Introduction
Cervical cancer is the most common malignancy of the
female genital tract and the second leading cause of mortality
among women worldwide, with an estimated global incidence of
>500,000 newly diagnosed cases and 260,000 mortalities annually
(1,2). Persistent infection with high-risk
human papillomavirus has been considered to be the primary risk
factor for developing cervical cancer and its precursor lesions
(3–6). Although surgical resection combined
with radiotherapy and chemotherapy has been used as a major
treatment for patients with cervical cancer, the overall survival
(OS) rate and disease-free survival rate for patients with
late-stage disease remain poor (1,5,7).
Therefore, understanding the molecular mechanisms underlying
cervical cancer and identifying factors involved in the progression
of the disease is important, in order to offer novel therapeutic
targets and improve patient survival.
Forkhead box protein C1 (FOXC1), a member of the FOX
family of transcription factors, is located on chromosome 6p25 and
regulates an array of biological processes, including metabolism,
development, differentiation, proliferation, apoptosis and cell
migration (8–11). In addition to its roles in normal
function and development, FOXC1 has been demonstrated to be a
possible master regulator in various types of human cancer,
including breast cancer, hepatocellular carcinoma, pancreatic and
non-small cell lung cancers (8,9,11–13).
In addition, high FOXC1 expression is correlated with poor clinical
outcome (9,13–15).
However, to the best of the authors' knowledge, expression of FOXC1
has not been investigated in cervical cancer. The aim of the
present study was to investigate alterations in the expression of
FOXC1 and the biological function of FOXC1 in cervical cancer cells
in vitro.
Materials and methods
Patients and tissue specimens
Samples from patients aged 48–73 years (n=76) with
cervical cancer who underwent curative surgical resection were
collected from The Fourth Affiliated Hospital of Harbin Medical
University (Harbin, China) between March 2009 and June 2011. A
total of 34 control samples were obtained from women who underwent
hysterectomy for nonmalignant conditions during the same period.
None of the patients were treated with any preoperative therapy.
The clinical and clinicopathological parameters, and staging, were
defined according to the 2009 International Federation of
Gynecology and Obstetrics (FIGO) criteria (16). The OS was defined as the time
between surgery and mortality or the last follow-up examination,
and the follow-up periods ranged between 19 and 84 months. Informed
consent was obtained from all enrolled individuals and the present
study was approved by the Ethics Committee of The Fourth Affiliated
Hospital of Harbin Medical University. All tissue specimens were
immediately snap-frozen in liquid nitrogen and stored at −80°C
until RNA extraction.
Cell lines
A total of four human cervical cancer cell lines
(CaSki, HeLa, ME-180 and SiHa) (17) and the human immortalized cervical
epithelial cell line (NC104) were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
All cell lines were cultured in Dulbecco's modified Eagle's medium
(Hyclone; GE Health Care Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified incubator containing 5%
CO2.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cells and fresh tissue samples was
extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A
total of 500 ng RNA was reversed transcribed into cDNA using the
ReverTra Ace-α kit (Toyobo Life Science, Osaka, Japan), according
to the manufacturer's protocol. qPCR was performed using
SYBR® Premix Ex Taq™ II (Takara Biotechnology
Co., Ltd., Dalian, China) with the ABI 7900 Fast Real Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used: 95°C for 30 sec;
followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
Primers were designed and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). The following primers were used: FOXC1 sense,
5′-CTGCCCGACTACTCTCTGC-3′ antisense, 5′-CACCGAGTGGAAGTTCTGC-3′; and
GAPDH sense, 5′-CGAGATCCCTCCAAAATCAA-3′ and antisense,
5′-TTCACACCCATGACGAACAT-3′. The fold-change in FOXC1 mRNA
expression was calculated using the 2−ΔΔCq method
following normalization to GAPDH expression (18).
Immunohistochemical (IHC)
staining
IHC staining was performed as previously described
(19). Collected tissue specimens
were paraffin-embedded (Gene Company Ltd., Hong Kong, China) and
cut into 4-µm-thick sections. Following deparaffinization with
xylene, the sections were submerged into citrate antigenic
retrieval buffer (pH 6.0) at 100°C for antigenic retrieval.
Endogenous peroxidase activity was blocked with 0.3% hydrogen
peroxide, followed by incubation with goat serum (Gene Company
Ltd.) to block non-specific binding for 2 h at room temperature.
The sections were incubated with rabbit anti-human FOXC1 antibody
(cat. no. HPA040670; 1:100; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) overnight at 4°C. The slides were sequentially incubated
with the appropriate secondary antibody (cat. no. A0545; 1:2,000,
Sigma-Aldrich; Merck KGaA) for 2 h at room temperature. Labeling
was detected by adding a peroxidase-conjugated avidin-biotin
complex (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)
and 3,3′-diaminobenzidine (Dako; Agilent Technologies, Inc.). The
immunostaining signals were counterstained with hematoxylin
(Beyotime Institute of Biotechnology, Haimen, China) for 2 min. As
a negative control, PBS was used in place of primary
antibodies.
Staining scores were evaluated by three independent
pathologists who were blind to the patients under an Olympus light
microscope (BX51; Olympus Corporation, Tokyo, Japan). Expression of
FOXC1 was calculated based on the proportion of positively stained
tumor cells and the intensity of staining. The proportion of tumor
cells was scored as follows: 0, No positive tumor cells; 1, <10%
positive; 2, 10–50% positive; 3, >50% positive. The staining
extent was scored according to the area percentages as follows: 0,
No staining; 1, weak staining (light yellow); 2, moderate staining
(yellow brown); 3, strong staining (brown). The staining index was
calculated as the staining intensity score multiplied by the
proportion of positive tumor cells.
Lentiviral short hairpin (sh)RNA
transduction
In order to knock down FOXC1 expression, an shRNA
sequence targeting FOXC1 (Forward oligonucleotide,
5′CCGGGAGCTTTCGTCTACGACTGTACTCGAGTACAGTCGTAGACGAAAGCTCTTTTTG3′;
reverse oligonucleotide,
5′AATTCAAAAAGAGCTTTCGTCTACGACTGTACTCGAGTACAGTCGTAGACGAAAGCTC3′) was
designed and synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). The sequence targeting FOXC1 was subcloned into
the pLKO.1-TRC vector (Sigma-Aldrich; Merck KGaA). A scrambled
non-target shRNA (shRNA; Forward oligonucleotide,
5′CCGGAATGCCTACGTTAAGCTATACCTCGAGGTATAGCTTAACGTAGGCATTTTTTTG3′;
reverse oligonucleotide,
5′AATTCAAAAAAATGCCTACGTTAAGCTATACCTCGAGGTATAGCTTAACGTAGGCATT3′) was
used as a negative control.
To produce lentiviral particles, 8 µg
pLKO.1-shControl (scramble shRNA sequence) and pLKO.1-shFOXC1 were
transfected into 293T cells (the Type Culture Collection of the
Chinese Academy of Sciences, Shanghai, China) with lentiviral
packaging vectors psPAX2 and pMD2.G using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Lentiviral particles were harvested by
collecting media from 293T cells after 48 h. Hela and SiHa cells
were infected with lentiviral particles using 8 µg/ml Polybrene
(Sigma-Aldrich; Merck KGaA), and stable shRNA-expressing cell lines
were selected by the addition of 2 µg/ml puromycin (Sigma-Aldrich;
Merck KGaA) to the growth medium.
Cell Counting Kit-8 (CCK-8)
assays
Cell proliferation was examined using the CCK-8
assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan),
according to the manufacturer's protocol. A total of
3×103 cells were seeded into each well in 96 well
plates. A total of 10 µl CCK-8 was added into each well at 24, 48,
72 and 96 h. Following 2 h incubation, the absorbance was measured
at a wavelength of 450 nm using a spectrophotometer (Tecan Infinite
M200 Pro; Tecan Group, Ltd., Mannedorf, Switzerland).
Analysis of apoptosis
Cell apoptosis analysis was performed using Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit I (BD
Pharmingen; BD Biosciences, San Jose, CA, USA). For cell apoptosis
analysis, cells (1×104) were harvested with trypsin
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), centrifuged
at 12,000 × g for 5 min at 4°C and incubated with 5 µl Annexin
V-FITC and 5 µl propidium iodide for 15 min at 4°C in the dark.
Following staining, cell apoptosis distribution was analyzed using
a flow cytometer (Cytomics FC 500; Beckman Coulter, Inc., Brea, CA,
USA) with FlowJo (version 10; Beckman Coulter, Inc.).
Wound-healing assay
A total of 8×104 cells were seeded in a
6-well plate and incubated to form a confluent monolayer. Scratches
were made using a 200-µl pipette tip. Cells were washed with PBS
and replaced with complete medium (10% FBS). Following incubation
for 36 h, the closure of the scratch was analyzed under the
microscope and images were captured using an Olympus light
microscope (BX51; Olympus Corporation, Tokyo, Japan). Experiments
were performed in triplicate and repeated three times.
Invasion assay
For the cell invasion assay, a filter membrane with
an 8-µm pore size (EMD Millipore, Billerica, MA, USA) was coated
with Matrigel (BD Biosciences). A total of 4×104 cells
in 100 µl serum-free media were added to the upper chamber and the
aforementioned complete medium (600 µl) was added to the lower
chamber. Following incubation for 36 h, the cells that invaded
through the Matrigel membrane were fixed with 100% methanol for 30
min and stained with 0.5% crystal violet for 30 min at room
temperature. A total of six random fields of each insert were
counted using an Olympus light microscope at magnification
×200.
Western blot analysis
Cells were lysed in ice-cold
radioimmunoprecipitation assay buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA). Protein concentration was determined using
the bicinchoninic acid assay. A total of 40 µg protein/lane was
separated by SDS-PAGE on a 10% gel and transferred to a
nitrocellulose membrane (EMD Millipore). The membrane was blocked
with 5% non-fat milk in 0.1% TBS-Tween-20 at room temperature for 2
h and incubated with the following primary antibodies: Rabbit
anti-human FOXC1 antibody (1:1,000), rabbit anti-human
phosphorylated (p)-RAC-α serine/threonine-protein kinase (AKT)
antibody (Ser473; cat. no. 9271; 1:1,000; Cell Signaling
Technology, Inc.), rabbit anti-human AKT antibody (cat. no. 9272;
1:1,000; Cell Signaling Technology, Inc.), rabbit anti-human
proto-oncogene c-Myc (c-Myc) antibody (cat. no. 9402; 1:1,000; Cell
Signaling Technology, Inc.), rabbit anti-human apoptosis regulator
B cell lymphoma-2 (Bcl-2) antibody (cat. no. 2872; 1:1,000; Cell
Signaling Technology, Inc.) or rabbit anti-human GAPDH antibody
(cat. no. 2118; 1:1,000; Cell Signaling Technology, Inc.) at 4°C
overnight. The specific binding on the membrane was probed with
horseradish peroxidase-conjugated anti-rabbit secondary antibody
(cat. no. 7071; 1:1,000; Cell Signaling Technology, Inc.) for 2 h
at room temperature. Bands were visualized using a nitrotyrosine
enzyme linked immunosorbent assay kit (cat. no. 17–376; Merck
KGaA).
Statistical analysis
The paired sample t-test was used to make
comparisons between two groups and one-way analysis of variance
followed by Tukey's post-hoc test were performed to assess the
difference between >2 groups. The χ2 test was
utilized to evaluate the association between FOXC1 mRNA expression
and clinicopathological characteristics. The Kaplan-Meier estimator
and log-rank test were used to evaluate the OS of cervical cancer
patients. Data are presented as the mean ± standard deviation of
three experimental repeats and all statistical analyses were
performed using GraphPad Prism (version 5.01; GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
significant difference.
Results
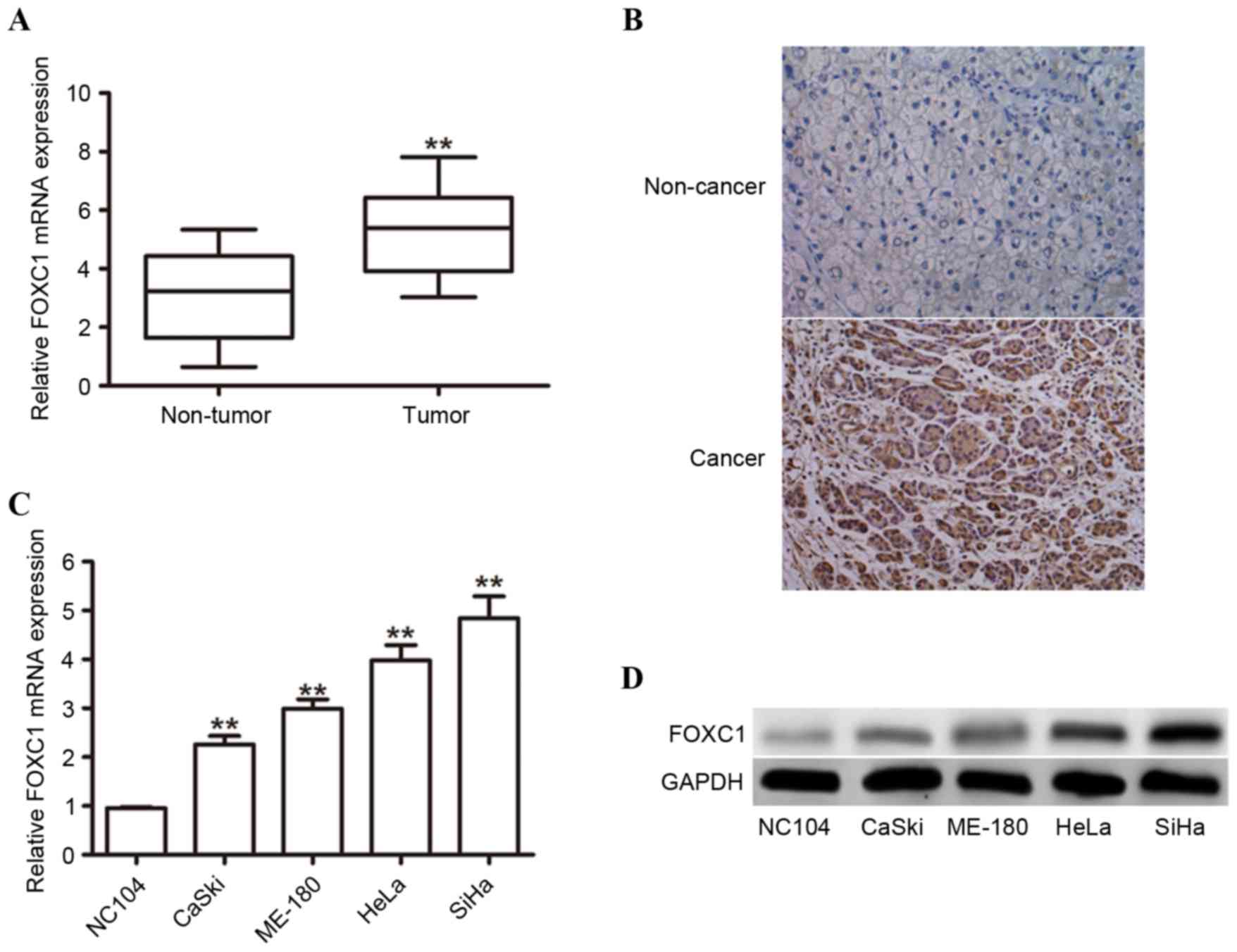
FOXC1 expression is upregulated in
human cervical cancer tissues and cell lines
To investigate the role of FOXC1 in human cervical
cancer tissues, the expression levels between 76 cervical cancer
tissues and 34 non-malignant control samples were compared using
RT-qPCR. The mRNA expression level of FOXC1 was observed to be
significantly increased in cancerous tissues compared with control
samples (Fig. 1A). IHC staining
detected FOXC1 protein in 76.3% (58/76) of paraffin-embedded
cervical cancer tissues, whereas a lower staining index was
calculated in adjacent non-cancerous tissues (Fig. 1B). The mRNA expression levels of
FOXC1 in four cervical cancer cell lines (CaSki, HeLa, ME-180 and
SiHa) and the non-malignant cell line NC104 were evaluated using
RT-qPCR. The results demonstrated that the expression levels of
FOXC1 in all four cell lines were significantly upregulated
compared with NC104 cells (Fig.
1C). In addition, western blotting was performed to confirm
that the protein expression level of FOXC1 was increased in
cervical cancer cell lines (Fig.
1D). These results suggested that FOXC1 may serve important
roles in human cervical cancer.
Association between expression of
FOXC1 and clinical characteristics in cervical cancer
To determine the clinical relevance of FOXC1 in
cervical cancer, the present study examined the association between
FOXC1 mRNA expression and various clinicopathological factors
(Table I). The median expression
level of FOXC1 was used as a cut-off point to divide all patients
into two groups: Patients who expressed FOXC1 at levels above the
cut-off value were assigned to the high expression group (n=38),
and those with expression less than the cut-off value were assigned
to the low expression group (n=38). A high FOXC1 expression level
was demonstrated to be significantly associated with lymph node
metastasis (P=0.0178) and FIGO stage (P=0.0093). However, a high
FOXC1 expression level was not associated with other
clinicopathological factors, including age, vaginal involvement,
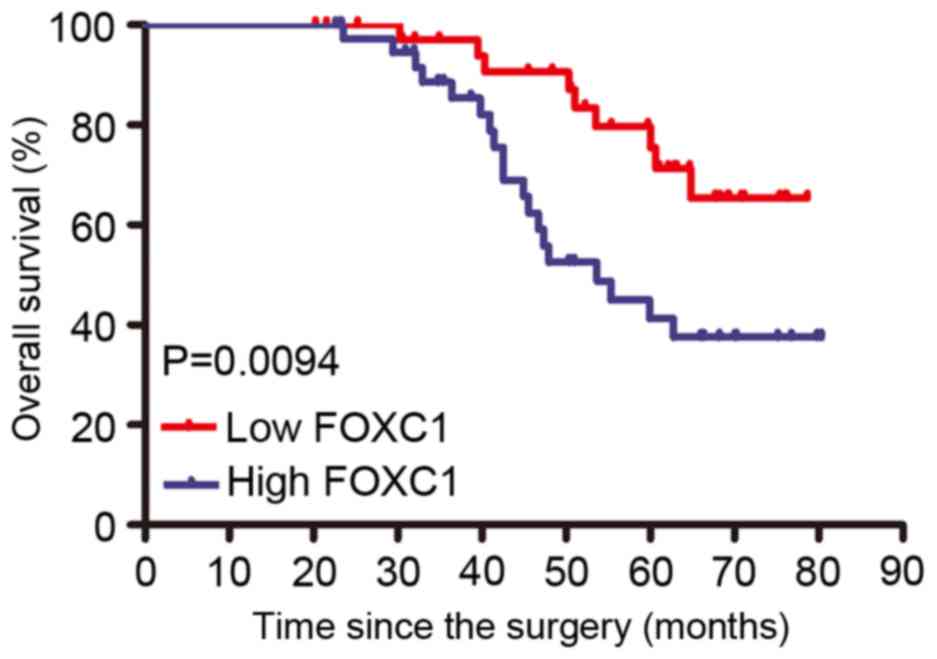
tumor histology, and tumor size. Furthermore, the Kaplan Meier
analysis revealed that patients with a high FOXC1 expression have a
poorer survival compared with those exhibiting a decreased
expression of FOXC1 (Fig. 2). The
results revealed that FOXC1 expression served as a potential
independent prognostic factor in patients with cervical cancer.
 | Table I.Association between FOXC1 expression
and clinicopathological parameters of cervical cancer. |
Table I.
Association between FOXC1 expression
and clinicopathological parameters of cervical cancer.
|
|
| FOXC1 expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Total (n=76) | High (n=38) | Low (n=38) | P-value |
|---|
| Age, years |
|
|
| 0.2055 |
| ≦45 | 22 | 8 | 14 |
|
|
>45 | 54 | 30 | 24 |
|
| FIGO stage |
|
|
| 0.0093 |
| I | 48 | 19 | 29 |
|
| II | 28 | 20 | 8 |
|
| Differentiation
grade |
|
|
| 0.3506 |
|
Well/Moderate | 45 | 20 | 25 |
|
| Poor | 31 | 18 | 13 |
|
| Tumor size (cm) |
|
|
| 0.2264 |
| ≤4 | 50 | 22 | 28 |
|
|
>4 | 26 | 16 | 10 |
|
| Lymph node
metastasis |
|
|
| 0.0178 |
| Yes | 20 | 15 | 5 |
|
| No | 56 | 23 | 33 |
|
| Vascular
involvement |
|
|
| 0.1332 |
| Yes | 23 | 15 | 8 |
|
| No | 53 | 23 | 30 |
|
| Stromal
invasion |
|
|
| 0.3801 |
|
<66% | 61 | 26 | 25 |
|
|
≧66% | 15 | 10 | 5 |
|
| Vaginal
involvement |
|
|
| 0.7361 |
|
Yes | 10 | 6 | 4 |
|
| No | 66 | 32 | 34 |
|
| Parametrial
infiltration |
|
|
| 0.6745 |
|
Yes | 6 | 4 | 2 |
|
| No | 70 | 34 | 36 |
|
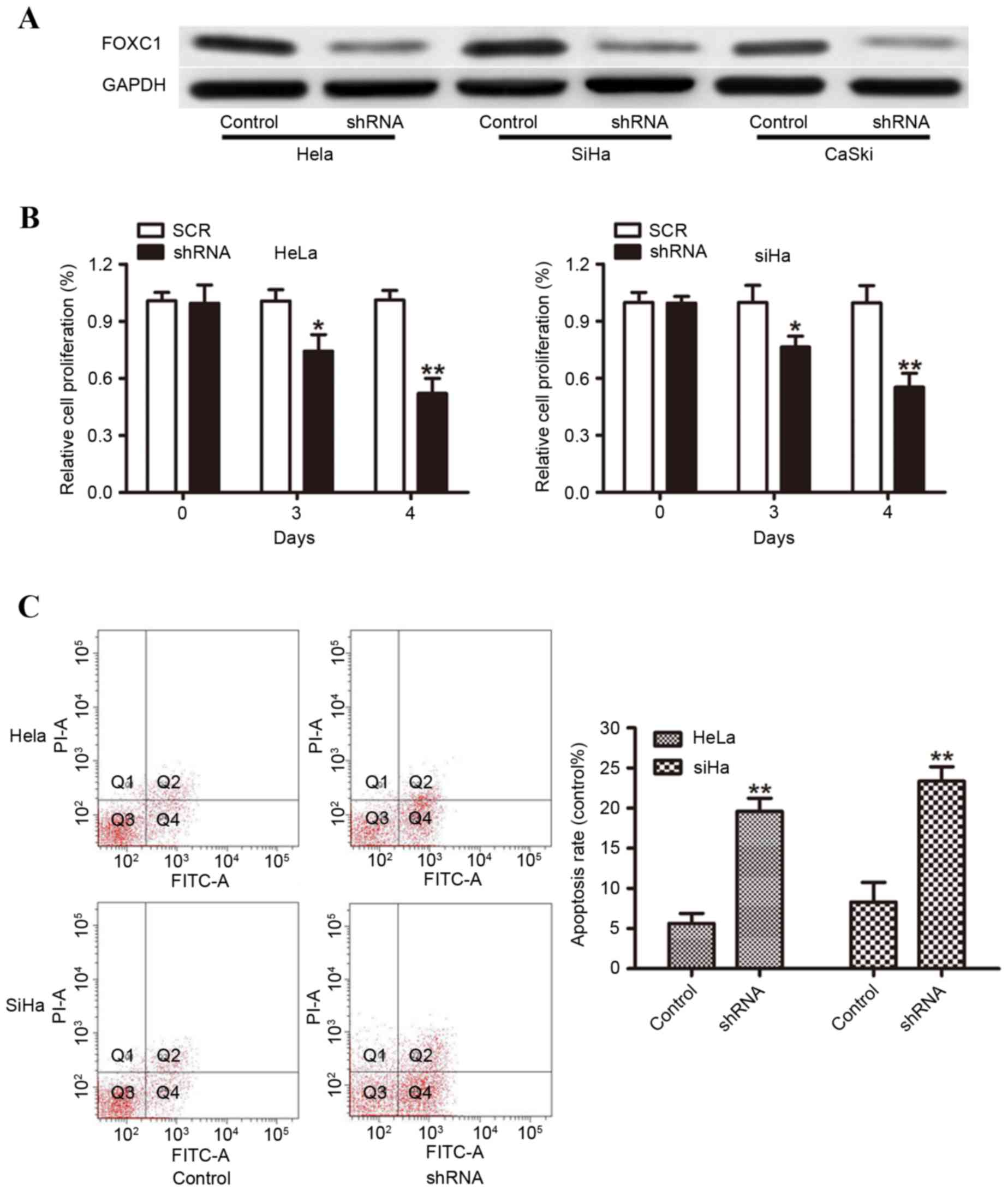
Knockdown of FOXC1 inhibits cervical
cancer cell proliferation and induces apoptosis
As FOXC1 was demonstrated to be increased in
cervical cancer tissues, the present study then knocked down FOXC1
using shRNA to investigate the biological function of FOXC1 in
vitro. Hela, SiHa and CaSki cells with high endogenous FOXC1
expression were selected for the knockdown. Western blot analysis
was performed to detect the knockdown efficiencies, and the protein
expression of FOXC1 was significantly decreased in FOXC1
shRNA-transduced cells compared with control shRNA-transduced cells
(Fig. 3A). Cellular proliferation
was assessed using a CCK-8 assay and the results demonstrated that
FOXC1 silencing resulted in a significant inhibition of Hela and
SiHa cell proliferation at 72 and 96 h (Fig. 3B). To further investigate the
effect of FOXC1 on cell survival, the present study measured cell
apoptosis using flow cytometry. As presented in Fig. 3C, knockdown of FOXC1 led to a
significant increase in apoptosis (Fig
3C). These data suggested that FOXC1 exhibited a key role in
cervical cancer cell survival.
FOXC1 silencing attenuates cell
invasion
To further evaluate the effect of FOXC1 on cervical
cancer progression, the present study detected the influence on
cervical cancer cell migration and invasion. Migration was assessed
via a wound-healing assay, whereas invasion was assessed using a
Matrigel invasion assay. FOXC1 silencing significantly delayed
wound healing of Hela, SiHa and CaSki compared with
control-transduced cells (Fig.
4A). Furthermore, FOXC1 knockdown in Hela, SiHa and CaSki cells
significantly decreased the invasion of cells through the Matrigel
basement membrane (Fig. 4B). To
study the mechanism implicated in the reduction of growth rate and
invasion in cervical cancer cell, the relative levels of proteins
that are direct or indirect targets of FOXC1 were analyzed. As
presented in Fig. 4C, the
expression of p-AKT, c-Myc and Bcl-2 protein in FOXC1
shRNA-transduced cells was decreased compared with control
shRNA-transduced cells (Fig.
4C).
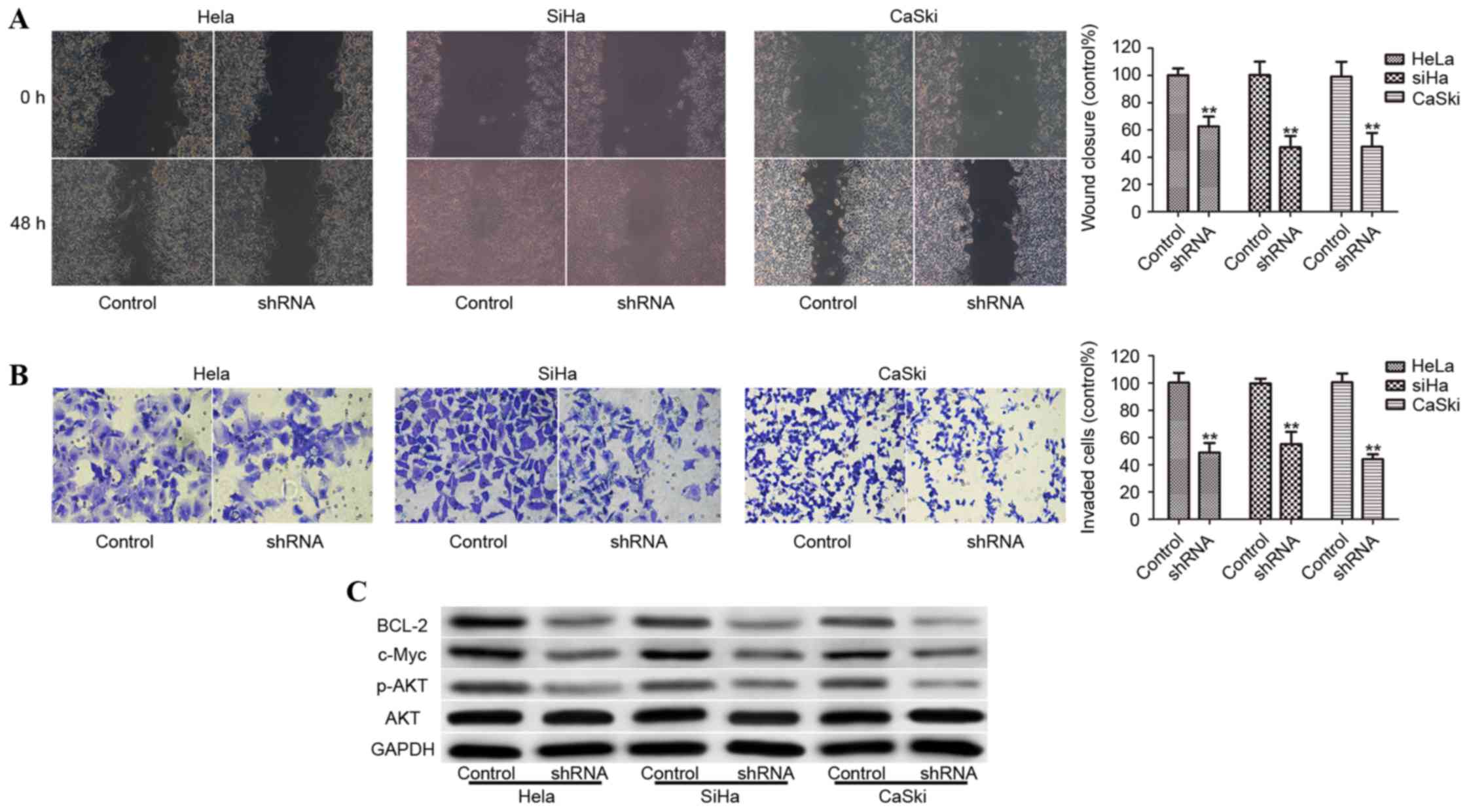 | Figure 4.Knockdown of FOXC1 decreases migratory
and invasive abilities of cervical cancer cells. (A) Wound-healing
capacity of Hela, SiHa and CaSki cells transduced with control
shRNA or FOXC1 shRNA. (B) Matrigel-coated Transwell cell invasion
assays of control cells and FOXC1 shRNA stably transduced cervical
cancer cells. (C) p-AKT, c-Myc, and Bcl-2 protein levels were
downregulated by FOXC1 shRNA in Hela, SiHa and CaSki cells.
**P<0.01 vs. shRNA control. shNRA, short hairpin RNA; FOXC1,
forkhead box protein C1; p, phosphorylated; AKT, RAC-α
serine/threonine-protein kinase (AKT); c-Myc, proto-oncogene c-Myc;
Bcl-2, apoptosis regulator B cell lymphoma-2. |
Discussion
Several reports have demonstrated that aberrant
FOXC1 expression is associated with the development and progression
of a variety of cancers, including breast, hepatocellular
carcinoma, pancreatic and non-small cell lung cancers (9,12,14,15).
However, FOXC1 expression and its potential role in cervical cancer
remains unclear. The present study aimed to explore the expression
of FOXC1 in cervical cancers compared with benign cervical tissues,
assess its association with clinicopathological parameters, and
investigate its prognostic value for cervical cancer patients.
The present study demonstrated that the mRNA level
of FOXC1 in cervical cancer tissues was significantly increased
compared with non-cancerous cervical tissues. Consistent with
previous studies, an increased FOXC1 expression was revealed to be
positively associated with lymph node metastasis, FIGO stage, and
poor prognosis (9,13,15).
It has previously been demonstrated that FOXC1 is overexpressed in
human cancer and acts as an oncogene to promote proliferation and
metastasis. A previous study suggests that non-canonical Hedgehog
signaling is mediated by FOXC1 to determine the basal-like breast
cancer stem-like phenotype and anti-Hedgehog sensitivity (19). FoxC1 has additionally been
demonstrated to act as a transcriptional factor involved in tumor
cell growth via regulating the cell cycle (12,20,21).
In accordance with the results of previous studies, the present
study demonstrated that FOXC1 silencing decreased cell
proliferation and induced cell apoptosis. The involvement of FOXC1
in regulating tumor metastasis has been previously described in
breast cancer, hepatocellular carcinoma, melanoma, nasopharyngeal
carcinoma, and non-small cell lung cancer (8,9,17,19,22).
The present study demonstrated that shRNA-mediated FOXC1
downregulation yielded a significant decrease in cell migration and
invasion. The phosphoinositide 3-kinase (PI3K)-Akt signaling
pathway has been previously demonstrated to be important in cancer
cell proliferation and invasion (23–25).
Depletion of FOXC1 in cervical cancer cells significantly reduced
expression levels of p-AKT, c-Myc and Bcl-2, which are known to
exhibit important roles in tumor progression. The data suggested
that activation of PI3K/AKT signaling pathways were involved in
FOXC1-mediated cell proliferation, migration, and invasion of
cervical cancer cells.
In conclusion, the results of the present study
demonstrated that FOXC1 was highly expressed in cervical cancer and
increased FOXC1 expression was positively associated with
metastasis, FIGO stage, and OS. Functional studies suggested that
knockdown of FOXC1 suppressed cell proliferation, migration and
invasion by regulating the AKT signaling pathway. The results
suggested that FOXC1 exhibits an important role in cervical cancer
and may act as a potential therapeutic target for future treatment
of the disease.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Heilongjiang Province, China (grant no.
H201385).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Denny L, de Sanjose S, Mutebi M, Anderson
BO, Kim J, Jeronimo J, Herrero R, Yeates K, Ginsburg O and
Sankaranarayanan R: Interventions to close the divide for women
with breast and cervical cancer between low-income and
middle-income countries and high-income countries. Lancet.
389:861–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molano M, Moreno-Acosta P, Morales N,
Burgos M, Buitrago L, Gamboa O, Alvarez R, Garland SM, Tabrizi SN,
Steenbergen RD and Mejía JC: Association between type-specific HPV
infections and hTERT DNA methylation in patients with invasive
cervical cancer. Cancer Genomics Proteomics. 13:483–491. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiffman M and Solomon D: Clinical
practice. Cervical-cancer screening with human papillomavirus and
cytologic cotesting. N Engl J Med. 369:2324–2331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muñoz N, Franceschi S, Bosetti C, Moreno
V, Herrero R, Smith JS, Shah KV, Meijer CJ and Bosch FX;
International Agency for Research on Cancer, ; Multicentric
Cervical Cancer Study Group, : Role of parity and human
papillomavirus in cervical cancer: The IARC multicentric
case-control study. Lancet. 359:1093–1101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rauh-Hain JA, Clemmer JT, Bradford LS,
Clark RM, Growdon WB, Goodman A, Boruta DM II, Schorge JO and del
Carmen MG: Racial disparities in cervical cancer survival over
time. Cancer. 119:3644–3652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sizemore ST and Keri RA: The forkhead box
transcription factor FOXC1 promotes breast cancer invasion by
inducing matrix metalloprotease 7 (MMP7) expression. J Biol Chem.
287:24631–24640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia L, Huang W, Tian D, Zhu H, Qi X, Chen
Z, Zhang Y, Hu H, Fan D, Nie Y and Wu K: Overexpression of forkhead
box C1 promotes tumor metastasis and indicates poor prognosis in
hepatocellular carcinoma. Hepatology. 57:610–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun J, Ishii M, Ting MC and Maxson R:
Foxc1 controls the growth of the murine frontal bone rudiment by
direct regulation of a Bmp response threshold of Msx2. Development.
140:1034–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin Y, Han B, Chen J, Wiedemeyer R,
Orsulic S, Bose S, Zhang X, Karlan BY, Giuliano AE, Cui Y and Cui
X: FOXC1 is a critical mediator of EGFR function in human
basal-like breast cancer. Ann Surg Oncol. 21 Suppl 4:S758–S766.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu ZY, Ding SM, Zhou L, Xie HY, Chen KJ,
Zhang W, Xing CY, Guo HJ and Zheng SS: FOXC1 contributes to
microvascular invasion in primary hepatocellular carcinoma via
regulating epithelial-mesenchymal transition. Int J Biol Sci.
8:1130–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei LX, Zhou RS, Xu HF, Wang JY and Yuan
MH: High expression of FOXC1 is associated with poor clinical
outcome in non-small cell lung cancer patients. Tumour Biol.
34:941–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song Y, Washington MK and Crawford HC:
Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal
transition in pancreatic cancer. Cancer Res. 70:2115–2125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J,
Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, et al: FOXC1 is a
potential prognostic biomarker with functional significance in
basal-like breast cancer. Cancer Res. 70:3870–3876. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saslow D, Solomon D, Lawson HW, Killackey
M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur
DC, et al: American Cancer Society, American Society for Colposcopy
and Cervical Pathology, and American Society for Clinical Pathology
screening guidelines for the prevention and earlydetection of
cervical cancer. Am J Clin Pathol. 137:516–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu T, Chen X, Peng R, Liu H, Yin P, Peng
H, Zhou Y, Sun Y, Wen L, Yi H, et al: Let7a suppresses cell
proliferation via the TGF-β/SMAD signaling pathway in cervical
cancer. Oncol Rep. 36:3275–3282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ou-Yang L, Xiao SJ, Liu P, Yi SJ, Zhang
XL, Ou-Yang S, Tan SK and Lei X: Forkhead box C1 induces
epithelial-mesenchymal transition and is a potential therapeutic
target in nasopharyngeal carcinoma. Mol Med Rep. 12:8003–8009.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han B, Qu Y, Jin Y, Yu Y, Deng N,
Wawrowsky K, Zhang X, Li N, Bose S, Wang Q, et al: FOXC1 activates
smoothened-independent hedgehog signaling in basal-like breast
cancer. Cell Rep. 13:1046–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Jiao S, Jia Y and Li Y: Effects of
targeted silencing of FOXC1 gene on proliferation and in vitro
migration of human non-small-cell lung carcinoma cells. Am J Transl
Res. 8:3309–3318. 2016.PubMed/NCBI
|
|
22
|
Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP,
Lee AV, Lin X, Bagaria SP, Giuliano AE and Cui X: FOXC1 regulates
the functions of human basal-like breast cancer cells by activating
NF-κB signaling. Oncogene. 31:4798–4802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Li L, Liu S, Zhao Y, Wang L and Du
G: FOXC1 promotes melanoma by activating MST1R/PI3K/AKT.
Oncotarget. 7:84375–84387. 2016.PubMed/NCBI
|
|
24
|
Wang Z, Qu L, Deng B, Sun X, Wu S, Liao J,
Fan J and Peng Z: STYK1 promotes epithelial-mesenchymal transition
and tumor metastasis in human hepatocellular carcinoma through
MEK/ERK and PI3K/AKT signaling. Sci Rep. 6:332052016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Qin G, Luo M, Chen J, Zhang Q, Li
L, Pan L and Qin S: Reciprocal positive regulation between Cx26 and
PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC
cells via GJIC-independent induction of EMT. Cell Death Dis.
6:e18292015. View Article : Google Scholar : PubMed/NCBI
|