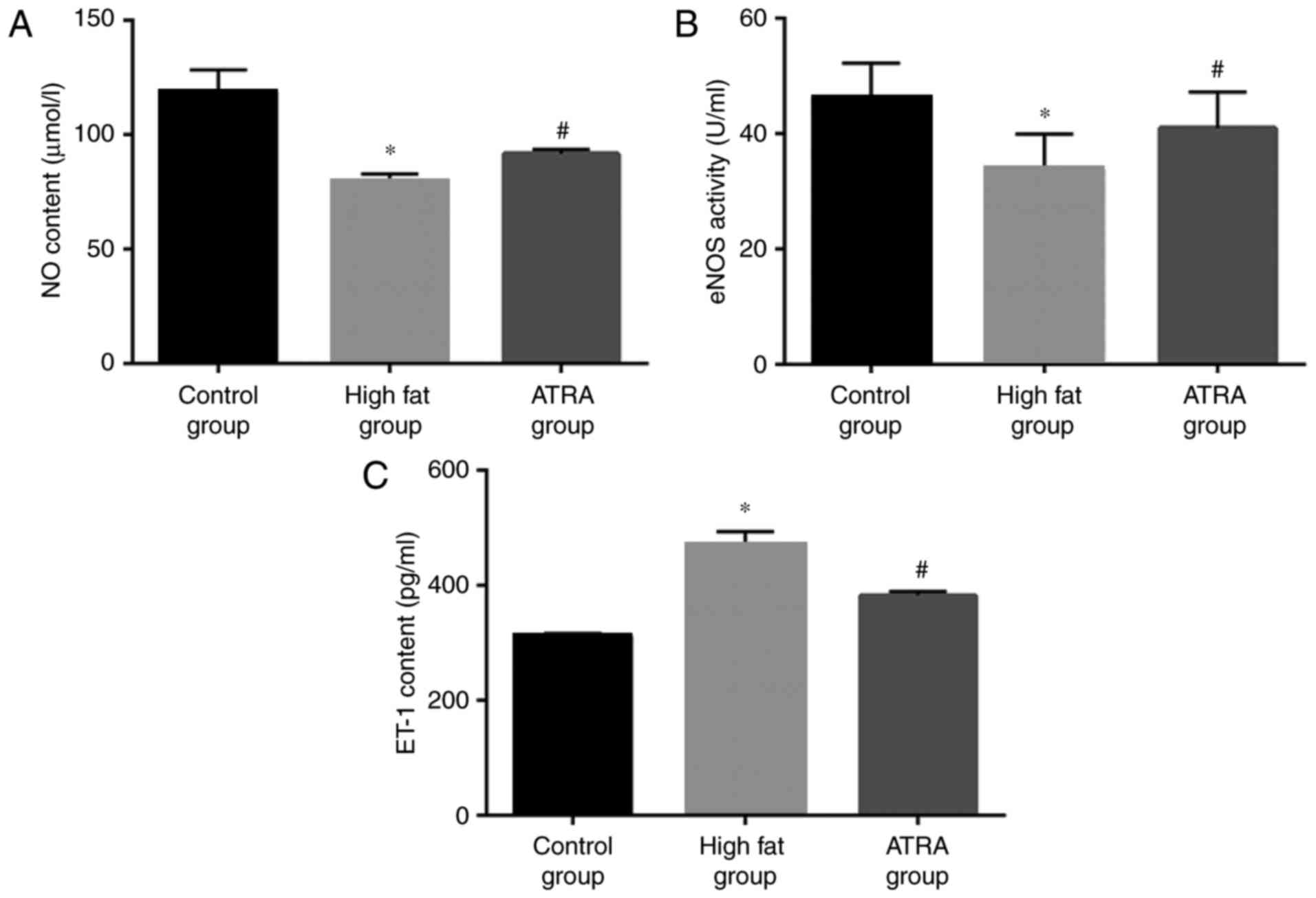 | Figure 5.The levels of ET-1 and NO, and the
activity of eNOS, in the plasma in the three treatment groups
(n=8). (A) NO levels, (B) eNOS activity and (C) ET-1 expression in
the plasma of control, high fat and ATRA groups. ATRA increased the
activity of eNOS and the levels of NO, and decreased the levels of
ET-1, in the plasma compared with rabbits in the high fat group.
Data are presented as the mean ± standard deviation. *P<0.05 vs.
control group; #P<0.05 vs. high fat group. ET-1,
endothelin-1; NO, nitric oxide; eNOS, endothelial nitric oxide
synthase; ATRA, all-trans retinoic acid. |
Discussion
The formation mechanisms of AS are complex and
numerous, and the structural and functional damage of the vascular
endothelium is regarded as the initial step for the genesis of AS
(21,22), which influences the AS lesion
degree (23,24). It is established that under the
action of numerous AS risk factors, including hyperlipidemia,
hypertension, diabetes and smoking, the endothelial function is
damaged and may be aggravated gradually with AS development
(25). Vascular relaxation and
contraction is regulated by various vasoactive substances in
vivo, including NO, ET, prostaglandins and angiotensin. The
levels of ET and NO are important in the regulation of endothelial
function (26).
ET-1 is a type of polypeptide consisting of 21 amino
acids that was extracted from cultured swine aortic EC supernatant
by Yanagisawa and Lefer (27), and
it is the strongest endogenous vasoconstrictor substance to be
identified thus far. ET has three isomers, ET-1, ET-2 and ET-3. ECs
primarily secrete ET-1, which is the major ET type in blood
circulation (28,29). Increased ET-1 may induce vascular
endothelial dysfunction, which manifests as vasomotor dysfunction
due to various stimuli, including acetylcholine, catecholamine,
mechanical pressure, extension and cold stimulation (30). It may also promote platelet
aggregation and platelet-leukocyte adhesion, and accelerate the
proliferation of smooth muscle cells and fibroblasts. ET-1 is a
chemoattractant for monocytes and initiates endothelial lesions,
while the uptake of lipid by macrophages may cause ECs to
synthesize ET-1 (31). It was
demonstrated in previous studies that the application of an ET-1
receptor antagonist restored the NO-mediated control of endothelial
function and inhibited AS genesis (32,33),
while NO inhibitors weakened such effects; therefore, it was
considered that ET-1 may promote AS. In the present study, the
plasma ET-1 levels in the high fat group were notably increased
compared with the control group, and these levels were reduced when
comparing the ATRA group with the high fat group, indicating that
the anti-AS function of ATRA may, at least partially, occur through
protecting the endothelial function and acting against the
synthesis, release and function of ET-1.
Previous studies have demonstrated that in vascular
ECs, the expression and activity of eNOS may contribute to NO
synthesis and release (11,34).
Asymmetric dimethylarginine (ADMA), as an endogenous eNOS
inhibitor, can reduce the production of NO by inhibiting eNOS
function (35) and, as a result,
the expression and activity of eNOS determine the synthesis and
secretion of NO, and influence the endothelial-dependent relaxation
reaction. NO serves an important role in sustaining basic vascular
tension and inhibiting the proliferation of vascular smooth muscle
cells (36,37). NO also exerts an anti-AS function
through mechanisms that include the combination of ET-1 and the
ET-B receptor, which induces the release of NO and thus leads to
the feedback inhibition of ET-1 synthesis and release, in addition
to free radical scavenging (38).
In the present study, the activity of eNOS in the high fat group
was reduced in plasma when compared with the control group, and
notably increased in the ATRA group. ATRA was able to increase the
expression of p-eNOS in arterial tissue, which had been decreased
in the high fat group, indicating that the anti-AS function of ATRA
may be exercised by protecting the endothelial function, and at
least partially by acting against the synthesis, release and the
functioning of ET-1.
ATRA is a natural derivative of vitamin A that
possesses extensive biological effects. As indicated in previous
studies, ATRA inhibits the migration and proliferation of smooth
muscle cells, and also exerts anti-inflammatory and antifibrosis
effects (39,40). With regard to the association
between ATRA and AS, on the one hand, as was reported in a previous
study, ATRA may alter the levels of the endogenous NO synthase
inhibitor ADMA, thus increasing the synthesis of NO (41). On the other hand, ATRA was reported
to inhibit the expression of ET-1 mRNA in ECs (40,42),
thus leading to anti-AS effects. The present study indicated that
ATRA may alleviate AS by reducing ET-1 expression and increasing
eNOS phosphorylation in AS rabbits.
Regulation of the balance between NO and ET is an
important approach to prevent and treat AS. It was demonstrated in
the present study that ATRA may reduce the levels of ET-1 in the
plasma, increase NO content through increasing eNOS phosphorylation
and regulate the balance between the two, thus improving vascular
relaxation, alleviating atherosclerotic plaque formation and
exerting an anti-AS function. ATRA may provide a novel direction
for future clinical treatment of AS.
Acknowledgements
The present study was supported by the grants from
the National Natural Science Foundation of China (grant nos.
81570419, 81270372 and 81300223) and Key Personnel Training Program
of Education Department in Anhui Province (grant no.
gxfxZD2016047). The Anhui Academic and Technology Leader Candidate
Scientific Research Fund and the Reserve Talented Person Fund of
the First Affiliated Hospital of Anhui Medical University.
References
|
1
|
Bays HE: ‘Sick fat,’ metabolic disease,
and atherosclerosis. Am J Med. 122 Suppl 1:S26–S37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennett MR: Life and death in the
atherosclerotic plaque. Curr Opin Lipidol. 21:422–426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baumgartner C, Brandl J, Münch G and
Ungerer M: Rabbit models to study atherosclerosis and its
complications-Transgenic vascular protein expression in vivo. Prog
Biophys Mol Biol. 121:131–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Genasetti A, Vigetti D, Viola M, Karousou
E, Moretto P, Rizzi M, Bartolini B, Clerici M, Pallotti F, De Luca
G and Passi A: Hyaluronan and human endothelial cell behavior.
Connect Tissue Res. 49:120–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Itakura H, Yokoyama M, Matsuzaki M, Saito
Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Kita T, et
al: Relationships between plasma fatty acid composition and
coronary artery disease. J Atheroscler Thromb. 18:99–107. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Libby P, Lichtman AH and Hansson GK:
Immune effector mechanisms implicated in atherosclerosis: From mice
to humans. Immunity. 38:1092–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harjes U, Bensaad K and Harris AL:
Endothelial cell metabolism and implications for cancer therapy. Br
J Cancer. 107:1207–1212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Fu L, Gao Q and Ma D: Valsartan
alleviates atherosclerotic lesions in pulmonary arteries of rabbits
via an endothelium-dependent mechanism. Acta Cardiol. 65:23–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo DX, Cheng J, Xiong Y, Li J, Xia C, Xu
C, Wang C, Zhu B, Hu Z and Liao DF: Static pressure drives
proliferation of vascular smooth muscle cells via caveolin-1/ERK1/2
pathway. Biochem Biophys Res Commun. 391:1693–1697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gkaliagkousi E and Ferro A: Nitric oxide
signalling in the regulation of cardiovascular and platelet
function. Front Biosci (Landmark Ed). 16:1873–1897. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang B and Rizzo V: TNF-alpha potentiates
protein-tyrosine nitration through activation of NADPH oxidase and
eNOS localized in membrane rafts and caveolae of bovine aortic
endothelial cells. Am J Physiol Heart Circ Physiol. 292:H954–H962.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Atochin DN and Huang PL: Endothelial
nitric oxide synthase transgenic models of endothelial dysfunction.
Pflugers Arch. 460:965–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bkaily G, Avedanian L, Al-Khoury J,
Provost C, Nader M, D'Orléans-Juste P and Jacques D: Nuclear
membrane receptors for ET-1 in cardiovascular function. Am J
Physiol Regul Integr Comp Physiol. 300:R251–R263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noy N: Between death and survival:
Retinoic acid in regulation of apoptosis. Annu Rev Nutr.
30:201–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou B, Pan Y, Hu Z, Wang X, Han J, Zhou
Q, Zhai Z and Wang Y: All-trans-retinoic acid ameliorated high fat
diet-induced atherosclerosis in rabbits by inhibiting platelet
activation and inflammation. J Biomed Biotechnol. 2012:2596932012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng Z, Li X, Diao S, et al: Effect of
all-trans retinoic acid on the atherosclerotic plaque and
inflammatory response in carotid artery of rabbits. J Guangdong
Pharm Univ. 32:639–642. 2016.(In Chinese).
|
|
18
|
Wang X, Han J, Hu Z, et al: Effects of all
trans retinoic acid on vascular endothelial function in
atherosclerotic rabbits. Acta Univ Med Anhui. 47:150–153. 2012.(In
Chinese).
|
|
19
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
20
|
Zhu HQ, Zhou Q, Jiang ZK, Gui SY and Wang
Y: Association of aorta intima permeability with myosin light chain
kinase expression in hypercholesterolemic rabbits. Mol Cell
Biochem. 347:209–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizuno Y, Jacob RF and Mason RP:
Inflammation and the development of atherosclerosis. J Atheroscler
Thromb. 18:1–358. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bilbija D, Elmabsout AA, Sagave J, Haugen
F, Bastani N, Dahl CP, Gullestad L, Sirsjö A, Blomhoff R and Valen
G: Expression of retinoic acid target genes in coronary artery
disease. Int J Mol Med. 33:677–686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grace VM, Siddikuzzaman and Rimashree B:
Liposome encapsulated all trans retinoic acid (ATRA) has enhanced
immunomodulatory and inflammation reducing activities in mice
model. Anticancer Agents Med Chem. 15:196–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reriani M, Raichlin E, Prasad A, Mathew V,
Pumper GM, Nelson RE, Lennon R, Rihal C, Lerman LO and Lerman A:
Long-term administration of endothelin receptor antagonist improves
coronary endothelial function in patients with early
atherosclerosis. Circulation. 122:958–966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wågsater D, Jatta K, Ocaya P, Dimberg J
and Sirsjo A: Expression of IL-1beta, IL-1 receptor type I and IL-1
receptor antagonist in human aortic smooth muscle cells: Effects of
all-trans-retinoic acid. J Vasc Res. 43:377–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanagisawa A and Lefer AM: Vasoactive
effects of eicosapentaenoic acid on isolated vascular smooth
muscle. Basic Res Cardiol. 82:186–196. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Granger JP, Abram S, Stec D, Chandler D
and LaMarca B: Endothelin, the kidney, and hypertension. Curr
Hypertens Rep. 8:298–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li MW, Mian MO, Barhoumi T, Rehman A, Mann
K, Paradis P and Schiffrin EL: Endothelin-1 overexpression
exacerbates atherosclerosis and induces aortic aneurysms in
apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol.
33:2306–2315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W and Li Y: Regulation of dHAND protein
expression by all-trans retinoic acid through ET-1/ETAR signaling
in H9c2 cells. J Cell Biochem. 99:478–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu YR, Li PW, Suo JJ, Sun Y, Zhang BA, Lu
H, Zhu HC and Zhang GB: Catalpol provides protective effects
against cerebral ischaemia/reperfusion injury in gerbils. J Pharm
Pharmacol. 66:1265–1270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan XB, Peng TC and Huang D: Correlations
between plasma endothelin-1 levels and breakthrough pain in
patients with cancer. Onco Targets Ther. 8:3703–3706.
2015.PubMed/NCBI
|
|
33
|
Xu Y, Buikema H, van Gilst WH and Henning
RH: Caveolae and endothelial dysfunction: Filling the caves in
cardiovascular disease. Eur J Pharmacol. 585:256–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salvi E, Kuznetsova T, Thijs L, Lupoli S,
Stolarz-Skrzypek K, D'Avila F, Tikhonoff V, De Astis S, Barcella M,
Seidlerová J, et al: Target sequencing, cell experiments, and a
population study establish endothelial nitric oxide synthase (eNOS)
gene as hypertension susceptibility gene. Hypertension. 62:844–852.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Böger RH: When the endothelium cannot say
‘NO’ anymore. ADMA, an endogenous inhibitor of NO synthase,
promotes cardiovascular disease. Eur Heart J. 24:1901–1902. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Facemire CS, Nixon AB, Griffiths R,
Hurwitz H and Coffman TM: Vascular endothelial growth factor
receptor 2 controls blood pressure by regulating nitric oxide
synthase expression. Hypertension. 54:652–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoon JJ, Lee YJ, Han BH, Choi ES, Kho MC,
Park JH, Ahn YM, Kim HY, Kang DG and Lee HS: Protective effect of
betulinic acid on early atherosclerosis in diabetic
apolipoprotein-E gene knockout mice. Eur J Pharmacol. 796:224–232.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Cui J, Yang H, Liu W and Li Q:
Reno-protective effects of all trans retinoic acid on adriamycin
induced nephropathy in mice. J Chongqing Med Univ. 37:484–488.
2012.
|
|
39
|
Yuan LP, Chen ZW, Li F, Dong LY and Chen
FH: Protective effect of total flavones of rhododendra on ischemic
myocardial injury in rabbits. Am J Chin Med. 34:483–492. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rhee EJ, Nallamshetty S and Plutzky J:
Retinoid metabolism and its effects on the vasculature. Biochim
Biophys Acta. 1821:230–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Achan V, Tran CT, Arrigoni F, Whitley GS,
Leiper JM and Vallance P: All-trans-Retinoic acid increases nitric
oxide synthesis by endothelial cells: A role for the induction of
dimethylarginine dimethylaminohydrolase. Circ Res. 90:764–769.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yokota J, Kawana M, Hidai C, Aoka Y,
Ichikawa K, Iguchi N, Okada M and Kasanuki H: Retinoic acid
suppresses endothelin-1 gene expression at the transcription level
in endothelial cells. Atherosclerosis. 159:491–496. 2001.
View Article : Google Scholar : PubMed/NCBI
|