Introduction
Diabetes mellitus (DM) is one of the top 10 most
prevalent diseases worldwide, ranking fourth in Taiwan. Individuals
with DM are at an increased risk of developing eye disease, foot
lesions, nerve degeneration, cerebrovascular disease,
cardiovascular disease, hypertension and renal disease (1). The pathology of type II DM results
from insulin resistance and/or a lack of insulin secretion, which
ultimately increases plasma glucose levels. This hyperglycemic
state stimulates insulin secretion and induces hyperinsulinemia
(2). The clinical symptoms of
insulin resistance include dyslipidemia, hypertension, glucose
intolerance, hyperuricemia or gout, central obesity, impaired blood
coagulation or hyper-coagulation, hyperandrogenism like symptoms,
fatty liver and coronary vascular disease (3). These chronic complications not only
threaten the life of those diagnosed with type II DM, but also puts
a heavy burden on medical resources and the social economy
(4).
To date, the best methods for improving insulin
resistance are exercise (5),
smoking cessation (6) and
medications including metformin, a biguanide (7) and thiazolidinediones (TZDs), a class
of oral hypoglycemic agents (8).
In complementary and alternative medicine, it has also been
demonstrated that electroacupuncture on the Zhongwan (CV12)
acupoint or the bilateral Zusanli (ST36) acupoints may lower plasma
glucose and enhance insulin sensitivity (9–11).
Among the aforementioned methods, the use of medication is the most
effective and convenient for the management of type II DM, but can
result in adverse side effects. Therefore, it is important to
identify other methods with fewer side effects that can improve
insulin resistance.
Antrodia cinnamomea (AC) is a Taiwanese
fungus species that grows in the hollow trunk of the Cinnamomum
kanehirai tree. Previous investigations have indicated that AC
may suppress tumor formation (12), enhance the immune system (13), inhibit viral activity and protect
the liver (14). It is often used
in Taiwanese folk medicine. Some evidence has also indicated that
AC can act as an anti-oxidant, improve hypertension and decrease
plasma lipids (15–17). Ergostatrien-3β-ol (EK100) from the
AC was evaluated for its hypoglycemic effects and was demonstrated
to improve diabetes and dyslipidemia in mice fed a high-fat diet.
EK100 treatment also resulted in decreased visceral adipocyte size
and reduced the ballooning degeneration of hepatocytes. Levels of
glucose transporter 4 (GLUT-4) protein and Akt phosphorylation in
skeletal muscle are also significantly increased in EK100 treated
mice (18). In addition,
antroquinonol extracted from the mycelium of AC effectively
inhibited dipeptidyl peptidase-4 activity and AMP-activated protein
kinase (AMPK) activation (19).
Dehydroeburicoic acid from AC prevented the development of diabetic
and dyslipidemic states in streptozotocin-induced diabetic mice,
through the regulation of GLUT-4, peroxisome proliferator activated
receptor α, fatty acid synthase and AMPK phosphorylation (20). An extract made from the fruiting
body of AC lowered liver triglyceride and total cholesterol levels
(17) and enhanced the production
of superoxide dismutase, catalase and glutathione peroxidase
(21).
In addition to the aforementioned effects of AC, its
therapeutic effects on insulin resistance have also been
investigated (22–24), but the mechanism by which AC
enhances insulin sensitivity has not been completely elucidated,
particularly for steroid-induced insulin-resistance (SIIR)
(25,26). Steroids are widely prescribed and
are known to cause insulin resistance. Patients with DM treated
with steroids commonly require an increased dose of insulin. Thus,
the aim of the present study was to use the SIIR rat as a model to
explore the hypoglycemic and insulin resistance improving effects
of orally administered AC and to investigate the mechanisms
underlying its hypoglycemic and insulin resistance improving
properties.
Materials and methods
Preparation of AC mycelium
AC mycelium was cultured on solid-state cereal
medium (provided by the Chair Professor Wai-Jane Ho, Da-Yeh
University, Changhua, Taiwan) for 85–90 days at 21–23°C in the
dark. The solid medium was mainly barley-supplemented with yeast
extract and glucose. At the end of the culturing period, cultures
were harvested, dried and ground into powder for subsequent
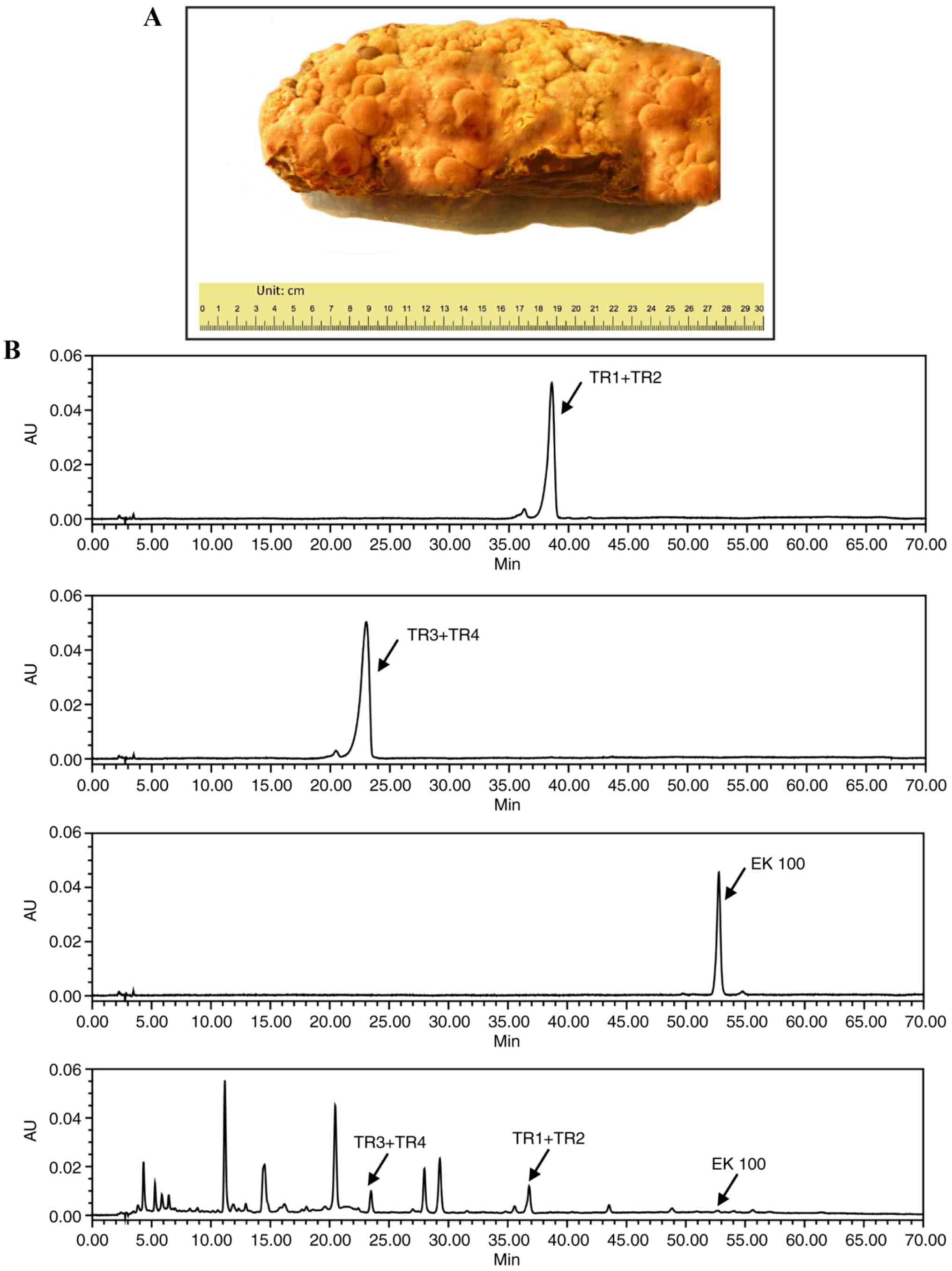
experiments. The final yield from the solid-state culture was ~20%
of the original dry weight of cereal medium (Fig. 1A). The powdered culture was then
mixed with normal saline to produce oral solutions at the
concentrations of 100, 200 and 500 mg/kg body weight (BW). The
solutions were subsequently stored in a 4°C refrigerator ready for
use.
High-performance liquid chromatography
(HPLC) assay
Components of the AC powder methanol extract were
assessed by HPLC assay with a configured module using a Waters
Alliance 2695 with auto-sample injection (Waters XTerra column,
4.6×250 mm, 5 µm; mobile phase A, water; mobile phase B,
acetonitrile; injection volume, 10 µl; detector, PDA; detector
wavelength, qualitative 250 nm, quantitative 280 nm; temperature,
25°C; flow rate, 1.0 ml/min; Waters Corporation, Milford, MA, USA).
The peaks of the retention time from the analysis of the AC
methanol extract were compared with the peaks of the internal
standard dissolved in methanol solution (provided by Professor
Yueh-Hsiung Kuo, China Medical University, Taichung, Taiwan).
Animal models
A total of 24 Male Wistar rats aged 8–10 weeks,
250–300 g BW, were obtained from the BioLASCO (Taipei, Taiwan).
They were housed in rooms at 25±1°C with relative humidity of
65±5%. The rats were acclimatized in an alternating 12 h light/12 h
dark cycle with free access to water and standard rat chow. After 1
week of adaptation, the rats were randomly assigned into
experimental and control groups. The Institutional Animal Care and
Use Committee of Da-Yeh University approved the methods of this
study according to the national guidelines for the Care and Use of
Animals.
A previously published research protocol was
followed to establish the SIIR rat model (26). At 8 weeks, rats were
intraperitoneally (i.p.) injected with dexamethasone at a dose of 1
mg/kg daily for 5 days. It was concluded that insulin resistance
was successfully induced when their measured fasting plasma glucose
levels were higher than 150 mg/dl. Tests were performed at 8:00
a.m. and all SIIR rats were fasted before each test and
anesthetized with pentobarbital (40 mg/kg i.p.) (11).
Experimental protocols
The SIIR rats were randomly divided into four groups
and administered the following treatments once: Group A, control
group (n=6), 1 ml/kg oral saline; group B (n=6), 100 mg/kg AC;
group C (n=6), 200 mg/kg; and group D (n=6), 500 mg/kg AC. The
three experimental groups (EGs) were force-fed the AC solution.
Every AC sample was shock homogenized prior to each feeding. The
plasma glucose levels were assayed with a commercial enzymatic
method using Glucose HK stable liquid reagent (Randox Laboratories
Ltd., Crumlin, UK). The percentage hypoglycemic activity was
calculated as follows: [plasma glucose levels at 30 min (or 60 min)
- plasma glucose levels at 0 min/plasma glucose levels at 30 min
(or 60 min)] × 100. The optimal dose group for inducing
hypoglycemia was determined and used in subsequent experimental
procedures.
Assay of plasma free fatty acid (FFA)
levels
Plasma FFA levels were measured using a
non-esterified fatty acid kit (Randox Laboratories Ltd.). FFA
levels were measured via an ELISA. As described in our previous
study (25), plasma FFA was
transformed into a purple adduct for subsequent detection by
automatic spectrophotometer (COBAS MIRA Plus system; Roche
Diagnostics, Basel, Switzerland).
ELISA of plasma insulin levels and
resistance test
A Mercodia ultrasensitive rat insulin ELISA kit
(cat. no. 10-1251-01; Mercodia AB, Uppsala, Sweden) was used to
detect plasma insulin levels. The homeostatic model assessment of
insulin resistance (HOMA-IR) was calculated using the following
formula: [fasting plasma insulin levels (µU/ml) × fasting plasma
glucose (mmol/l)]/22.5 (27,28).
Western blot analysis
The samples were minced coarsely and homogenized by
an ultrasonic processor (VCX 750; Sonics and Materials Inc.,
Newtown, CT, USA) in a radioimmunoprecipitation assay lysis buffer
with the protease inhibitor, phenylmethylsulfonyl fluoride (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The muscle extracts
were centrifuged at 16,440 × g at 4°C for 1 h, and the supernatants
were measured using a spectrophotometer. A total of 90 µg/ml
protein was separated by 8% SDS-PAGE, and the proteins were
transferred to a polyvinylidene fluoride (PVDF) membrane for
western blotting. The PVDF membrane was then submerged in 5%
non-fat milk to block the nonspecific binding sites in the membrane
at 25°C for 1 h. The membrane was incubated overnight with
anti-insulin receptor substrate-1 (IRS-1; 1:200, sc-559),
anti-GLUT-4 (1:200, sc-7938) and anti-phosphoinositide 3-kinase
(PI3K; 1:200, sc-376112) antibodies (all from Santa Cruz
Biotechnology, Inc.) at 4°C in the refrigerator. Finally, the
membranes were incubated with goat anti-rabbit immunoglobulin
G-horseradish peroxidase antibodies (GTX213110-01, 1:2,000;
GeneTex, Inc., Irvine, CA, USA) for at 25°C for 1 h, and specific
bands were detected by an enhanced chemiluminescence kit (Clarity™
and Clarity Max™ Western ECL Blotting Substrates; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) The bands were quantified
using optical densitometry (Gel-Pro analysis version 4.0; Media
Cybernetics, Rockville, MD, USA). The actin bands were used as an
internal loading control, the results are presented as a ratio of
signal-to-actin (11,26).
Statistical analysis
The experimental results are presented as the mean ±
standard error in each group (n=6). The statistical analysis of the
results was performed using the Student's t-test or one-way
analysis of variance, with a least significant difference post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
HPLC assay
HPLC analysis detected eburicoic acid +
dehydroeburicoic acid (TR1+TR2), sulphurenic acid +
dehydrosulphurenic acid (TR3+TR4) and EK100 (standard retention
times: 36–38, 20–23, and 52.8 min, respectively). The AC sample
contained TR1+TR2, TR3+TR4, and EK100 (Fig. 1B).
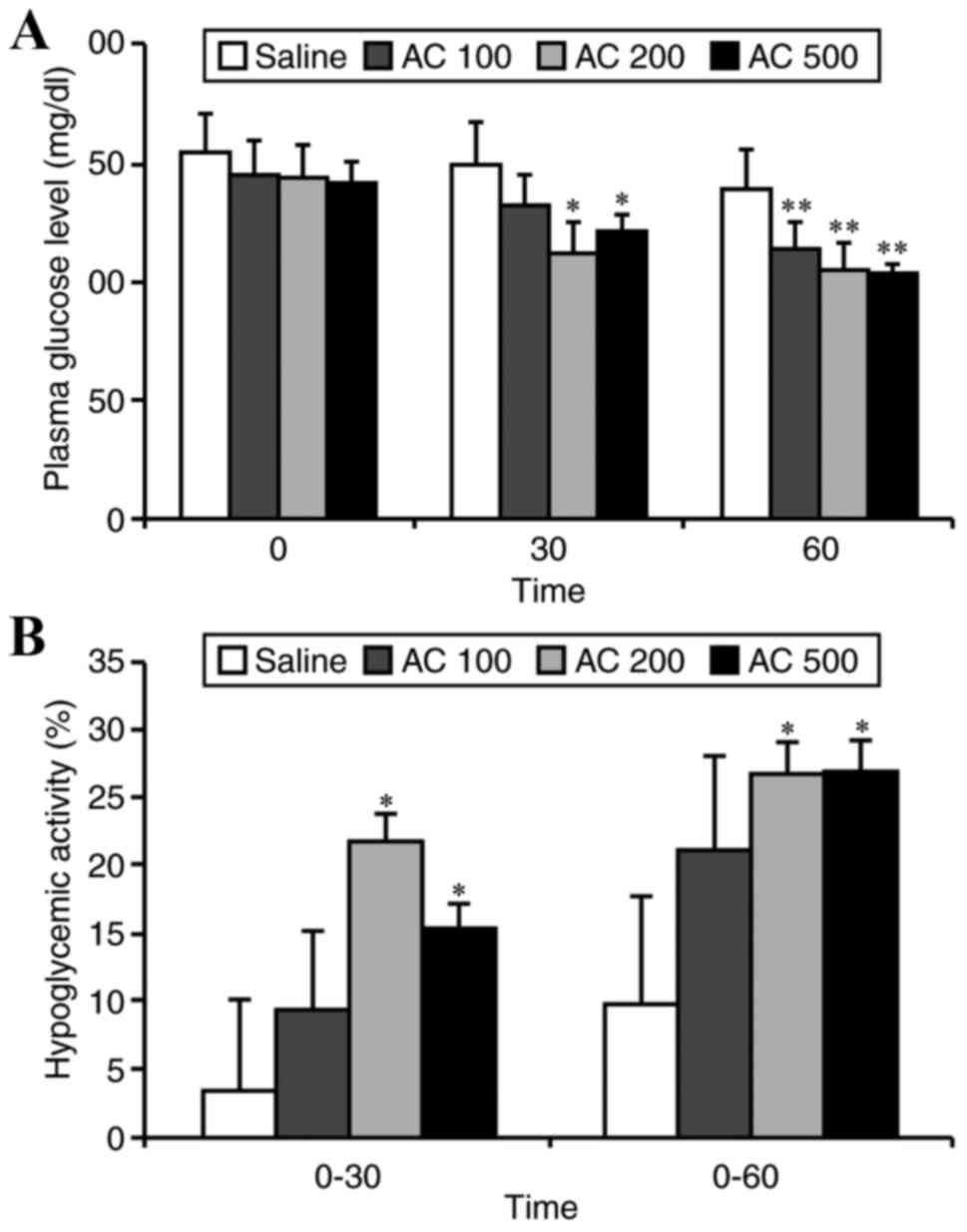
Hypoglycemic effect of AC in SIIR
The SIIR rats in the EGs were administered different
doses of AC (100, 200 or 500 mg/kg) and the CG rats were
administered normal saline. At 30 min after treatment, the plasma
glucose levels in the rats administered 200 and 500 mg/kg of AC was
significantly lower than those in the CG (P<0.05). After 60 min,
the plasma glucose levels in all EGs was significantly lower than
those in the CG (P<0.01; Fig.
2A). At 30 min after treatment administration, the hypoglycemic
activity in the 200 and 500 mg/kg groups was 21.69 and 15.22%
respectively, which was significantly greater than that of the CG
(3.33%, P<0.05). After 60 min, the hypoglycemic activity of the
200 and 500 mg/kg groups was 26.59 and 26.86%, respectively, both
of which were significantly greater than that of the CG (9.74%,
P<0.05; Fig. 2B). No specific
adverse events, including no mortality, normal activity, water and
food intake were observed following each experiment at any
dose.
Hypoglycemic effect of the optimal
dose of AC
The above results indicated that 200 mg/kg AC was
optimal to achieve a hypoglycemic effect. Therefore, this
particular dose was used in the subsequent experiments. Thirty
minutes after administration of 200 mg/kg of AC, the plasma glucose
level decreased from 155.33±23.12 to 125.81±29.81 mg/dl (P<0.05
vs. baseline), and to 113.69±14.15 mg/dl after 60 min (P<0.05
vs. baseline).
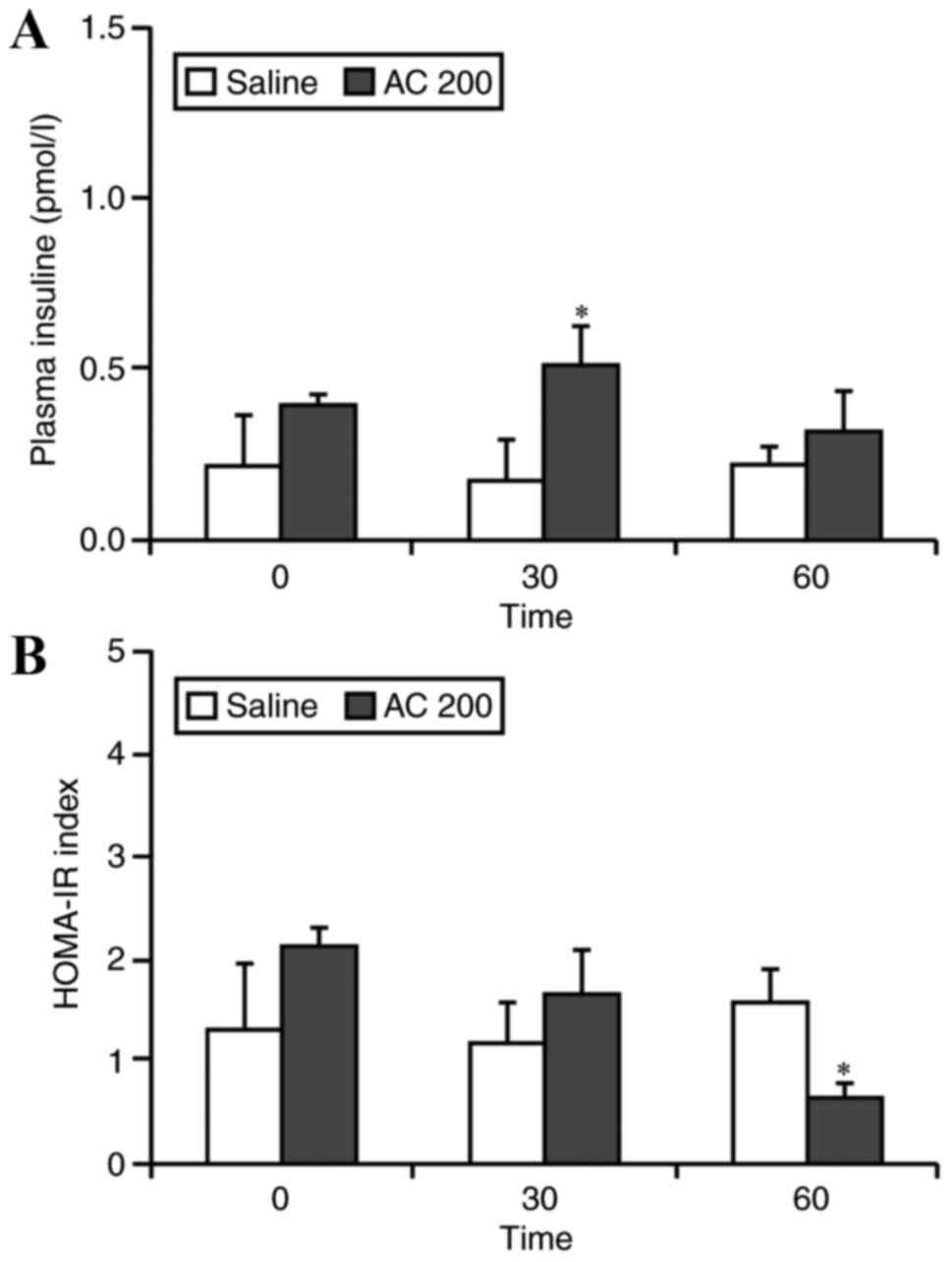
Plasma insulin and HOMA-IR levels in
SIIR rats following administration of 200 mg/kg AC
Following oral administration of 200 mg/kg AC,
plasma insulin levels were detected in serum samples taken from the
femoral vein using an ELISA kit. Plasma insulin levels increased
from 0.35±0.41 to 0.51±0.30 pmol/l at 30 min after AC
administration, with a significant elevating trend compared to
those of the CG, in which plasma insulin levels changed from
0.11±0.07 to 0.07±0.02 pmol/l after 30 min. There was a significant
difference between the levels of insulin in the EG and the CG at 30
min (Fig. 3A).
There were no significant differences in HOMA-IR
levels between the EG and the CG at baseline and 30 min after oral
administration of 200 mg/kg of AC. However, HOMA-IR levels in the
EG were significantly decreased compared with the CG at 60 min
after AC administration (1.53±0.22 vs. 0.83±0.50 respectively,
P<0.05; Fig. 3B).
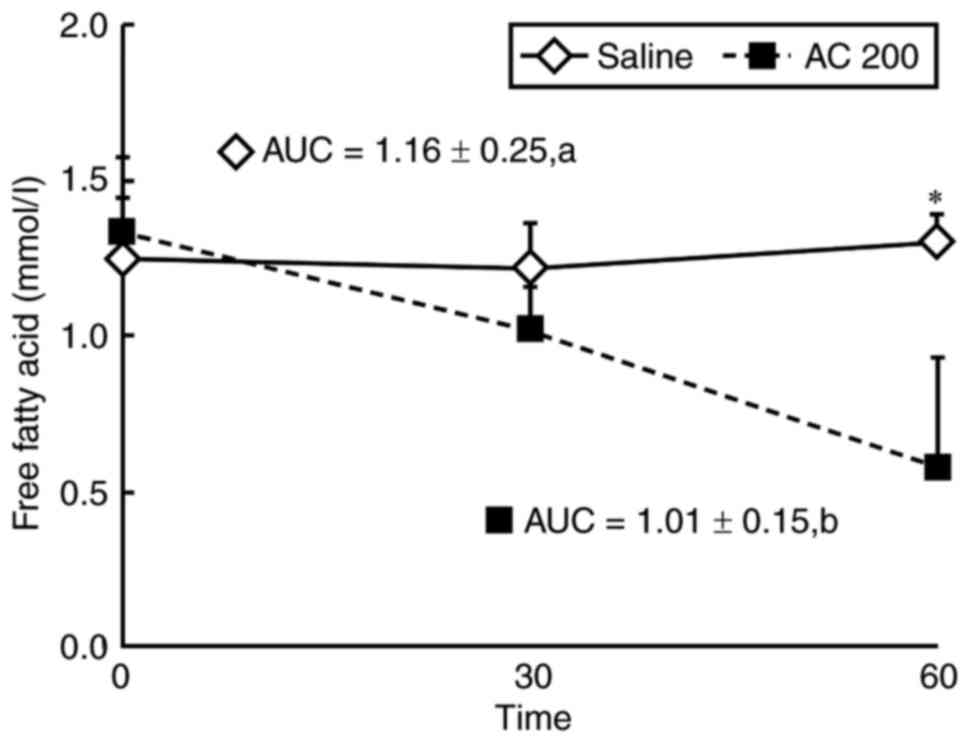
Plasma FFA levels in SIIR rats
following 200 mg/kg AC
At 60 min, plasma FFA levels in the EG had decreased
from 1.33±0.24 to 0.58±0.35 mmol/l, and were significantly
decreased (15.69±3.97%) compared with in the CG, in which plasma
FFA levels were increased from 1.25±0.19 to 1.30±0.09 mmol/l
(5.5±0.80%). Additionally, the area under the curve of plasma FFA
in the EG (1.01±0.15) was significantly smaller than that of the CG
(1.16±0.25, P<0.05; Fig.
4).
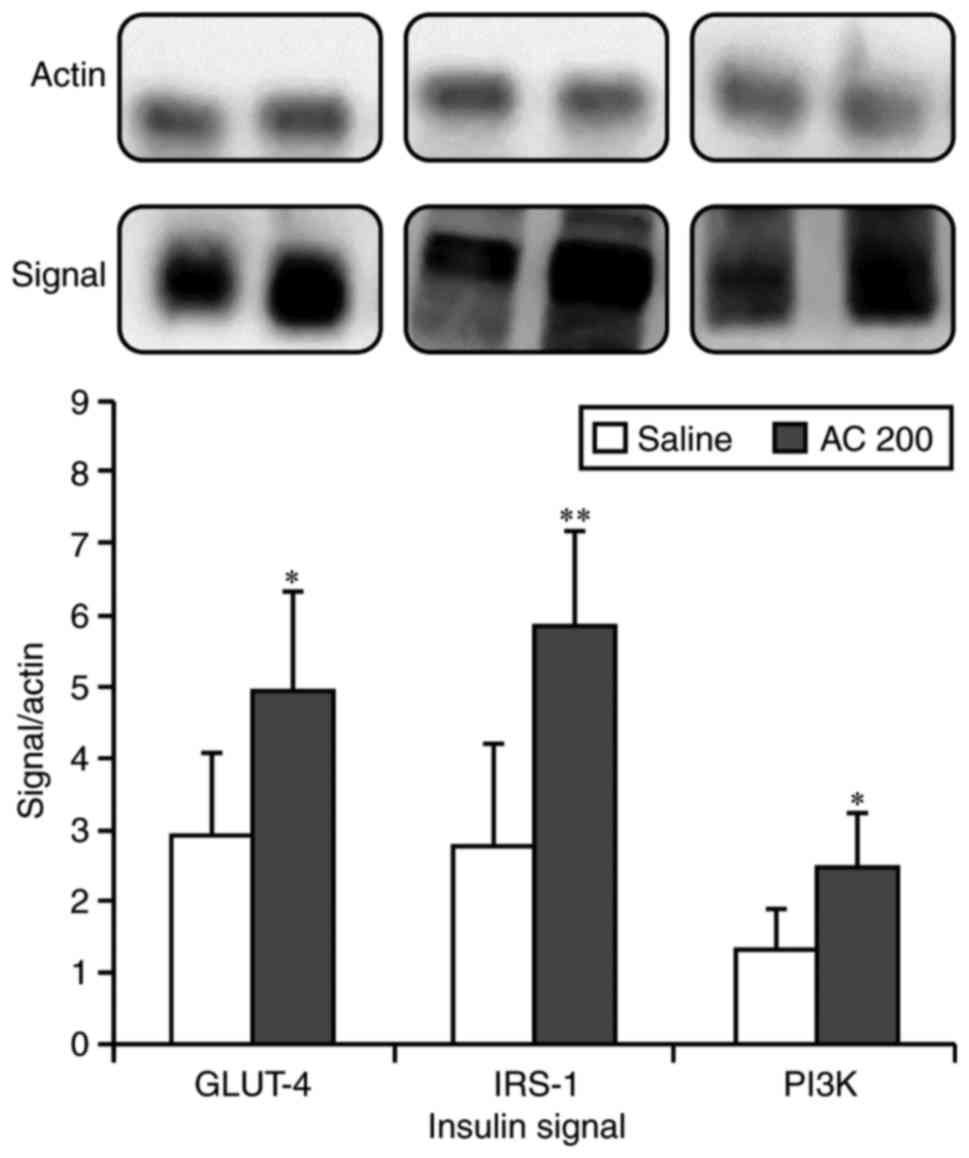
Expression of insulin signal proteins
following AC administration
The levels of insulin signal proteins, IRS-1,
GLUT-4, and PI3K, were significantly elevated compared to the CG
following AC administration. The fold changes (EG/CG) were 1.71,
2.10 and 1.87, respectively (Fig.
5).
Discussion
Metabolic syndrome has gained growing attention in
recent years, particularly in developed countries. Overeating and
an aging society contributes to the increasing incidence of
metabolic syndrome, and insulin resistance may be a major factor
that contributes to the development of type II DM (29). Therefore, preventing metabolic
syndrome or correcting insulin resistance may help to slow the
development of type II DM. There are many oral hypoglycemic agents
and insulin sensitizers like TZDs available, but the side effects
of these drugs limit their use (30). An agent with fewer side effects
that can manage of plasma glucose levels is desirable for patients,
particularly one that can also improve insulin resistance.
AC is a valuable medicinal fungus found in the
forests of Taiwan. It grows naturally in the hollow trunk of the
old Cinnamomum kanehirai tree or through artificial culture
on host wood sections. Due to its versatile biomedical activities,
AC is highly valued (12–16) and Taiwanese forests are threatened
with the illegal logging of Cinnamomum kanehirai trees. This
study developed a solid-state culture method for AC product to
combat this serious illegal logging problem. Additionally, a
standard operating procedure was developed for the AC solid-state
culture method, to verify the quality of each batch (provided by
Chair Professor Wai-Jane Ho from Da-Yeh University; Fig. 1A). The possible toxicity or
potential side effects of the AC mycelium power may be relative to
the dose, but in the present study, 100% of the rats survived
following administration of the highest dose (500 mg/kg).
Physiological signs including respiration, hair color and food and
water intake, were all normal after 1 week of AC administration. As
metabolic syndrome is a chronic condition requiring long-term
treatment, the potential toxic effects of AC are very important and
should be investigated. However, acute and subacute toxicity tests
were not the aim of the present study.
The AC powder methanol extract was analyzed using
HPLC to verify the presence of active hypoglycemic components,
EK100, TR1+TR2 and TR3+TR4 (Fig.
1B). These components were previously reported to be bioactive
and present in AC (18,20,31).
There is concern that the solid-state culture method may result in
variation of the components between batches and different strengths
of bioactivity, but this HPLC analysis method allows for the
quality control of each batch. Prior to the study, experimental
doses were determined in preliminary tests by administering various
doses to a SIIR rat once a week to establish a small, medium, and
high dose (100, 200, and 500 mg/kg) appropriate for rats. Thus, the
doses used are different than what would be appropriate in a
clinical situation.
The hypoglycemic effect of AC was subsequently
evaluated and supported by the findings of the present study. The
optimal oral dose to achieve a hypoglycemic effect was determined
to be 200 mg/kg AC, and this was used to explore the mechanism of
action of AC.
Previous research has confirmed the hypolipidemic
effect of AC (17,20,31,32),
supporting the results of the present study that indicated a
decrease in plasma FFA levels. To establish the SIIR animal model,
steroids were administered, causing an increase in plasma FFA
levels and the development of insulin resistance (11,25).
AC improved insulin resistance by lowering plasma FFA levels in the
SIIR state. Steroids are frequently used to treat inflammatory
diseases and the impairment of insulin sensitivity is a problematic
side effect, particularly in patients with type II DM. The results
of this study demonstrate that AC may improve insulin resistance
caused by the steroid administration.
The increase in plasma insulin in the EG was not
significant at the 60 min time-point compared with the CG, which
might have been due to the duration of plasma insulin secretion
within this studied animal model due to oral administration of the
AC powder. However, HOMA-IR at the 60 min time-point indicated an
improvement in insulin resistance following AC treatment; enhanced
insulin secretion induced by AC was recently reported in MIN6 cells
(33). Therefore, this AC may
elevate plasma insulin and improve insulin resistance.
With respect to signal transduction proteins, AC
caused an increase in IRS-1, PI3K and GLUT-4. Previous studies have
indicated that AC stimulates AMPK to enhance GLUT-4 translocation
and activates the peroxisome proliferator activated receptor α
(PPARα) to decrease plasma FFA levels, which may complement the
insulin signaling pathway to result in a hypoglycemic effect and
improvement in insulin resistance (18,20,32,34).
In our previous studies, we also used the SIIR animal model to test
the hypoglycemic effect of the Xylaria nigripes (Xn) fungus
and the Gardenia jasminoides (GJ) plant (26,35).
However, Xn was found to exert a serotonin-associated hypoglycemic
effect, and PPAR activation was key to the hypoglycemic effect of
GJ. This differs from the mechanism of AC identified in this study,
in which a decrease in plasma FFA levels was observed.
Administration of 200 mg/kg AC to SIIR rats resulted
in a decrease in plasma glucose levels, which was closely
associated with a decrease in plasma FFA levels. Furthermore, an
increase in the expression of insulin signaling proteins (GLUT-4,
IRS-1 and PI3K) was observed with improved insulin resistance.
These results indicate that AC acts as an insulin sensitizer in
insulin resistant animals. Due to the use of an animal model in
this study, results cannot be applied to a clinical situation.
Thus, a randomized controlled trial of AC mycelium powder should be
performed to determine the clinical dosage and enhance its effect
on insulin resistance.
Acknowledgements
This study was supported by grants provided by the
Ministry of Science and Technology (grant no. 104-2632-E-212-001),
Taichung Veterans General Hospital and Da-Yeh University joint
project (grant no. TCVGH-DYU-1058304) and Cheng Ching Hospital and
Da-Yeh University Joint Project (grant no. CCGH-DYU-106-001 &
CCGH-DYU-104-001) in Taiwan. This study was also supported by a
grant from China Medical University under the Aim for Top
University Plan of the Ministry of Education, Taiwan (grant no.
CHM106-5-2) and Taiwan Ministry of Health and Welfare Clinical
Trial Center, Taiwan (grant no. MOHW106-TDU-B-212-113004) for the
analysis of HPLC.
Glossary
Abbreviations
Abbreviations:
|
AC
|
Antrodia cinnamomea
|
|
CG
|
control group
|
|
DM
|
diabetes mellitus
|
|
EG
|
experimental group
|
|
FFA
|
free fatty acid
|
|
GLUT-4
|
glucose transporter 4
|
|
HOMA-IR
|
homeostasis model assessment-estimated
insulin resistance
|
|
IRS-1
|
insulin receptor substrate-1
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
SIIR
|
steroid-induced insulin resistant
|
|
TZD
|
thiazolidinedione
|
References
|
1
|
Annadurai T, Vasanthakumar A, Geraldine P
and Thomas PA: Variations in erythrocyte antioxidant levels and
lipid peroxidation status and in serum lipid profile parameters in
relation to blood haemoglobin A1c values in individuals with type 2
diabetes mellitus. Diabetes Res Clin Pract. 105:58–69. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colussi G, Catena C, Lapenna R, Nadalini
E, Chiuch A and Sechi LA: Insulin resistance and hyperinsulinemia
are related to plasma aldosterone levels in hypertensive patients.
Diabetes Care. 30:2349–2354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeFronzo RA and Ferrannini E: Insulin
resistance. A multifaceted syndrome responsible for NIDDM, obesity,
hypertension, dyslipidemia, and atherosclerotic cardiovascular
disease. Diabetes Care. 14:173–194. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamounier-Zepter V, Ehrhart-Bornstein M
and Bornstein SR: Insulin resistance in hypertension and
cardiovascular disease. Best Pract Res Clin Endocrinol Metab.
20:355–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hecksteden A, Grütters T and Meyer T:
Associations between acute and chronic effects of exercise on
indicators of metabolic health: A pilot training trial. PLoS One.
8:e811812013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eliasson B, Attvall S, Taskinen MR and
Smith U: Smoking cessation improves insulin sensitivity in healthy
middle-aged men. Eur J Clin Invest. 27:450–456. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inzucchi SE, Maggs DG, Spollett GR, Page
SL, Rife FS, Walton V and Shulman GI: Efficacy and metabolic
effects of metformin and troglitazone in type II diabetes mellitus.
N Engl J Med. 338:867–872. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jermendy G and Csermely P:
Thiazolidinediones-a new class of oral antidiabetic drugs. Orv
Hetil. 142:1547–1554. 2001.(In Hungarian). PubMed/NCBI
|
|
9
|
Chang SL, Lin JG, Chi TC, Liu IM and Cheng
JT: An insulin-dependent hypoglycaemia induced by
electroacupuncture at the Zhongwan (CV12) acupoint in diabetic
rats. Diabetologia. 42:250–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang SL, Lin KJ, Lin RT, Hung PH, Lin JG
and Cheng JT: Enhanced insulin sensitivity using electroacupuncture
on bilateral Zusanli acupoints (ST 36) in rats. Life Scie.
79:967–971. 2006. View Article : Google Scholar
|
|
11
|
Tzeng CY, Lee YC, Chung JJ, Tsai JC, Chen
YI, Hsu TH, Lin JG, Lee KR and Chang SL: 15 Hz electroacupuncture
at ST36 improves insulin sensitivity and reduces free fatty acid
levels in rats with chronic dexamethasone-induced insulin
resistance. Acupunct Med. 34:296–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li SL, Huang ZN, Hsieh HH, Yu WC, Tzeng
WY, Lee GY, Chen YP, Chang CY and Chuu JJ: The augmented anti-tumor
effects of Antrodia camphorata co-fermented with Chinese medicinal
herb in human hepatoma cells. Am J Chin Med. 37:771–783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song AR, Qin D, Zhao C, Sun XL, Huang F,
Kong C and Yang S: Immunomodulatory effect of polysaccharides
extracted from the medicinal mushroom Antrodia camphorata (higher
Basidiomycetes) in specific pathogen-free chickens. Int J Med
Mushrooms. 16:95–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue PY, Wong YY, Wong KY, Tsoi YK and
Leung KS: Current evidence for the hepatoprotective activities of
the medicinal mushroom Antrodia cinnamomea. Chin Med. 8:212013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY,
Chou DS, Lin CH, Su CH and Sheu JR: Antioxidative and
hepatoprotective effects of Antrodia camphorata extract. J Agric
Food Chem. 51:3302–3308. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yue PY, Wong YY, Chan TY, Law CK, Tsoi YK
and Leung KS: Review of biological and pharmacological activities
of the endemic Taiwanese bitter medicinal mushroom, Antrodia
camphorata (M. Zang et C. H. Su) Sh. H. Wu et al. (higher
Basidiomycetes). Int J Med Mushrooms. 14:241–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai MN, Ko HJ and Ng LT: Hypolipidemic
effects of antrodia cinnamomea extracts in high-fat diet-fed
hamsters. J Food Biochem. 36:233–239. 2012. View Article : Google Scholar
|
|
18
|
Kuo YH, Lin CH and Shih CC:
Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and
hyperlipidemia in high-fat-diet treated mice via regulation of
hepatic related genes, glucose transporter 4, and AMP-activated
protein kinase phosphorylation. J Agric Food Chem. 63:2479–2489.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weng CF, Chen CP, Shivaji SR and Hsu CY:
Compounds from antrodia camphorate and their use in treatment of
diabetes mellitus. US Patent 20150203430 A1. Filed January 22,
2014; issued July 23. 2015.
|
|
20
|
Kuo YH, Lin CH and Shih CC: Antidiabetic
and antihyperlipidemic properties of a triterpenoid compound,
dehydroeburicoic acid, from antrodia camphorata in vitro and in
streptozotocin-induced mice. J Agric Food Chem. 63:10140–10151.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wen CL, Chang CC, Huang SS, Kuo CL, Hsu
SL, Deng JS and Huang GJ: Anti-inflammatory effects of methanol
extract of Antrodia cinnamomea mycelia both in vitro and in vivo. J
Ethnopharmacol. 137:575–584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuo MT, Tseng WP, Tseng TL and Kuo YY:
Method for promoting insulin secretion by using compounds and
extracts isolated from antrodia camphorata US Patent 20160038549
A1. Filed August 7, 2015; issued February 11. 2016
|
|
23
|
Tsai PJ, Wang C, Chou CJ and Huang WC:
Method for controlling obesity using Antrodia camphorata US Patent
20150157673 A1. Filed December 5, 2013; issued June 11. 2015
|
|
24
|
Park TS: Composition for prevention or
treatment of obesity, dyslipidemia, fatty liver or insulin
resistance syndrome comprising camphene as active ingredients US
Patent 20120035274 A1. Filed March 18, 2009; issued February 9.
2012
|
|
25
|
Lin RT, Tzeng CY, Lee YC, Ho WJ, Cheng JT,
Lin JG and Chang SL: Acute effect of electroacupuncture at the
Zusanli acupoints on decreasing insulin resistance as shown by
lowering plasma free fatty acid levels in steroid-background male
rats. BMC Complement Altern Med. 9:262009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen YI, Cheng YW, Tzeng CY, Lee YC, Chang
YN, Lee SC, Tsai CC, Chen JC, Tzen JT and Chang SL: Peroxisome
proliferator-activated receptor activating hypoglycemic effect of
Gardenia jasminoides Ellis aqueous extract and improvement of
insulin sensitivity in steroid induced insulin resistant rats. BMC
Complement Altern Med. 14:302014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takai T and Sakura H: Insulinogenic index,
HOMA-beta, disposition index. Nihon Rinsho. 70 Suppl 3:S459–S464.
2012.(In Japanese).
|
|
28
|
Haffner SM, Miettinen H and Stern MP: The
homeostasis model in the San Antonio Heart Study. Diabetes Care.
20:1087–1092. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chillarón JJ, Flores-Le-Roux JA, Goday A,
Benaiges D, Carrera MJ, Puig J, Cano-Pérez JF and Pedro-Botet J:
Metabolic syndrome and type-1 diabetes mellitus: Prevalence and
associated factors. Rev Esp Cardiol. 63:423–429. 2010.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rizos CV, Elisaf MS, Mikhailidis DP and
Liberopoulos EN: How safe is the use of thiazolidinediones in
clinical practice? Expert Opin Drug Saf. 8:15–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng CH, Yang MY, Yang YS, Yu CC and Wang
CJ: Antrodia cinnamomea prevents obesity, dyslipidemia, and the
derived fatty liver via regulating AMPK and SREBP signaling. Am J
Chin Med. 45:67–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuo YH, Lin CH, Shih CC and Yang CS:
Antcin K, a triterpenoid compound from Antrodia camphorata,
displays antidiabetic and antihyperlipidemic effects via glucose
transporter 4 and AMP-activated protein kinase phosphorylation in
muscles. Evid Based Complement Alternat Med. 2016:48670922016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vong CT, Tseng HH, Kwan YW, Lee SM and Hoi
MP: Antrodia camphorata increases insulin secretion and protects
from apoptosis in MIN6 Cells. Front Pharmacol. 7:672016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuo YH, Lin CH and Shih CC:
Dehydroeburicoic acid from Antrodia camphorata prevents the
diabetic and dyslipidemic state via modulation of glucose
transporter 4, peroxisome proliferator-activated receptor alpha
expression and AMP-activated protein kinase phosphorylation in
high-fat-fed mice. Int J Mol Sci. 17:E8722016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen YI, Tzeng CY, Cheng YW, Hsu TH, Ho
WJ, Liang ZC, Hsieh CW, Tzen JT and Chang SL: The involvement of
serotonin in the hypoglycemic effects produced by administration of
the aqueous extract of Xylaria nigripes with steroid-induced
insulin-resistant rats. Phytother Res. 29:770–776. 2015. View Article : Google Scholar : PubMed/NCBI
|