Introduction
In the history of world civilization and with the
evolution of wine culture, wine has become a common
pharmacologically active beverage in society, and is consumed for
social and recreational purposes. However, the consumption alcohol
also increases the risk of health problems, and particularly
affects future generations. In the late 1960s and early 1970s,
clinical studies in the United States and France showed that
drinking alcohol during pregnancy can lead to a variety of
abnormalities, namely fetal alcohol syndrome (1). Fetal alcohol syndrome refers to the
birth defects resulting from alcohol consumption by both parents,
particularly when the mother consumes alcohol prior to or during
pregnancy (2). A number of studies
have confirmed that exposure to alcohol during pregnancy affects
fetal growth and development, predominantly in various organ
deformities of the central nervous system (2,3).
The mechanism underlying the neurotoxicology of
alcohol remains to be fully elucidated. It has been suggested that
alcohol may have an effect by inhibiting the N-methyl-D-aspartate
receptor (NMDA) receptor and activating GABA receptors, and can
promote apoptosis by affecting the activities of
Na+/K+-ATP enzymes, the adenosine acid
cyclization enzyme, and opening of Ca2+ channels. Others
have suggested that the pharmacological and toxicological
mechanisms of alcohol may work through ceramide (4,5). As
an important cellular signal transduction carrier, ceramide is
involved in a variety of cellular signal transduction pathways, and
has multiple cellular physiological functions, including regulation
of the cell cycle, cell differentiation and proliferation,
regulation of apoptosis, and being involved in stress, immunity and
inflammation (6,7).
The quantity of resveratrol produced by plants is
associated with the stress experienced by the plants. The function
universality of resveratrol lies in the diversity of the targets,
of which the acting sites may include membrane and intracellular
receptors, signal molecules and various enzymes, the oxidation
system, DNA repair systems and transcription factors, cell
proliferation, the cell cycle, differentiation and cell death
(8). The signal transduction
function of cells converts stimuli into signals, and the process
usually consists of a series of biochemical reactions. Resveratrol
can activate or inhibit a series of signal transduction pathways
(9). In vitro experiments,
in which resveratrol regulates the activity of sirtuin 1 (SIRT1),
are difficult to replicate completely in vivo, therefore,
resveratrol may act on SIRT1 through an indirect mechanism.
Resveratrol can regulate glucose homeostasis in mammals by the
deacetylation mediated by SIRT1 (9). The aim of the present study was to
examine whether the protective effect of resveratrol protects
against alcohol-induced neurodegeneration in rats and humans. In
addition, the present study aimed to examine the association among
the AMP-activated protein kinase (AMPK)/SIRT1/p38 MAPK signaling
pathway and the protective effect of resveratrol on alcohol-induced
neurodegeneration.
Materials and methods
Animals
All animal experiments were approved by the
Institute Animal Care and Use Committee of Dalian University and
were performed humanely. Male Sprague-Dawley rats (8–10-weeks-old,
weighing 250–300 g, n=28) were housed at 22–24°C and 55–60%
humidity under a 12 h light/dark cycle with free access to food and
water. Rats were randomly assigned into a control group (n=8),
alcohol-induced group (n=10) and resveratrol-treated group (n=10).
A total of 20 Sprague-Dawley rats from the alcohol-induced group
and resveratrol-treated group were administered orally with 5 g/kg
ethanol (25% v/v) once a day for 10 days. The eight Sprague-Dawley
rats in the control group were administered orally with 200 µl
normal saline. The 20 Sprague-Dawley rats in the
resveratrol-treated group were administered orally with 30 mg/kg of
resveratrol for 4 days.
Brain tissue collection
Rat was anesthetized with 35 mg/kg of pentobarbital
sodium and sacrificed using decollation. Whole brain samples were
obtained and sectioned to 50 mm thickness. Rat brain samples from
rats in every group were fixed in 10% formalin buffer overnight at
4°C and then dehydrated using 90% ethanol for 30–60 min and 100%
ethanol for 2 h. The tissues were cleared with xylene for 2 h and
embedded in paraffin at 60°C.
Cell culture
The human neuroblastoma SH-SY5Y cells were purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China) and grown in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C under 5% CO2.
The SH-SY5Y cells were exposed to 100 mM of ethanol (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 24 h at 37°C in the
alcohol-induced group and resveratrol-treated group. The SH-SY5Y
cells in the resveratrol-treated group were exposed to 50 mg/ml
resveratrol for 1 day at 37°C.
ELISA
Blood samples were collected from rats in every
group into a serum separator tube and centrifuged at 1,000 × g for
15 min at room temperature. Commercial ELISA kits (Nanjing
Jiancheng Bioengineering Institute, Jiangsu, China) were used to
analyze the levels of nuclear factor (NF)-κB (H202), tumor necrosis
factor (TNF)-α (H052), superoxide dismutase (SOD, A001-3) and
glutathione (GSH, A006-2) levels, according to the manufacturer's
protocols, at 450 nm absorbance using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Caspase-3 activity assay
The activity of caspase-3 was measured using a
caspase-3 activity assay kit (Sangon Biotech Co., Ltd., Shanghai,
China). The protein concentration was determined using a
Bicinchoninic Acid kit (Beyotime Institute of Biotechnology). The
proteins (50 µg) were incubated with solution buffer at room
temperature for 1 h and then incubated with 100 µl
Asp-Glu-Val-Asp-p-nitroaniline at 37°C for 6 h. The activity of
caspase-3 was measured using a microplate reader (Bio-Rad
Laboratories, Inc.) at an absorbance of 405 nm.
Western blot analysis
The rat brain slices from rats in every group were
homogenized in 100 µl tissue lysis buffer (Beyotime Institute of
Biotechnology) with protease inhibitor cocktail (Sigma-Aldrich;
Merck KGaA) on ice for 30 min and centrifuged at 12,000 × g for 10
min at 4°C. The protein concentration was determined using a
Bicinchoninic Acid kit (Beyotime Institute of Biotechnology). The
proteins (50 µg) was separated by sodium dodecyl sulphate
polyacrylamide gel electrophoresis (10–12%) and transferred onto
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked with 5% (w/v) non-fat dry milk in
Tris-buffered saline with 0.1% Tween 20 for 2 h at room temperature
and incubated with the following primary antibodies: B-cell
lymphoma 2 (Bcl-2; sc-783, 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) Bcl-2-associated X protein (Bax; sc-6236, 1:2,000;
Santa Cruz Biotechnology, Inc.), AMPK (sc-25792, 1:1,500, Santa
Cruz Biotechnology, Inc.), SIRT1 (sc-15404, 1:2,000; Santa Cruz
Biotechnology, Inc.), p-p38 (sc-17852-R, 1:2,000; Santa Cruz
Biotechnology, Inc.) and β-actin (sc-7210, 1:5,000; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. The membranes were then
blocked with secondary antibodies (D110058, 1:20,000; Sangon
Biotech Co., Ltd.) for 2 h at room temperature and developed with
an enhanced chemiluminescence substrate solution (Applygen
Technologies, Inc., Beijing, China) and analyzed using
Image-ProPlus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Small interfering RNA (siRNA)
knockdown of AMPK
The SH-SY5Y cells (3×105/well) in 12-well
plates were incubated with Lipofectin (Gibco; Thermo Fisher
Scientific, Inc.) with 200 nM AMPK-specific siRNA (sc-270395, Santa
Cruz Biotechnology, Inc.) or control siRNA (sc-270395, Santa Cruz
Biotechnology, Inc.) for 48 h at 37°C. The effects of siRNA
transfection was measured by western blot analysis as
aforementioned.
Statistical analysis
All data are presented as the mean ± standard error
of the mean using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Statistical analyses were performed using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference. All data show results from at least three independent
experiments.
Results
Resveratrol protects against
alcohol-induced neurodegeneration
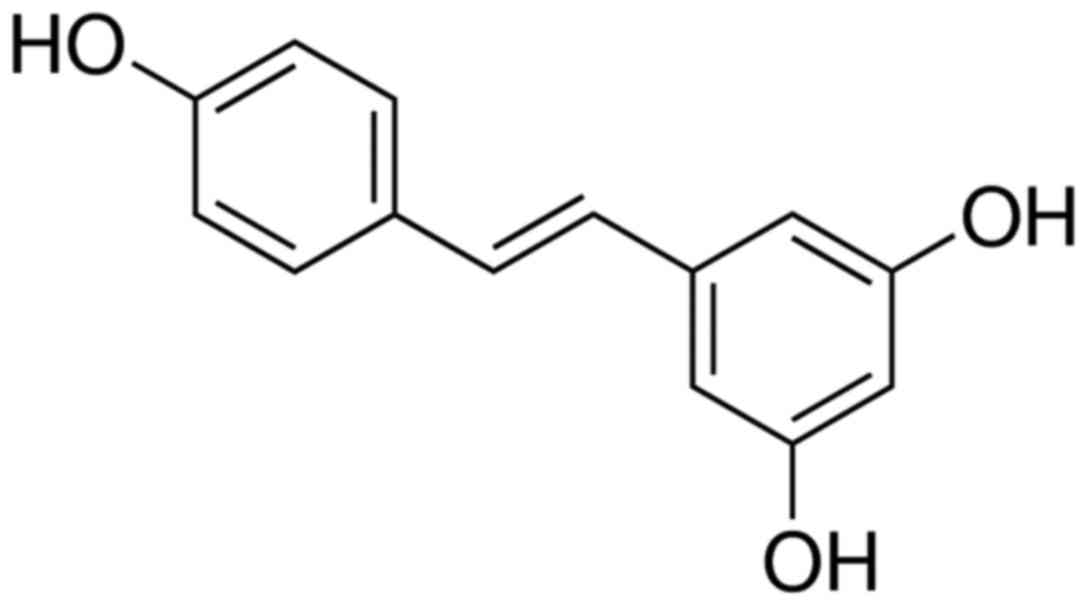
The chemical structure of resveratrol is shown in
Fig. 1. The neuroprotective
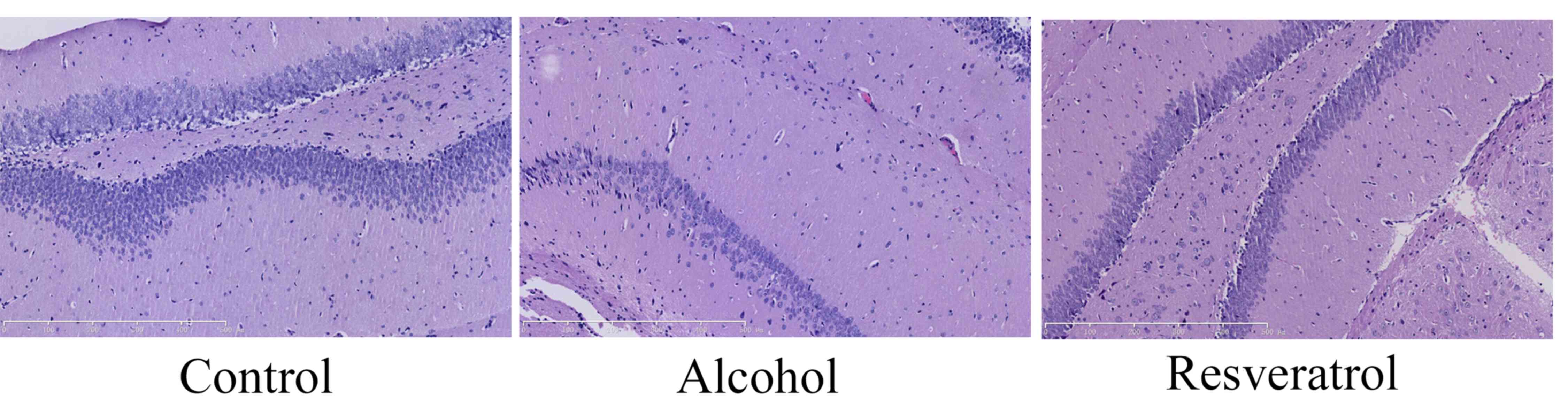
effects of resveratrol on alcohol-induced neurodegeneration were
measured, in which the number of neuron cells were observed in
every group. Alcohol inhibited the number of neuron cells in the
rats, compared with the number in the control group (Fig. 2). Resveratrol increased the number
of neuron cells in the rats, compared with the number in the
alcohol-induced model group (Fig.
2).
Resveratrol protects against the
alcohol-induced inflammatory response
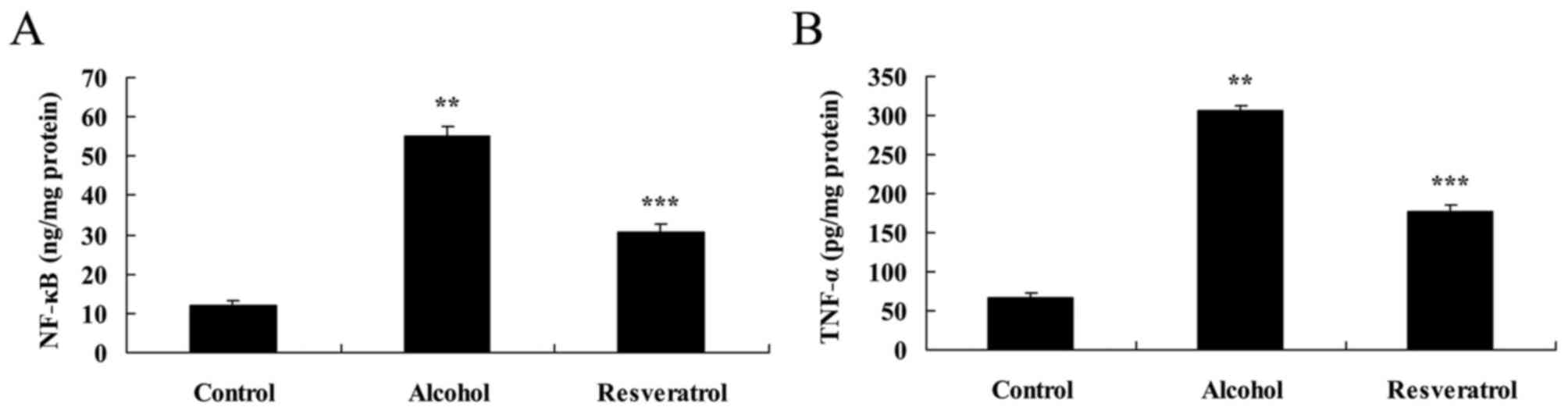
The present study then examined the effect of
resveratrol on the alcohol-induced inflammatory response in rats.
The results showed that the expression levels of NF-κB and TNF-α
were markedly increased in the alcohol-induced model group,
compared with levels in the control group (Fig. 3A and B). Treatment with resveratrol
for 4 days reduced the alcohol-induced levels of NF-κB and TNF-α in
the alcohol-induced rats (Fig. 3A and
B).
Resveratrol protects against
alcohol-induced oxidative stress
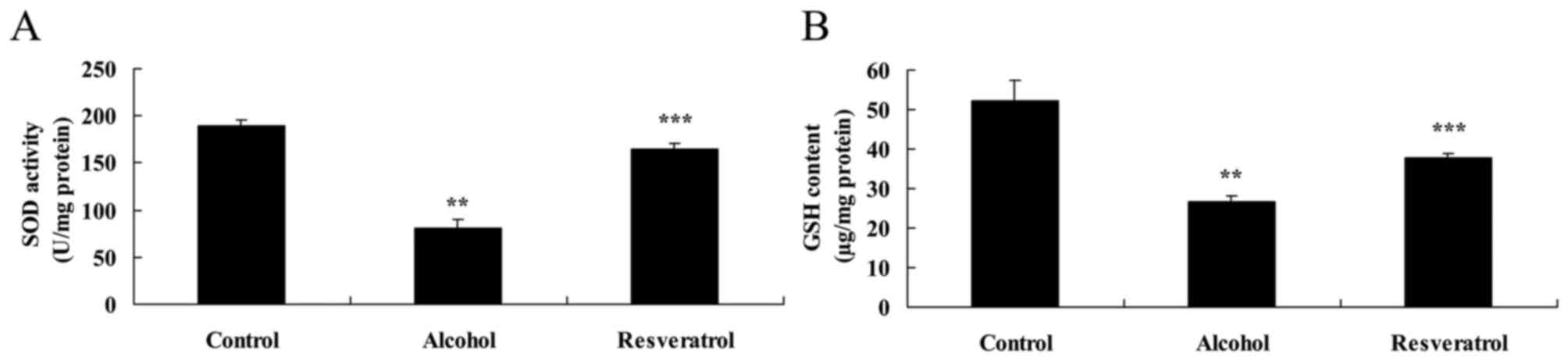
The present study also examined the expression
levels of SOD and GSH using ELISA kits. In the model group, the
expression levels of SOD and GSH were significantly suppressed,
compared with the levels in the control group (Fig. 4A and B). Treatment of the
alcohol-induced rats with resveratrol significantly increased the
expression levels of SOD and GSH in the rats, compared with the
number in the model group (Fig. 4A and
B).
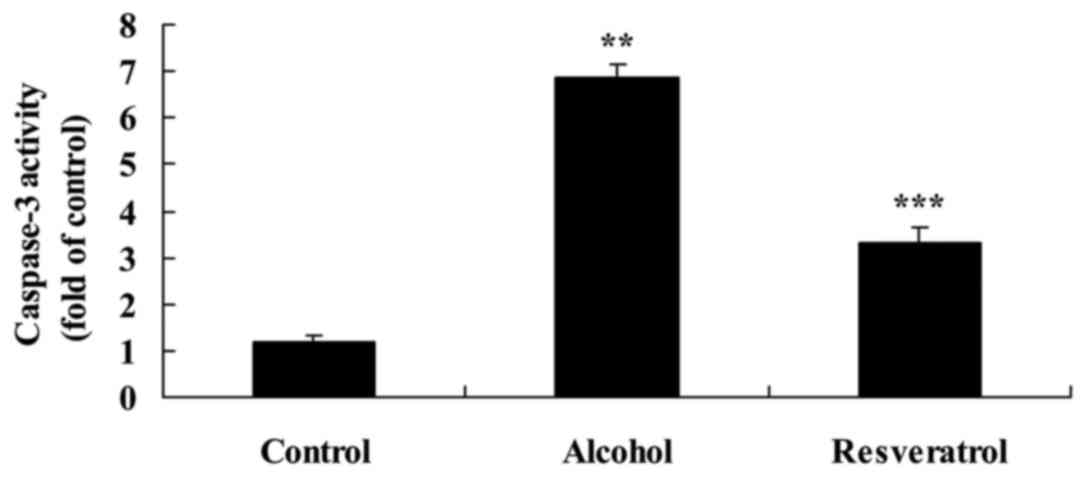
Resveratrol protects against
alcohol-induced caspase-3 activity
The microplate reader analysis of the activity of
caspase-3 activity following alcohol exposure revealed that the
activity of caspase-3 was increased significantly, compared with
that in the control group. However, the activity of caspase-3 was
significantly decreased 5 days following treatment with resveratrol
(Fig. 5).
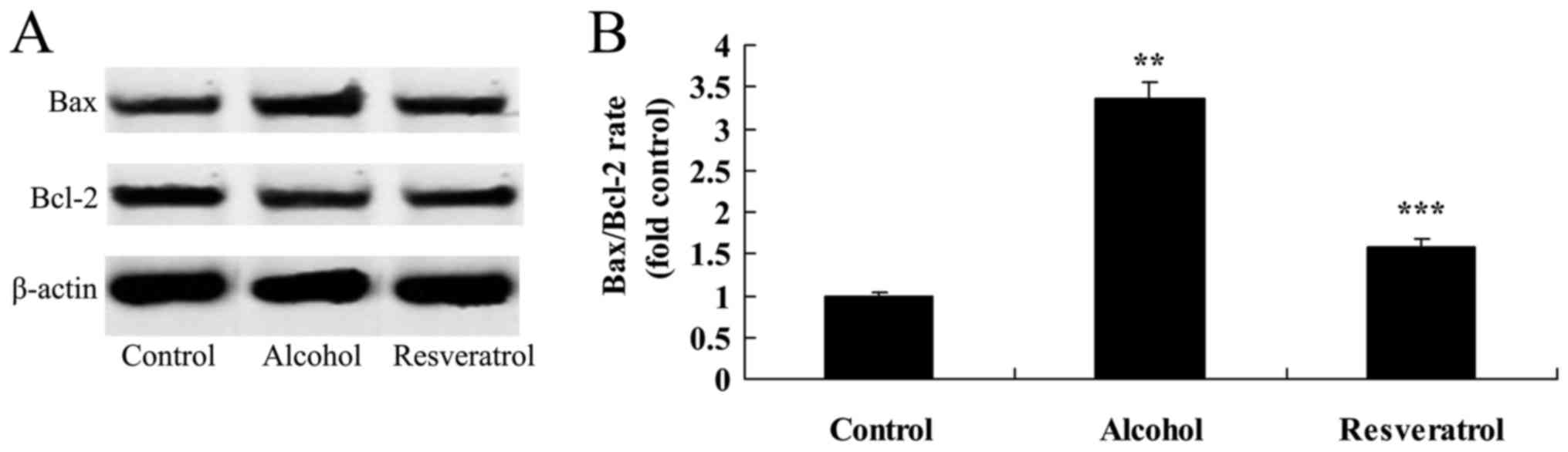
Resveratrol protects against
alcohol-induced expression of Bax/Bcl-2
To examine the mechanisms underlying the
neuroprotective effects of resveratrol on alcohol-induced Bax/Bcl-2
ratio in rats, the present study measured the expression of
Bax/Bcl-2 using western blot analysis. Alcohol significantly
increased the ratio of Bax/Bcl-2, compared with that in the control
group (Fig. 6). Following the
administration of resveratrol, the ratio of Bax/Bcl-2 was
significantly decreased in the rats, compared with that in the
model group (Fig. 6).
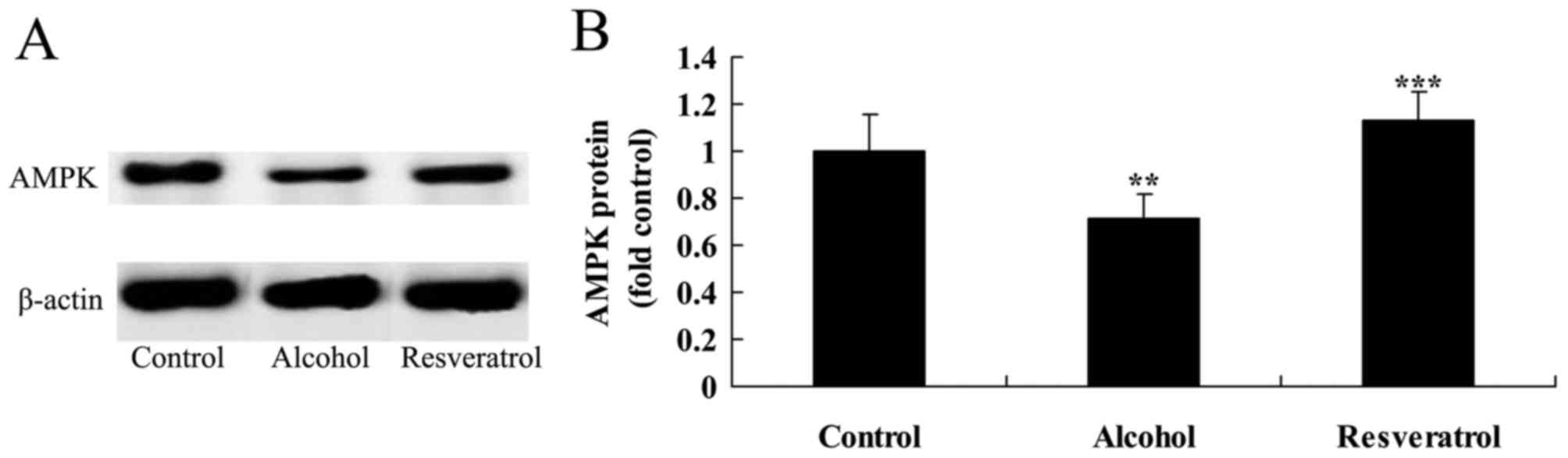
Resveratrol upregulates the expression
of AMPK
The present study also assessed whether the
neuroprotective effects of resveratrol affected the expression of
AMPK in the alcohol-induced rats. The results of the western blot
analysis showed that the protein expression of AMPK was
significantly suppressed following alcohol exposure, compared with
that in the control group (Fig. 7A and
B). By contrast, treatment with resveratrol upregulated the
expression of AMPK in the alcohol-induced rats, compared with that
in the alcohol-induced model group (Fig. 7A and B).
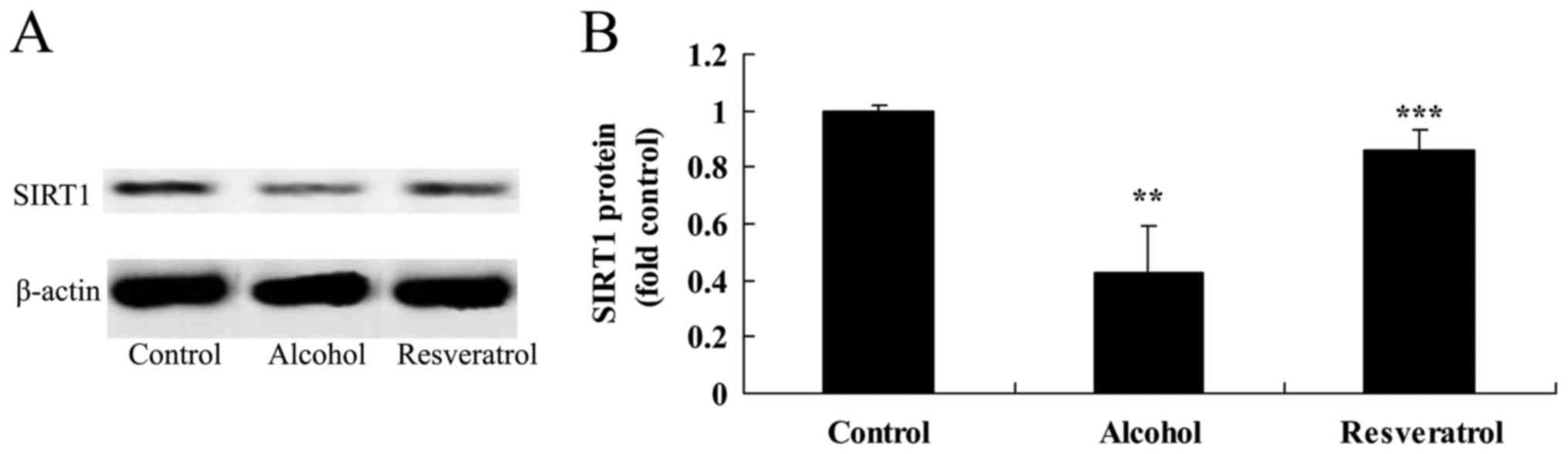
Resveratrol upregulates the expression
of SIRT1
The results of the western blot analysis showed that
alcohol inhibited the protein expression of SIRT1 in the rats,
compared with that in the control group (Fig. 8A and B). However, pretreatment with
resveratrol significantly upregulated the inhibited protein
expression of SIRT1 induced by alcohol, compared with the
expression in the model group without resveratrol (Fig. 8A and B).
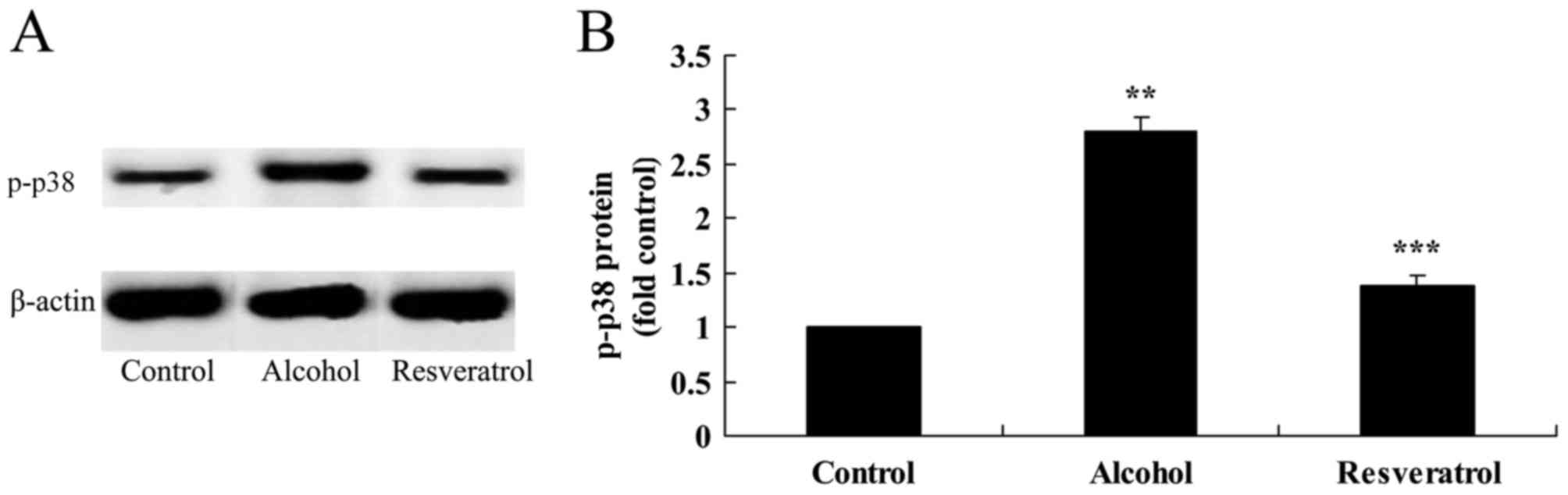
Resveratrol downregulates the
expression of p-p38
The results showed that the protein expression of
p-p38 was increased following exposure to alcohol, compared with
that in the control group (Fig. 9A and
B). Treatment with resveratrol significantly decreased the
alcohol-induced protein expression of p-p38, compared with the
expression in the model group without resveratrol (Fig. 9A and B).
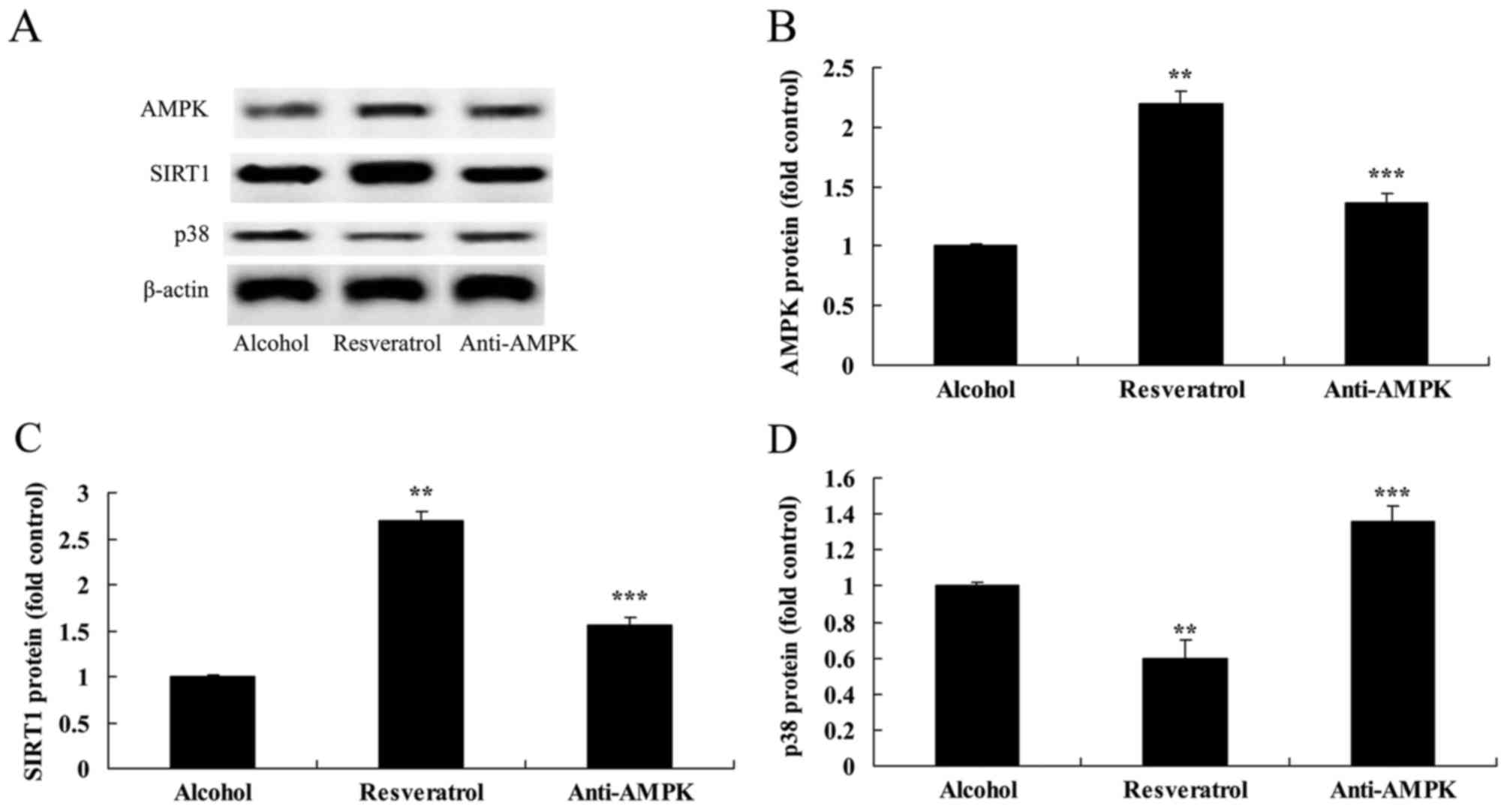
Downregulation of AMPK affects the
expression of SIRT1 and p38
In order to provide additional support for the
results described above, the present study analyzed the effect of
the downregulation of AMPK on the expression of SIRT1 and p38 in
the SH-SY5Y cells. The results demonstrated that the downregulation
of AMPK suppressed the expression of SIRT1 and activated the
expression of p38 in the SH-SY5Y cell model (Fig. 10A-D).
Discussion
Alcohol has become a commonly consumed beverage.
Although a small quantity of alcohol may be beneficial to humans,
long-term heavy alcohol intake can cause damage to the digestive
system, cardiovascular system, immune system, nervous system and
other organs in the body, and can induce cancer (6,10).
The potential damage of alcohol to the nervous system is relatively
high; it has a direct neurotoxic effect on the central nervous
system through the blood brain barrier, induces the apoptosis of
nerve cells, reduces the number of synapses, and can lead to
organic change in brain function (11). Cognitive impairment is a mild
symptom, however, serious consequences include the induction of
neurodegenerative diseases, including Alzheimer's disease and
Parkinson's disease (12,13). The present study found that
resveratrol reduced the number of alcohol-induced microglial cells
and neuron cells, and inhibited the increased levels of NF-κB,
TNF-α, SOD and GSH in alcohol-induced rats. Zhang et al
reported the neuroprotective effects of resveratrol against
glutamate-induced excitotoxicity in neurodegenerative diseases
(14), and Abengózar-Vela et
al demonstrated that resveratrol reduced inflammatory and
oxidative damage in human ocular surface epithelial cells (15).
AMPK has protective and reparative effects on
developing and mature neurons, which can enhance the uptake of
neurons cultured in vitro and promote the differentiation of
neurons (16). Studies have shown
that the expression of AMPK is significantly decreased in the
substantia nigra of patients (17). In open clinical trials, intraspinal
and putamen injections of AMPK have exhibited significant efficacy.
The partial protective effect of AMPK protein on glucose-deprived
PC12 cells is realized by the direct binding with p38, which
partially inhibits p38 nuclear translocation, and inhibits its
transcription-dependent pro-apoptotic process; in addition to the
binding site of 253–282 bits, sites between may exist (18). The overexpression of AMPK can also
inhibit the expression of p38, which can weaken p38-induced
pro-apoptosis (19). In the
present study, it was found that treatment with resveratrol
upregulated the expression of AMPK in the alcohol-induced rats. Guo
et al also demonstrated that resveratrol protects against
cardiomyocytes through the AMPK-associated pathway (20).
SIRT1 is the most unique homologous protein of SIRT2
in mammals, involved in chromatin remodeling, gene silencing and
DNA damage repair processes (21).
SIRT1 has a substrate, which is widely used, including
transcription factors p53, forkhead box O and NF-kB (22). The activity of SIRT1 can be
regulated by oxidized nicotinamide adenine dinucleotide and
nicotinamide and phosphorylation (23). SIRT1 is involved in the regulation
of age-related metabolic processes, including fat storage, insulin
secretion, glucose metabolism, neuroprotection and apoptosis;
therefore, it is possible that Sirt1 is associated with the life
span of mammals (24). The present
study showed that resveratrol significantly upregulated the
alcohol-inhibited protein expression of SIRT1 in rats. Sonnemann
et al also suggested that resveratrol significantly promotes
the protein expression of SRT1 in SK-N-MC cells (9).
Tumor suppressor p38 can inhibit cell proliferation
through cell cycle arrest, apoptosis and the response to cell aging
induced by stress from different cells (25). There is data showing that tumors
without mutant p38 are likely to have other defects in the p38
signaling pathway, which has an important effect on the form of
cancer (26). In addition,
experiments have shown that p38 is a non-histone target gene, which
is deacetylated by Sirt1 and the first to have been found (27). The present found that treatment
with resveratrol significantly reduced the alcohol-induced protein
expression of p-p38. Wu et al (28) reported that resveratrol induces the
apoptosis of human chronic myelogenous leukemia cells through p38.
Of note, the downregulation of AMPK suppressed the expression of
SIRT1 and activation of p38 in the SH-SY5Y cell model.
In conclusion, the data obtained in the present
study showed that resveratrol protected against alcohol-induced
neurodegeneration. The results also provided novel mechanistic
insights by which SH-SY5Y cells are important in mediating
AMPK/SIRT1/p38 signaling.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81673727).
References
|
1
|
Goodlett CR and Eilers AT: Alcohol-induced
Purkinje cell loss with a single binge exposure in neonatal rats: A
stereological study of temporal windows of vulnerability. Alcohol
Clin Exp Res. 21:738–744. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qin L and Crews FT: NADPH oxidase and
reactive oxygen species contribute to alcohol-induced microglial
activation and neurodegeneration. J Neuroinflammation. 9:52012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeuchi M and Saito T: Cytotoxicity of
acetaldehyde-derived advanced glycation end-products (AA-AGE) in
alcoholic-induced neuronal degeneration. Alcohol Clin Exp Res. 29
12 Suppl:220S–224S. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiss B: The first 83 and the next 83:
Perspectives on neurotoxicology. Neurotoxicology. 30:832–850. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Myers J, London L and Lucchini RG:
Neurotoxicology and development: Human, environmental and social
impacts. Neurotoxicology. 45:217–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berman JW, Carson MJ, Chang L, Cox BM, Fox
HS, Gonzalez RG, Hanson GR, Hauser KF, Ho WZ, Hong JS, et al:
NeuroAIDS, drug abuse, and inflammation: Building collaborative
research activities. J Neuroimmune Pharmacol. 1:351–399. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trickler WJ, Lantz SM, Murdock RC, Schrand
AM, Robinson BL, Newport GD, Schlager JJ, Oldenburg SJ, Paule MG,
Slikker W Jr, et al: Silver nanoparticle induced blood-brain
barrier inflammation and increased permeability in primary rat
brain microvessel endothelial cells. Toxicol Sci. 118:160–170.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park I, Lee Y, Kim HD and Kim K: Effect of
resveratrol, a SIRT1 activator, on the interactions of the
CLOCK/BMAL1 complex. Endocrinol Metab (Seoul). 29:379–387. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonnemann J, Kahl M, Siranjeevi PM,
Blumrich A, Blümel L, Becker S, Wittig S, Winkler R, Krämer OH and
Beck JF: Reverse chemomodulatory effects of the SIRT1 activators
resveratrol and SRT1720 in Ewing's sarcoma cells: Resveratrol
suppresses and SRT1720 enhances etoposide- and vincristine-induced
anticancer activity. J Cancer Res Clin Oncol. 142:17–26. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allen JW, Mutkus LA and Aschner M: Chronic
ethanol produces increased taurine transport and efflux in cultured
astrocytes. Neurotoxicology. 23:693–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan W and DePetrillo PB: Ritonavir
protects hippocampal neurons against oxidative stress-induced
apoptosis. Neurotoxicology. 23:301–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Overstreet DH: Organophosphate pesticides,
cholinergic function and cognitive performance in advanced age.
Neurotoxicology. 21:75–81. 2000.PubMed/NCBI
|
|
13
|
Polizzi S, Pira E, Ferrara M, Bugiani M,
Papaleo A, Albera R and Palmi S: Neurotoxic effects of aluminium
among foundry workers and Alzheimer's disease. Neurotoxicology.
23:761–774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang LN, Hao L, Wang HY, Su HN, Sun YJ,
Yang XY, Che B, Xue J and Gao ZB: Neuroprotective effect of
resveratrol against glutamate-induced excitotoxicity. Adv Clin Exp
Med. 24:161–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abengózar-Vela A, Calonge M, Stern ME,
González-Garcia MJ and Enriquez-De-Salamanca A: Quercetin and
resveratrol decrease the inflammatory and oxidative responses in
human ocular surface epithelial cells. Invest Ophthalmol Vis Sci.
56:2709–2719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leyton L, Hott M, Acuña F, Caroca J, Nuñez
M, Martin C, Zambrano A, Concha MI and Otth C: Nutraceutical
activators of AMPK/Sirt1 axis inhibit viral production and protect
neurons from neurodegenerative events triggered during HSV-1
infection. Virus Res. 205:63–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi JS, Park C and Jeong JW:
AMP-activated protein kinase is activated in Parkinson's disease
models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Biochem Biophys Res Commun. 391:147–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao W, Takano T, Guillemette J, Papillon
J, Ren G and Cybulsky AV: Induction of apoptosis by the Ste20-like
kinase SLK, a germinal center kinase that activates apoptosis
signal-regulating kinase and p38. J Biol Chem. 281:3075–3084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu K, Yang Y and Mansbridge J: Comparison
of the stress response to cryopreservation in monolayer and
three-dimensional human fibroblast cultures: Stress proteins, MAP
kinases, and growth factor gene expression. Tissue Eng. 6:539–554.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo S, Yao Q, Ke Z, Chen H, Wu J and Liu
C: Resveratrol attenuates high glucose-induced oxidative stress and
cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol.
412:85–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida K, Bacal J, Desmarais D, Padioleau
I, Tsaponina O, Chabes A, Pantesco V, Dubois E, Parrinello H,
Skrzypczak M, et al: The histone deacetylases sir2 and rpd3 act on
ribosomal DNA to control the replication program in budding yeast.
Mol Cell. 54:691–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zill OA, Scannell D, Teytelman L and Rine
J: Co-evolution of transcriptional silencing proteins and the DNA
elements specifying their assembly. PLoS Biol. 8:e10005502010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallagher JE, Babiarz JE, Teytelman L,
Wolfe KH and Rine J: Elaboration, diversification and regulation of
the Sir1 family of silencing proteins in Saccharomyces. Genetics.
181:1477–1491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou Z, Bernstein DA, Fox CA and Keck JL:
Structural basis of the Sir1-origin recognition complex interaction
in transcriptional silencing. Proc Natl Acad Sci USA. 102:pp.
8489–8494. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Donauer J, Schreck I, Liebel U and Weiss
C: Role and interaction of p53, BAX and the stress-activated
protein kinases p38 and JNK in benzo(a)pyrene-diolepoxide induced
apoptosis in human colon carcinoma cells. Arch Toxicol. 86:329–337.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dziegielewska B, Brautigan DL, Larner JM
and Dziegielewski J: T-type Ca2+ channel inhibition induces
p53-dependent cell growth arrest and apoptosis through activation
of p38-MAPK in colon cancer cells. Mol Cancer Res. 12:348–358.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hishida T, Nozaki Y, Nakachi Y, Mizuno Y,
Iseki H, Katano M, Kamon M, Hirasaki M, Nishimoto M, Okazaki Y and
Okuda A: Sirt1, p53, and p38(MAPK) are crucial regulators of
detrimental phenotypes of embryonic stem cells with Max expression
ablation. Stem Cells. 30:1634–1644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu XP, Xiong M, Xu CS, Duan LN, Dong YQ,
Luo Y, Niu TH and Lu CR: Resveratrol induces apoptosis of human
chronic myelogenous leukemia cells in vitro through p38 and
JNK-regulated H2AX phosphorylation. Acta Pharmacol Sin. 36:353–361.
2015. View Article : Google Scholar : PubMed/NCBI
|