Introduction
Pseudomonas aeruginosa is a gram-negative,
rod-shaped, belonging to the family of Pseudomonadaceae. It
can be found in many natural environments including warm and moist
atmospheres containing very low levels of organic material
(1). Therefore, P.
aeruginosa can contaminate contact lens, cosmetic and several
hospital niches, including taps, drains, water pipes, medical
equipment and several other devices leading to nosocomial
infections (2). This organism is
an opportunistic pathogen that can cause serious infections
including septicemia, pneumonia, endocarditis, otitis and keratitis
(3). P. aeruginosa is an
increasingly frequent organism found in humans with bacterial
keratitis particularly among contact lens wearers (4). This organism can adhere on the
surface of contact lens and form the biofilm, as a result, ability
to grow is increasing and it is difficult to dispose. P.
aeruginosa is an important cause of keratitis and may lead to
permanent loss of vision if not treated promptly and appropriately
(5). Therefore, the rapid and
accurate method for determination of P. aeruginosa is
required.
Identification of P. aeruginosa in the
clinical laboratory is generally performed by growing the bacteria
on nalidixic acid-cetrimide (NAC) agar or cetrimide agar,
Pseudomonas CN selective agar (PCN) and Pseudomonas isolation agar
(PIA). Although this method is credible but the time required for
performing is 24–72 h, which is a long time and other
Pseudomonas species may display similar growth that limit
the identification of species (6).
Using polymerase chain reaction (PCR) methods, which allow for more
rapid identification of P. aeruginosa, have been shown to be
highly specific and less time-consuming than classical method
(3,7,8) and
PCR have also been reported (9,10).
However, the PCR based methods require expensive device and skilled
personnel to avoid cross contamination between samples.
A novel nucleic acid amplification method termed
loop-mediated isothermal amplification (LAMP) using a set of four
oligonucleotide primers and the strand displacement enzyme for
amplification of target gene at constant temperature ranging from
60 to 65°C was invented (11).
LAMP method has been successfully used to detect various bacterial
pathogens such as Vibrio vulnificus (12), Vibrio parahaemolyticus
(13), and Salmonella spp.
(14). In the case of P.
aeruginosa detection, traditional LAMP methods based on various
genes such as outer membrane lipoprotein I (opr I) gene
(15), outer membrane lipoprotein
L (opr L) gene (16),
ecfX gene in bottled water samples (17), exoS and exoU of Type
III Secretion System (18) were
reported. In order to increase specificity, the visualization of
LAMP product using a labelled-hybridization probe is an alternative
method. The LAMP assay combined with DNA-labeled gold nanoparticles
(AuNP) probe were established to detect various viral pathogens
such as shrimp yellow head virus (YHV) (19), white spot syndrome virus (WSSV)
(20), or identification of human
DNA in forensic evidence (21). In
this system, the DNA-AuNP probe aggregates with a color shift from
red to blue or colorless at optimal salt concentration if there is
no hybridization reaction (18).
However, hybridization of the AuNP probe to a complementary target
DNA prevents aggregation in salt environment and solution remained
red.
Since LAMP method is highly sensitive for nucleic
acid amplification; therefore, the assay is likely susceptible to
carryover contamination. To overcome this problem, a one-pot,
closed-vessel enzymatic assay that prevent carryover contamination
during LAMP reaction was reported (22). This system used
uracil-DNA-glycosylase (UDG) to digest uracil-containing LAMP
amplicons from previous reaction prior to performing LAMP
amplification in the same tube. This system had been successfully
used with traditional LAMP assay for detection of Salmonella
Typhimurium (22). In the present
study, a modified UDG-LAMP combined with colorimetric AuNP probe
assay (UDG-LAMP-AuNP) for specific identification of P.
aeruginosa based on ecfX gene was developed. The
specificity and sensitivity for detection of P. aeruginosa
was also investigated.
Materials and methods
Bacterial isolates and DNA
extraction
A total of 39 bacterial isolates including 16
isolates of P. aeruginosa, 8 isolates of Pseudomonas
spp. and 15 isolates of other bacteria were used in the present
study (Table I). The 16S rRNA gene
amplifications were performed to verify the bacterial
identification as described in a previous report (23). The origins and sources of all 40
bacterial isolates tested were indicated in Table I. All bacterial isolates were grown
for overnight at 37°C in trypticase soy agar (TSA). A single colony
from TSA was picked and inoculated into trypticase soy broth (TSB)
at 37°C for overnight.
 | Table I.Bacterial isolates used in the
present study. |
Table I.
Bacterial isolates used in the
present study.
|
|
| DNA amplification
ecfX |
|
|---|
|
|
|
|
|
|---|
| Bacterial
isolates | Origin | LAMP | PCR | Source |
|---|
| A, Pseudomonas
aeruginosa (n=16) |
|
|
|
|
|
| P.
aeruginosa (PA02) | Unknown | + | + | DMST |
| P.
aeruginosa (PA04) | Urine | + | + | DMST |
| P.
aeruginosa (PA05) | Sputum | + | + | DMST |
| P.
aeruginosa (PA06) | Compost | + | + | DMST |
| P.
aeruginosa (PA07) | Outer-ear
infection | + | + | ATCC |
| P.
aeruginosa (PA08) | Animal room water
bottle | + | + | ATCC |
| P.
aeruginosa (PA10) | Bacterial
resistance testing of latex paint | + | + | ATCC |
| P.
aeruginosa (PA11) | Sputum | + | + | DMST |
| P.
aeruginosa (PA12) | Blood culture | + | + | DMST |
| P.
aeruginosa (PA14) | Pus | + | + | TISTR |
| P.
aeruginosa (PA16) | Intercostal
Drainage | + | + | DMST |
| P.
aeruginosa (PA17) | Cerebrospinal
fluid | + | + | DMST |
| P.
aeruginosa (PA19) | Heart blood | + | + | DMST |
| P.
aeruginosa (PA20) | Endo tracheal | + | + | DMST |
| P.
aeruginosa (PA21) | Unknown | + | + | DMST |
| P.
aeruginosa (PA22) | Unknown | + | + | TISTR |
|
| B,
Pseudomonas spp. (n=8) |
|
|
|
|
|
| P. japonica
1526 | Flower of
Haliconia sp. | − | − | TISTR |
| P. putida
23201 | Unknown | − | − | DMST |
| P.
fluorescens 358 | Unknown | − | − | TISTR |
| P. olevorans
1097 | Epoxidizes terminal
olefins | − | − | TISTR |
| P. syringae
19310 | Syringa
vulgaris, Great Britain | − | − | ATCC |
| P. stutzeri
22487 | Blood culture | − | − | DMST |
| P.
boreopolis 33662 | Unknown | − | − | ATCC |
| P.
acidovorans | Unknown | − | − | Unknown |
|
| C, Other bacteria
(n=15) |
|
|
|
|
|
| Escherichia
coli 25922 | Unknown | − | − | ATCC |
| Plesiomonas
shigelloides 22107 | Rectal swab | − | − | DMST |
| Photobacterium
damselae sub piscicida | Unknown | − | − | Unknown |
| Vibrio
ordalii VIB02 | Unknown | − | − | DABU |
| Vibrio
anguillarum AVL01 | Unknown | − | − | GB |
| Vibrio
campbellii 21361 | Unknown | − | − | GB |
| Vibrio
alginolyticus 24047 | Stool | − | − | DMST |
| Vibrio
cholerae 22117 | Stool | − | − | DMST |
| Vibrio
shilonii 4907012 | Penaeus
vannamei | − | − | SWU |
| Vibrio
harveyi 639 | Penaeus
monodon | − | − | CENTEX |
| Salmonella
Typhimurium 14029 | Unknown | − | − | ATCC |
| Salmonella
Enteritidis 7108 | Unknown | − | − | DMST |
| Staphylococcus
aureus 25923 | Unknown | − | − | ATCC |
| Aeromonas
veronii 21255 | Unknown | − | − | DMST |
| Yersinia
rukeri | Unknown | − | − | DABU |
DNA was extracted from bacteria cultured in TSB by
using QIAamp DNA mini kit (Qiagen, Inc., Valencia, CA, USA)
according to the manufacture's manual. The extracted DNA was stored
at −20°C until use and an isolate of P. aeruginosa (PA07)
ATCC was used for the assay of optimization and sensitivity testing
with pure culture.
General PCR
The DNA extracted from bacterial samples was used as
a template for PCR amplification. The PCR amplification of
ecfX gene was performed as described in previous reports
(3). Briefly, the PCR was carried
out with primers ECF 1 (ATGGATGAGCGCTTCCGT) and ECF 2
(TCATCCTTCGCCTCCCTG) for 35 cycles, each of which consisted of
denaturation at 94°C for 45 sec, annealing at 58°C for 45 sec and
extension at 72°C for 30 sec. The PCR amplicon size was 528 bp. PCR
products were separated by electrophoresis on 1% (w/v) agarose gel
and visualized using a gel documentation system (Dynamica GelView
Master).
Design of LAMP primers and probe for
the LAMP assay
A set of four primers consisting of 2 outer primers
(F3 and B3) and 2 inner primers (FIP and BIP) recognized six
distinct regions on ecfX gene (GenBank, accession no. DQ
996559.1) of P. aeruginosa was designed using PrimerExplorer
ver. 4 (http://primerexplorer.jp/elamp4.0.0/index.html). The
FITC-labeled oligonucleotide probe was synthesized and labeled with
a thiol group at 5′-end (Bio Basic Inc., Markham, Ontario, Canada).
The sequences of primers and probe are shown in Table II.
 | Table II.Primers and the probe for
loop-mediated isothermal amplification designed from the
ecfX gene of Pseudomonas aeruginosa. |
Table II.
Primers and the probe for
loop-mediated isothermal amplification designed from the
ecfX gene of Pseudomonas aeruginosa.
| Primer | Sequence
(5′-3′) |
|---|
| Forward outer
primer (F3) |
TCCGTGGTTCCGTCTCG |
| Backward outer
primer (B3) |
AAGTTGCGGGCGATCTG |
| Forward inner
primer (FIP) |
TGCCCAGGTGCTTGCGCATTTTCATGCCTATCAGGCGTTCC |
| Backward inner
primer (BIP) |
GCCGACCTCGCCCAGGATATTTTGCTCGACCGATTGCCG |
| Thiol probe | (SH-)
A10-GGATACTTTCGACCAGTGGC |
Optimization of UDG-LAMP
conditions
In order to prevent the carryover contamination of
LAMP product, the UDG-LAMP reactions were performed as described by
a previous study with some modification. The dTTP in a standard
dNTP mix was partially replaced with dUTP and UDG was used to
degrade uracil-labeled LAMP amplicons (22).
The UDG-LAMP assay was carried out in a total of 25
µl reaction mixture consisted of 40 pmol each of the inner primers
(FIP and BIP), 5 pmol each of the outer primers (F3 and B3), 1.4 mM
each of dATP, dGTP, dCTP and dUTP: dTTP (different ratios at 100%
dUTP; 80% dUTP + 20% dTTP; 60% dUTP + 40% dTTP; 40% dUTP + 60%
dTTP; 20% dUTP + 80% dTTP; and 100% dTTP) (New England Biolabs,
Inc., Ipswich, MA, USA), 5.0 mM of MgSO4, 0.8 M Betaine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 8 U of Bst
2.0 DNA polymerase (New England Biolabs), 5 U of UDG (New England
Biolabs), 1X of supplied buffer and DNA template. The reaction
mixture was incubated at 37°C for 5 min. and then further incubated
at 60, 63 and 65°C for 60 min to determine the optimal temperature.
To establish the optimal time, the UDG-LAMP reaction was carried
out at pre-determined temperature of 65°C for 30, 45, 60, 75 and 90
min and heated at 80°C for 10 min to terminate the reaction. The
products were analyzed by 2% agarose gel electrophoresis and
visualized using a gel documentation system (Dynamica GelView
Master).
Preparation of ssDNA-labeled gold
nanoparticles probe (AuNPs-probe)
The AuNPs probe was prepared according to a previous
report (21). Briefly, 4 ml of
colloidal AuNPs with diameter of 10 nm (Sigma-Aldrich; Merck KGaA)
was added to 2.5 nmol of the thiol-probe and incubated with shaking
at 150 rpm, 45°C for 16 h. The following solutions consisted of 4
µl of 10% SDS, 400 µl of 100 mM phosphate buffer, pH 7.5 (0.02 M
NaH2PO4·H2O, 0.08 M
Na2HPO4·7 H2O) and 200 µl of 2 M
NaCl were added to the mixture and incubated at 45°C, 150 rpm
shaking for 48 h. The AuNPs-probe was precipitated with
centrifugation at 20,000 × g, 4°C for 30 min. The pellet was washed
twice with 700 µl of solution containing 10 mM phosphate buffer,
100 mM NaCl, and 0.01% (w/v) SDS. Finally, the pellet was
resuspended in 700 µl of 10 mM phosphate buffer, monitored for
absorbance at 525 nm in the range of 0.3–0.4, stored at 4°C and
protected from light.
Optimization of the AuNPs-probe for
detection of UDG-LAMP products
To establish the optimal salt concentration for
induction of free probe aggregation, 5 µl of MgSO4 was
added to the mixture (the total volume of 15 µl) to achieve the
final concentration of 5, 10, 20, 40, 100 and 200 mM. The positive
reaction (red-purple color) and negative reaction (blue-gray or
colorless) were observed and recorded by naked eyes and by
UV-visible analysis (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
To optimize the hybridization temperature for
detection of UDG-LAMP products, 2.5 nmol of AuNPs-probe solution
was added to the UDG-LAMP products in a 1:1 ratio at 57, 62 and
65°C for 5 min. The optimal hybridization time was investigated at
5, 10 and 20 min under pre-determined temperature (65°C).
Specificity testing of PCR and
UDG-LAMP-AuNPs assays
The specificity of UDG-LAMP-AuNPs and PCR based on
ecfX gene was performed to examine the 39 bacterial isolates
including 16 isolates of P. aeruginosa, 8 isolates of
Pseudomonas spp. and 15 isolates of other bacteria as shown
in Table I. DNA templates isolated
from bacterial cultures by QIAamp DNA mini kit (Qiagen) were used
to evaluate the specificity test.
Sensitivity of UDG-LAMP-AuNPs and PCR
assays in pure culture
The sensitivity of the UDG-LAMP-AuNPs assay for the
detection of P. aeruginosa in pure culture was performed
according to a previous study (24) with some modifications using known
amounts of P. aeruginosa (PA 07). Briefly, bacterial cells
from a single colony on TSA were inoculated into 4 ml of trypticase
soy broth (TSB; Difco) and incubated at 37°C for overnight.
Approximately 40 µl of TSB culture was added to a new 4 ml of TSB
and incubated at 37°C with shaking at 225 rpm to mid-log phase (OD
600 nm=0.5–0.6). Then, 10-fold serial dilutions of the cultures
were prepared in phosphate-buffered saline solution (PBS).
To extract DNA from pure culture, 100 µl of each
dilution was transferred into a new microcentrifuge tube and
centrifuged at 20,000 × g for 5 min. Then, the supernatant was
removed and the pellet was resuspended in 50 µl of 25 mM NaOH and
subsequently heated at 95°C for 5 min. After neutralization with 4
µl of 1 M Tris-HCl buffer (pH 7.5), the suspension was centrifuged
at 4°C, 20,000 × g for 5 min and used as a template (1 µl) with
optimization condition for UDG-LAMP-AuNPs and PCR assays. The
detection limit of UDG-LAMP-AuNPs detection methods was compared
with that of PCR assay.
In parallel, to count the bacteria colony number,
100 µl of each dilution was spread on TSA in duplicate and
incubated at 37°C for overnight. The bacterial colonies were
counted at the dilution yielding 30–300 colony-forming units (CFUs)
and then the CFU ml−1 of bacterial suspension was
calculated.
Sensitivities of UDG-LAMP-AuNPs and
PCR with spiked contact lens
Contact lens samples (Pretty lens; VASSEN Co., Ltd.,
Pyeongtaek-si gyeonggido, Republic of Korea) were purchased at a
store in Bangkok, Thailand. The contact lens samples were washed
with 4 ml of sterile PBS. The DNA template was prepared as
described below and tested with PCR specific to ecfX gene as
indicated in the above section to confirm that they were negative
for P. aeruginosa. The bacterial suspension and adherence of
P. aeruginosa to contact lens was employed according to a
previous report (25) with some
modifications using known amounts of P. aeruginosa (PA 07).
Briefly, a single bacterial colony on TSA was inoculated into a 5
ml of trypticase soy broth and incubated for overnight at 37°C.
Then, bacterial cells were collected by centrifugation at 2,000 x
g for 10 min and the pellet was washed with sterile PBS
twice before resuspended in PBS. The absorbance at 660 nm was
adjusted to the range of 0.1 which is ~108 CFU
ml−1. Sterile contact lenses were transferred into 2 ml
of 108 CFU ml−1 of PA07 and incubated at 37°C
with shaking at 125 rpm for 24 h.
After 24 h, the contact lenses were removed
aseptically and washed gently with PBS to remove loosely attached
microorganisms before transferred to 4 ml sterile PBS and vortexed
for 1 min to remove the adhered microorganisms. Then, 10-fold
serial dilutions of the cultures were prepared in PBS. For DNA
extraction, the 100 µl of each dilution was centrifuged at 20,000 ×
g for 5 min, the supernatant was removed and the pellet was
resuspended in 50 µl of 25 mM NaOH and subsequently heated at 95°C
for 5 min. After neutralization with 4 µl of 1 M Tris-HCl buffer
(pH 7.5), the suspension was centrifuged at 4°C, 20,000 × g for 5
min and the supernatant was used as a template (1 µl) for
UDG-LAMP-AuNPs and PCR assay. The sensitivity of UDG-LAMP-AuNPs was
compared with that of PCR assay. In parallel, the bacterial
colonies were counted as described above.
Statistical analysis
The results were analysed using the descriptive
statistics tests of SPSS version 23.0 software (IBM Corp., Armonk,
NY, USA). Data are presented as the mean ± standard deviation of
three independent experiments.
Results
Optimization of the temperature,
reaction time and ratio of dUTP to dTTP for Pseudomonas aeruginosa
detection by UDG-LAMP
To determine the optimal conditions for UDG-LAMP
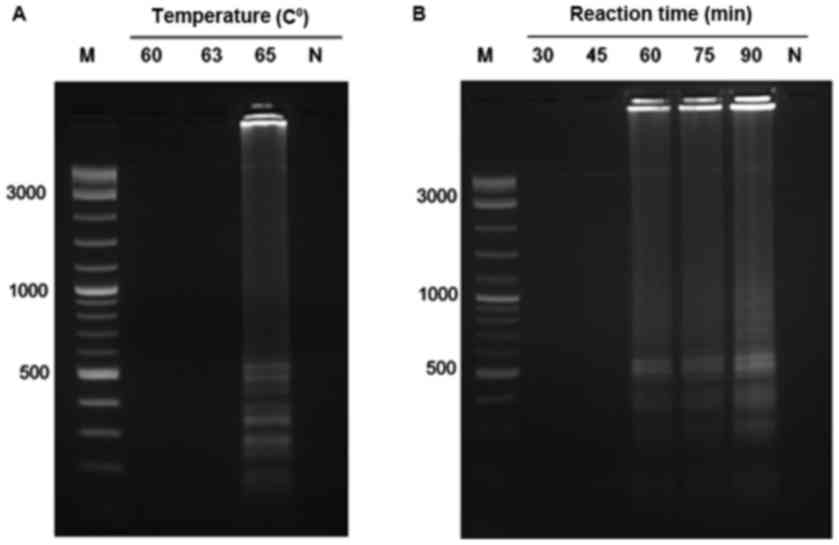
assay, various temperatures for the UDG-LAMP assay were conducted
at 60, 63 and 65°C for 60 min. The ladder-like pattern
characteristic of LAMP reaction was only observed at 65°C tested
temperature; therefore, the temperature at 65°C was chosen for the
subsequent UDG-LAMP assays (Fig.
1A).
To determine the optimal time for UDG-LAMP assay,
the reaction was conducted at 65°C for 30, 45, 60, 75 and 90 min.
The LAMP amplicons could be clearly observed at 60, 75 and 90 min
but no amplification was detected at 30 and 45 min. Therefore, the
reaction time at 60 min was chosen as optimal time for the UDG-LAMP
assay to minimize the reaction time (Fig. 1B).
The LAMP reaction using Bst 2.0 DNA
polymerase was well progressed using dUTP in place of dTTP;
however, the concentration of dUTP affected the visible of
ladder-like pattern characteristic on agarose gel. When dTTP was
totally replaced with dUTP, the UDG-LAMP reaction produced no
ladder-like pattern (Fig. 2, lane
1). Therefore, the ratio between dUTP to dTTP was varied from 80%
dUTP + 20% dTTP; 60% dUTP + 40% dTTP; 40% dUTP + 60% dTTP; 20% dUTP
+ 80% dTTP; and 100% dTTP. The ladder-like pattern was observed at
the ratios of 60% dUTP + 40% dTTP; 40% dUTP + 60% dTTP; 20% dUTP +
80% dTTP; and 100% dTTP (Fig. 2).
Therefore, the ratio of 40% dUTP to 60% dTTP was chosen for the
subsequent UDG-LAMP assays to study the specificity and
sensitivity.
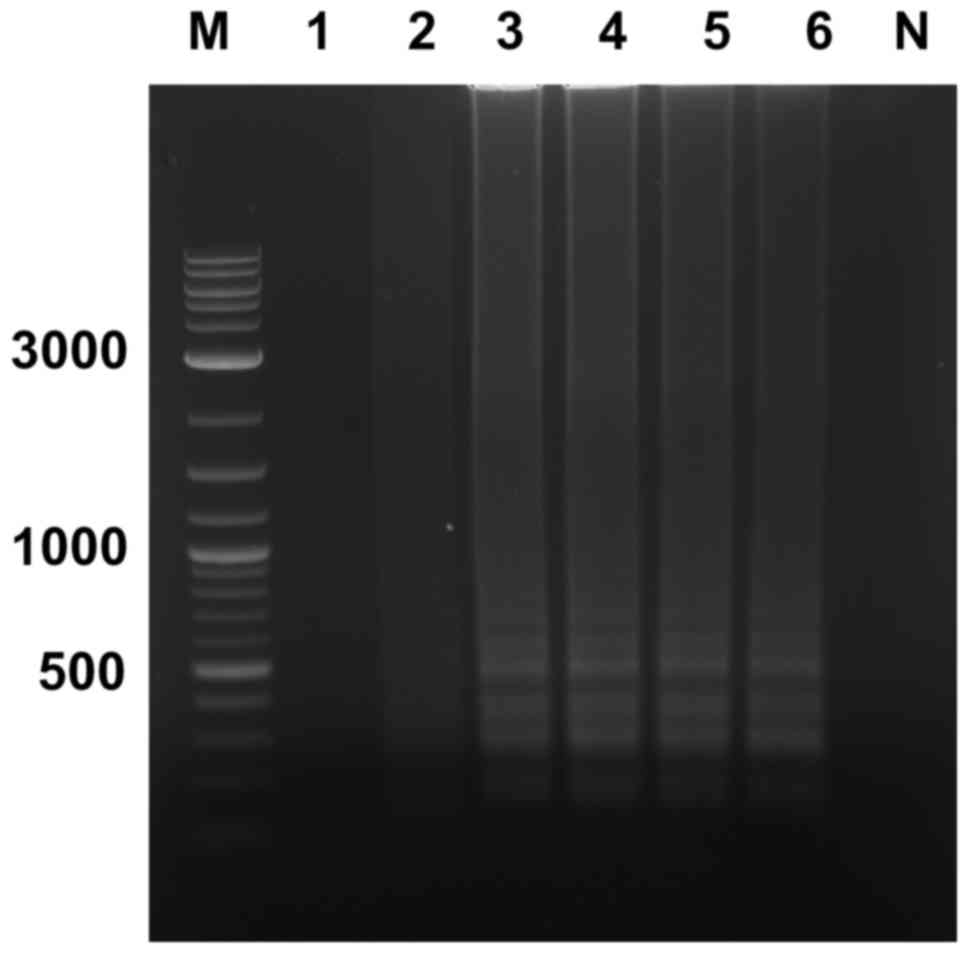 | Figure 2.Optimization of dUTP to dTTP for
loop-mediated isothermal amplification. The ratios of dUTP to dTTP
were as follows: Lane 1, 100% dUTP; lane 2, 80% dUTP + 20% dTTP;
lane 3, 60% dUTP + 40% dTTP; lane 4, 40% dUTP + 60% dTTP; lane 5,
20% dUTP + 80% dTTP; and lane 6, 100% dTTP. Lane M, molecular
weight marker; lane N, negative control (no DNA template); dUTP,
deoxyuridine triphosphate; dTTP, deoxythymidine triphosphate. |
Optimization of the AuNPs-probe for
detection of UDG-LAMP products
To study the effect of salt concentration
(MgSO4), the UDG-LAMP product were hybridized to AuNPs
probe under various concentrations of MgSO4 including 5,
10, 20, 40, 100 and 200 mM. The results showed that 40 mM
MgSO4 yielded the clear difference between positive
sample (red-purple) and negative control (blue-gray or colorless)
(Fig. 3A and B). These results
were corresponded to LAMP reactions followed by UV-visible analysis
(Fig. 3C and D) and the absorbance
at 525 nm clearly confirmed that results (Fig. 3E). Therefore, 40 mM
MgSO4 was used for all subsequent assays.
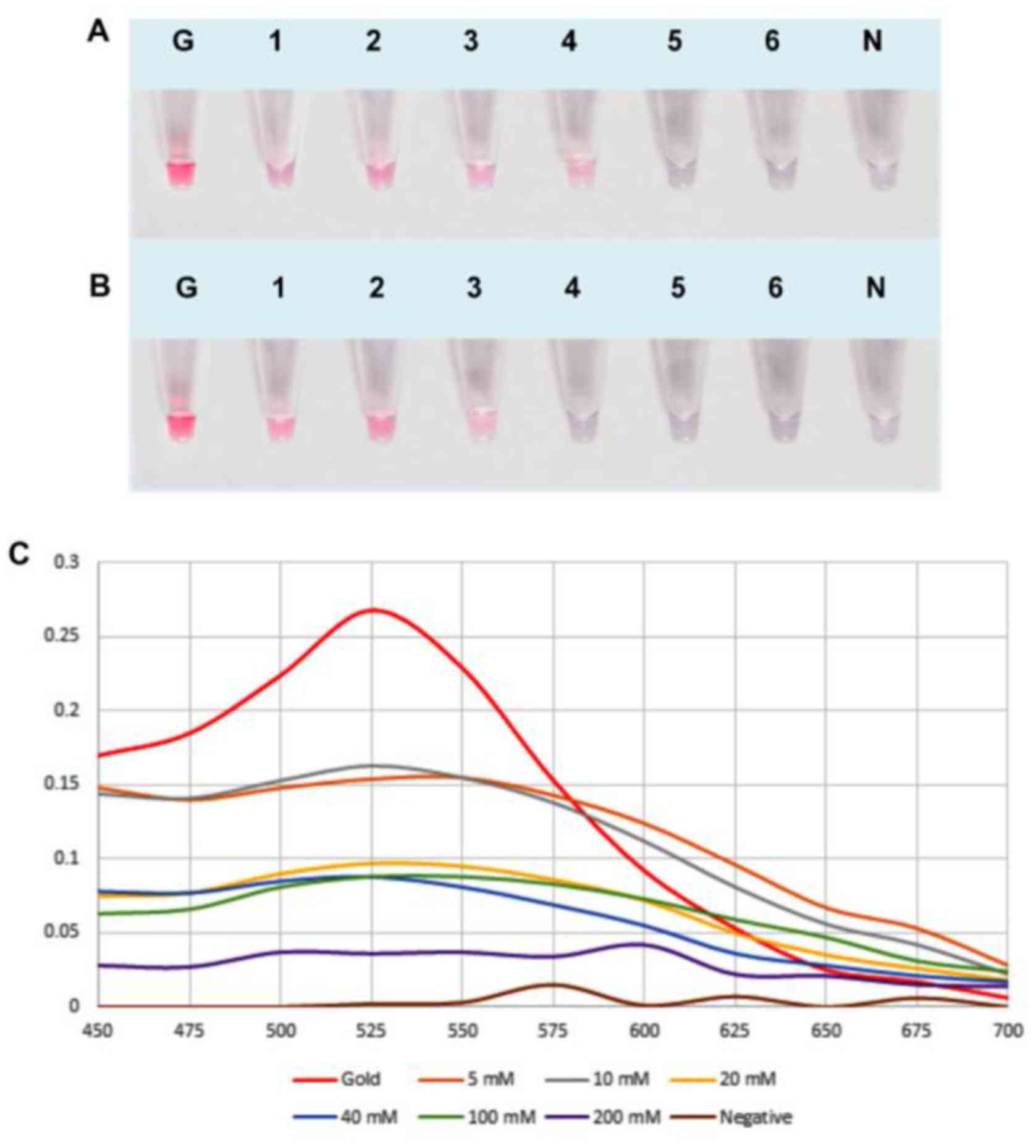 | Figure 3.Optimization of the MgSO4
concentration for UDG-LAMP-AuNPs. (A) Various concentrations of
MgSO4 were added with the AuNPs probe to P.
aeruginosa UDG-LAMP products and (B) non-P. aeruginosa
UDG-LAMP products (negative control). Hybridization was performed
at 65°C for 5 min. Lane G, AuNPs probe only (without salt
solution); lane 1–6, MgSO4 concentration at 5, 10, 20,
40, 100 and 200 mM, respectively; lane N, negative control (no DNA
template). Absorption spectra of the colloidal AuNP probe in the
presence of (C) P. aeruginosa UDG-LAMP products and (D)
non-P. aeruginosa UDG-LAMP products under various
concentrations of MgSO4. (E) Absorption of UV-visible
spectrophotometer at the wavelength of 525 nm. Blue bar indicates
positive samples and the orange bar indicates negative samples.
LAMP, loop-mediated isothermal amplification; UDG-LAMP-AuNPs,
uracil-DNA-glycosylase-supplemented LAMP coupled with nanogold
probe; AuNPs, gold nanoparticles; PA/P. aeruginosa, Pseudomonas
aeruginosa. |
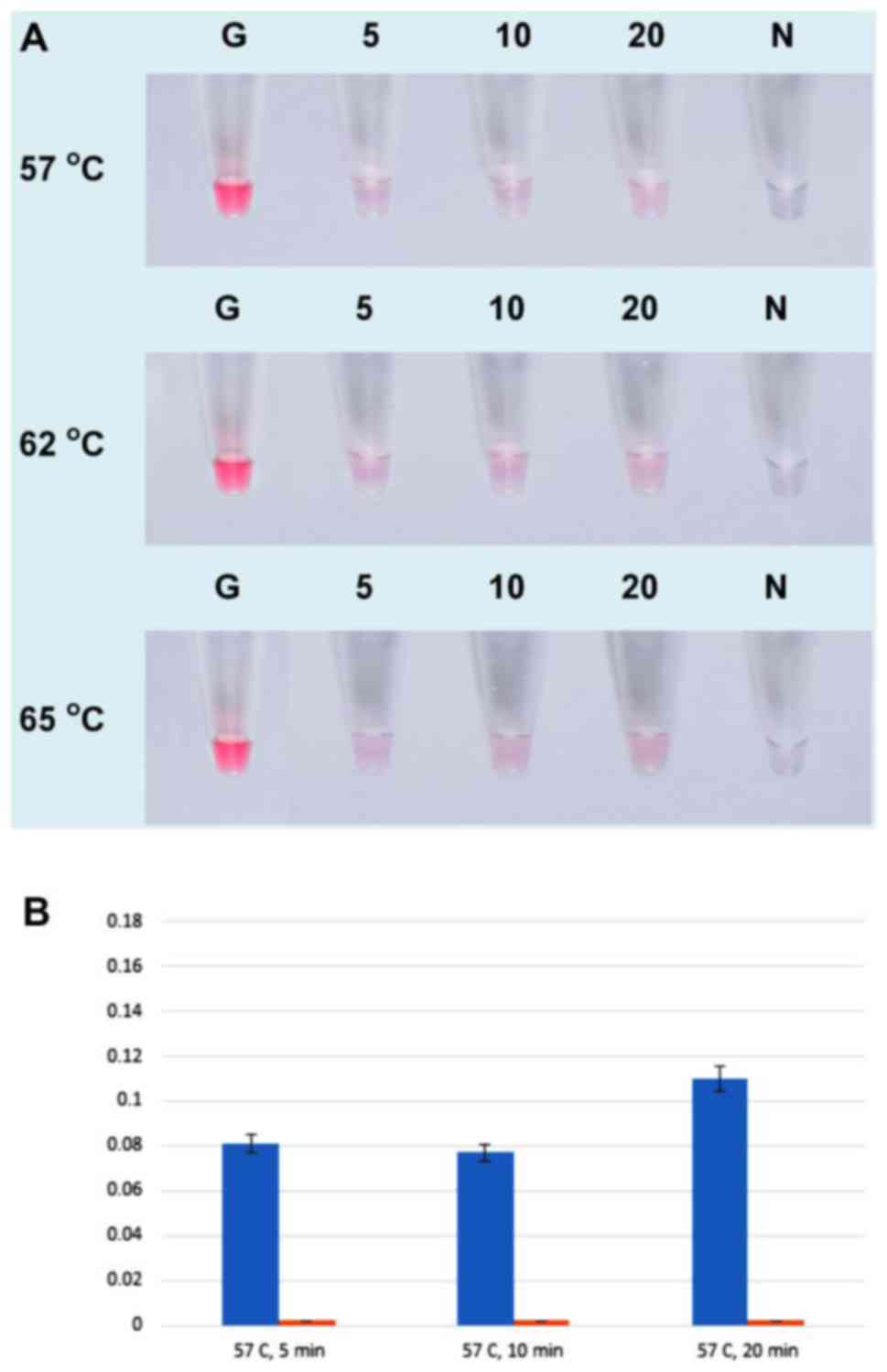
To determine the optimal temperature and time for
hybridization between AuNPs probe and UDG-LAMP product, the
hybridization was conducted at 57, 62 and 65°C for 5, 10, and 20
min at each temperature. The color reactions demonstrated similar
results in all ranges of the tested temperature and time (Fig. 4A). However, the UV-visible analysis
revealed that the hybridization at 65°C for 5 min yielded the
highest absorbance at 525 nm (Fig.
4D). Therefore, the hybridization performed at the temperature
of 65°C for 5 min was chosen for the subsequent reactions.
Specificity of the UDG-LAMP-AuNPs and
PCR assays
The specific testing demonstrated that
UDG-LAMP-AuNPs and PCR specific to ecfX gene revealed
positive results to all tested isolates of P. aeruginosa.
While 8 other Pseudomonas species and all
non-Pseudomonas bacteria yielded negative results (Table I).
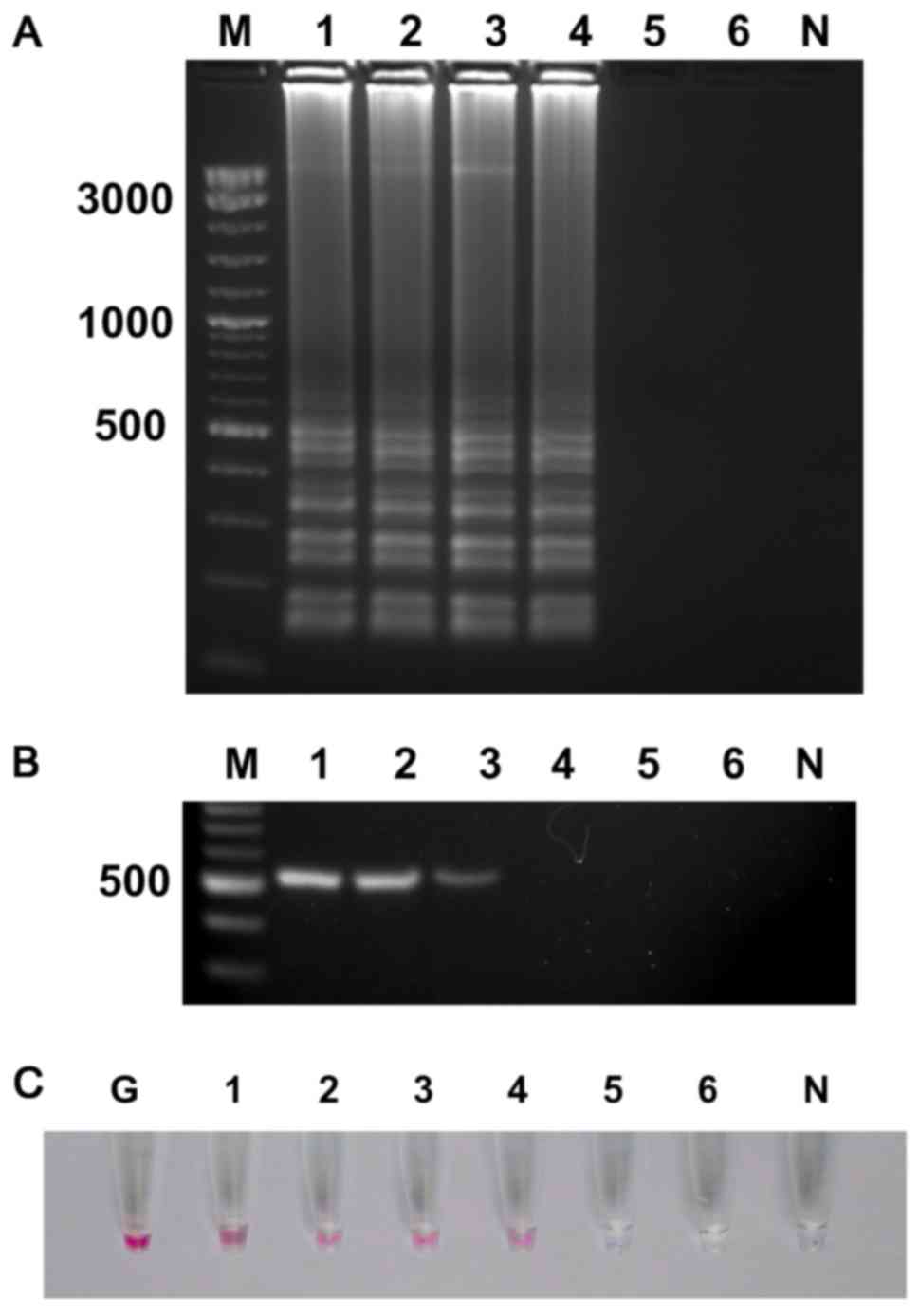
Detection limit of UDG-LAMP-AuNPs and PCR assays
with pure culture. To determine the sensitivity of UDG-LAMP-AuNPs
and PCR assays for detection of P. aeruginosa (PA07), the
stock solution of bacterial culture (1.6×108 CFU
ml−1) was diluted in 10-fold serial dilution and DNA
extracted from each dilution was used in the UDG-LAMP-AuNPs and PCR
assays. The UDG-LAMP and UDG-LAMP-AuNPs assay exhibited the
positive result at 1.6×103 CFU ml−1 or
equivalent to ~3 CFU per reaction (Fig. 5A and C), whereas, the PCR assay
showed the detection limit at 1.6×104 CFU ml-1 or
equivalent to 30 CFU per reaction (Fig. 5B).
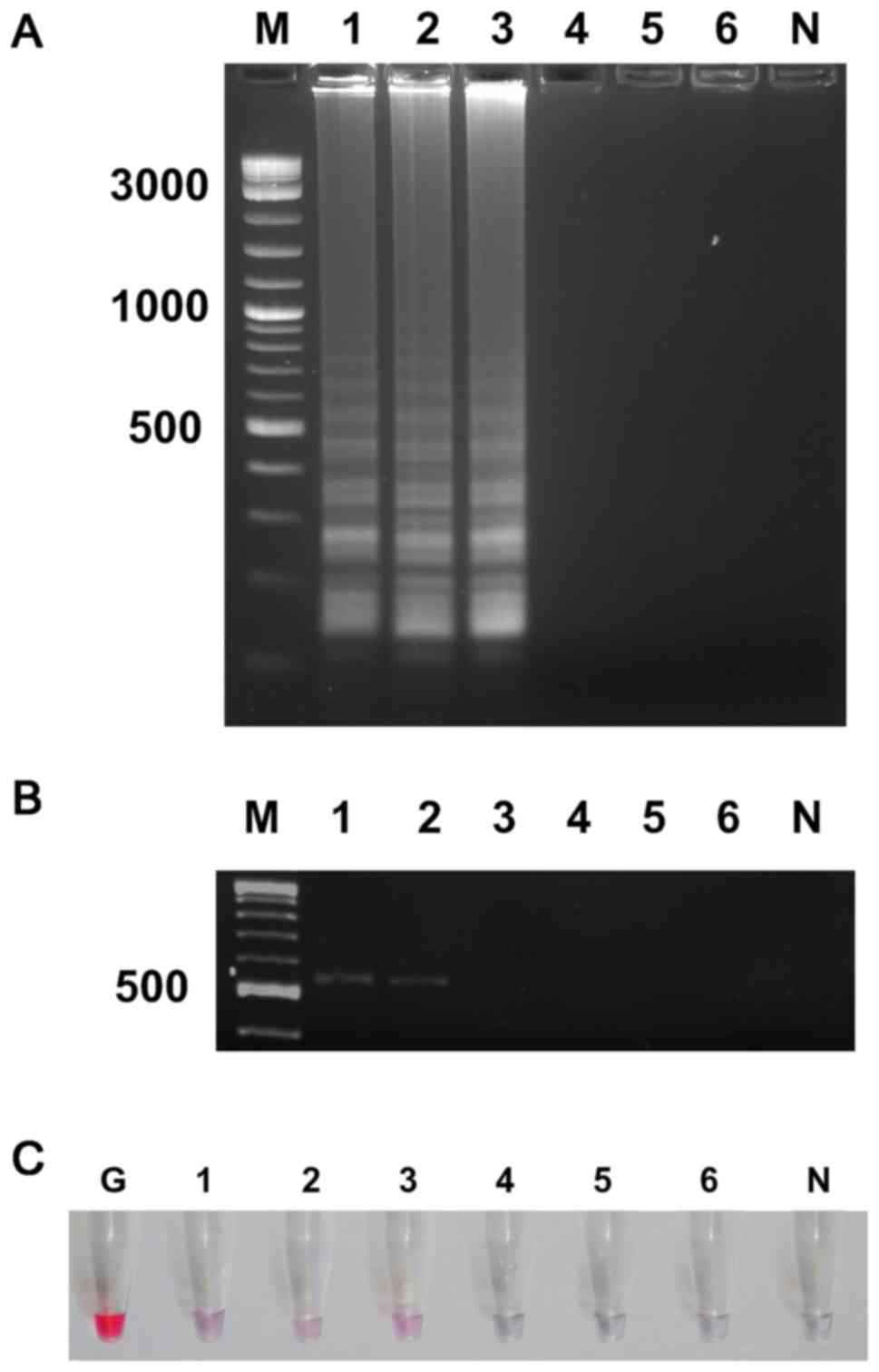
Sensitivity of UDG-LAMP-AuNPs and PCR
assay with spiked contact lens samples
The detection limit of UDG-LAMP-AuNPs and PCR assays
for PA07 spiked into contact lenses was examined. After removal of
loosely attached bacteria, the determined number of bacteria in
washed solution was 1.1×106 CFU ml−1. After
10-fold serial dilutions of washed solution was performed, the
total DNA extracted from each dilution was used to investigate the
detection limit. The results showed the sensitivity of UDG-LAMP and
UDG-LAMP-AuNPs at 1.1×103 CFU ml−1 or
equivalent to 2 CFU per reaction (Fig.
6A and C). Whereas, the PCR assay showed detection limit at
1.1×104 CFU ml−1 or equivalent to 20 CFU per
reaction (Fig. 6B).
Discussion
P. aeruginosa is one of the most important
pathogens causing human infections such as pneumonia, bacteremia,
urinary tract infections and keratitis (26,27).
Microbial keratitis is a serious complication of contact lens wear
and P. aeruginosa is a common causative agents associated
with infectious keratitis reported in many countries (28). In order to manage patient
infection, a rapid and specific detection method is required to
differentiate keratitis caused by P. aeruginosa from other
microorganism infections.
In the present study, a UDG-LAMP-AuNP method
targeting to the ecfX gene of P. aeruginosa was
successfully developed. The optimum temperature and time for
UDG-LAMP reaction were 65°C and 60 min, respectively. Upon
hybridization with nanogold labeled probe and salt addition, the
positive reaction (red-purple color) of UDG-LAMP products could be
observed within 5–10 min. This is more rapid than the PCR method
and more suitable for small laboratory since the sophisticated
equipment is not required. The LAMP assay is highly sensitive for
DNA amplification; therefore, it is prone to carryover
contamination. In our study, the UDG was effectively used to digest
the uracil incorporated amplicons in a one-tube, closed vessel
reaction. This UDG-LAMP strategy was successfully employed with
Salmonella Typhimurium (22) and multiplex LAMP for simultaneous
detection of white spot syndrome virus (WSSV) and infectious
hypodermal and hematopoietic necrosis virus (IHHNV) in shrimp
(29). In the present study, the
optimum ratio of 40% dUTP to 60% dTTP was used. This ratio was in
agreement with that of previous reports for detection of
Salmonella Typhimurium (the ratio of 1:1) (22) and WSSV-IHHNV (the ratio of 5/8)
(29).
The gold nanoparticle can interact with disulfide
modified DNA probe and the ability to change color (from red to
blue) on self-assembly is due to aggregation at an optimal salt
concentration. In our study, under the low-salt concentration (5 to
10 mM MgSO4 solution), the AuNP probes were still
stabilized in both control samples and the samples containing P.
aeruginosa. However, at 40 mM MgSO4 the aggregation
of AuNP probes in the control sample was clearly observed
(blue-gray or colorless) and could be distinguishable from positive
sample containing P. aeruginosa (red color). This result was
in good agreement with previous report on specific detection of
WSSV (20).
Previous studies on detection of P.
aeruginosa showed that the false-negative results could be
obtained from PCR assays targeting to the toxA and
algD genes due to the highly polymorphic characteristic and
sequence variation of P. aeruginosa (3,30).
However, the PCR assay targeting to ecfX for specific
detection of P. aeruginosa has been reported (3). In our UDG-LAMP-AuNPs assay, four
specific LAMP primers and DNA probe targeting to ecfX gene
of P. aeruginosa were designed and employed to ensure high
specificity of nucleic acid amplification (3). Positive results were obtained for all
tested P. aeruginosa isolates without cross-reaction to
other bacterial species, demonstrating that these LAMP primers and
DNA probe were specific for identification of P.
aeruginosa.
In the present study, the sensitivity of
UDG-LAMP-AuNPs assay in pure culture was 1.6×103 CFU
ml−1 or equivalent to 3 CFU per reaction, 10 times more
sensitive than that of PCR. The detection limit of UDG-LAMP-AuNPs
at 3 CFU per reaction was more sensitive than traditional LAMP
method for detection of P. aeruginosa based on oprI
gene (10 CFU per reaction) (15).
In the case of artificially inoculated contact
lenses, the detection limit of UDG-LAMP-AuNPs was
1.1×103 CFU ml−1 or equivalent to 2 CFU per
reaction, 10 times more sensitive than that of PCR assay
(1.1×104 CFU ml−1 or equivalent to 20 CFU per
reaction). The sensitivity of UDG-LAMP-AuNPs in both pure culture
and spiked contact lenses were more sensitive than that of the
conventional PCR assay. These results agreed with previous studies
that demonstrate a better sensitivity of LAMP compared to that of
PCR (13,15,16,31).
The detection limit of UDG-LAMP-AuNPs for spiked contact lenses at
2 CFU per reaction was comparable to that of colorimetric LAMP
detection of P. aeruginosa inoculated in mouse feces at 3.25
CFU per reaction (16) and LAMP
assay for direct detection of P. aeruginosa from equine
genital swabs at 11 DNA copies per reaction (32).
Recently, a microchip electrical sensing for
detection of P. aeruginosa was developed. The detection
limit of spiked eye wash samples at 10 CFU ml−1 was
reported (33). Another study
based on magnetic relaxation switch (MRSw) aptasensor for detection
of P. aeruginosa with the sensitivity of 50 CFU
ml−1 was also reported (34). In our study, an inferior detection
limit of 1.1×103 CFU per ml was obtained. However, the
UDG-LAMP-AuNPs reaction could be performed with simple and
inexpensive heating block while the MRSw method required a large
size and high cost of magnetic relaxation scanners which is
improper for field study.
In conclusion, the first UDG-LAMP-AuNPs method for
detection of P. aeruginosa based on ecfX gene was
successfully established. The assay demonstrated high specificity
and high sensitivity of ~3 CFU per reaction with pure culture and 2
CFU per reaction with spiked contact lens samples. The developed
UDG-LAMP-AuNPs assay is a sensitive, simple, rapid and valuable
tool for specific diagnosis of P. aeruginosa in contaminated
biological samples.
Acknowledgements
The present study was supported by the Higher
Education Research Promotion, Office of the Higher Education
Commission, Thailand. The authors are also indebted to all of the
institutes listed in Table I for
providing the bacteria used in the present study.
References
|
1
|
van der Kooij D, Oranje JP and Hijnen WA:
Growth of Pseudomonas aeruginosa in tap water in relation to
utilization of substrates at concentrations of a few micrograms per
liter. Appl Environ Microbiol. 44:1086–1095. 1982.PubMed/NCBI
|
|
2
|
Romling U, Kader A, Sriramulu DD, Simm R
and Kronvall G: Worldwide distribution of Pseudomonas aeruginosa
clone C strains in the aquatic environment and cystic fibrosis
patients. Environ Microbiol. 7:1029–1038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lavenir R, Jocktane D, Laurent F, Nazaret
S and Cournoyer B: Improved reliability of Pseudomonas aeruginosa
PCR detection by the use of the species-specific ecfX gene target.
J Microbiol Methods. 70:20–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onlen Y, Tamer C, Oksuz H, Duran N, Altug
ME and Yakan S: Comparative trial of different anti-bacterial
combinations with propolis and ciprofloxacin on Pseudomonas
keratitis in rabbits. Microbiol Res. 162:62–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McLaughlin-Borlace L, Stapleton F,
Matheson M and Dart JK: Bacterial biofilm on contact lenses and
lens storage cases in wearers with microbial keratitis. J Appl
Microbiol. 84:827–838. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiser R, Donoghue D, Weightman A and
Mahenthiralingam E: Evaluation of five selective media for the
detection of Pseudomonas aeruginosa using a strain panel from
clinical, environmental and industrial sources. J Microbiol
Methods. 99:8–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jimenez L, Smalls S and Ignar R: Use of
PCR analysis for detecting low levels of bacteria and mold
contamination in pharmaceutical sample. J Microbiol Methods.
41:259–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deschaght P, Van daele S, De Baets F and
Vaneechoutte M: PCR and the detection of Pseudomonas aeruginosa in
respiratory samples of CF patient. A literature review. J Cyst
Fibros. 10:293–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anuj SN, Whiley DM, Kidd TJ, Bell SC,
Wainwright CE, Nissen MD and Sloots TP: Identification of
Pseudomonas aeruginosa by a duplex real-time polymerase chain
reaction assay targeting the ecfX and gyrB genes. Diagn Microbiol
infect Dis. 63:127–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCulloch E, Lucas C, Ramage G and
Williams C: Improved early diagnosis of Pseudomonas aeruginosa by
real-time PCR to prevent chronic colonization in a pediatric cystic
fibrosis population. J Cyst Fibros. 10:21–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Notomi T, Okayama H, Masubuchi H, Yonekawa
T, Watanabe K, Amino N and Hase T: Loop-mediated isothermal
amplification of DNA. Nucleic Acids Res. 28:e632000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Surasilp T, Longyant S, Rukpratanporn S,
Sridulyakul P, Sithigorngul P and Chaivisuthangkura P: Rapid and
sensitive detection of Vibrio vulnificus by loop-mediated
isothermal amplification combined with lateral flow dipstick
targeted to rpoS gene. Mol Cell Probes. 25:158–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prompamorn P, Sithigorngul P,
Rukpratanporn S, Longyant S, Sridulyakul P and Chaivisuthangkura P:
The development of loop-mediated isothermal amplification combined
with a lateral flow dipstick for detection of Vibrio
parahaemolyticus. Lett Appl Microbiol. 52:344–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Zhai L, Bie X, Lu Z, Kong X, Yu Q,
Lv F, Zhang C and Zhao H: A novel visual loop-mediated isothermal
amplification assay targeting gene 62181533 for the detection of
Salmonella spp. in foods. Food control. 60:230–236. 2016.
View Article : Google Scholar
|
|
15
|
Zhao X, Wang L, Li Y, Xu Z, Li L, He X,
Liu Y, Wang J and Yang L: Development and application of a
loop-mediated isothermal amplification method on rapid detection of
Pseudomonas aeruginosa strains. World J Microbiol Biotechnol.
27:181–184. 2011. View Article : Google Scholar
|
|
16
|
Goto M, Shimada K, Sato A, Takahashi E,
Fukasawa T, Takahashi T, Ohka S, Taniguchi T, Honda E, Nomoto A, et
al: Rapid detection of Pseudomonas aeruginosa in mouse feces by
colorimetric loop-mediated isothermal amplification. J Microbiol
Methods. 81:247–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Xu X, Wu Q and Zhang J: Rapid and
sensitive detection of Pseudomonas aeruginosa in bottled water by
loop-mediated isothermal amplification. Eur Food Res Technol.
236:209–215. 2013. View Article : Google Scholar
|
|
18
|
Shi H, Chen Z and Kan J: Development of
loop-mediated isothermal amplification assays for genotyping of
Type III Secretion System in Pseudomonas aeruginosa. Lett Appl
Microbiol. 61:361–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaroenram W, Arunrut N and Kiatpathomchai
W: Rapid and sensitive detection of shrimp yellow head virus using
loop-mediated isothermal amplification and a colorogenic nanogold
hybridization probe. J Virol Methods. 186:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seetang-Nun Y, Jaroenram W, Sriurairatana
S, Suebsing R and Kiatpathomchai W: Visual detection of white spot
syndrome virus using DNA-functionalized gold nanoparticles as
probes combined with loop-mediated isothermal amplification. Mol
Cell Probes. 27:71–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watthanapanpituck K, Kiatpathomchai W, Chu
E and Panvisavas N: Identification of human DNA in forensic
evidence by loop-mediated isothermal amplification combined with a
colorimetric gold amplification combined with a colorimetric gold
nanoparticle hybridization probe. Int J Legal Med. 128:923–931.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh K, Mage PL, Csordas AT, Eisenstein M
and Soh HT: Simultaneous elimination of carryover contamination and
detection of DNA with uracil-DNA-glycosylase-supplemented loop
mediated isothermal amplification (UDG-LAMP). Chem Commun.
50:3747–3749. 2014. View Article : Google Scholar
|
|
23
|
Weisburg WG, Barns SM, Pelletier DA and
Lane DJ: 16S ribosomal DNA amplification for phylogenic study. J
Bacteriol. 173:697–703. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamazaki W, Seto K, Taguchi M, Ishibashi M
and Inoue K: Sensitive and rapid detection of cholera toxin
producing Vibrio cholerae using a loop-mediated isothermal
amplification. BMC Microbiol. 8:942008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan KY, Cho P and Boost M: Microbial
adherence to cosmetic contact lenses. Cont Lens Anterior Eye.
37:267–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jarvis WR and Martone WJ: Predominant
pathogens in hospital infections. J Antimicrob Chemother. 29 Suppl
A:S19–S24. 1992. View Article : Google Scholar
|
|
27
|
Schwartz T, Volkmann H, Kirchen S, Kohnen
W, Schön-Hölz K, Jansen B and Obst U: Real-time PCR detection of
Pseudomonas aeruginosa in clinical and municipal wastewater and
genotyping of the ciprofloxacin-resistant isolates. FEMS Microbiol
Ecol. 57:158–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willcox MD: Management and treatment of
contact lens-related Pseudomonas keratitis. Clin Ophthalmol.
6:919–924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He L and Xu HS: Development of a multiplex
loop-mediated isothermal amplification (mLAMP) method for the
simultaneous detection of white spot syndrome virus and infectious
hypodermal and hematopoietic necrosis virus in penaeid shrimp.
Aquaculture. 311:94–99. 2011. View Article : Google Scholar
|
|
30
|
Qin X, Emerson J, Stapp J, Stapp L, Abe P
and Burns JL: Use of real-time PCR with multiple targets to
identify Pseudomonas aeruginosa and other nonfermenting
gram-negative bacilli from patients with cystic fibrosis. J Clin
Microbiol. 41:4312–4317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Srisuk C, Chaivisuthangkura P,
Rukpratanporn S, Longyant S, Sridulyakul P and Sithigorngul P:
Rapid and sensitive detection of Vibrio cholerae by loop-mediated
isothermal amplification targeted to the gene of outer membrane
protein ompW. Lett Appl Microbiol. 50:36–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diribe O, Fitzpatrick N, Sawyer J, La
Ragione R and North S: A Rapid and simple loop-mediated isothermal
amplification assay for the detection of Pseudomonas aeruginosa
from equine genital swabs. J Equine Vet Sci. 35:929–934. 2015.
View Article : Google Scholar
|
|
33
|
Pandya HJ, Kanakasabapathy MK, Verma S,
Chug MK, Memic A, Gadjeva M and Shafiee H: Label-free electrical
sensing of bacteria in eye wash samples: A step towards
point-of-care detection of pathogens in patients with infectious
keratitis. Biosens Bioelectron. 91:32–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia F, Xu L, Yan W, Wu W, Yu Q, Tian X,
Dai R and Li X: A magnetic relaxation switch aptasensor for the
rapid detection of Pseudomonas aeruginosa using superparamagnetic
nanoparticles. Microchim Acta. 184:1539–1545. 2017. View Article : Google Scholar
|