Introduction
Psoriasis is a chronic and systemic inflammatory
disease, with an estimated population prevalence of 2–5% worldwide
(1,2). Psoriasis is characterized by red,
demarcated skin lesions with adherent silver scales (2). The scales are formed by
hyperproliferative keratinocytes. In psoriatic skin, the fraction
of proliferating keratinocytes is ~100%, whereas in normal skin it
is ~20% (3). The epidermal cell
turnover in psoriasis is increased 8-fold compared with normal
skin, which may lead to altered keratinocyte differentiation
(1,3,4). The
increased rate of keratinocyte proliferation may be induced by
cytokines secreted by activated resident immune cells, by
keratinocytes themselves; or by infiltrating inflammatory cells,
including T cells, dendritic cells (5,6).
Keratinocytes can respond to dendritic cell-derived and T
cell-derived cytokines, including interferon (IFN), tumour necrosis
factor (TNF), interleukin (IL)-17, and the IL-20 family.
Furthermore, these T cell-derived cytokines can then produce
pro-inflammatory cytokines (IL-1, IL-6, TNF-α, as well as
chemokines such as IL-8, C-X-C motif chemokine 10, chemokine (C-C
motif) ligand 20 (6).
The Wnt protein family is involved in cell
proliferation, differentiation, polarity, adhesion and motility
(7). Wnt family member 5a (Wnt5a)
is one of the most extensively studied of the Wnt protein family
members and represents a prototypical non-canonical Wnt family
member (7). Wnt5a has a complex
biological activity and can positively or negatively affect cell
proliferation in different cell types (8,9). In
addition, Wnt5a can both activate and inhibit the canonical Wnt
signalling pathway (10,11). A previous study revealed that Wnt5a
expression is significantly upregulated in the lesional skin of
patients with psoriasis (12).
Therefore, Wnt5a may contribute to the abnormal proliferation,
differentiation and inflammatory response of keratinocytes in
psoriasis. The present study aimed to determine the molecular
mechanisms underlying the effect of Wnt5a in human epithelial cells
and to investigate the role of Wnt5a in psoriasis.
Materials and methods
Cell culture
HaCaT cells were purchased from China Infrastructure
of Cell Line Resources (Beijing, China). All cells were cultured in
minimum essential medium/Earle's balanced salt solution (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA) supplemented with
foetal bovine serum (FBS; Corning Life Sciences, New York, NY,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin (HyClone, GE
Healthcare Life Sciences). All cells were maintained at 37°C in a
humidified incubator with 5% CO2. HaCaT cells in
logarithmic growth phase were treated with either 40 or 80 ng/ml
recombinant human Wnt5a (R&D Systems, Inc., Minneapolis, MN,
USA).
Cell viability assay
HaCaT cells were seeded into 96-well plates at a
density of 1×105 cells/ml and incubated in medium
containing 0.1% FBS overnight prior treatment with either 40 or 80
ng/ml recombinant human Wnt5a for 24 h. The viability of HaCaT
cells was investigated using Cell Counting Kit-8 (CCK8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) according to the
manufacturer's instructions. Survival rates were calculated
according to the following formula: Cell survival rate (%)=[optical
density (OD) of treatment group-OD of blank group]/(OD of control
group-OD of blank group)x100. All experiments were repeated four
times.
Cell cycle analysis using flow
cytometry
The cell cycle of HaCaT cells was investigated using
flow cytometry as previously described (12). HaCaT cells were seeded into 6-well
plates at a density of 1×105 cells/ml. The cells were
treated with different concentrations of 0, 40 and 80 ng/ml Wnt5a
for 24 h. The cells were then collected, washed twice with PBS,
fixed with 70% ethanol overnight at 4°C, treated with 1% RNase A
for 30 min at 37°C, and then stained with propidium iodide (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) for 30 min at 4°C. A
total of 20,000 cells were counted per sample, and the cell cycle
was analysed using an Accuri C6 cytometer (BD Biosciences, San
Jose, CA, USA). The cell cycle profiles were analyzed using the
ModFit program version 3.1 (Verity Software House, Inc., Topsham,
ME, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from different groups of
cultured HaCaT cells using the TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). cDNA was synthesized
from 0.5 µg of total RNA using the Goscript™ Reverse Transcription
System (Thermo Fisher Scientific, Inc., USA). The RT reaction was
performed at 42°C for 60 min and then at 70°C for 5 min. The qPCR
reaction was performed according to the protocol of the FastStart
Universal SYBR Green Master (Roche Applied Science, Penzberg,
Germany) using an Applied Biosystems 7500 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc., USA). The
amplification program for all primers was 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The results
were then analysed using the 2−ΔΔCq method (13), and determined values were then
normalized to GAPDH expression as an internal control.
Primers are listed in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| Filaggrin |
TGAAGCCTATGACACCACTGA |
TCCCCTACGCTTTCTTGTCCT |
| Keratin 1 |
GGCAGTTCCAGCGTGAAGTTTGTT |
TTCTCCGGTAAGGCTGGGACAAAT |
| Keratin 10 |
GAGCAAGGAACTGACTACAG |
CTCGGTTTCAGCTCGAATCT |
| Cyclin D1 |
AACTACCTGGACCGCTTCCT |
CCACTTGAGCTTGTTCACCA |
| PCNA |
GGCGTGAACCTCACCAGTAT |
TTCTCCTGGTTTGGTGCTTC |
| Ki-67 |
TGACAAGCCCACGACTGATGAGAA |
CTTTGCCTGCTGATGGTGTTCGTT |
| β-catenin |
AAAATGGCAGTGCGTTTAG |
TTTGAAGGCAGTCTGTCGTA |
| GAPDH |
CGGAGTCAACGGATTTGGTCGTAT |
AGCCTTCTCCATGGTGGTGAAGAC |
Enzyme-linked immunosorbent assay
(ELISA)
The concentration of IL-8, IL-17A and IFN-γ in
conditioned medium from cultured cells was detected using ELISA
kits (EHC008, EHC170 and EHC102; Xin Bo Sheng Biological Technology
Co., Ltd., Shenzhen, China). ELISAs were performed according to the
manufacturer's protocol. The concentrations of IL-8, IL-17A and
IFN-γ were calculated via comparison of the relative absorbance of
the samples with the standards.
Statistical analysis
Statistical analyses were performed using SPSS
version 21.0 (IBM Corporation, Armonk, NY, USA). The data are
presented as the mean ± standard error of mean. One-way analysis of
variance with a least square difference (LSD) was used to determine
the statistical significance of results. P<0.05 was considered
to indicate a statistically significant difference.
Results
Wnt5a reduces keratinocyte
viability
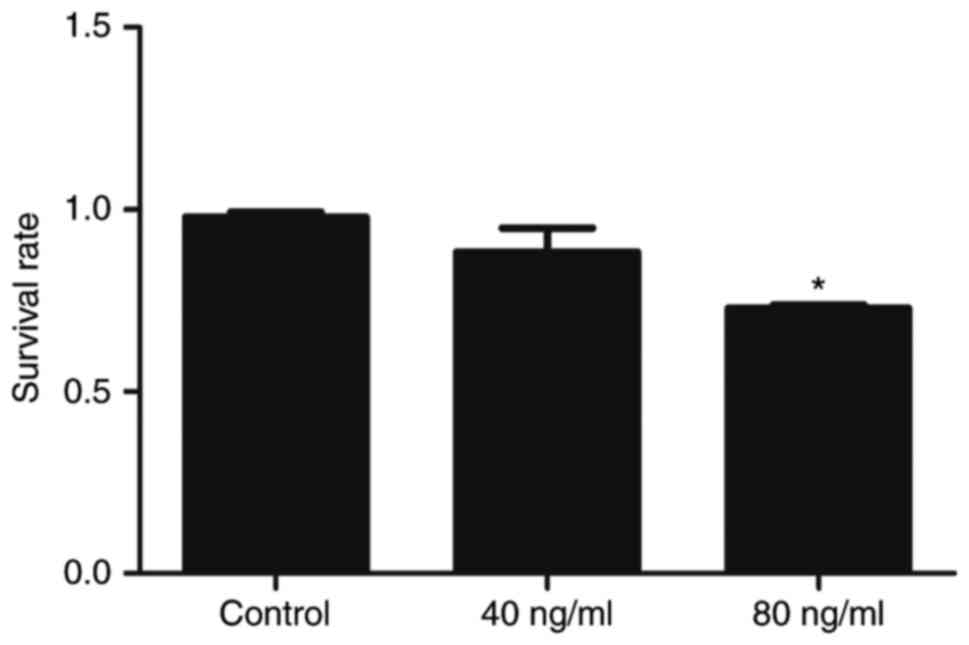
The proliferative inhibitory effect of Wnt5a on
HaCaT cell viability was investigated using the CCK8 assay. No
significant difference in cell viability between the 40 ng/ml Wnt5a
and the control group was observed (Fig. 1); thus, no cytotoxicity was
considered to be present at this concentration. However, a higher
concentration of Wnt5a (80 ng/ml) was significantly toxic to the
cells (Fig. 1). Therefore, Wnt5a
effectively inhibited the proliferation of HaCaT cells at the
concentration of 80 ng/ml.
Effect of Wnt5a on keratinocyte cell
cycle
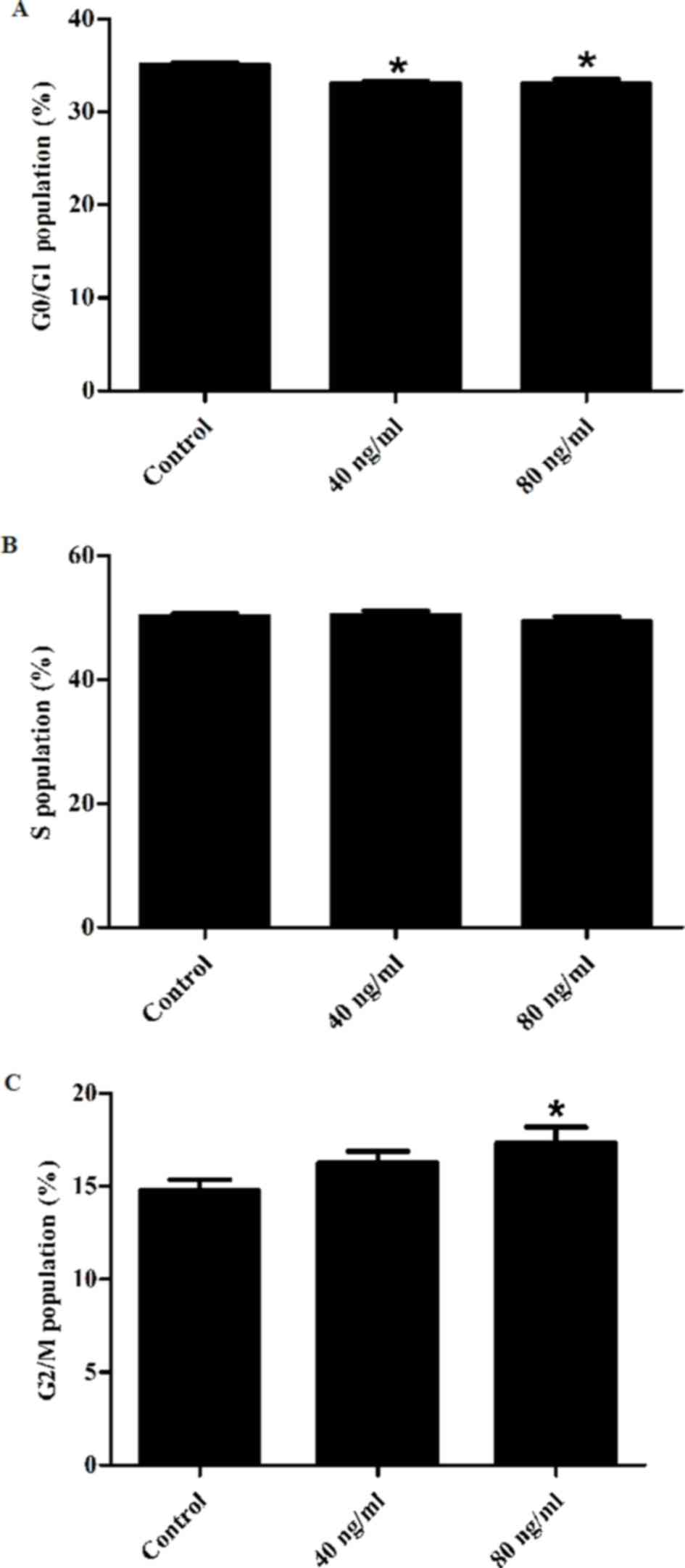
Flow cytometry was used in order to investigate the
effect of Wnt5a on HaCaT cell cycle progression (Fig. 2). Compared with the control group,
Wnt5a significantly decreased the proportion of cells in the G0/G1
phase at both 40 and 80 ng/ml concentrations, whereas it
significantly increased the proportion of cells in the G2/M phase
at 80 ng/ml. These results suggest that Wnt5a arrested the
keratinocyte cell cycle at the G2/M phase.
Wnt5a negatively regulates the
expression of keratinocyte differentiation markers
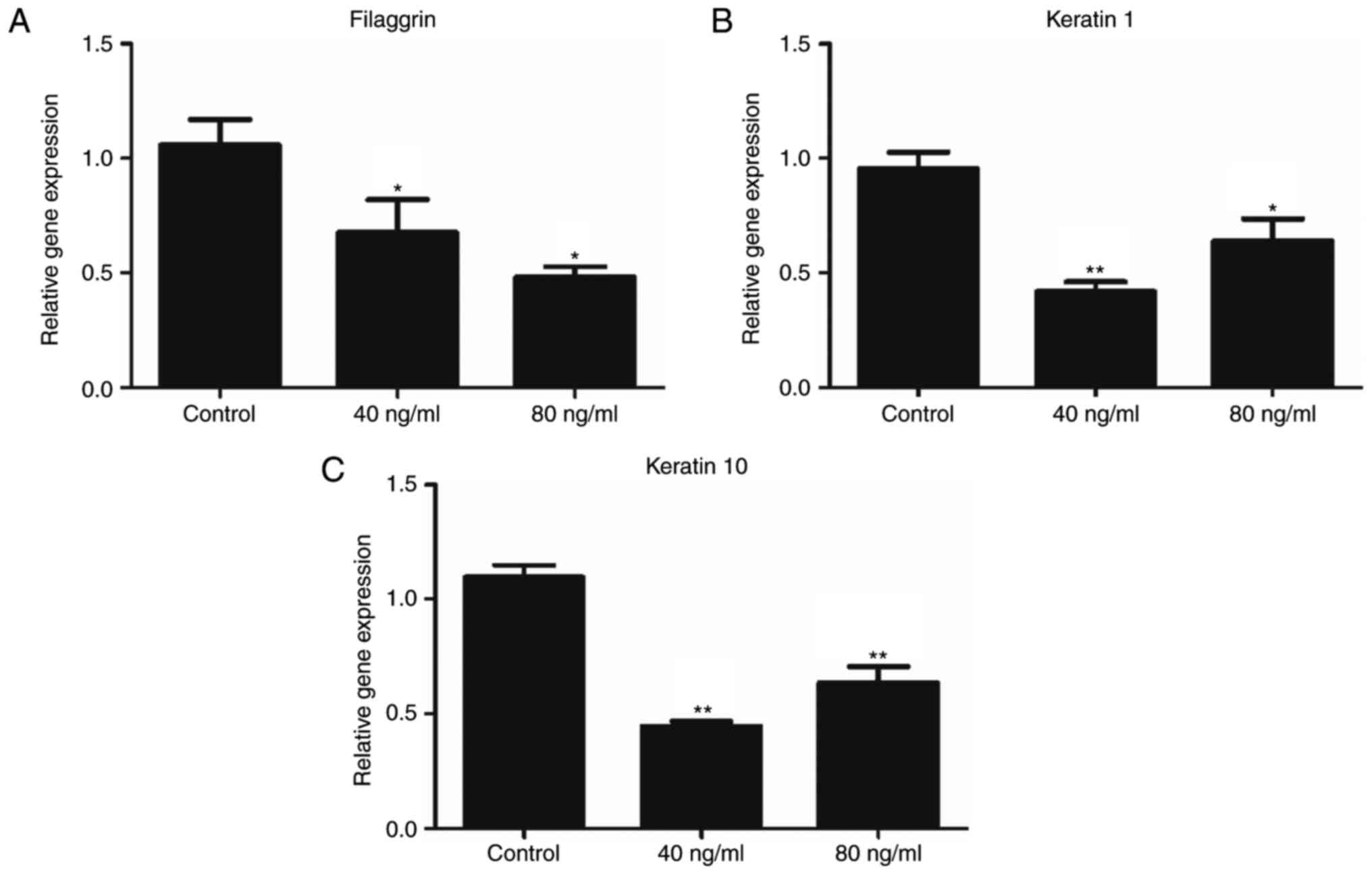
In order to investigate the effect of Wnt5a on
keratinocyte differentiation, the expression levels of filaggrin,
keratin 1 and keratin 10 were investigated. The mRNA expression of
these markers was determined using RT-qPCR (Fig. 3). The results demonstrated
significant differences in the expression levels of filaggrin,
keratin 1 and keratin 10 mRNA expression between the Wnt5a
treatment groups (both 40 and 80 ng/ml) and the control group, thus
suggesting that Wnt5a inhibited keratinocyte differentiation.
Wnt5a increases the expression of
inflammatory-associated proteins in keratinocytes
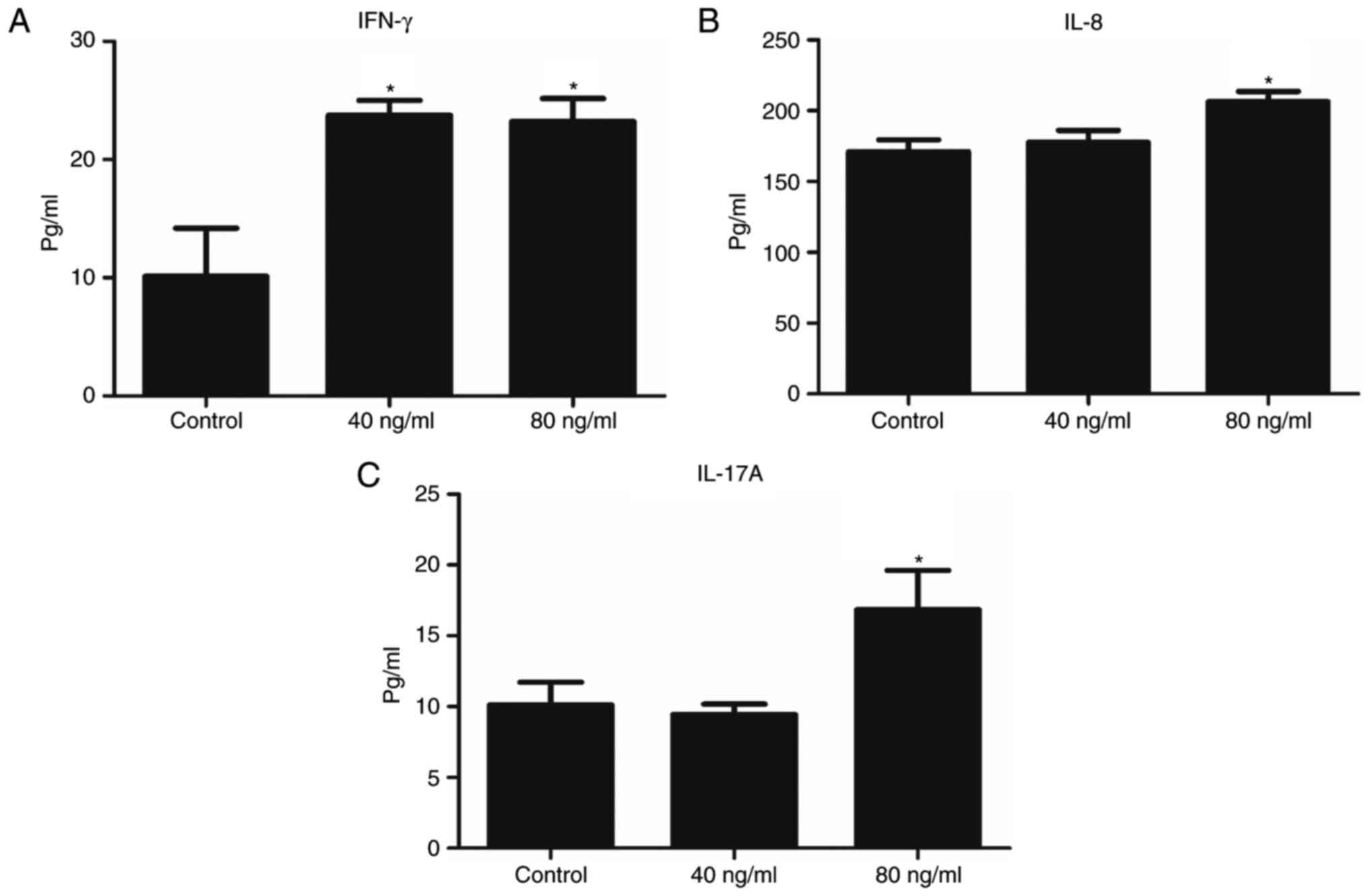
The involvement of Wnt5a in the regulation of the
inflammatory response in keratinocytes was investigated. ELISA was
used to detect the expression of IFN-γ, IL-8 and IL-17A proteins in
conditioned medium from cultured cells. Wnt5a significantly
attenuated IFN-γ expression at both 40 and 80 ng/ml concentrations
(Fig. 4A). Expression levels of
inflammatory factors IL-8 and IL-17A were significantly enhanced by
Wnt5a administration at the concentration of 80 ng/ml (Fig. 4B and C). These findings suggest
that Wnt5a is an upstream mediator of the inflammatory response in
keratinocytes.
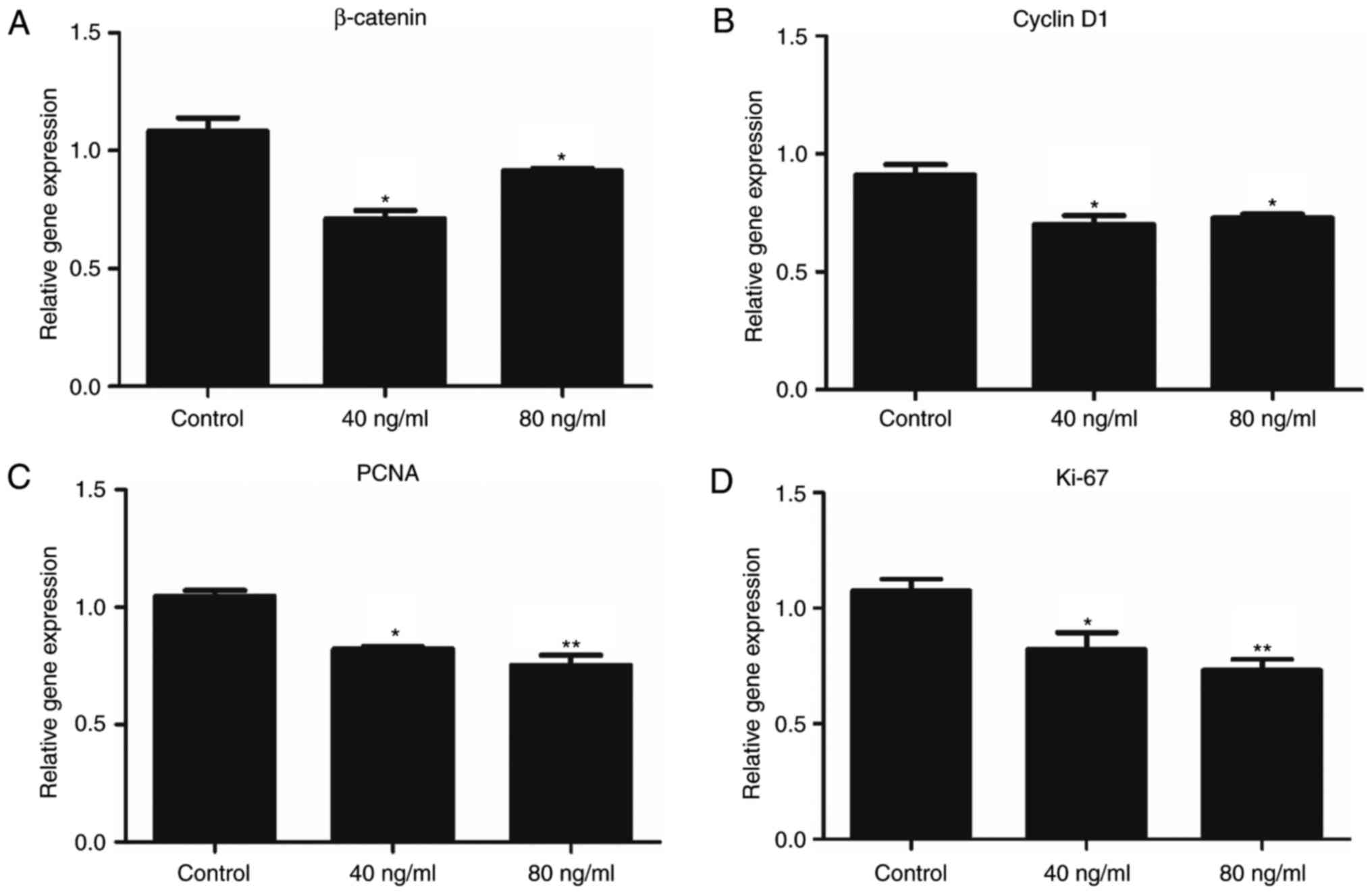
Wnt5a downregulates the expression of
β-catenin in keratinocytes
Exposure to Wnt5a for 24 h resulted in the
suppression of β-catenin expression (Fig. 5A). Considering that Wnt5a was
revealed to suppress canonical Wnt signalling, the expression
levels of downstream genes in the canonical Wnt pathway, as well as
proliferation markers, were also investigated (Fig. 5B-D). RT-qPCR demonstrated that
treatment with Wnt5a significantly reduced the expression of cyclin
D1, Ki-67 and proliferating cell nuclear antigen (PCNA), thus
suggesting that Wnt5a antagonized the canonical Wnt signalling
pathway in keratinocytes.
Discussion
Wnt5a, a member of the Wnt family, has been
determined to have diverse biological functions in organ
development, cellular functioning, inflammatory responses and
innate immunity (14,15). It has previously been demonstrated
that Wnt5a is associated with numerous diseases, including cancer,
diabetes, metabolic disorders and inflammatory diseases,
specifically sepsis, atherosclerosis (16), rheumatoid arthritis (17), psoriasis vulgaris (18). Zhang et al (12) revealed that Wnt5a is significantly
upregulated in psoriatic lesions compared with healthy skin. In the
present study, it was demonstrated that treatment with Wnt5a
inhibits cell proliferation and promotes an inflammatory response
in HaCaT keratinocytes. Therefore, the present study suggests that
Wnt5a may have an involvement in the abnormal cell differentiation
and inflammatory responses of keratinocytes in patients with
psoriasis.
Previous studies have demonstrated the association
between Wnt5a and inflammatory diseases, including psoriasis
vulgaris, atherosclerosis and rheumatoid arthritis, suggests that
Wnt5a may be involved in the development and pathogenesis of
inflammatory disease (19,20). In addition, Wnt5a has previously
been revealed to induce inflammatory responses in a variety of cell
types, such as macrophages, cultured endothelial cells and synovial
fibroblasts (20,21). However, the role of Wnt5a in the
inflammatory process associated with psoriasis remains unclear. In
the present study, HaCaT cells that were treated with Wnt5a, which
imitated the inflammatory response observed in patients with
psoriasis. Furthermore, it was also revealed that Wnt5a stimulated
the production of IFN-γ, IL-8 and IL-17A inflammatory factors. The
expression levels of IFN-γ and IL-8 have previously been
demonstrated to be upregulated in psoriatic lesions and anti-IL-17
therapeutic agents have previously been used for the treatment of
psoriasis (1,6,22).
Therefore, the findings of the present study suggest that Wnt5a has
a pro-inflammatory function in HaCaT cells.
Histologically, psoriatic lesions are characterized
by epidermal hyperplasia and altered epidermal differentiation,
which are associated with the downregulation of keratinocyte
differentiation markers (6,22,23).
Vitamin D derivatives have been the first-line treatment for
psoriasis for several years (24).
A previous study demonstrated that administration of vitamin D can
inhibit cell proliferation and promote differentiation in
keratinocytes (24). Previous
studies have also revealed that several late differentiation
markers in keratinocytes, such as filaggrin and loricrin, are
downregulated in psoriatic lesions (22,25,26).
Furthermore, altered keratinocyte cell differentiation can lead to
skin-barrier impairment in lesional skin (27). Currently available treatments for
psoriasis, including retinoid and vitamin D administration,
primarily target keratinocyte differentiation. Thus, keratinocytes
may be involved in psoriasis pathogenesis. In the present study,
filaggrin, keratin 1 and keratin 10 expression levels were reduced
following treatment with Wnt5a, which suggests a role for Wnt5a in
the differentiation of keratinocytes.
It has been previously established that Wnt5a is
involved in the upregulation and the downregulation of cell
proliferation (28,29). For example, Wnt5a can promote the
proliferation of chronic lymphocytic leukaemia cells (28) and endothelial cells, whereas it
suppresses the proliferation of prostate cancer cells (29), B lymphocytes and epithelial ovarian
cancer cells (9). In the present
study, it was demonstrated that Wnt5a suppressed the growth of
keratinocytes and regulated cell cycle progression at the G2/M
phase. The findings of the present study are in contrast to the
findings of Zhang et al (12), as they revealed that a Wnt5a
knockdown using small interfering RNA suppressed cell
proliferation, induced apoptosis, and arrested cell cycle
progression at the G0/G1 phase in keratinocytes. Therefore, further
studies regarding the role of Wnt5a in keratinocyte proliferation
in patients with psoriasis are required in order to verify the
results of the present study.
Due to the diversity of Wnt5a-binding receptors and
target cells/tissues, Wnt5a may activate and inhibit the β-catenin
signalling pathway (10,11). Previous studies have demonstrated
that Wnt5a activates the β-catenin signalling pathway in pancreatic
cancer cells, osteoblast-lineage cells and dermal fibroblasts
(11). However, a previous study
also revealed that Wnt5a suppressed β-catenin signalling during
hair follicle regeneration (30).
In the present study, it was revealed that Wnt5a negatively
regulated the expression of β-catenin in HaCaT cells.
Furthermore, canonical Wnt signalling genes (β-catenin and cyclin
D1) and proliferation markers (Ki-67 and PCNA), were downregulated
following treatment with Wnt5a. Cyclin D1 is an important regulator
of cell cycle progression, and PCNA and Ki-67 are markers of cell
proliferation (31,32). These findings suggest that Wnt5a
may inhibit the β-catenin signalling pathway in keratinocytes.
However, there were several limitations in the
present study. Firstly, in order to determine the exact role of
Wnt5a, experimentation using a wider range of Wnt5a concentrations
would be required. In addition, the gene expression, protein level
and cellular distribution of β-catenin were not investigated in the
present study. With regards to psoriasis, the exact role of Wnt5a
and its relationship with β-catenin signalling remain to be
elucidated. In previous studies, increased nuclear β-catenin
staining in the suprabasal epidermis in psoriatic lesions compared
with normal skin has been identified (33). In contrast, Yamazaki et al
(34) demonstrated that there was
no β-catenin activation in psoriatic skin. Zhang et al
(12); however, reported that
Wnt5a is significantly upregulated in all of the epidermal layers
in psoriasis lesions. Therefore, further investigation is required
in order to determine the exact molecular mechanisms underlying the
role of Wnt5a in psoriasis.
In conclusion, the present study has identified the
roles of Wnt5a in keratinocyte responses that are relevant to
psoriasis. In addition, the present study has demonstrated that
exogenous Wnt5a may induce an inflammatory response, inhibit cell
differentiation, downregulate β-catenin signalling and suppress the
proliferation of keratinocytes. However, further studies are
required in order to determine the in vivo function and
mechanism of Wnt5a in the occurrence, development and relapse of
psoriasis.
References
|
1
|
Baliwag J, Barnes DH and Johnston A:
Cytokines in psoriasis. Cytokine. 73:342–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raychaudhuri SK, Maverakis E and
Raychaudhuri SP: Diagnosis and classification of psoriasis.
Autoimmun Rev. 13:490–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gudjonsson JE, Johnston A, Stoll SW,
Riblett MB, Xing X, Kochkodan JJ, Ding J, Nair RP, Aphale A,
Voorhees JJ and Elder JT: Evidence for altered Wnt signaling in
psoriatic skin. J Invest Dermatol. 130:1849–1859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinstein GD, McCullough JL and Ross PA:
Cell kinetic basis for pathophysiology of psoriasis. J Invest
Dermatol. 85:579–583. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rabeony H, Petit-Paris I, Garnier J,
Barrault C, Pedretti N, Guilloteau K, Jegou JF, Guillet G, Huguier
V, Lecron JC, et al: Inhibition of keratinocyte differentiation by
the synergistic effect of IL-17A, IL-22, IL-1α, TNFα and oncostatin
M. PLoS One. 9:e1019372014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Endo M, Nishita M, Fujii M and Minami Y:
Insight into the role of Wnt5a-induced signaling in normal and
cancer cells. Int Rev Cell Mol Biol. 314:117–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asem MS, Buechler S, Wates RB, Miller DL
and Stack MS: Wnt5a Signaling in Cancer. Cancers. 8:E792016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang H, Chen Q, Coles AH, Anderson SJ,
Pihan G, Bradley A, Gerstein R, Jurecic R and Jones SN: Wnt5a
inhibits B cell proliferation and functions as a tumor suppressor
in hematopoietic tissue. Cancer cell. 4:349–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y
and Gu Q: Wnt5a suppresses colon cancer by inhibiting cell
proliferation and epithelial-mesenchymal transition. J Cell
Physiol. 229:1908–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumawat K and Gosens R: WNT-5A: Signaling
and functions in health and disease. Cell Mol Life Sci. 73:567–587.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Tu C, Zhang D, Zheng Y, Peng Z,
Feng Y, Xiao S and Li Z: Wnt/β-Catenin and Wnt5a/Ca pathways
regulate proliferation and apoptosis of keratinocytes in psoriasis
lesions. Cell Physiol Biochem. 36:1890–1902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatt PM and Malgor R: Wnt5a: A player in
the pathogenesis of atherosclerosis and other inflammatory
disorders. Atherosclerosis. 237:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: Wnt5a: Its signalling, functions and implication in
diseases. Acta physiol(Oxf). 204:17–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malgor R, Bhatt PM, Connolly BA, Jacoby
DL, Feldmann KJ, Silver MJ, Nakazawa M, McCall KD and Goetz DJ:
Wnt5a, TLR2 and TLR4 are elevated in advanced human atherosclerotic
lesions. Inflamm Res. 63:277–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sen M, Chamorro M, Reifert J, Corr M and
Carson DA: Blockade of Wnt-5A/frizzled 5 signaling inhibits
rheumatoid synoviocyte activation. Arthritis Rheum. 44:772–781.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suarez-Farinas M, Fuentes-Duculan J, Lowes
MA and Krueger JG: Resolved psoriasis lesions retain expression of
a subset of disease-related genes. J Invest Dermatol. 131:391–400.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wright M, Aikawa M, Szeto W and Papkoff J:
Identification of a Wnt-responsive signal transduction pathway in
primary endothelial cells. Biochem Biophys Res Commun. 263:384–388.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao Y, Zheng Q, Wang W, Xin N, Song X and
Zhao C: Biological functions of macrophage-derived Wnt5a and its
roles in human diseases. Oncotarget. 7:67674–67684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim J, Chang W, Jung Y, Song K and Lee I:
Wnt5a activates THP-1 monocytic cells via a β-catenin-independent
pathway involving JNK and NF-κB activation. Cytokine. 60:242–248.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tschachler E: Psoriasis: The epidermal
component. Clin Dermatol. 25:589–595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buchau AS and Gallo RL: Innate immunity
and antimicrobial defense systems in psoriasis. Clin Dermatol.
25:616–624. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soleymani T, Hung T and Soung J: The role
of vitamin D in psoriasis: A review. Int J Dermatol. 54:383–392.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hohl D: Expression patterns of loricrin in
dermatological disorders. Am J Dermatopathol. 15:20–27. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bernard BA, Asselineau D,
Schaffar-Deshayes L and Darmon MY: Abnormal sequence of expression
of differentiation markers in psoriatic epidermis: Inversion of two
steps in the differentiation program? J Invest Dermatol.
90:801–805. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gschwandtner M, Mildner M, Mlitz V, Gruber
F, Eckhart L, Werfel T, Gutzmer R, Elias PM and Tschachler E:
Histamine suppresses epidermal keratinocyte differentiation and
impairs skin barrier function in a human skin model. Allergy.
68:37–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu J, Chen L, Cui B, Widhopf GF II, Shen
Z, Wu R, Zhang L, Zhang S, Briggs SP and Kipps TJ: Wnt5a induces
ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and
proliferation. J Clin Invest. 126:585–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thiele S, Göbel A, Rachner TD, Fuessel S,
Froehner M, Muders MH, Baretton GB, Bernhardt R, Jakob F, Glüer CC,
et al: WNT5A has anti-prostate cancer effects in vitro and reduces
tumor growth in the skeleton in vivo. J Bone Miner Res. 30:471–480.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing Y, Ma X, Guo H, Deng F, Yang J and Li
Y: Wnt5a Suppresses β-catenin signaling during hair follicle
regeneration. Int J Med Sci. 13:603–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tarapore RS, Siddiqui IA, Saleem M, Adhami
VM, Spiegelman VS and Mukhtar H: Specific targeting of
Wnt/β-catenin signaling in human melanoma cells by a dietary
triterpene lupeol. Carcinogenesis. 31:1844–1853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi W, Hu J, Zhu S, Shen X, Zhang X, Yang
C, Gao H and Zhang H: Expression of MTA2 and Ki-67 in
hepatocellular carcinoma and their correlation with prognosis. Int
J Clin Exp Pathol. 8:13083–13089. 2015.PubMed/NCBI
|
|
33
|
Hampton PJ, Ross OK and Reynolds NJ:
Increased nuclear beta-catenin in suprabasal involved psoriatic
epidermis. Br J Dermatol. 157:1168–1177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamazaki F, Aragane Y, Kawada A and Tezuka
T: Immunohistochemical detection for nuclear beta-catenin in
sporadic basal cell carcinoma. Br J Dermatol. 145:771–777. 2001.
View Article : Google Scholar : PubMed/NCBI
|