Introduction
Renal cell carcinoma (RCC) is a common malignancy
and is the third leading cause of cancer-associated mortality in
cancer of the urinary system (1).
The morbidity rates of RCC continue to increase rapidly, whereas
the overall five-year survival rate has not improved significantly,
resulting in unsatisfactory prognosis (2). Therefore, examining the molecular
interactions occurring in the initiation and progression of RCC may
assist in identifying more therapeutic targets and investigating
novel approaches in prognosis.
High mobility group AT-hook 2 (HMGA2) is a member of
the HMGA group, which functions as a transcriptional enhancer in
the alteration of chromatin structure via binding to AT-rich
regions in DNA sequences (3).
HMGA2 is an oncofetal protein, the expression of which is at low
levels or absent with the differentiation of tissues, which is in
contrast to its high level of expression with the dedifferentiation
of several types of malignant tumor (4,5).
Notably, it has been observed that the abnormal expression of HMGA2
shows high correlation with various malignancies, including cancer
of the colon, breast, kidney, pancreas, liver and lung (3,6–8).
Previous studies have demonstrated that the level of HMGA2 is
positively correlated with tumor size and Fuhrman grade in RCC, and
the high expression of HMGA2 leads to poor prognosis of patients,
which indicates that HMGA2 is important in the progression of RCC
(7).
MicroRNAs (miRNAs) are a series of non-coding,
single-stranded RNA molecules, consisting of 20–24 nucleotides
(9). They are endogenously
expressed and negatively correlated with the transcription of genes
through binding to their 3′-untranslated-region (3′UTR) (10). Previous studies have demonstrated
that the aberrant expression of miRNAs contributes to tumor growth
and is involved in regulating the physiological behavior of tumor
cells through the modulation of target genes at mRNA or protein
levels. For example, miRNA-129 (miR-129) inhibits the metastasis of
prostate cancer by binding to centriolar coiled-coil protein 110
(11), miR-200a regulates the
proliferation and metastasis of pancreatic cancer through
modulating the DEK gene (12), and
miR-543 is downregulated in colorectal cancer samples, acting as
tumor suppressor by targeting KRAS, MTA1 and HMGA2 (13).
In the present study, the discrepant expression
between miR-539 and HMGA2 was analyzed in RCC tumor tissues, and it
was predicted and confirmed that miR-539 binds to HMGA2.
Subsequently, the regulatory effect of miR-539 on the proliferation
and apoptosis of RCC cells was confirmed. These findings suggest
that miR-539 may be important in the progression of RCC and may be
used as a biomarker for predicting the growth of RCC.
Materials and methods
Clinical specimens
The present study was approved by the Institutional
Ethics Committee of Ren'min Hospital Affiliated to Wuhan University
(Wuhan, China) and performed according to the guidelines on ethical
management. Tissue specimens from a 23 cases of RCC and 19 paired
normal tissues were collected from the Department of Urology,
Ren'min Hospital of Wuhan University between 2015 and 2017. Written
informed consent was signed by all participants prior to the study.
The tumor stage and grade were classified according to the
tumor-node-metastasis staging system of the American Joint
Committee on Cancer (14). All
tissues were divided into two sections, one of which was fixed in
4% paraformaldehyde, and the other was immediately frozen and
stored at −80°C for subsequent analysis. The clinical data of the
participants are shown in Table
I.
 | Table I.Clinicopathological features of
participants. |
Table I.
Clinicopathological features of
participants.
|
|
| HMGA2 expression
(n) |
|
|---|
|
|
|
|
|
|---|
| Variables | Group | High | Low | Total | P-value |
|---|
| Gender | Male | 7 | 6 | 13 | 0.509 |
|
| Female | 4 | 6 | 10 |
|
| Age (years) | <60 | 8 | 9 | 17 | 0.901 |
|
| ≥60 | 3 | 3 | 6 |
|
| Clinical stage | Stage I–II | 5 | 10 | 15 | 0.056 |
|
| Stage III–IV | 6 | 2 | 8 |
|
| T classification | T1-2 | 4 | 10 | 14 | 0.021 |
|
| T3-4 | 7 | 2 | 9 |
|
| Lymph node
metastasis | N0 | 7 | 11 | 18 | 0.103 |
|
| N1-2 | 4 | 1 | 5 |
|
| M
classification | M0 | 9 | 11 | 20 | 0.483 |
|
| M1-2 | 2 | 1 | 3 |
|
| Differentiation
degree | Well-moderate | 4 | 12 | 16 | 0.007 |
|
| Low | 6 | 1 | 7 |
|
Cell lines and cell culture
The 786-O human kidney cancer cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in minimal Roswell Parker Memorial Institute-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% FBS (Wuhan Biofavor Biotech Services Co.,
Ltd., Wuhan, China) at 37°C under normoxic conditions (5%
CO2, 95% O2).
Transfection and plasmid
construction
The 786-O cells were transfected with miR-539 mimics
and miR-539 inhibitors (Biossci Biotechnology Co., Ltd., Wuhan,
China) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A total
of 1.0×106 cells/ml cells were transferred to 24-well
plates for group comparison experiments. The cells were
serum-starved for 24 h for further analyses prior to reaching a
confluence of 90%.
The wild-type sequence of the HMGA2 3′UTR containing
predicted miR-539 binding site was amplified from the 786-O cells
using the polymerase chain reaction (PCR) method. The mutant 3′UTR
sequence of HMGA2 was produced using an overlap-extension PCR
method. Subsequently, the wild-type and mutant sequences were
subcloned into a psiCHECK-2 vector (Promega Corporation, Madison,
WI, USA).
In silico prediction
The binding of miR-539 to HMGA2 was predicted using
open access databases TargetScan (www.targetscan.org), miRanda (www.microrna.org). and miRwalk2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/),
and a putative binding site in the 3′UTR of HMGA2 for miR-539 was
identified (Fig. 2C).
Luciferase reporter assays
For the luciferase reporter assay, the 786-O cells
were seeded into a 24-well plate (2×103 cells per well),
and then co-transfected with the miR-539 mimics and
HMGA2-3′UTR-luciferase plasmids. At 48 h post-transfection, the
cells were collected and lysed. The luciferase activities were
analyzed using a Dual-Luciferase Reporter Assay system (Promega
Corporation).
Immunohistochemistry
The expression of HMGA2 was examined using
immunohistochemical staining. The tissues were sliced into 3-µm
sections and deparaffinized in xylene, followed by dehydration in
gradient ethanol and blocking with 3% hydrogen peroxide for 15 min
at room temperature. The sections were then incubated with rabbit
polyclonal anti-HMGA2 (cat. no. ab97276; 1:500; Abcam, Cambridge,
UK) antibodies at 4°C overnight. Following washing three times with
PBS, the sections were stained using diaminobenzidine reagents and
counterstained with hematoxylin and the results were observed with
an Olympus BX50 light microscope (Olympus Corporation, Tokyo,
Japan).
Western blot analysis
Total cellular proteins were extracted with
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Jiangsu, China). The protein concentration was
measured using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology). Briefly, equivalent quantities of protein sample
(40 µg/lane) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
electrotransferred onto polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Subsequently, the membrane
was blocked at 4°C for 1 h with TBS containing 0.05% Tween-20
(TBST) buffer with 5% non-fat milk and then incubated with the
following primary antibodies against HMGA2 (1:1,000; cat. no.
ab97276; Abcam), AKT (1:1,000; cat. no. sc-8312; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), phosphorylated (p)-AKT
(1:1,000; cat. no. sc-33437; Santa Cruz Biotechnology, Inc.),
mammalian target of rapamycin (1:500, mTOR; cat. no. sc-8319; Santa
Cruz Biotechnology, Inc.) and p-mTOR (1:500, cat. no. sc-101738;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. Following
incubation with secondary antibodies conjugated with horseradish
peroxidase (LK2001 and LK2003; 1:100; Sungene Biotech, Co., Ltd.,
Tianjin, China) for 1 h at room temperature, the bands were
examined using an enhanced chemiluminescence system (MultiSciences
Biotech Co., Ltd., Hangzhou, China).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the clinical specimens
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Subsequently, the RNAs
were reversed transcribed into cDNA using a reverse transcription
reagent kit (Takara Biotechnology Co., Ltd., Dalian China).
Subsequently, cDNA was amplified using an SYBR Green mix kit and
the ABI 7900 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) following the manufacturer's instructions.
PCR was performed with the following thermocycling conditions:
Initial denaturation at 94°C for 4 min, 40 cycles of 94°C for 30
sec, 56°C for 30 sec and 72°C for 25 sec, using the ABI 7900
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific
Inc.). The primer sequences are shown in Table II. Relative levels of miR-539 and
mRNA expression levels of HMGA2 were normalized to that of small
nuclear RNA U6 (for miRNAs) or GAPDH (for mRNAs) respectively. The
relative expression of miRNA or mRNA was calculated using the
2−ΔΔCt method (15).
 | Table II.Primers for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II.
Primers for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) |
|---|
| HMGA2 | F:
CGAAAGGTGCTGGGCAGCTCCGG |
|
| R:
CCATTTCCTAGGTCTGCCTCTTG |
| miR-539 | F:
GGAGAAAUUUCCUUGUGUGU |
|
| R:
UUUCUUUAAAGGAACAUACA |
| U6 snRNA | F:
CTCGCTTCGGCAGCACATATACT |
|
| R:
ACGCTTCACGAATTTGCGTGTC |
| GAPDH | F:
TGAAGGTCGGTGTGAACGGATTTGGTC |
|
| R:
CATGTAGGCCATGAGGTCCACCAC |
Cell proliferation assay
The 786-O cells were cultured in 96-well plates
(2×103 cells per well) for 24 h. The cells were then
stained with 10 µl of 5 mg/ml MTT per well (Sigma-Aldrich; Merck
Millipore; Darmstadt, Germany) for 4 h at 37°C. The culture medium
was then discarded and 150 µl of dimethyl sulfoxide was added. The
absorbance was detected at 490 nm with an ELX-800 spectrometer
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Cell apoptosis assay
Cell apoptosis was measured using Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
(BD Pharmingen, San Diego, CA, USA) according to the manufacturer's
protocol. In brief, the 786-O cells were collected in 6-well plates
at a concentration of 105 cells/ml. Annexin V-FITC (5
µl) and PI (5 µl) were then distributed into each well, and the
cells were incubated in the dark for 15 min to undergo flow
cytometry (BD LSRII; BD Pharmingen).
Statistical analysis
All data are presented as the mean ± standard
deviation. Differences were assessed by two-tailed Student's t-test
and χ2 test, as appropriate. P≤0.05 was considered to
indicate a statistically significant difference. All experiments
were performed at least three times. Statistical analyses were
performed using SPSS 19.0 (IBM SPSS, Armonk, NY, USA).
Results
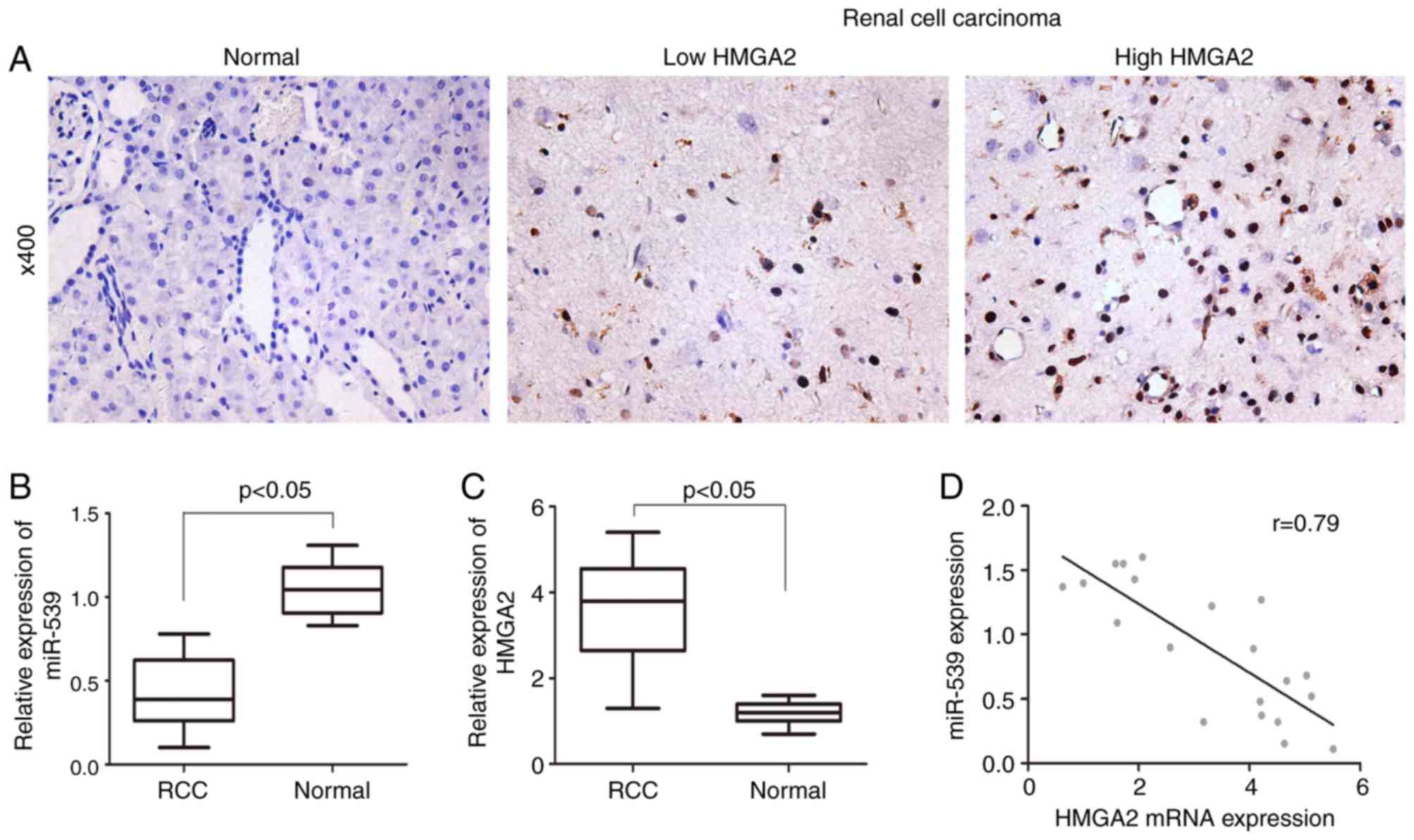
Expression of miR-539 negatively
correlates with the expression of HMGA2 in RCC samples
To examine the expression of HMGA2 in RCC samples
and adjacent normal control samples, immunohistochemistry was
performed to stain tissues from 23 cases of RCC tissues and 19
paired normal tissues. As shown in Fig. 1A, HMGA2 was predominantly expressed
in the nucleus of cells and the expression of HMGA2 was
significantly increased in the RCC tissues, compared with that in
the adjacent normal tissues. In addition to the progression of RCC,
HMGA2 was expressed at a higher level in advanced samples. By
contrast, the RT-qPCR results revealed a lower expression of
miR-539 in RCC samples, compared with that in paired normal samples
(Fig. 1B and C). Pearson's
correlation analysis was performed, and the result showed that the
expression of miR-539 was negatively correlated with the expression
of HMGA2 (Fig. 1D).
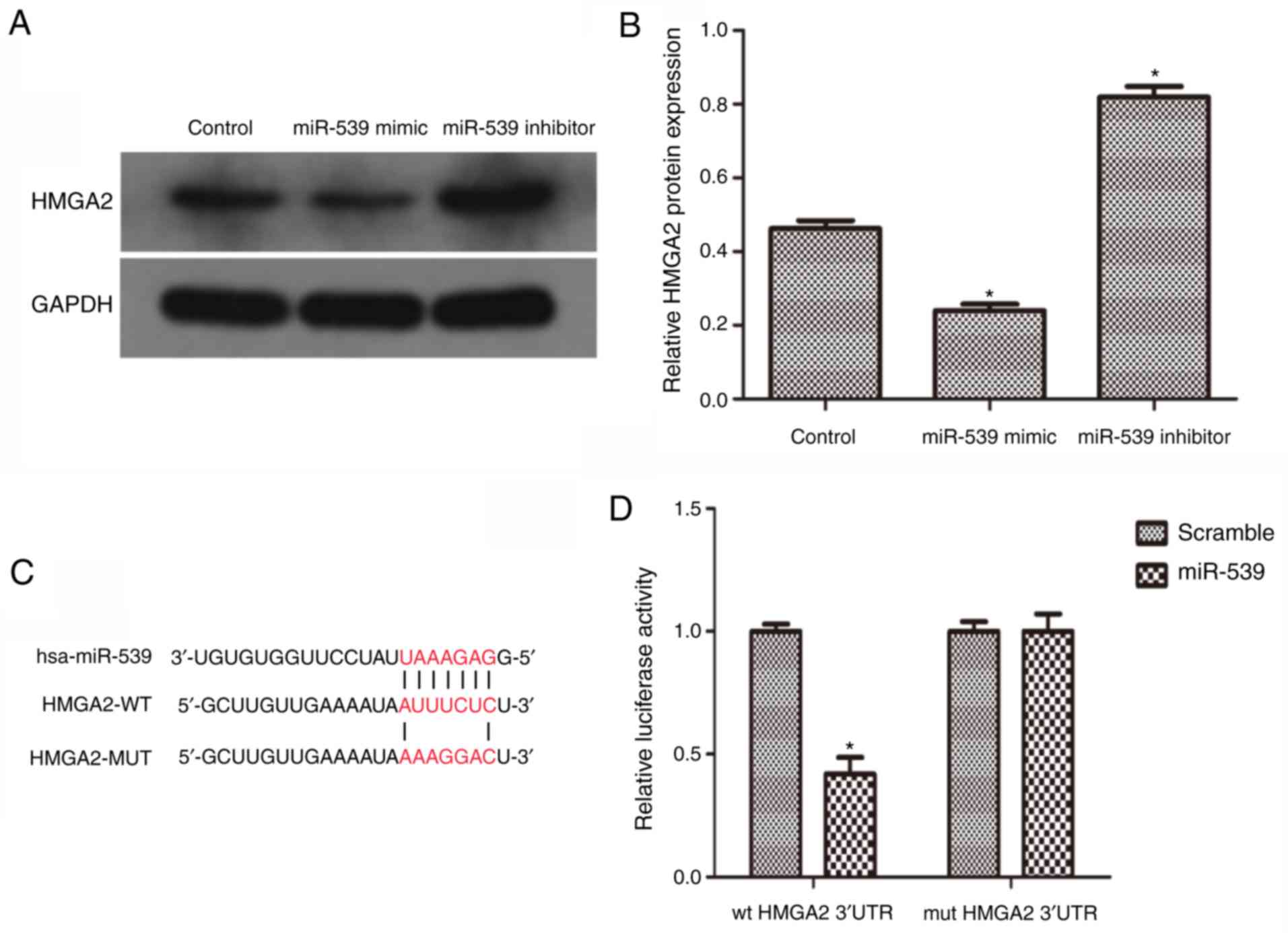
miR-539 directly regulates the
expression of HMGA2
To further confirm the hypothesis that miR-539
downregulates the expression of HMGA2, the 786-O RCC cell line was
used. The 786-O cells were transfected with miR-539 mimics or
inhibitors to obtain cells with miR-539 overexpression or
knockdown. Using western blot analysis, the expression of HMGA2 was
measured. As the data revealed, the expression of HMGA2 was
significantly downregulated in the cells overexpressing miR-539,
but was moderately increased in the miR-539-knockdown cells
(Fig. 2A and B). Subsequently, it
was predicted that miR-539 was an upstream regulator of HMGA2 using
open access databases and a putative binding site in the 3′UTR of
HMGA2 for miR-539 was identified (Fig.
2C). To confirm this prediction, a luciferase reporter assay
was performed. The result revealed that the reporter activity of
the HMGA2 3′UTR was significantly suppressed in the cells
overexpressing miR-539. However, this effect was abrogated when the
putative binding site in the 3′UTR of HMGA2 was mutated (Fig. 2D). Taken together, these results
suggested that miR-539 negatively regulated the expression of HMGA2
by directly targeting it.
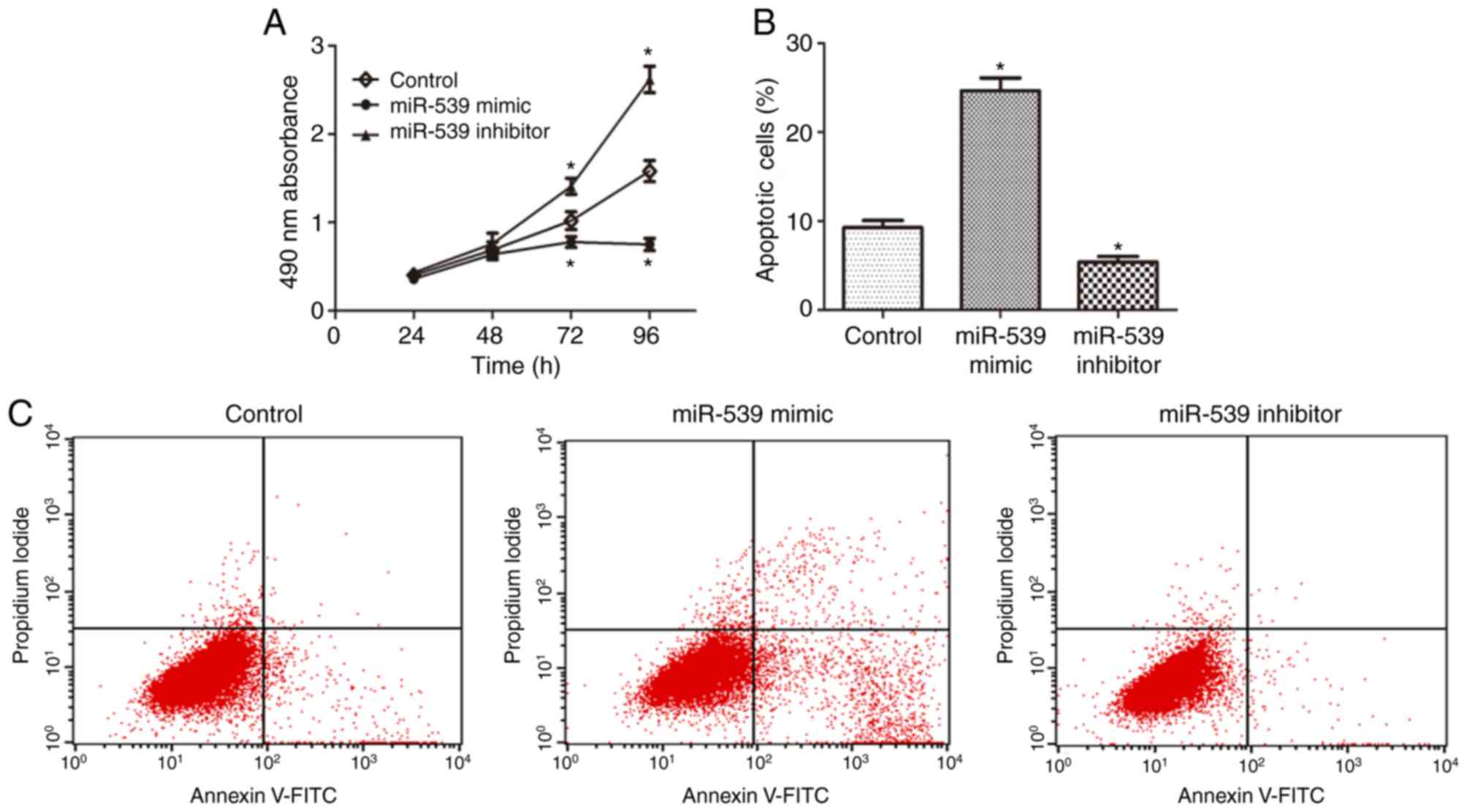
miR-539 suppresses proliferation and
promotes apoptosis of RCC cells
To investigate the effect of miR-539 on the
proliferation of RCC cells, an MTT assay was performed and the data
showed that the viability of 786-O cells transfected with miR-539
mimics was markedly suppressed, compared with that in the control
group, whereas transfection with miR-539 inhibitors increased cell
viability (Fig. 3A). Subsequently,
the effect of miR-539 on the apoptosis of RCC cells was observed.
The results of the flow cytometry revealed that the overexpression
of miR-539 led to a significant increase in apoptotic rate,
compared with that in the control group, and this promotion of cell
apoptosis was reversed following the use of miR-539 inhibitor
(Fig. 3B and C). Collectively,
these results indicated that miR-539 regulated the proliferation
and apoptosis of RCC cells.
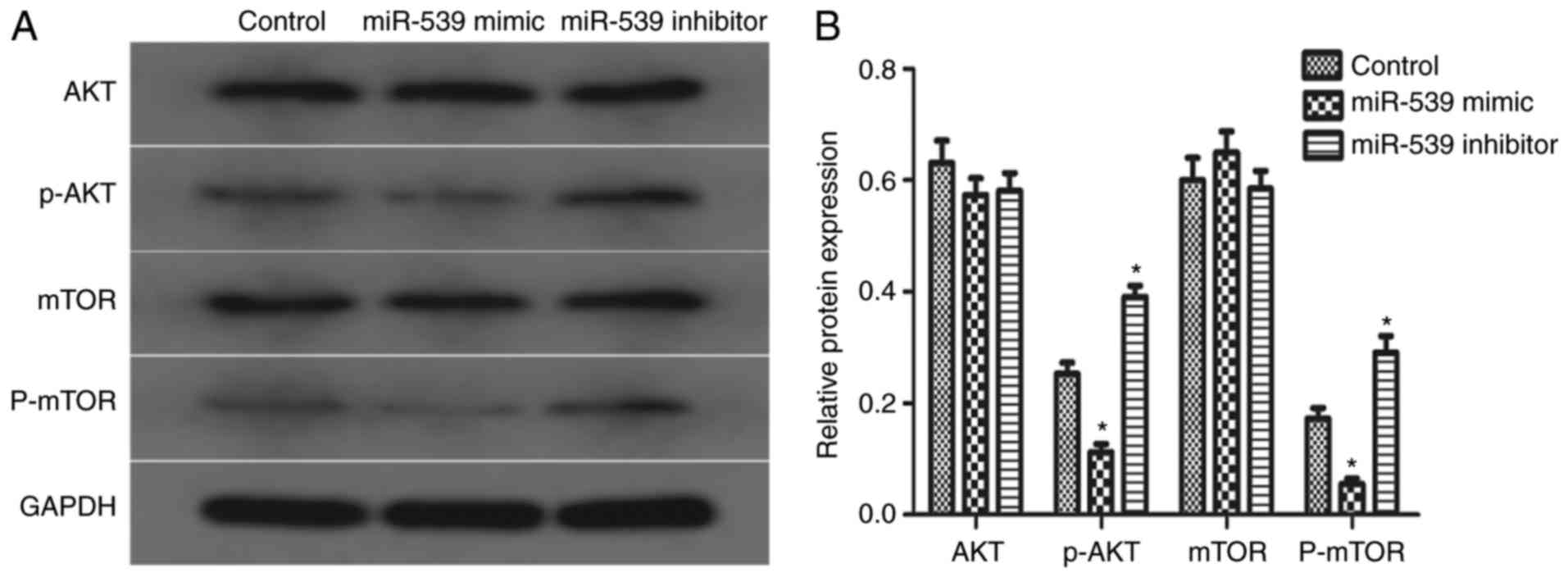
Effects of miR-539 on the AKT
signaling pathway
To investigate whether the AKT signaling pathway was
involved in the potential mechanism of miR-539, the present study
detected the phosphorylation levels of AKT and mTOR. The results of
the western blot analysis showed that the overexpression of miR-539
suppressed the expression of p-AKT and p-mTOR, compared with the
expression in the control group, whereas the knockdown of miR-539
led to the opposite results. The total levels of AKT and mTOR were
not affected by miR-539 (Fig. 4A and
B). These data indicated that miR-539 is involved in regulation
of the AKT signaling pathway, which may be an important factor to
the growth of 786-O cells.
Discussion
RCC is not sensitive to radiotherapy or
chemotherapy; therefore, partial or radical nephrectomy is the
preferred treatment for localized RCC in patients (16,17).
Unfortunately, RCC usually occurs without typical symptoms,
therefore, it is difficult to diagnose prior to its development
into advanced stages or distant metastasis (18). According to previous studies, up to
one-third of patients with RCC exhibit metastatsis at the time of
diagnosis, whereas almost 40% of patients with a localized lesion
have been shown to experience recurrence following surgical
therapies (19,20). Therefore, it is important to
elucidate the mechanism underlying the progression of RCC and
examine novel molecular interactive targets to develop more
effective therapeutic approaches. Previous studies have indicated
that miRNA can regulate tumor proliferation and apoptosis in cancer
cells by targeting specific genetic markers (21). Based on these results, the present
study aimed to identify miRNAs involved in regulating the
progression of RCC.
Several studies have confirmed that miRNA is
important in tumorigenesis and metastasis. It has been elucidated
that miRNA regulates gene expression post-transcriptionally and
acts as an oncogene or tumor suppressor in different types of
cancer (22,23). miR-539 has been reported to be
downregulated in osteosarcoma and suppresses tumor metastasis by
targeting MMP-8 (24). It has also
been shown that miR-539 functions as a tumor suppressor in prostate
cancer through binding to sperm-associated antigen 5 (25). A previous study revealed that
miR-539 can be used as a prognostic biomarker for colon cancer
(26). However, the effect of
miR-539 on RCC and its underlying mechanisms had not been
elucidated. In the present study, a negative correlation was found
between miR-539 and HMGA2 in RCC specimens. Compared with adjacent
normal tissues, miR-539 was significantly downregulated, whereas
HMGA2 was upregulated in tumors. Subsequently, the binding
correlation between miR-539 and HMGA2 was predicted using open
access databases, and HMGA2 was confirmed as a direct downstream
target of miR-539 using a luciferase reporter assay.
HMGA2 has been reported to be abnormally expressed
in various types of cancer, and to regulate the proliferation and
apoptosis of tumor cells through multiple pathways (27,28).
For example, HMGA2 promotes the proliferation and metastasis of
nasopharyngeal cancer cells through activation by the transforming
growth factor-β/small mothers against decapentaplegic 3 signaling
pathway (29). In addition, the
downregulation of HMGA2 effectively inhibits the proliferation
process and promotes apoptosis in prostate cancer (30). In the present study, in
vitro experiments were performed using the 786-O renal
carcinoma cell line to simulate the progression of RCC. As
expected, the overexpression of miR-539 suppressed the expression
of HMGA2, whereas the inhibition of miR-539 caused a significant
upregulation in the expression of HMGA2. These data demonstrated
that miR-539 negatively regulated the expression of HMGA2 in RCC
cells, which was in agreement with the observations from clinical
samples.
A previous study showed that the
phosphoinositide-3-kinase/-Akt signaling pathway is involved in the
regulation of cell proliferation and apoptosis (31,32).
It has also been reported that HMGA2 promotes cell proliferation by
activating the AKT pathway (33).
Accordingly, the present study aimed to investigate whether the AKT
signaling pathway mediated the tumor suppression induced by
miR-539. The data revealed that the overexpression of miR-539
inhibited the phosphorylation of AKT and mTOR, rather than altering
the expression of total AKT and mTOR. Taken together, the above
results suggested that miR-539 may be important in regulating the
AKT pathway. This regulatory effect may be initiated via the
modulation of the expression of HMGA2 by miR-539.
In conclusion, the present study indicated that
miR-539 acted as a tumor suppressor in RCC cells by suppressing
cell proliferation and inducing cell apoptosis, and this
suppression may be due, at least in part, to the modulation of
HMGA2 through the AKT signaling pathway. These results suggested
that miR-539 may be used as a diagnostic biomarker for RCC
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZY was responsible for conception and design of the
study, data collection and analysis, and manuscript writing. DG,
designed the study, performed critical revision and supervised all
phases of the study.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of Ren'min Hospital Affiliated to Wuhan University
(Wuhan, China) and performed according to the guidelines on ethical
management. Written informed consent was signed by all participants
prior to the study.
Consent for publication
Written informed consent was signed by all
participants prior to the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang CM, Ji S, Li Y, Fu LY, Jiang T and
Meng FD: β-Catenin promotes cell proliferation, migration, and
invasion but induces apoptosis in renal cell carcinoma. Onco
Targets Ther. 10:711–724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu J, Zhang S, Shan J, Hu Z, Liu X, Chen
L, Ren X, Yao L, Sheng H, Li L, et al: Elevated HMGA2 expression is
associated with cancer aggressiveness and predicts poor outcome in
breast cancer. Cancer Lett. 376:284–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piscuoglio S, Zlobec I, Pallante P, Sepe
R, Esposito F, Zimmermann A, Diamantis I, Terracciano L, Fusco A
and Karamitopoulou E: HMGA1 and HMGA2 protein expression correlates
with advanced tumour grade and lymph node metastasis in pancreatic
adenocarcinoma. Histopathology. 60:397–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parameswaran S, Xia X, Hegde G and Ahmad
I: Hmga2 regulates self-renewal of retinal progenitors.
Development. 141:4087–4097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pallante P, Sepe R, Puca F and Fusco A:
High mobility group a proteins as tumor markers. Front Med
(Lausanne). 2:152015.PubMed/NCBI
|
|
7
|
Na N, Si T, Huang Z, Miao B, Hong L, Li H
and Qiu J and Qiu J: High expression of HMGA2 predicts poor
survival in patients with clear cell renal cell carcinoma. Onco
Targets Ther. 9:7199–7205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang W, Li J, Guo X, Zhao Y and Yuan X:
miR-663a inhibits hepatocellular carcinoma cell proliferation and
invasion by targeting HMGA2. Biomed Pharmacother. 81:431–438. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kala R, Peek GW, Hardy TM and Tollefsbol
TO: MicroRNAs: An emerging science in cancer epigenetics. J Clin
Bioinforma. 3:62013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bijnsdorp IV, Hodzic J, Lagerweij T,
Westerman B, Krijgsman O, Broeke J, Verweij F, Nilsson RJ,
Rozendaal L, van Beusechem VW, et al: miR-129-3p controls
centrosome number in metastatic prostate cancer cells by repressing
CP110. Oncotarget. 7:16676–16687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X, Wu G, Wu Z, Yao X and Li G: MiR-200a
suppresses the proliferation and metastasis in pancreatic ductal
adenocarcinoma through downregulation of DEK gene. Transl Oncol.
9:25–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan C, Lin Y, Mao Y, Huang Z, Liu AY, Ma
H, Yu D, Maitikabili A, Xiao H, Zhang C, et al: MicroRNA-543
suppresses colorectal cancer growth and metastasis by targeting
KRAS, MTA1 and HMGA2. Oncotarget. 7:21825–21839. 2016.PubMed/NCBI
|
|
14
|
Martínez-Salamanca JI, Huang WC, Millán I,
Bertini R, Bianco FJ, Carballido JA, Ciancio G, Hernández C,
Herranz F, Haferkamp A, et al: Prognostic impact of the 2009
UICC/AJCC TNM staging system for renal cell carcinoma with venous
extension. Eur Urol. 59:120–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2^(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
16
|
Chow TF, Youssef YM, Lianidou E, Romaschin
AD, Honey RJ, Stewart R, Pace KT and Yousef GM: Differential
expression profiling of microRNAs and their potential involvement
in renal cell carcinoma pathogenesis. Clin Biochem. 43:150–158.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan XD, Gu DH, Mao JH, Zhu H, Chen X,
Zheng B and Shan Y: Concurrent inhibition of mTORC1 and mTORC2 by
WYE-687 inhibits renal cell carcinoma cell growth in vitro and in
vivo. PLoS One. 12:e1725552017.
|
|
18
|
Wu SW, Chen PN, Lin CY, Hsieh YS and Chang
HR: Everolimus suppresses invasion and migration of renal cell
carcinoma by inhibiting FAK activity and reversing epithelial to
mesenchymal transition in vitro and in vivo. Environ Toxicol.
32:1888–1898. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI,
Knox JJ, Bjarnason GA, Srinivas S, Pal SK, Yuasa T, et al: The
international metastatic renal cell carcinoma database consortium
model as a prognostic tool in patients with metastatic renal cell
carcinoma previously treated with first-line targeted therapy: A
population-based study. Lancet Oncol. 16:293–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chatzizacharias NA, Rosich-Medina A,
Dajani K, Harper S, Huguet E, Liau SS, Praseedom RK and Jah A:
Surgical management of hepato-pancreatic metastasis from renal cell
carcinoma. World J Gastrointest Oncol. 9:70–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang T, Li M, Li Q, Guo Z, Sun X, Zhang
X, Liu Y, Yao W and Xiao P: MicroRNA-98-5p inhibits cell
proliferation and induces cell apoptosis in hepatocellular
carcinoma via targeting IGF2BP1. Oncol Res. 25:1117–1127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okato A, Goto Y, Kurozumi A, Kato M,
Kojima S, Matsushita R, Yonemori M, Miyamoto K, Ichikawa T and Seki
N: Direct regulation of LAMP1 by tumor-suppressive microRNA-320a in
prostate cancer. Int J Oncol. 49:111–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang
C, Wu S, Yu D, Huang Z, Liu F, et al: MicroRNA-33b Inhibits Breast
Cancer Metastasis by Targeting HMGA2, SALL4 and Twist1. Sci Rep.
5:99952015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin H and Wang W: MicroRNA-539 suppresses
osteosarcoma cell invasion and migration in vitro and targeting
Matrix metallopeptidase-8. Int J Clin Exp Pathol. 8:8075–8082.
2015.PubMed/NCBI
|
|
25
|
Zhang H, Li S, Yang X, Qiao B, Zhang Z and
Xu Y: miR-539 inhibits prostate cancer progression by directly
targeting SPAG5. J Exp Clin Cancer Res. 35:602016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bobowicz M, Skrzypski M, Czapiewski P,
Marczyk M, Maciejewska A, Jankowski M, Szulgo-Paczkowska A,
Zegarski W, Pawłowski R, Polańska J, et al: Prognostic value of
5-microRNA based signature in T2-T3N0 colon cancer. Clin Exp
Metastasis. 33:765–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou Q, Xiong L, Yang Z, Lv F, Yang L and
Miao X: Expression levels of HMGA2 and CD9 and its
clinicopathological significances in the benign and malignant
lesions of the gallbladder. World J Surg Oncol. 10:922012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Zhao Z, Xu C, Zhou Z, Zhu Z and You
T: HMGA2 induces transcription factor Slug expression to promote
epithelial-to-mesenchymal transition and contributes to colon
cancer progression. Cancer Lett. 355:130–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia YY, Yin L, Jiang N, Guo WJ, Tian H,
Jiang XS, Wu J, Chen M, Wu JZ and He X: Downregulating HMGA2
attenuates epithelial-mesenchymal transition-induced invasion and
migration in nasopharyngeal cancer cells. Biochem Biophys Res
Commun. 463:357–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi Z, Wu D, Tang R, Li X, Chen R, Xue S,
Zhang C and Sun X: Silencing of HMGA2 promotes apoptosis and
inhibits migration and invasion of prostate cancer cells. J Biosci.
41:229–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie HX, Xu ZY, Tang JN, DU YA, Huang L, Yu
PF and Cheng XD: Effect of Huaier on the proliferation and
apoptosis of human gastric cancer cells through modulation of the
PI3K/AKT signaling pathway. Exp Ther Med. 10:1212–1218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng W, Zhang Y, Gu L, Cui J, Duan B, Wang
Y and Du J: Heat shock protein 27 downstream of P38-PI3K/Akt
signaling antagonizes melatonin-induced apoptosis of SGC-7901
gastric cancer cells. Cancer Cell Int. 16:52016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan L, Wei X, Zheng L, Zeng J, Liu H, Yang
S and Tan H: Amplified HMGA2 promotes cell growth by regulating Akt
pathway in AML. J Cancer Res Clin Oncol. 142:389–399. 2016.
View Article : Google Scholar : PubMed/NCBI
|