Introduction
Cervical cancer is one of the most common
malignancies in woman worldwide, which occurs and develops on the
surface of the cervix (1). A total
of >510,000 patients are diagnosed with cervical cancer, and
>288,000 patients die from the disease each year worldwide
(1). The primary cause of cervical
cancer is infection with human papillomavirus (HPV) (2). Although vaccines to high-risk human
HPV (hrHPV) strains may prevent infection with hrHPV, cervical
cancer remains a primary cause of cancer-associated mortality,
particularly in under-developed countries (3,4).
Tumor metastasis is the primary cause of
cancer-associated death. Numerous studies have suggested that
epithelial mesenchymal transition (EMT) contributes to migration,
invasion and metastasis of cancer (5,6).
During the EMT process, epithelial cells undergo a cell morphology
alteration from the polarized epithelial phenotype to mesenchymal
phenotype, loss of cell-cell junction and epithelial markers
including E-cadherin, acquirement of mesenchymal markers
N-cadherin, vimentin and febronectin, and cytoskeleton
rearrangement, which leads to gain of mesenchymal traits including
cell migration and invasion (7,8). The
EMT process is regulated by several transcription factors including
zinc finger E-box-binding homeobox 1 (Zeb1), snail1, snail2 (Slug)
and Twist, which repress the expression of E-cadherin and other
cell adhesion and cytokeratin genes, and increase expression of
mesenchymal markers vimentin, fibronectin and N-cadherin (9). Several signaling pathways are
activated during EMT, including nuclear factor (NF)-κB, glycogen
synthase kinase (GSK)3β, Notch, protein kinase B (Akt) and mitogen
activated protein kinase (MAPK), which have been demonstrated to
promote metastasis of lung, breast, ovarian and prostate cancers
(10–13). In addition, EMT is involved in
anti-cancer drug resistance (14).
Therefore, EMT is a target of cancer therapy, particularly in
metastasized cancer types.
Currently, chemotherapy and radiotherapy are the
principal therapeutic strategies for cervical cancer. However,
these two therapeutic approaches have severe side effects including
headache, abnormal respiration, cirrhosis and cardiac dysfunction.
There is therefore increasing interest in the study of safe and
effective anti-cancer compounds from natural plants (15). Ginseng is considered as a panacea
and is frequently used in traditional Chinese medicine (16,17).
Ginsenosides are one of the primary bioactive ingredients in
ginseng, which have various pharmacological effects including
anti-obesity, anti-diabetic, regulation of blood pressure,
anti-aging, immune regulation and anti-tumor functions (18–22).
Ginsenosides are classified into three types, including
protopanaxatriol-type, oleanolic acid-type and protopanaxadiol-type
ginsenosides (23). Ginsenoside
20(S)-Rh2 (GRh2) belongs to the protopanaxadiol-type ginsenoside
group. Several studies have reported that GRh2 exhibits anticancer
activities in glioblastoma, skin squamous cell carcinoma,
pancreatic cancer, prostatic cancer and leukemia (24–28).
A previous study used GRh2 to inhibit cancer cell proliferation and
induce transformation to normal cells in patients with cervical
cancer (29). However, the effect
of GRh2 on cervical cancer, the EMT process and its underlying
mechanism remains unclear.
In the present study, it was demonstrated that GRh2
effectively inhibited proliferation, migration, invasion and EMT by
targeting the Akt/GSK3β signaling pathway in human cervical cancer
cells. Therefore, the results suggested that GRh2 may have the
potential to be a novel anti-cancer agent for cervical cancer.
Materials and methods
Reagents and antibodies
Commercial GRh2 was obtained from Sichuan Weike
Biotechnology Co., Ltd. (Chengdu, China). GRh2 was prepared in a
stock of 100 mg/ml and applied to cultured HeLa cells at 10, 25 and
50 µM. Antibodies for N-cadherin (cat. no. 13116), E-cadherin (cat.
no. 3195), vimentin (cat. no. 5741), Zeb1 (cat. no. 3396), snail1
(cat. no. 3879), phosphorylated (p)-Akt (cat. no. 4060), Akt (cat.
no. 4691), p-GSK3β (cat. no. 9327), GSK3β (cat. no. 5676) and
β-actin (cat. no. 4970) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Cell culture
Human cervical carcinoma HeLa cells were purchased
from Shanghai Institute of Pharmaceutical Industry (Shanghai,
China). Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
streptomycin and penicillin at 37°C in an environment containing 5%
CO2. DMEM and FBS were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA).
Cell proliferation assay
Cell proliferation was detected by 3-(4,
5-dimethylthiazol-2-yl)-5
3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)
assay. 1×103/ml HeLa were seeded into each well of a
96-well microplate. Then, cells were incubated with media
containing normal saline (control) or different concentrations of
GRh2 (10, 25 and 50 µM) respectively. Cells were incubated for 6,
24, 48, 72, 96 and 120 h and then incubated with 10 µl MTS reagent
(Promega Corporation, Madison, WI, USA) at 37°C for 4 h according
to the manufacturer's protocol. The absorbance was measured at a
wavelength of 490 nm using a microplate reader. Proliferation
inhibition rates were determined following treatment for 120 h. %
proliferation inhibition rate=(1-drug group/control group)
×100.
Transwell invasion assays
For the matrigel assay, 5×104 HeLa cells
at the logarithmic growth phase were supplemented with 100 µl
serum-free DMEM and seeded into the upper chamber of a transwell
plate with 8-mm pore size (BD Biosciences, Franklin Lakes, NJ,
USA). The upper side of the filter member of the chamber was coated
with Matrigel (BD Biosciences) that was diluted with DMEM media
(1:3). A total of 600 µl DMEM media supplemented with 1% FBS was
added in the lower chamber, and GRh2 at a concentration of 0 µM
(control) or 10, 25 and 50 µM was added into the upper chamber.
Following incubation for 24 h at 37°C, the cells in the upper
membrane were discarded and cells on the lower membrane were fixed
using 4% paraformaldehyde for 30 min and stained with crystal
violet (Beyotime Institute of Biotechnology, Haimen, China) for 10
min at room temperature. Next, five random fields were captured
with a phase contrast video microscope (ELWD 0.3; Nikon
Corporation, Tokyo, Japan; magnification, ×40) and then the cells
were counted. Each experiment was performed in triplicate.
Wound healing assay
For wound healing assay, 3×105 HeLa cells
at the logarithmic growth phase were seeded into 6-well plates. At
80% confluence, cells were incubated with GRh2 at a concentration
of 0 µM (control), or 10, 25 and 50 µM, and the monolayer was
disrupted with a cell scraper and captured at 0 and 48 h with a
phase contrast video microscope (model ELWD 0.3; Nikon Corporation,
Tokyo, Japan; magnification, ×100). Each experiment was performed
in triplicate. The percentage of wound closure between the wound
edges at different time points was measured with Image-Pro Plus
version 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
% wound closure=(1-distance at 0 h/distance at 48 h) ×100.
Protein extraction and western
blotting
For detection of N-cadherin, E-cadherin, vimentin,
zeb1 and snail1 expression, HeLa cells were treated with GRh2 at a
concentration of 0 µM (control), or 25, and 50 µM for 48 h at 37°C
in an environment containing 5% CO2. To investigate the
activity of the Akt and GSK3β signaling pathway, HeLa cells were
treated with GRh2 at the concentration of 0 µM (control) or 25 and
50 µM for 12 h at 37°C in an environment containing 5%
CO2. Cells were lysed using a radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology) supplemented
with complete EDTA-free protease inhibitor cocktail tablets (Roche
Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's protocol. Total protein concentrations were
determined with the bicinchoninic acid protein assay kit (Applygen
Technologies Inc., Beijing, China). A total of 40 µg protein in
each sample was loaded onto 8% SDS-PAGE gels, and transferred onto
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Following blocking with 5% nonfat milk for 30
min at room temperature, the membranes were incubated with primary
antibodies including N-cadherin (1:1,000 dilution), E-cadherin
(1:1,000 dilution), vimentin (1:1,000 dilution), Zeb1 (1:1,000
dilution), snail1 (1:1,000 dilution), phosphorylated (p)-Akt
(1:2,000 dilution), Akt (1:1,000 dilution), p-GSK3β (1:1,000
dilution), GSK3β (1:1,000 dilution) and β-actin (1:1,000 dilution)
at 4°C overnight. The membranes were next incubated with
horseradish peroxidase-conjugated anti-rabbit IgG (cat. no. A0545;
1:5,000 dilution; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 1 h at room temperature. Signals were visualized using enhanced
chemiluminescence reagent (EMD Millipore, Billerica, USA) and
protein expression levels normalized to β-actin were calculated by
using ImageJ software (version 1.48; National Institutes of Health,
Bethesda, MD, USA). Detection of the protein of interest was
repeated three times. The quantification of the western blots
presented in the figures are representative of three independent
experiments.
Statistical analysis
All tests were performed in triplicate and the
experiments were repeated three times independently. Data was
expressed as mean ± standard deviation. A one-way analysis of
variance followed by Tukey's post hoc test was conducted to assess
differences among multiple groups. All statistical calculations
were carried out using SPSS software, version 19.0 (IBM Corp.,
Armonk, NY, USA) and P<0.05 was considered to indicate a
statistically significant difference.
Results
GRh2 inhibits cell viability and
proliferation of cervical cancer
To evaluate the function of GRh2 on cervical cancer
cells, HeLa cells were exposed to various concentrations of GRh2
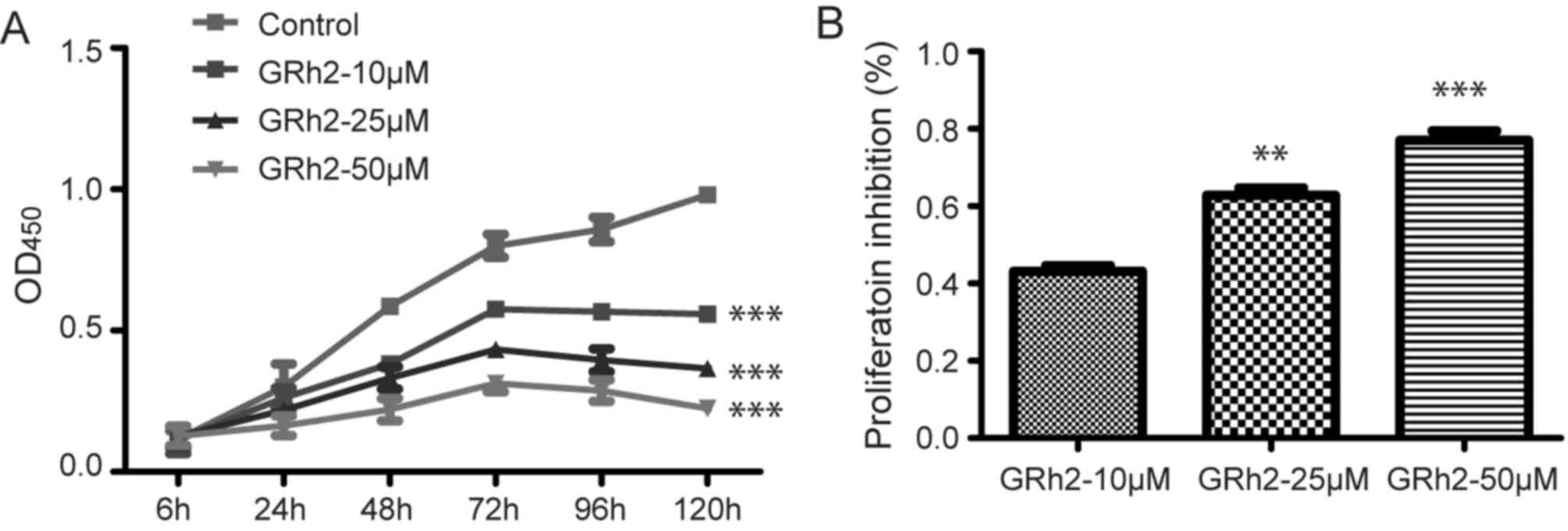
and the cell proliferation was assessed. Compared with the control
group, GRh2 treatments inhibited HeLa cell proliferation in a dose-
and time-dependent manner (P<0.001; Fig. 1A). Compared with the control group,
proliferation inhibition rates were 40, 60 and 80% at GRh2
concentrations of 10, 25 and 50 µM, respectively, at 120 h
(Fig. 1B). These results suggested
that GRh2 inhibited the proliferation of cervical cancer cells.
GRh2 inhibits cell migration and
invasion of cervical cancer
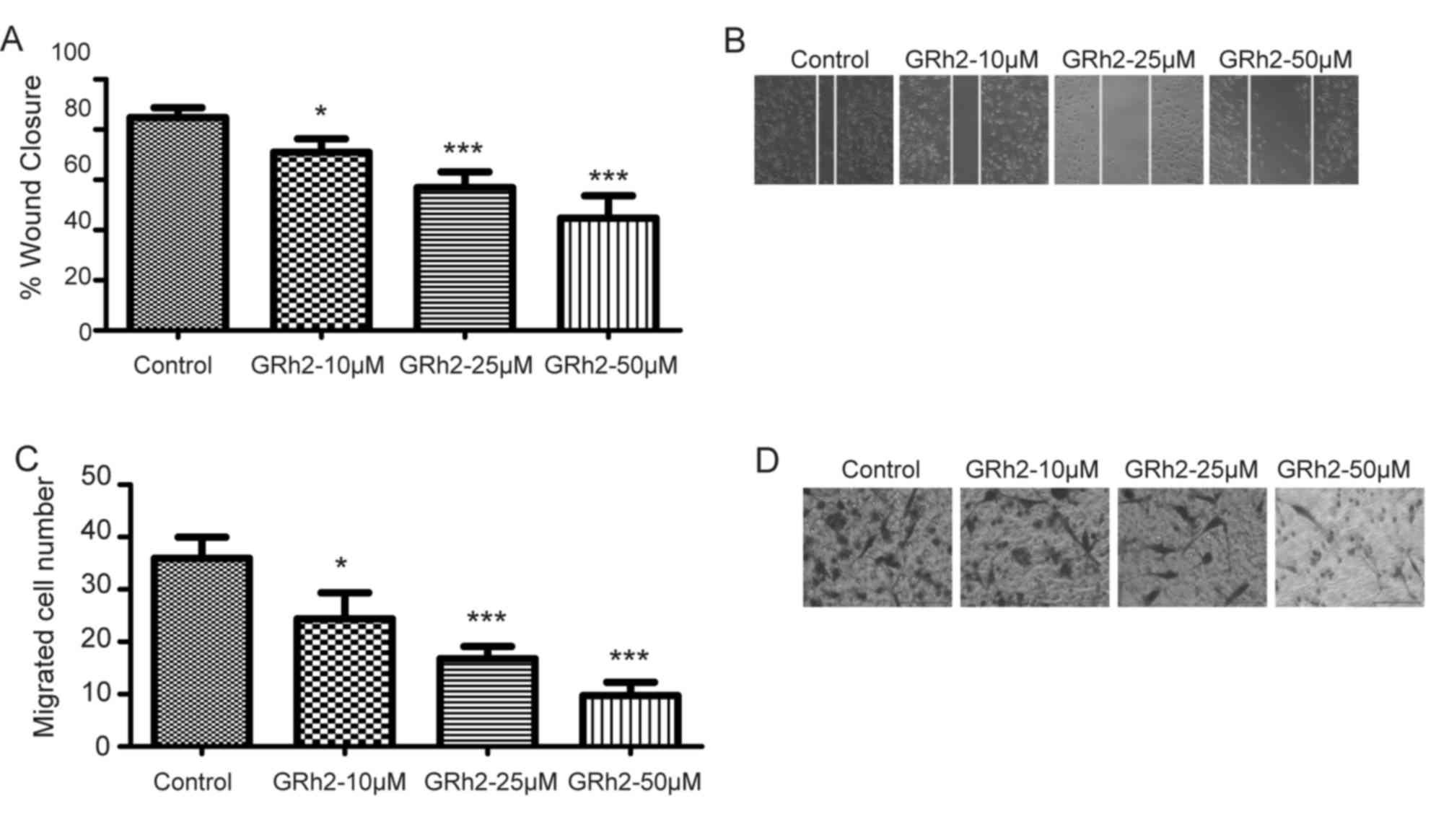
To ascertain the role of GRh2 in cervical cancer
cell migration, wound healing and transwell invasion assays were
performed in Hela cells treated with GRh2 at concentrations of 0 µM
(control), or 10, 25 and 50 µM. The migration abilities were
inhibited in HeLa cells treated with GRh2 in a dose-dependent
manner (P<0.05 vs. control; Fig. 2A
and B). Similar inhibitory effects were observed when detecting
the invasion ability of HeLa cells by a Matrigel-coated transwell
invasion assay (P<0.05 vs. control; Fig. 2C and D). These results suggested
that GRh2 inhibited cell migration and invasion of cervical
cancer.
GRh2 inhibits epithelial to
mesenchymal transition of cervical cancer
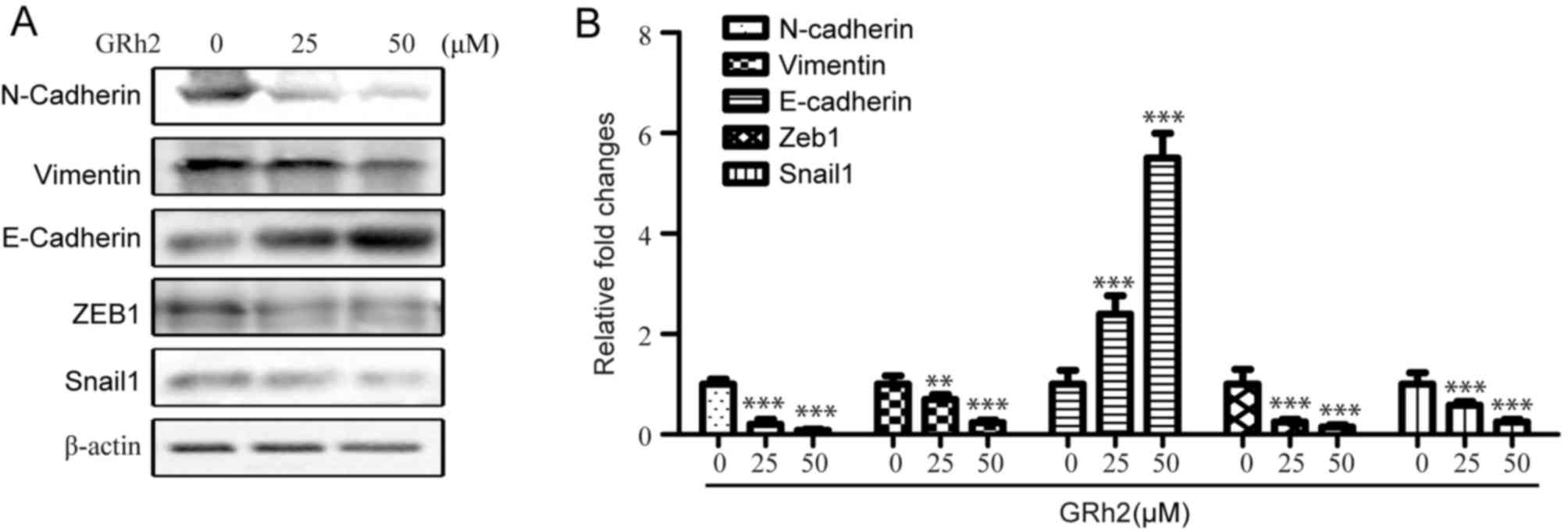
To investigate whether GRh2 inhibits the EMT
process, EMT markers were detected in HeLa cells treated with
different concentrations of GRh2 (0, 25 and 50 µM) for 48 h. As
expected, GRh2 treatment decreased the expression levels of
N-cadherin and vimentin, and increased the protein expression
levels of epithelial marker E-cadherin (Fig. 3A and B). The effects of GRh2 on the
expression levels of EMT control transcription factors, Zeb1 and
snail1, were also determined. The results revealed a downregulation
in the expression levels of Zeb1 and snail1 in the GRh2-treated
groups (Fig. 3A and B). These data
suggested that GRh2 effectively inhibited EMT in a dose-dependent
manner in cervical cancer cells.
GRh2 inhibits the Akt signaling
pathway in cervical cancer cells
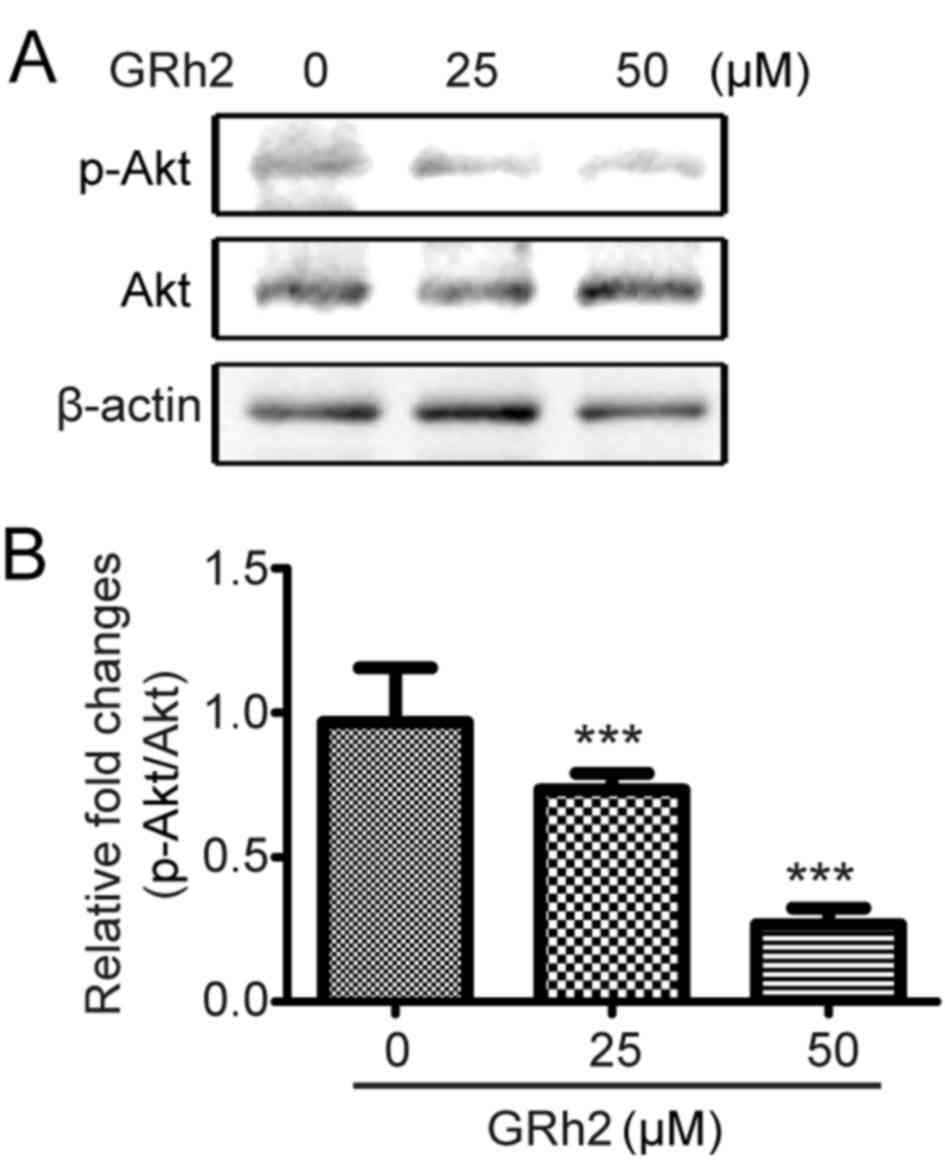
The effect of GRh2 on the Akt signaling pathway was
investigated. HeLa cells were treated with different concentrations
of GRh2 (0, 10, 25 and 50 µM) for 12 h. The phosphorylation and
protein expression levels of Akt were then detected. GRh2 treatment
significantly inhibited the phosphorylation of Akt compared with
cells treated with 0 µM GRh2 (Fig. 4A
and B), which suggested that GRh2 inhibited the activation of
the Akt pathway.
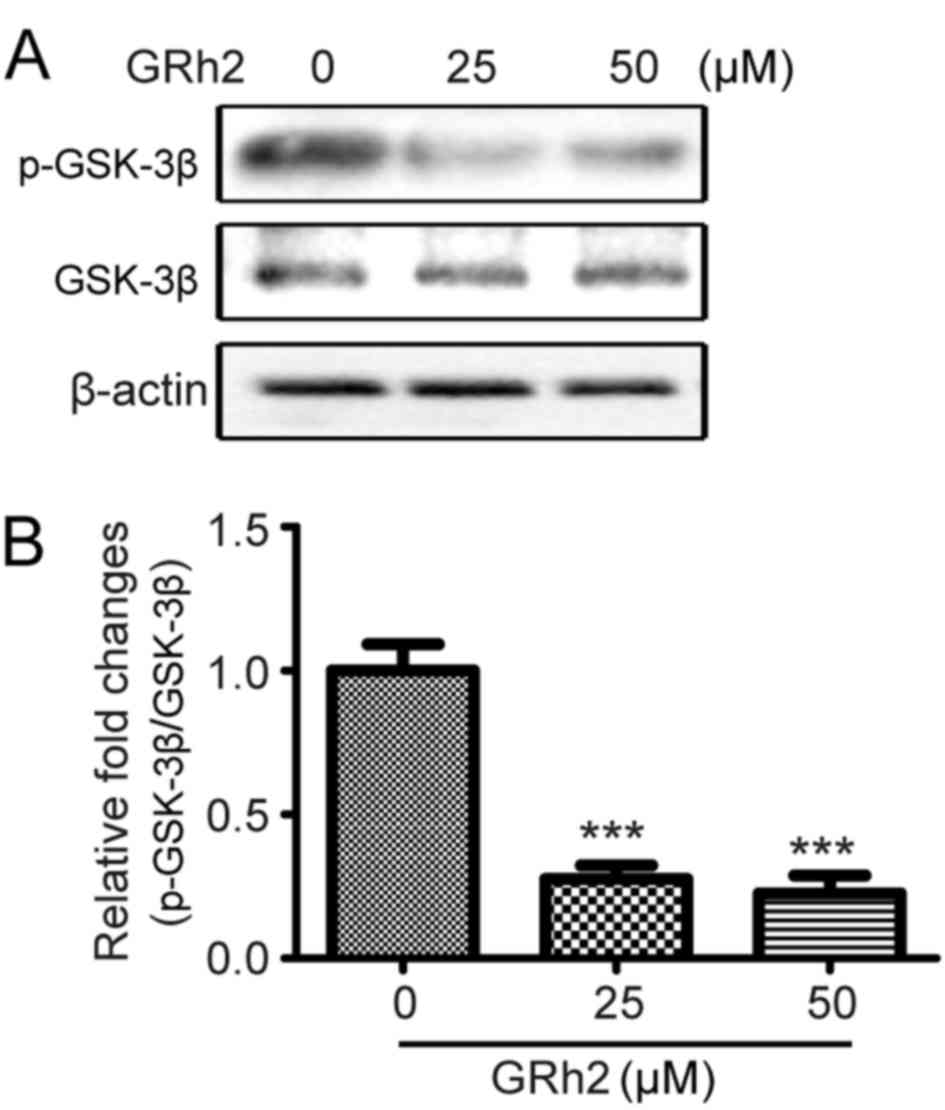
GRh2 inhibits the GSK3β signaling
pathway in cervical cancer cells
GSK3β is a downstream pathway of Akt. It has been
reported that the Akt/GSK3β pathway regulates EMT (30). Therefore, the effect of GRh2 on the
GSK3β pathway was investigated. HeLa cells were treated with
different concentrations of GRh2 (0, 10, 25 and 50 µM) for 12 h.
The activation of GSK3β was then detected. GRh2 treatment
significantly suppressed the phosphorylation of GSK3β compared with
cells treated with 0 µM GRh2 (Fig. 5A
and B), which suggested that GRh2 inhibited the activation of
the Akt/GSK3β pathway.
Discussion
Currently, cancer is one of the most common diseases
that leads to mortality in humans (31). Chemotherapy and radiotherapy are
the primary clinical therapeutic approaches. However, a limitation
of these treatment strategies is toxicity to normal cells in
addition to the tumor cells. The identification of safe and
efficacious anticancer drugs is of primary concern (32). Natural products are the primary
source for the development of anti-cancer agents (33). The anticancer activities of Panax
ginseng have been demonstrated by several studies (34,35).
Ginsenosides, a group of bioactive compounds of Panax ginseng,
exhibit anticancer activities (36,37).
GRh2 has been demonstrated to inhibit cancer cell proliferation,
migration and invasion, and prevent tumor growth and metastasis
(24–28). However, only a small number of
studies have explored the effects of GRh2 in human cervical
cancer.
In the present study, the effect of GRh2 on cell
proliferation of human cervical cancer cells was investigated. The
results revealed that GRh2 impaired HeLa cell proliferation in a
dose- and time-dependent manner. These findings are in accordance
with previous reports that demonstrated that ginseng Rh2 prevents
cell proliferation in colon, lung, liver, and gastric cancers, in
addition to leukemia and glioblastoma (38–42).
In addition, it was observed that GRh2 inhibited the activation of
the Akt pathway in HeLa cells. Akt mediates various biological
processes, including glucose metabolism, cell survival,
proliferation and differentiation (43,44).
Collectively, the results of the present study, and previous
studies, suggest that GRh2 may inhibit human cervical cancer cell
proliferation via impairment of the Akt signaling pathway.
Furthermore, the present study revealed that GRh2
decreased the migration and invasion of human cervical cancer
cells, which concurred with previous reports that Rh2 inhibits cell
migration and invasion in C2C12 skeletal muscle cells (45,46).
EMT is an important cellular process that promotes tumor
progression and metastasis (47).
To ascertain the underlying molecular mechanism of GRh2 inhibition
of cervical cancer migration and invasion, the present study
investigated the effects of GRh2 on EMT. The results demonstrated
that Rh2 treatment increased the expression levels of epithelial
marker E-cadherin and decreased the expression levels of
mesenchymal N-cadherin and vimentin, in addition to EMT-control
transcription factors Zeb1 and snail1. In addition, GRh2 treatment
impaired the activation of Akt and the phosphorylation of GSK3β.
Aberrant activation of the Akt signaling pathway was observed
during tumor proliferation and metastasis as well as during the
progression of EMT (45,48,49).
GSK3β is activated when it is dephosphorylated. The inhibition of
Akt pathway can activateGSK3β by preventing its phosphorylation
(50,51). The activation of GSK3β induces the
degradation of Snail. As a transcriptional factor, Snail inhibits
the expression of epithelial marker E-cadherin and activates the
transcription of mesenchymal N-cadherin and vimentin (47,52–54).
Thus, degradation of Snail increases the expression of epithelial
marker E-cadherin and decreases the expression of mesenchymal
N-cadherin and vimentin. Accordingly, the results of the present
study demonstrated that GRh2 inhibited AKT pathway,
dephosphorylated and activated GSK3β and inhibited the expression
of Snail, which lead to upregulation of E-cadherin and
downregulation of N-cadherin and vimentin. Taken together with the
data from the present study and previous observations, it is
suggested that GRh2 inhibited the expression of Snail and prevented
EMT by inhibiting AKT and activating GSK3β.
In conclusion, the present study demonstrated that
GRh2 inhibits cell proliferation of cervical cancer by suppressing
the Akt signaling pathway. In addition, the data suggested that
GRh2 prevents cell migration, invasion and EMT via inhibition of
the Akt/GSK3β signaling pathway. Therefore, these findings
suggested that GRh2 may be a potential treatment strategy for
cervical cancer treatment.
References
|
1
|
Saslow D, Castle PE, Cox JT, Davey DD,
Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscicki
AB, et al: American cancer society guideline for human
papillomavirus (HPV) vaccine use to prevent cervical cancer and its
precursors. CA Cancer J Clin. 57:7–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steben M and Duarte-Franco E: Human
papillomavirus infection: Epidemiology and pathophysiology. Gynecol
Oncol. 107 2 Suppl 1:S2–S5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ginsburg O, Bray F, Coleman MP, Vanderpuye
V, Eniu A, Kotha SR, Sarker M, Huong TT, Allemani C, Dvaladze A, et
al: The global burden of women's cancers: A grand challenge in
global health. Lancet. 389:847–860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang A, Farmer E, Wu TC and Hung CF:
Perspectives for therapeutic HPV vaccine development. J Biomed Sci.
23:752016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng WY, Kandel JJ, Yamashiro DJ, Canoll
P and Anastassiou D: A multi-cancer mesenchymal transition gene
expression signature is associated with prolonged time to
recurrence in glioblastoma. PLoS One. 7:e347052012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian L, Shen D, Li X, Shan X, Wang X, Yan
Q and Liu J: Ginsenoside Rg3 inhibits epithelial-mesenchymal
transition (EMT) and invasion of lung cancer by down-regulating
FUT4. Oncotarget. 7:1619–1632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. BR
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaowinn S, Kim J, Lee J, Shin DH, Kang CD,
Kim DK, Lee S, Kang MK, Koh SS, Kim SJ and Chung YH: Cancer
upregulated gene 2 induces epithelial-mesenchymal transition of
human lung cancer cells via TGF-β signaling. Oncotarget.
8:5092–5110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J, Byun HJ, Lee MS, Jin YJ, Jeoung D,
Kim YM and Lee H: The metastasis suppressor CD82/KAI1 inhibits
fibronectin adhesion-induced epithelial-to-mesenchymal transition
in prostate cancer cells by repressing the associated integrin
signaling. Oncotarget. 8:1641–1654. 2017.PubMed/NCBI
|
|
12
|
Lu L, Wang J, Wu Y, Wan P and Yang G:
Rap1A promotes ovarian cancer metastasis via activation of ERK/p38
and notch signaling. Cancer Med. 5:3544–3554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ci Y, Qiao J and Han M: Molecular
mechanisms and metabolomics of natural polyphenols interfering with
breast cancer metastasis. Molecules. 21:E16342016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:E9652016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim HK, Bae W, Lee HS and Jung J:
Anticancer activity of marine sponge Hyrtios sp. extract in human
colorectal carcinoma RKO cells with different p53 status. BioMed
Res Int. 2014:4135752014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bent S and Ko R: Commonly used herbal
medicines in the United States: A review. Am J Med. 116:478–485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nocerino E, Amato M and Izzo AA: The
aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 71
Suppl 1:S1–S5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong H, Bai LP, Wong VK, Zhou H, Wang JR,
Liu Y, Jiang ZH and Liu L: The in vitro structure-related
anti-cancer activity of ginsenosides and their derivatives.
Molecules. 16:10619–10630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ,
Kim MJ, Kim HS, Ha J, Kim MS and Kwon DY: Anti-obesity effects of
ginsenoside Rh2 are associated with the activation of AMPK
signaling pathway in 3T3-L1 adipocyte. Biochemical Biophys Res
Commun. 364:1002–1008. 2007. View Article : Google Scholar
|
|
21
|
Jovanovski E, Bateman EA, Bhardwaj J,
Fairgrieve C, Mucalo I, Jenkins AL and Vuksan V: Effect of
Rg3-enriched Korean red ginseng (Panax ginseng) on arterial
stiffness and blood pressure in healthy individuals: A randomized
controlled trial. J Am Soc Hypertens. 8:537–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim J, Han BJ, Kim H, Lee JY, Joo I, Omer
S, Kim YS and Han Y: Th1 immunity induction by ginsenoside Re
involves in protection of mice against disseminated candidiasis due
to Candida albicans. Int Immunopharmacol. 14:481–486. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh SJ, Lee S, Choi WY and Lim CJ: Skin
anti-photoaging properties of ginsenoside Rh2 epimers in
UV-B-irradiated human keratinocyte cells. J Biosci. 39:673–682.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Gao Y, Ma W, Guo W, Zhou G, Cheng T
and Liu Y: EGFR signaling-dependent inhibition of glioblastoma
growth by ginsenoside Rh2. Tumour Biol. 35:5593–5598. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang XP, Tang GD, Fang CY, Liang ZH and
Zhang LY: Effects of ginsenoside Rh2 on growth and migration of
pancreatic cancer cells. World J Gastroenterol. 19:1582–1592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung KS, Cho SH, Shin JS, Kim DH, Choi
JH, Choi SY, Rhee YK, Hong HD and Lee KT: Ginsenoside Rh2 induces
cell cycle arrest and differentiation in human leukemia cells by
upregulating TGF-β expression. Carcinogenesis. 34:331–340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Q, Hong B, Wu S and Niu T:
Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour
Biol. 36:2377–2381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Chen M, Li P, Wu Y, Chang C, Qiu Y,
Cao L, Liu Z and Jia C: Ginsenoside rh2 inhibits cancer stem-like
cells in skin squamous cell carcinoma. Cell Physiol Biochem.
36:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Li Y, Wang X, Fang X, He K, Guo X,
Zhan Z, Sun C and Jin YH: Co-treatment with ginsenoside Rh2 and
betulinic acid synergistically induces apoptosis in human cancer
cells in association with enhanced capsase-8 activation, bax
translocation and cytochrome c release. Mol Carcinog. 50:760–769.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo H, Luo H, Yuan H, Xia Y, Shu P, Huang
X, Lu Y, Liu X, Keller ET, Sun D, et al: Litchi seed extracts
diminish prostate cancer progression via induction of apoptosis and
attenuation of EMT through Akt/GSK-3β signaling. Sci Rep.
7:416562017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toh DF, Patel DN, Chan EC, Teo A, Neo SY
and Koh HL: Anti-proliferative effects of raw and steamed extracts
of Panax notoginseng and its ginsenoside constituents on human
liver cancer cells. Chin Med. 6:42011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang ZG, Sun HX and Ye YP: Ginsenoside Rd
from Panax notoginseng is cytotoxic towards HeLa cancer cells and
induces apoptosis. Chem Biodivers. 3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Unlu A, Nayir E, Kirca O, Ay H and Ozdogan
M: Ginseng and cancer. J BUON. 21:1383–1387. 2016.PubMed/NCBI
|
|
35
|
Hafez MM, Hamed SS, El-Khadragy MF, Hassan
ZK, Al Rejaie SS, Sayed-Ahmed MM, Al-Harbi NO, Al-Hosaini KA,
Al-Harbi MM, Alhoshani AR, et al: Effect of ginseng extract on the
TGFβ1 signaling pathway in CCl4-induced liver fibrosis in rats. BMC
Complement Altern Med. 17:452017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng X, Chen W, Hou H, Li J, Li H, Sun X,
Zhao L and Li X: Ginsenoside 20(S)-Rg3 induced autophagy to inhibit
migration and invasion of ovarian cancer. Biomed Pharmacother.
85:620–626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lahiani MH, Eassa S, Parnell C, Nima Z,
Ghosh A, Biris AS and Khodakovskaya MV: Carbon nanotubes as
carriers of Panax ginseng metabolites and enhancers of ginsenosides
Rb1 and Rg1 anti-cancer activity. Nanotechnology. 28:0151012017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Yuan D, Xing T, Su H, Zhang S, Wen
J, Bai Q and Dang D: Ginsenoside Rh2 inhibiting HCT116 colon cancer
cell proliferation through blocking PDZ-binding kinase/T-LAK
cell-originated protein kinase. J Ginseng Res. 40:400–408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Sun Y, Yue L, Li S, Qi X, Zhao H,
Yang Y, Zhang C and Yu H: JNK pathway and relative transcriptional
factor were involved in ginsenoside Rh2-mediated G1 growth arrest
and apoptosis in human lung adenocarcinoma A549 cells. Genet Mol
Res. 15:2016. View Article : Google Scholar
|
|
40
|
Shi Q, Shi X, Zuo G, Xiong W, Li H, Guo P,
Wang F, Chen Y, Li J and Chen DL: Anticancer effect of
20(S)-ginsenoside Rh2 on HepG2 liver carcinoma cells: Activating
GSK-3β and degrading beta-catenin. Oncol Rep. 36:2059–2070. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qian J, Li J, Jia JG, Jin X, Yu DJ, Guo
CX, Xie B and Qian LY: Ginsenoside-Rh2 inhibits proliferation and
induces apoptosis of human gastric cancer SGC-7901 side population
cells. Asian Pac J Cancer Prev. 17:1817–1821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wanderi C, Kim E, Chang S, Choi C and Choi
K: Ginsenoside 20(S)-protopanaxadiol suppresses viability of human
glioblastoma cells via down-regulation of cell adhesion proteins
and cell-cycle arrest. Anticancer Res. 36:925–932. 2016.PubMed/NCBI
|
|
43
|
Lim S, Yoon JW, Choi SH, Cho BJ, Kim JT,
Chang HS, Park HS, Park KS, Lee HK, Kim YB and Jang HC: Effect of
ginsam, a vinegar extract from Panax ginseng, on body weight and
glucose homeostasis in an obese insulin-resistant rat model.
Metabolism. 58:8–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee HM, Lee OH, Kim KJ and Lee BY:
Ginsenoside Rg1 promotes glucose uptake through activated AMPK
pathway in insulin-resistant muscle cells. Phytother Res.
26:1017–1022. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ge Y and Chen J: Mammalian target of
rapamycin (mTOR) signaling network in skeletal myogenesis. J Biol
Chem. 287:43928–43935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Toker A and Yoeli-Lerner M: Akt signaling
and cancer: Surviving but not moving on. Cancer Res. 66:3963–3966.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Frame S and Cohen P: GSK3 takes centre
stage more than 20 years after its discovery. Biochem J. 359:1–16.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Harwood AJ: Regulation of GSK-3: A
cellular multiprocessor. Cell. 105:821–824. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thiery JP: Epithelial-mesenchymal
transitions in cancer onset and progression. Bull Acad Natl Med.
193:1969. 2009.(In French). PubMed/NCBI
|