Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent
endocrine and metabolic disease. Changes in life style and
accelerations in the aging process have contributed to the
increasing prevalence of T2DM. It is a chronic non-communicable
disease that particularly affects those with cardiovascular or
cerebrovascular diseases (1–3). In
addition, diabetic neuropathy may occur, which involves the
excessive excitation of primary afferent receptors and central
neurons, leading to pain, and other adverse effects (4). The activation of satellite glial
cells (SGCs) has been reported to be an essential factor in several
experimental models of pain (5–7).
Hyperglycemia and dyslipidemia are hallmark features of
pre-diabetes (8,9). Obesity-associated dysregulation of
glucose and lipid metabolism has been associated with diabetes, and
high blood sugar and free fatty acids (FFA) in serum are thought to
contribute to neurological disorder development (10,11).
Thus, cell injury inducing a high glucose high free fatty acid
(HGHF) environment may effectively model the condition of
neurological disorders in T2DM (12,13).
Adenosine 5′-triphosphate (ATP) is an important
messenger that is involved in numerous processes, including the
transmission of pain signals. It may also act as an acute
pro-inflammatory danger signal and a crucial mediator of
neuroinflammation. In an environment of inflammation or stress,
levels of extracellular ATP (eATP) rapidly approach near millimolar
levels and become the main stimulation of pro-inflammatory pathways
(14). Subclasses of purinergic 2
(P2) receptors include P2X and P2Y. P2X receptors, particularly the
P2X purinoceptor 7 (P2X7), are strongly associated with
immunity and inflammation (14).
P2X7 receptors are highly expressed in immune cells and
are activated as a result of pro-inflammatory cytokine release
(15). In SGCs, eATP may activate
the P2X7 receptor, thus possibly contributing to the
development of chronic inflammatory disease (16).
Long non-coding RNAs (lncRNAs) are
non-protein-coding RNA transcripts >200 nucleotides in length.
Increasing evidence has highlighted the role of lncRNAs in
physiology and disease (17,18).
LncRNAs are involved in diverse regulatory processes, including the
alteration of chromatin and transcriptional state, nuclear
architecture, splicing and mRNA translation (19,20).
LncRNA BC168687 is evolutionarily conserved across numerous species
and significantly increased levels have been detected in the dorsal
root ganglion (DRG) of type 2 diabetic rats (21). Therefore, BC168687 was selected for
examination. The present study revealed that lncRNA BC168687 small
interfering RNA (siRNA) may downregulate P2X7 receptor
expression induced by a HGHF environment in primary cultured
SGCs.
Materials and methods
Primary culture
The present study was approved by the Ethical
Committee of Nanchang University (Nanchang, China) and animals were
treated according to the Guidelines for the Care and Use of Animals
(22). Fetal Sprague-Dawley rats
(n=6; male; 7–9 g) were obtained from the Laboratory Animal Science
Department of Nanchang University (Nanchang, China). All rats were
housed in clean, standard metabolic cages and kept at a constant
temperature of 37°C with 35–65% humidity. The rats were kept in a
12 h light/dark cycle and had free access to food and water. On the
third day, rats were anesthetized using ether. The DRGs of fetal
rats were extracted with microforceps and rapidly transferred into
Dulbecco's modified Eagle Medium/F12 (DMEM/F12) medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) and incubated at 4°C for
30 min prior to the next step. Following the detachment of
redundant fibers with ophthalmic forceps, the DRGs were incubated
with collagenase type III (0.1 mg/ml; Beijing Solarbio Science and
Technology, Ltd., Beijing, China) for 15 min at 37°C. The
collagenase was removed by centrifugation at 168 × g for 5 min and
DRGs were pre-incubated with 0.25% trypsin-EDTA (0.5 mg/ml; Beijing
Solarbio Science and Technology, Ltd.) in a cell incubator for
35–40 min at 37°C. DMEM/F12 containing 10% fetal bovine serum (FBS;
Biological Industries, Kibbutz Bei-Haemek, Israel) was subsequently
used to terminate enzymatic digestion.
The DRGs were blown gently using sterile disposable
pipettes before being passed through a cell strainer (aperture, 70
µm; 200 mesh). Glial cells (5×105 cells/ml) were
inoculated on polylysine-coated coverslips into 24-well plates to
obtain cell climbing slides. SGCs were purified from glial cells by
replacing the medium twice every 24 h. The purified SGCs were
sustained in DMEM/F12 containing 10% FBS (Biological Industries),
100 U/ml penicillin and 100 mg/ml streptomycin sulfate at 37°C in a
humidified incubator with 5% CO2. To imitate
hyperglycemia and dyslipidemia, 40 mM D-glucose (Beijing Solarbio
Science and Technology, Ltd.) and 0.60 mM FFAs were added to
DMEM/F12 medium. FFAs were a mixture of oleate (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and palmitate (Sigma-Aldrich, Merck
KGaA) at a 2:1 ratio (w/w) (23,24).
In addition, 20 mM D-Mannitol (Beijing Solarbio Science and
Technology) was added into DMEM/F12 as an isotonic control.
SGCs were divided into five groups: Control, HGHF
(40 mM D-glucose and 0.60 mM FFAs), HGHF+BC168687 small interfering
RNA (siRNA), HGHF+negative control siRNA (NCsi) and HGHF+empty
vector control (VD; Entranster™-R4000; Engreen
Biosystem, Ltd., Beijing, China). SGCs were treated with HGHF for
72 h. When the cells reached 70–80% confluence, siRNAs (50 nM) were
transfected into SGCs in 24-well plates for further
experimentation.
siRNA transfection
The following BC168687 siRNA and negative control
siRNA sequences were synthesized (Novobio Scientific, Inc.,
Shanghai, China) and used: BC168687-rat-159
(5′-GAGAUUAUUAAGGUGUACUTT-3′), BC168687-rat-1172
(5′-GACGGUUGAUACUGACUCUTT-3′), BC168687-rat-2400
(5′-GUUGGAUCCUUCUCAAUCATT-3′) and negative control siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′). The three different BC168687 siRNA
duplexes were transfected into SGCs using the
Entranster™-R4000 (Engreen Biosystem, Ltd.) according to
the manufacturer's protocol. After 48 h, the expression levels of
BC168687 were evaluated by reverse transcription
quantitative-polymerase chain reaction (RT-qPCR).
Cell viability test
The viability of SGCs was analyzed with the
TransDetect Cell Counting kit (CKK; Beijing TransGen Biotech, Co.,
Ltd., Beijing, China). A suspension of 100 µl SGCs
(5×103 cells/ml) from each group was placed onto a
96-well microplate. Each group was tested in triplicate. Following
culture of SGCs at the different concentrations of D-glucose and
FFA for 72 h, 10% CCK diluent was added to each well. Cells were
subsequently maintained in a cell incubator for 2 h. A wavelength
of 450 nm was used to detect the absorbance using a multimode plate
reader. The data was analyzed with GraphPad Prism v6.0 (GraphPad
Software Inc., La Jolla, CA, USA).
RT-qPCR
RNA was extracted with Transzol Up (Beijing TransGen
Biotech, Ltd.) and reverse transcribed at 37°C for 1 h using a
RevertAid™ First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The concentration of
cDNA for each group was detected to be ~4×103 ng/µl
using the NanoDrop2000 (Thermo Fisher Scientific, Inc.). The primer
sequences used (Sangon Biotech Co., Ltd., Shanghai, China) were as
follows: BC168687 forward, 5′-GGACAAGTCCTTAGCCATGC-3′ and reverse,
5′-CAACACCGTTGGATCCTTCT-3′; P2X7 forward,
5′-GCACGAATTATGGCACCGTC-3′ and reverse, 5′-CCCCACCCTCTGTGACATTC-3′;
and β-actin forward, 5′-CCTAAGGCCAACCGTGAAAAGA-3′ and reverse,
5′-GGTACGACCAGAGGCATACA-3′. RT-qPCR was performed using the SYBR
Premix Ex Taq (Takara Biotechnology Co., Ltd., Dalian, China) and
the StepOnePlus™ Real-Time PCR system (Thermo Fisher
Scientific, Inc.). The thermo cycling conditions were as follows:
95°C for 30 sec, 60°C for 15 sec, 95°C for 15 sec, 60°C for 1 min
and 95°C for 15 sec. The melting curve was used to determine the
amplification specificity and results were analyzed using the
StepOnePlus Real-Time PCR system. The average threshold cycle (Cq)
value for the target minus the average value for β-actin was used
to calculate the ∆Cq value (∆Cq=Cq target-Cq reference). The ∆∆Cq
value was calculated as follows: ∆Cq test sample-∆Cq calibrator
sample. The relative quantity (RQ) of the gene expression was
calculated using the following equation: 2−ΔΔCq
(21).
Immunocytochemistry
Immunocytochemistry was performed with the SPlink
Detection kit (cat no. SP-9001; OriGene Technologies, Inc.,
Beijing, China) and the working solutions provided by the
manufacturer were used if not otherwise specified. Cell climbing
slides (diameter, 8 mm) were removed from DMEM/F12 and washed three
times in PBS and subsequently fixed in 4% paraformaldehyde (Beijing
Solarbio Science and Technology, Ltd.) for 15 min at room
temperature. Following three washes with PBS, slides were blocked
with normal goat serum at 37°C for 30 min. The slides were washed
in PBS and incubated with P2X7 primary antibody (cat no.
APR-004-AO; 1:200; Alomone Labs, Jerusalem, Israel) overnight at
4°C. Slides were washed in PBS and incubated with Biotin labeled
goat anti-rabbit IgG polymer secondary antibody for 15 min at 37°C.
Slides were washed again with PBS prior to incubation with alkaline
phosphatase-labeled streptavidin for 15 min at 37°C. Slides were
subsequently stained with 3,3′-diaminobenzindine solution (OriGene
Technologies, Inc.) at room temperature for 10 min and sealed by
neutral balsam (OriGene Technologies, Inc.). The expression of
P2X7 receptors was visualized with a fluorescence
inverted microscope (magnification, ×200) and the integrated
optical density (IOD) of the P2X7 receptors was
calculated using Image-Pro Plus v6.0 (Media Cybernetics Inc.,
Rockville, MD, USA).
Western blot analysis
SGCs total protein was extracted with the Mammal
Cell Protein Extraction reagent (Wuhan Boster Biological
Technology, Ltd., Wuhan, China). Protein concentrations were
detected with a multimode plate reader using a Bradford Protein
Assay kit (Beyotime Institute of Biotechnology, Shanghai, China).
Supernatant samples containing 20 µg of protein were separated by
10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF)
membranes. The PVDF membranes were blocked in 5% bovine serum
albumin (Beijing Solarbio Science and Technology, Ltd.) at room
temperature for 2 h and subsequently incubated with the following
primary antibodies overnight at 4°C: rabbit anti-P2X7
(cat. no. APR-004-AO; dilution, 1:500; Alomone Labs), mouse
anti-glial fibrillary acidic protein (GFAP; cat. no. 644701;
dilution, 1:200; BioLegend, Inc., San Diego, CA, USA), rabbit
anti-phosphorylated-extracellular signal-related kinase 1/2
(ERK1/2) (Thr202/Thr204) (cat. no. D13.24.4E; dilution, 1:1,000;
Cell Signaling Technology, Inc. Danvers, MA, USA), rabbit
anti-ERK1/2 (cat. no. 137F5; dilution, 1:1,000; Cell Signaling
Technology, Inc.), and mouse anti-β-actin antibody (cat. no.
TA-09l; dilution, 1:800; OriGene Technologies, Inc.). The following
day membranes were washed three times with TBST and incubated with
the following secondary antibodies: Peroxidase-conjugated goat
anti-rabbit IgG (1:2,000; cat no. ZB-2301; OriGene Technologies,
Inc.) and goat anti-mouse IgG (1:2,000; cat no. ZB-2305; OriGene
Technologies, Inc.) for 2 h at room temperature. Bands were
visualized using the Enhanced Chemiluminescent reagent kit (Wuhan
Boster Biological Technology, Ltd.) and the IOD was calculated
using Image-Pro Plus v6.0 (Media Cybernetics Inc.).
Double immunofluorescence
Cell climbing slides (diameter, 8 mm) were removed
from DMEM/F12 and washed three times with PBS. Slides were fixed
with 4% paraformaldehyde (Beijing Solarbio Science and Technology,
Ltd.) for 15 min at room temperature. Slides were washed three
times in PBS and subsequently blocked with normal goat serum
(OriGene Technologies, Inc.) at the working solution provided by
the manufacturer for 1 h in a thermostatic water bath at 37°C,
prior to incubation with rabbit anti-P2X7 (cat. no.
APR-004-AO; dilution, 1:200; Alomone Labs) and mouse anti-GFAP
(cat. no. 644701; dilution, 1:200; BioLegend, Inc.) overnight at
4°C. Slides were then washed three times with PBS, and incubated
with fluorescent goat anti-mouse fluorescein isothiocyante (1:200;
cat no. ZF-0311; OriGene Technologies, Inc.) and goat anti-rabbit
tetramethylrhodamine isothiocyanate secondary antibodies (1:200;
cat no. ZF-0313; OriGene Technologies, Inc.) in the dark at 37°C
for 1 h. Slides were washed three times with PBS and subsequently
stained with DAPI (cat. no. AR1176; dilution, 1:1,000; Wuhan Boster
Biological Technology, Ltd.) at 37°C for 60 sec. Slides were washed
three times with PBS, sealed with anti-fluorescent quencher (Wuhan
Boster Biological Technology, Ltd.) and visualized with a
fluorescence inverted microscope.
Measurement of eATP
The release of ATP from SGCs was measured using an
ATPlite 1 step luminescence assay system kit (10 ml; PerkinElmer,
Inc., Waltham, MA, USA). A suspension of 100 µl SGCs from each
group was placed onto a 96-well microplate. Each group was tested
in triplicate. The substrate vial and buffer solution were
equilibrated at room temperature. The lyophilized substrate
solution was then mixed with the buffer and left to stand at room
temperature for 5 min. The reconstituted reagent (100 µl) was
subsequently added into each well containing the 100 µl suspension.
The 96-well microplates were agitated for 2 min at 168 × g at 37°C
and the luminescence was measured using a multimode plate
reader.
Measurement of intracellular nitric
oxide (NO) and ROS
Intracellular NO was measured using the nitrate
reductase method (21). The NO
concentration in each group was calculated according to the formula
provided in the Nitric Oxide Assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The IOD values of each
group were calculated using a multimode plate reader.
Intracellular ROS levels were inspected with the ROS
Assay kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Following the removal of DMEM/F12 from SGCs, diluted
2′,7′-dichlorofluorescin diacetate (DCFH-DA; 1:1,000; 10 µM; cat
no. S0033, Beyotime Institute of Biotechnology) was added. Sample
were placed into 24-well plates and incubated for 20 min at 37°C,
then subsequently washed three times with serum-free DMEM/F12. The
fluorescence density was detected at an excitation wavelength of
488 nm and an emission wavelength of 525 nm with the multimode
plate reader.
Statistical analysis
Results are presented as the mean ± standard error.
GraphPad Prism v6.0 (GraphPad Software Inc.) and Image-Pro Plus
v6.0 (Media Cybernetics Inc.) were used to perform the statistical
tests. The unpaired Student's t-test was used when comparing two
groups and the one-way analysis of variance with the Bonferroni
correction was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
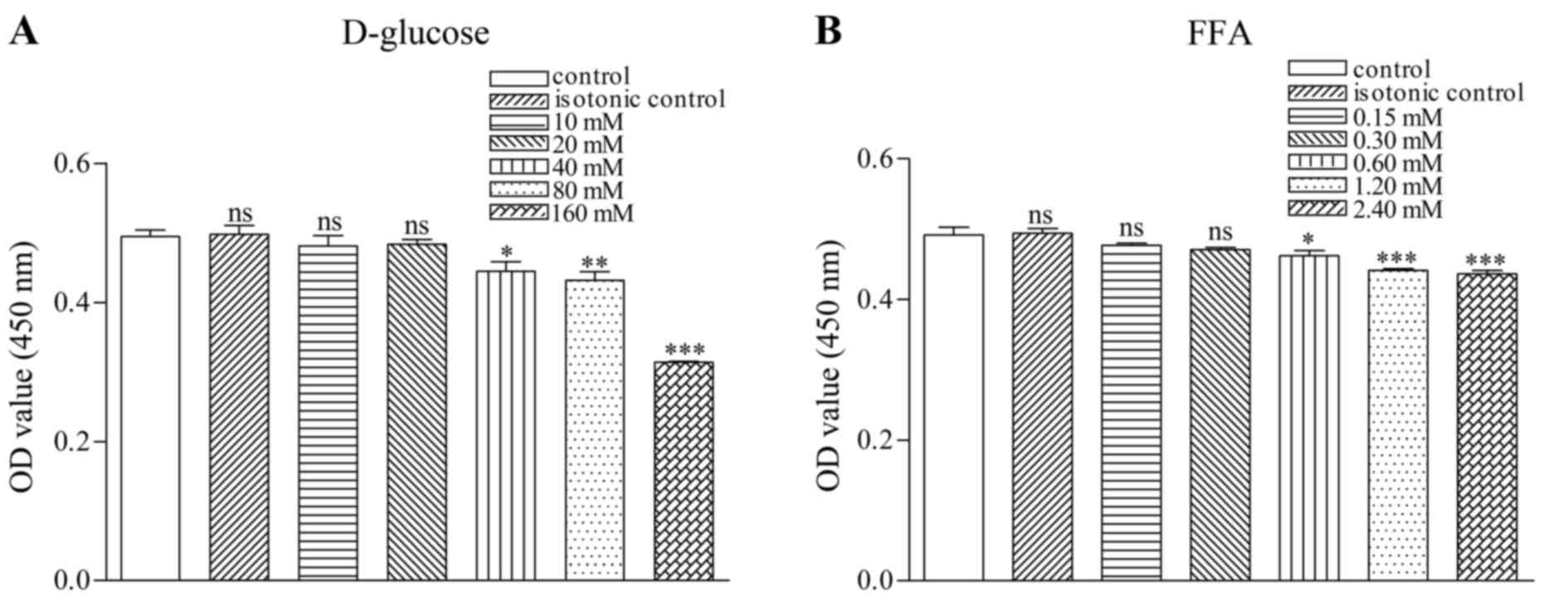
Screening of high D-glucose and FFAs
concentrations
SGC viability was measured with CCK. The results
indicated that the viability of SGCs in a D-glucose environment
significantly decreased at D-glucose concentrations of ≥40 mM:
Control, 0.49±0.02; isotonic control, 0.50±0.02; 10 mM D-glucose;
0.48±0.02; 20 mM D-glucose, 0.48±0.01; 40 mM D-glucose, 0.45±0.02;
80 mM D-glucose, 0.43±0.02; and 160 mM D-glucose 0.31±0.00
(Fig. 1A).
The viability of SGCs in a FFA environment was also
significantly decreased at FFA concentrations ≥0.60 mM: Control,
0.49±0.02; isotonic control, 0.49±0.01; 0.15 mM, 0.48±0.01; 0.30
mM, 0.47±0.00; 0.60 mM, 0.46±0.01; 1.2 mM, 0.44±0.01; and 2.4 mM,
0.44±0.02 (Fig. 1B). Based on the
aforementioned results, 40 mM D-glucose and 0.6 mM FFA were
selected as the final concentrations to produce a HGHF
environment.
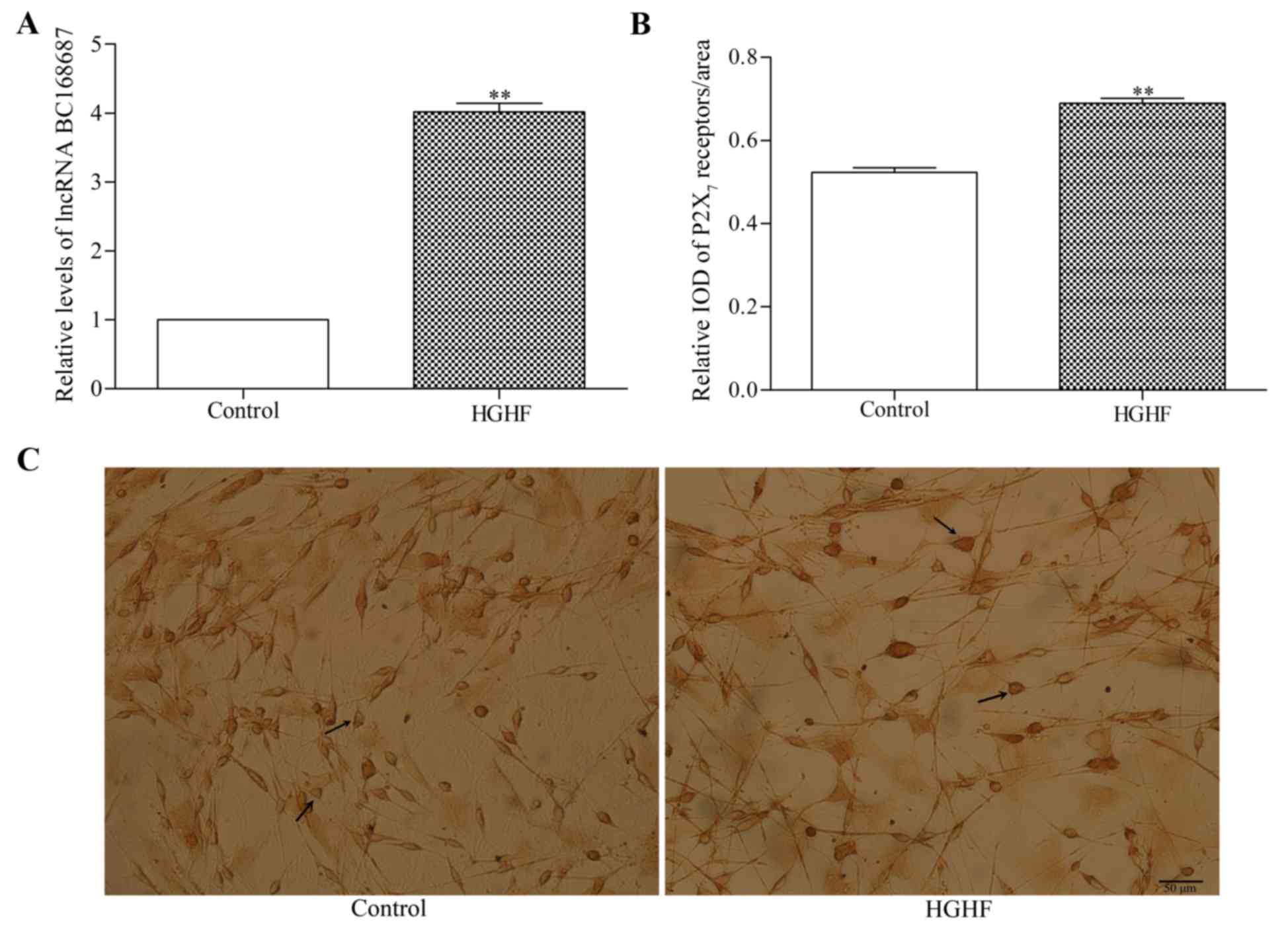
P2X7 receptor and lncRNA
BC168687 expression in SGCs in a HGHF environment
The relative expression level of BC168687 was
determined using RT-qPCR (Fig. 2A)
and the expression of P2X7 receptors was analyzed with
immunocytochemistry (Fig. 2B and
C). The results indicated that the relative expression levels
of BC168687 and P2X7 were higher in the HGHF environment
compared with the control group (Fig.
2A and B; P<0.01).
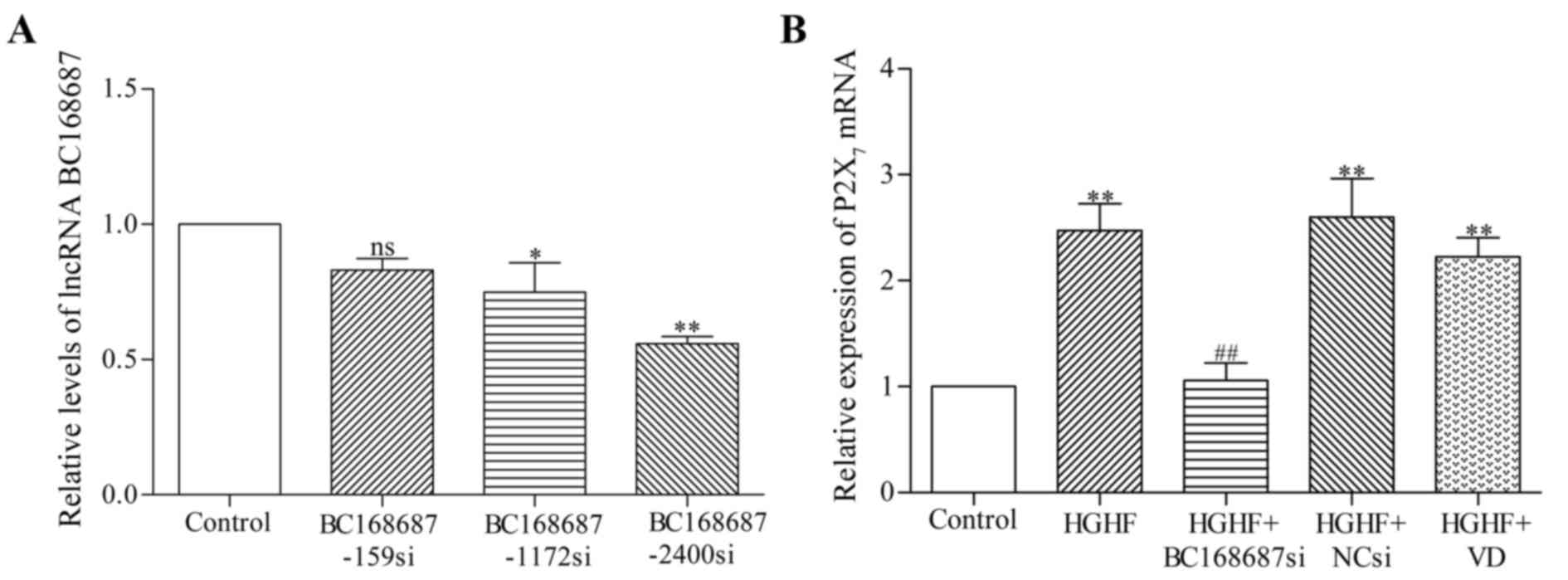
BC168687 siRNA screening and
P2X7 mRNA expression in SGCs
Following the transfection of siRNAs into SGCs for
48 h, duplexes from three different siRNA sequences were screened
for effective BC168687 silencing (Fig.
3A). The relative level of BC168687 and P2X7 mRNA
expression in SGCs was determined using RT-qPCR (Fig. 3B). The results revealed that the
relative level of BC168687 was significantly decreased by siRNAs
1172 (P<0.05) and 2400 (P<0.001): Control, 1.00±0.00;
BC168687-159si, 0.83±0.08; BC168687-1172si, 0.75±0.19;
BC168687-2400si, 0.56±0.04. Thus, BC168687-2400 was selected as the
targeting siRNA of BC168687.
The relative mRNA expression of P2X7 in
each group was as follows: Control, 1.00±0.00; HGHF, 2.47±0.44;
HGHF+BC168687si, 1.06±0.29; HGHF+NCsi, 2.60±0.64; and HGHF+VD,
2.23±0.31. The variance was statistically significant (P<0.01).
Compared with the control group, P2X7 mRNA expression in
the HGHF group was significantly increased. P2X7 mRNA
expression in the HGHF+BC168687si group was significantly lower
compared with the HGHF group (P<0.01). No significant
differences were observed among the HGHF, HGHF+NCsi and HGHF+VD
group. Based on the results obtained, it was concluded that
BC168687 siRNA was able to attenuate the upregulation of
P2X7 mRNA induced in a HGHF environment.
BC168687 siRNA downregulates the
expression of P2X7 and GFAP in SGCs
Following transfection of the siRNAs into SGCs for
72 h, P2X7 and GFAP expression was detected by western
blot analysis (Fig. 4A and B). The
relative expression of P2X7 in each group was as
follows: Control, 0.50±0.02; HGHF, 0.65±0.01; HGHF+BC168687si,
0.43±0.03; HGHF+NCsi, 0.63±0.02 and HGHF+VD 0.67±0.01. The variance
analysis was statistically significant between the HGHF+BC168687si
and HGHF group (P<0.001). The relative expression of GFAP in
each group was as follows: Control, 0.53±0.08; HGHF, 0.85±0.06;
HGHF+BC168687si, 0.59±0.10; HGHF+NCsi, 0.93±0.05; and HGHF+VD,
0.84±0.04. Expression of P2X7 protein and GFAP in the
HGHF group was signficantly increased compared with the control
group (P<0.01). Compared with the HGHF group, the
P2X7 protein and GFAP expression levels were
signficantly decreased in the HGHF+BC168687si group (P<0.01). No
significant differences were observed among the HGHF, HGHF+NCsi and
HGHF+VD groups. Therefore, BC168687 siRNA may attenuate the
upregulation of the P2X7 receptor and GFAP induced by a
HGHF environment in SGCs.
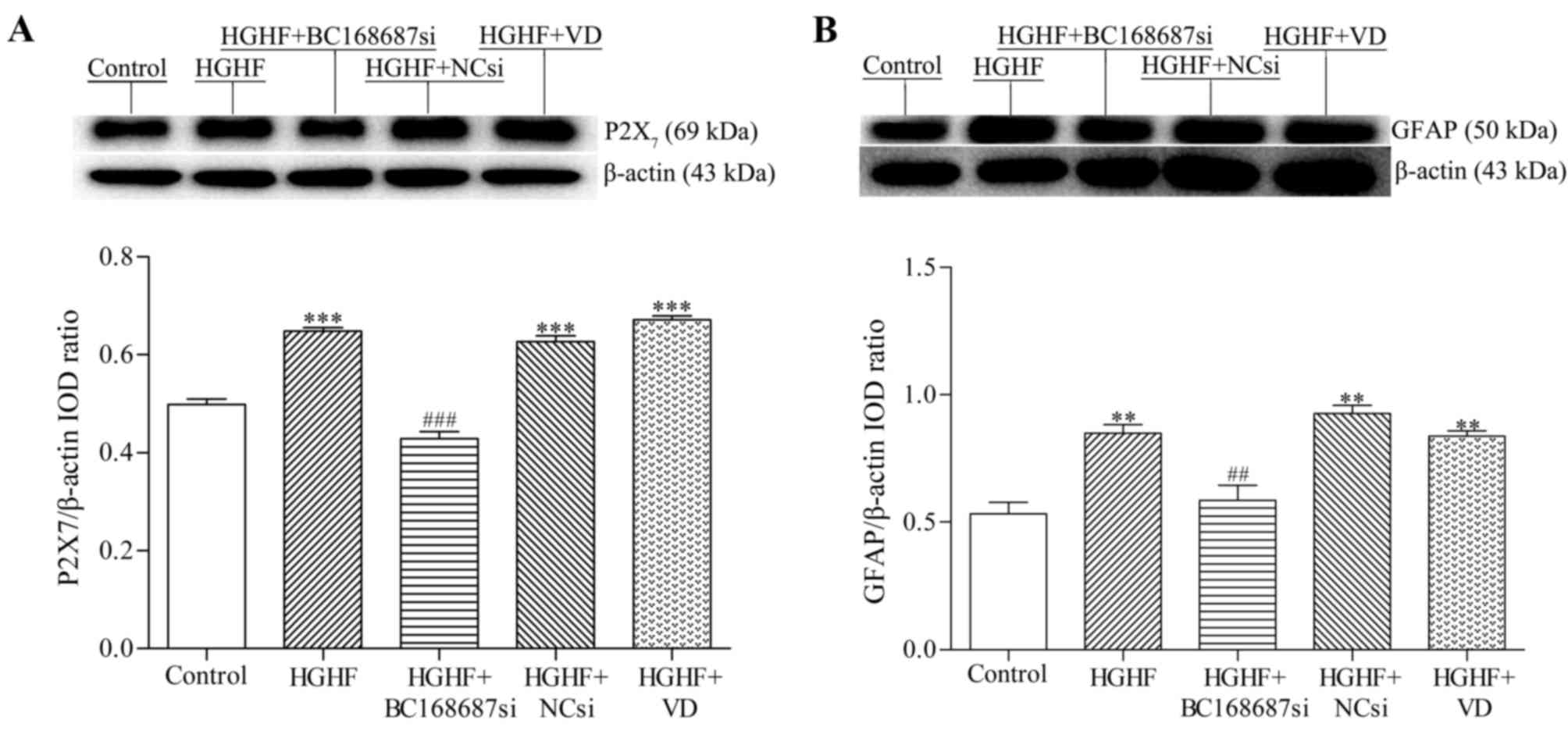 | Figure 4.BC168687 siRNA downregulates the
expression of P2X7 and GFAP induced by HGHF in SGCs. (A)
Representative western blot analysis and the relative expression
levels of P2X7 and (B) GFAP. β-actin from the same
sample was used as an internal control. The expression of
P2X7 protein and GFAP in the HGHF group was
significantly increased compared with the control group. The
expression levels of P2X7 protein and GFAP in the
BC168687si group significantly decreased compared with the HGHF
group. **P<0.05, ***P<0.001 vs. Control group.
##P<0.05, ###P<0.001 vs. HGHF group.
siRNA, small interfering RNA; P2X7, P2X purinoceptor 7;
GFAP, glial fibrillary acidic protein; HGHF, high glucose and high
free fatty acids; SGC, satellite glial cell; NCsi, negative control
siRNA; IOD, integrated optical density; VD, empty vector
control. |
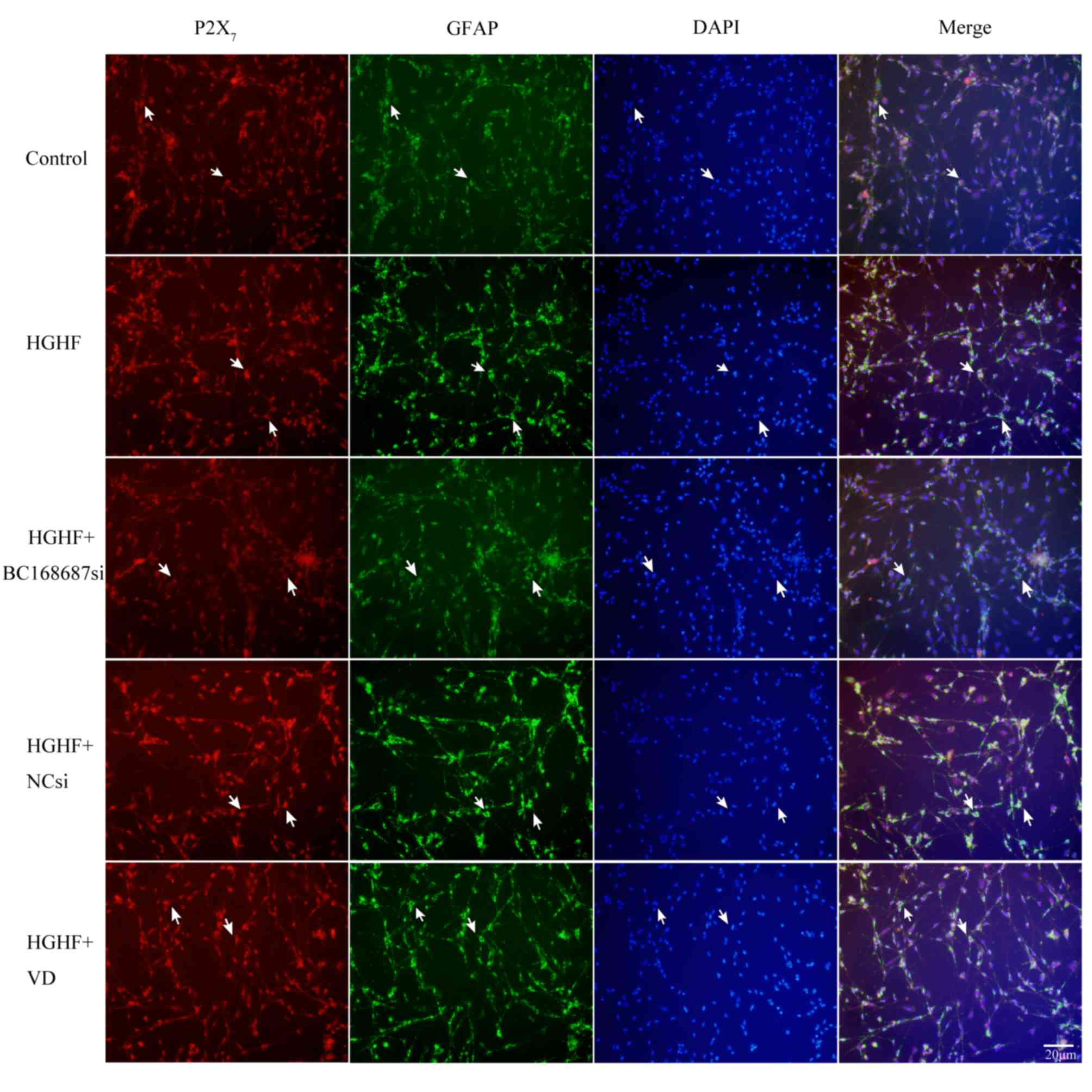
P2X7 and GFAP co-expression
is induced by a HGHF environment in SGCs
Immunofluorescence was used to detect the
co-expression of P2X7 receptor and GFAP in SGCs
(25). The co-expression
quantities of the P2X7 receptors and GFAP in the five
groups was detected following 72 h of siRNA transfection, based on
the co-localization of P2X7 and GFAP in SGCs (Fig. 5). Compared with the control group,
the P2X7 receptor and GFAP co-expression quantities were
increased in the HGHF group. The co-expression quantities of the
HGHF+BC168687si group were decreased compared with the HGHF group.
No apparent difference was observed among the HGHF, HGHF+NCsi and
HGHF+VD groups. Therefore, it was inferred that BC168687 siRNA may
reduce the P2X7 receptor upregulation induced by a HGHF
environment.
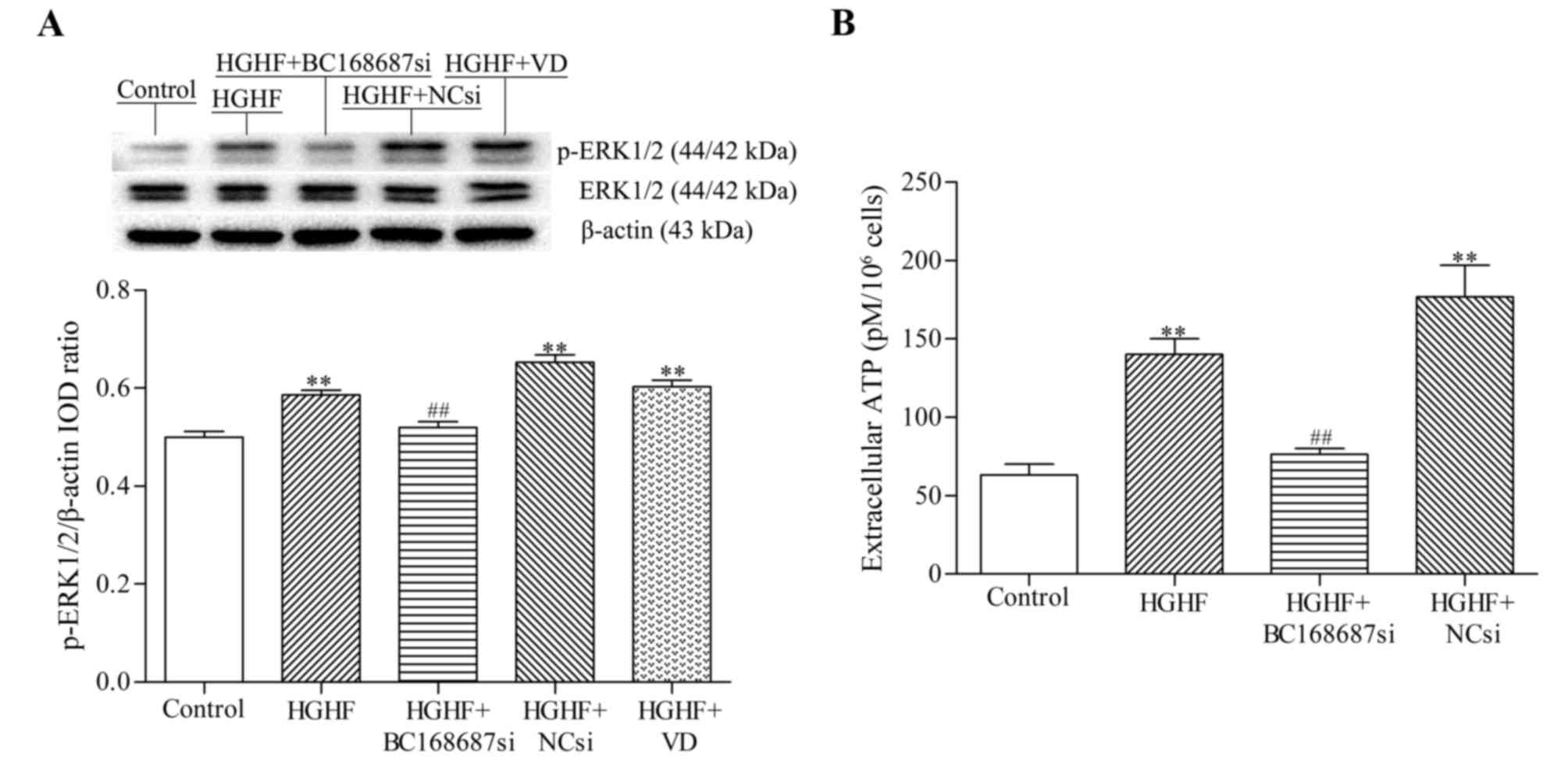
BC168687 siRNA reduces the
upregulation of p-ERK1/2 expression induced by a HGHF environment
in SGCs
The expression level of phosphorylated-ERK1/2
protein in SGCs was detected by western blot analysis. The relative
expression levels of p-ERK1/2 protein in each group were as
follows: Control, 0.50±0.02; HGHF, 0.58±0.02; HGHF+BC168687si,
0.52±0.10; HGHF+NCsi, 0.65±0.03; and HGHF+VD, 0.60±0.01. Compared
with the control group, the p-ERK1/2 protein expression in the HGHF
group was signficantly increased. The expression level of p-ERK1/2
protein in the HGHF+BC168687si group was significantly decreased
compared with the HGHF group (Fig.
6A; P<0.01). No significant difference was observed among
the HGHF, HGHF+NCsi and HGHF+VD groups. Based on the results
obtained, it was concluded that BC168687 siRNA was able to reduce
the upregulation of p-ERK1/2 signalling induced by a HGHF
environment in SGCs.
Effect of BC168687 siRNA on ATP levels
in SGCs induced by a HGHF environment
As a proinflammatory mediator released from SGCs,
ATP contributes to the initiation and maintainence of neuropathic
pain (26). The results revealed
that the concentrations of ATP (pM) in each group were as follows:
Control, 63.33±11.55; HGHF, 140±17.32; HGHF+BC168687si, 76.67±5.77;
and HGHF+NCsi, 176.67±35.12. ATP levels in the HGHF group were
signficantly increased compared with the control group (Fig. 6B; P<0.01) and the levels of ATP
in the HGHF+BC168687si group were significantly decreased compared
with the HGHF group (P<0.01). There was no significant
difference between the HGHF and HGHF+NCsi groups.
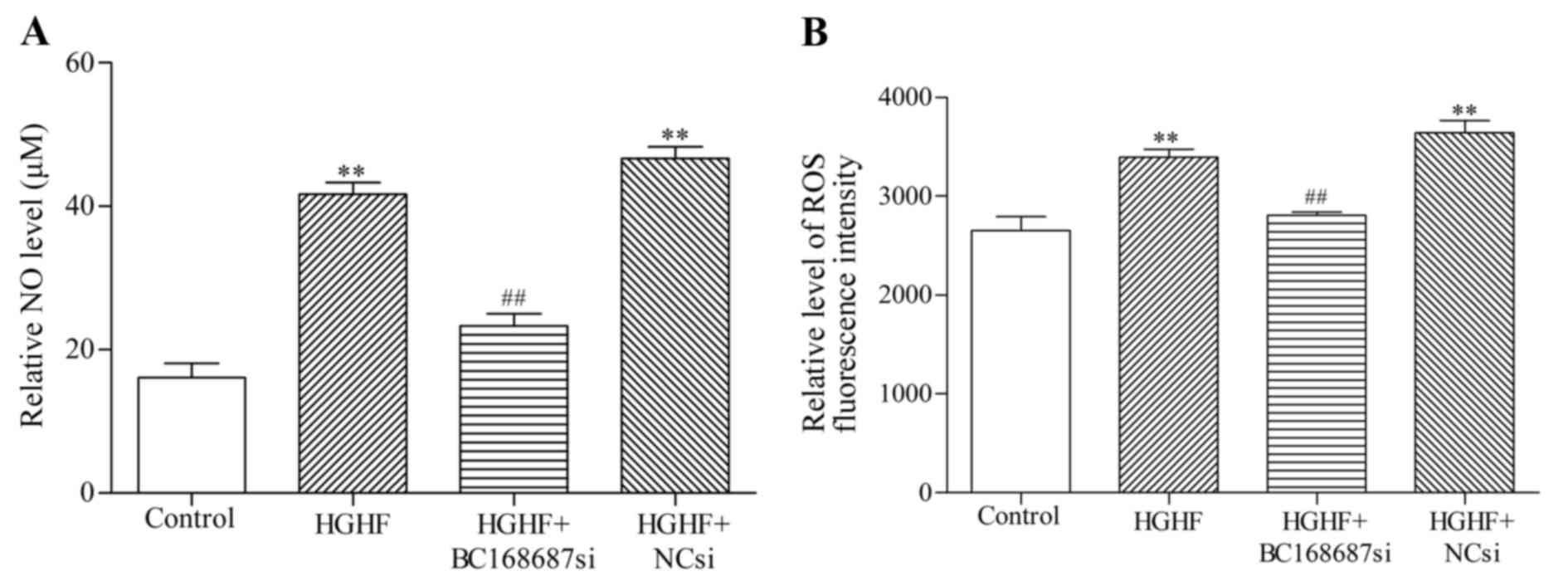
Effect of BC168687 siRNA on NO and ROS
levels in SGCs induced by a HGHF environment
NO and ROS are oxidative injury factors released
from SGCs that are also considered to contribute to the initiation
and maintainence of neuropathic pain (27–29).
The results revealed that the concentrations (µM) of NO in each
group were as follows: Control, 16.11±3.47; HGHF, 41.67±2.89;
HGHF+BC168687si, 23.33±2.89; and HGHF+NCsi, 46.67±2.89 (Fig. 7A). The variance was statistically
significant between the HGHF+BC168687si and HGHF group (P<0.01).
Intracellular ROS levels were measured by fluorescence density. The
results of the ROS assay kit were as follows: Control,
2,655±243.98; HGHF, 3,394±141.74; HGHF+BC168687si, 2,807±58.03; and
HGHF+Ncsi, 3,642±213.18 (Fig. 7B).
NO and ROS levels in the HGHF group were significantly increased
compared with the control group (P<0.01). NO and ROS levels in
the HGHF+BC168687 si group were signficantly decreased compared
with the HGHF group (P<0.01). There was no signifcant difference
between the HGHF and HGHF+NCsi groups.
Discussion
Compared with short-chain ncRNAs, including
microRNAs, siRNAs and Piwi-interacting RNAs, lncRNAs account for
the majority of ncRNAs that regulate biological mechanisms and
processes (30,31). They participate in the regulation
of transcription and intracellular signal transduction pathways,
including those involved in organism development (32). Therefore, dysregulated lncRNA
expression may contribute to the development of numerous human
diseases (33–35). P2X7 receptors are
expressed in SGCs and studies have demonstrated that
P2X7 receptors contribute to neuropathic pain (36–39).
High levels of glucose and FFAs have been identified as a primary
cause of nervous system dysfunction in diabetes (8,13).
NcRNAs lack the ability to encode proteins, but possess regulatory
functions, and are involved in almost all physiological and
pathological processes (30,31,40–42).
The present study demonstrated that BC168687 expression in SGCs in
a HGHF environment group was significantly increased compared with
the control group. P2X7 receptor expression was also
upregulated in SGCs in a HGHF environment, inferring the
involvement of BC168687 in pathological processes mediated by
P2X7 receptors in SGCs.
The P2 receptor family is comprised of ligand-gated
ion channel P2X receptors and G-protein coupled P2Y receptors
(43). Autocrine release of ATP by
glial cells activates P2X7 receptors and may amplify
pain signals through a cascade reaction (44–47).
Thus, inhibiting P2X7 receptors may relieve inflammatory
and chronic neuropathic pain (37,39).
The present study demonstrated that a HGHF environment increased
ATP release in SGCs and BC168687 siRNA was able to decrease this
release. P2X7 mRNA and protein expression in SGCs in the
HGHF group was significantly increased compared with the control
group. Expression of P2X7 mRNA and protein was
significantly decreased in the HGHF+BC168687si group compared with
the HGHF group, suggesting that BC168687 is associated with the
upregulation of P2X7 receptors observed in the HGHF
group.
The increasing incidence of T2DM along with its
comorbidities makes it urgent to understand the pathogenesis and
regulatory mechanisms of the disease. The specific involvement of
lncRNAs in diabetes is unclear (48,49).
Diabetes is a chronic inflammatory disease, and the expression
P2X7 receptors may be upregulated by inflammatory injury
(25,50). In an inflammatory state, ATP can be
released from sensory neurons and SGCs in an autocrine or a
paracrine fashion and activate P2 receptors (7,51).
Excessive P2X7 receptor excitation by ATP can promote
the opening of plasma membrane pores, and may increase the release
of pro-inflammatory cytokines, including interleukin-1β,
interleukin-6 and tumor necrosis factor-α (37,52).
These cytokines further induce glial cells to release
pro-inflammatory mediators and exacerbate neuronal damage (14,45).
Oxidative stress is one of the important factors
leading to diabetic neuropathy. Along with ATP, NO and ROS are
released from glial cells and contribute to chronic neuropathic
pain in diabetes (27,53,54).
The present study indicated that HGHF significantly increased the
release of NO and ROS, and these levels were decreased in the
HGHF+BC168687si group. This suggests that BC168687 contributes to
the pathological processes involving P2X7 receptors,
leading to neuropathic and peripheral inflammatory pain.
The mitogen-activated protein kinase (MAPK)
signaling pathway is involved in cell proliferation,
differentiation and adaptation, and may also contribute to the
development of neuronal injury and disease. The MAPK family
contains p38 MAPKs, ERK and c-Jun N-terminal kinases (55,56).
The signaling pathways of MAPKs are crucial to signal transduction
between neurons and glial cells, both of which are also essential
for persistent pain (57,58). However, different MAPKs have
distinct actions in glial cells following injury (58). Active ERK1/2 signaling occurs
between the nucleus and the cytoplasm, and ROS is able to influence
the ERK MAPK signaling pathway through phosphorylation (57,59).
In the present study, an upregulation of p-ERK1/2 signaling was
observed in SGCs in the HGHF group. Thus, it may be inferred that
the ERK MAPK signaling pathway is involved in the aberrant
activation of SGCs in a HGHF environment. Overall, it was concluded
that BC168687 may be involved in the increased expression of
P2X7 receptors in SGCs in a HGHF environment, and
BC168687 siRNA may have the potential to alleviate diabetic
neuropathic pain mediated by P2X7. These findings
suggest BC168687 siRNA as a novel treatment for P2X7
associated diseases including diabetic neuropathic pain. Further
research to elucidate the specific mechanisms of BC168687 siRNA are
required.
Acknowledgements
The present study was supported by The Youth Science
Foundation of the Educational Department of Jiangxi Province (grant
no. GJJ14146), The Cultivating Foundation of Young Scientists (Star
of Jinggang) of Jiangxi Province (grant no. 20153BCB23033) and The
Innovation Foundation of the Graduate School of Nanchang University
(grant no. cx2016361).
References
|
1
|
Szuszkiewicz-Garcia MM and Davidson JA:
Cardiovascular disease in diabetes mellitus: Risk factors and
medical therapy. Endocrinol Metab Clin North Am. 43:25–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rathmann W and Giani G: Global prevalence
of diabetes: Estimates for the year 2000 and projections for 2030.
Diabetes Care. 27:2568–2569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katsiki N, Purrello F, Tsioufis C and
Mikhailidis DP: Cardiovascular disease prevention strategies for
type 2 diabetes mellitus. Expert Opin Pharmacother. 18:1243–1260.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baron R: Peripheral neuropathic pain: From
mechanisms to symptoms. Clin J Pain. 16 2 Suppl:S12–S20. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu JR, Chen H, Yao YY, Zhang MM, Jiang K,
Zhou B, Zhang DX and Wang J: Local injection to sciatic nerve of
dexmedetomidine reduces pain behaviors, SGCs activation, NGF
expression and sympathetic sprouting in CCI Rats. Brain Res Bull.
132:118–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji RR, Chamessian A and Zhang YQ: Pain
regulation by non-neuronal cells and inflammation. Science.
354:572–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanani M: Role of satellite glial cells in
gastrointestinal pain. Front Cell Neurosci. 9:4122015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Wu B, Jiang F, Xiong S, Zhang B, Li
G, Liu S, Gao Y, Xu C, Tu G, et al: High fatty acids modulate
P2×(7) expression and Il-6 release via the P38 Mapk pathway in Pc12
cells. Brain Res Bull. 94:63–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Namekawa J, Takagi Y, Wakabayashi K,
Nakamura Y, Watanabe A, Nagakubo D, Shirai M and Asai F: Effects of
high-fat diet and fructose-rich diet on obesity, dyslipidemia and
hyperglycemia in the Wbn/Kob-Lepr(Fa) rat, a new model of type 2
diabetes mellitus. J Vet Med Sci. 79:988–991. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruan X: Long Non-Coding Rna central of
glucose homeostasis. J Cell Biochem. 117:1061–1065. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kornfeld JW, Baitzel C, Könner AC,
Nicholls HT, Vogt MC, Herrmanns K, Scheja L, Haumaitre C, Wolf AM,
Knippschild U, et al: Obesity-induced overexpression of miR-802
impairs glucose metabolism through silencing of Hnf1b. Nature.
494:111–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan B, Gu JQ, Yan R, Zhang H, Feng J and
Ikuyama S: High glucose, insulin and free fatty acid concentrations
synergistically enhance perilipin 3 expression and lipid
accumulation in macrophages. Metabolism. 62:1168–1179. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh H, Brindle NP and Zammit VA: High
glucose and elevated fatty acids suppress signaling by the
endothelium protective ligand angiopoietin-1. Microvasc Res.
79:121–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burnstock G: P2× ion channel receptors and
inflammation. Purinergic Signal. 12:59–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baudelet D, Lipka E, Millet R and Ghinet
A: Involvement of the P2×7 purinergic receptor in inflammation: An
update of antagonists series since 2009 and their promising
therapeutic potential. Curr Med Chem. 22:713–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Faria RX, Freitas HR and Reis RAM: P2×7
receptor large pore signaling in avian muller glial cells. J
Bioenerg Biomembr. 49:215–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwok ZH and Tay Y: Long noncoding RNAs:
Lincs between human health and disease. Biochem Soc Trans.
45:805–812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun M and Kraus WL: From discovery to
function: The expanding roles of long noncoding RNAs in physiology
and disease. Endocr Rev. 36:25–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Z, Liu X, Liu L, Deng H, Zhang J, Xu Q,
Cen B and Ji A: Regulation of lncRNA expression. Cell Mol Biol
Lett. 19:561–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor DH, Chu ET, Spektor R and Soloway
PD: Long non-coding RNA regulation of reproduction and development.
Mol Reprod Dev. 82:932–956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Tao J, Wu H, Yang Y, Chen Q, Deng
Z, Liu J and Xu C: Effects of LncRNA BC168687 SiRNA on diabetic
neuropathic pain mediated by P2X7 receptor on SGCs in DRG of rats.
Biomed Res Int. 2017:78312512017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jones-Bolin S: Guidelines for the care and
use of laboratory animals in biomedical research. Curr Protoc
Pharmacol Appendix. 4:Appendix 4B2012.
|
|
23
|
Hirose H, Lee YH, Inman LR, Nagasawa Y,
Johnson JH and Unger RH: Defective fatty acid-mediated beta-cell
compensation in Zucker diabetic fatty rats. pathogenic implications
for obesity-dependent diabetes. J Biol Chem. 271:5633–5637. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu H, He L, Liu C, Tang L, Xu Y, Xiong M,
Yang M, Fan Y, Hu F, Liu X, et al: LncRNA NONRATT021972 SiRNA
attenuates P2X7 receptor expression and inflammatory cytokine
production induced by combined high glucose and free fatty acids in
PC12 Cells. Purinergic Signal. 12:259–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu S, Zou L, Xie J, Xie W, Wen S, Xie Q,
Gao Y, Li G, Zhang C, Xu C, et al: LncRNA NONRATT021972 siRNA
regulates neuropathic pain behaviors in type 2 diabetic rats
through the P2X7 receptor in dorsal root ganglia. Mol Brain.
9:442016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoue K: Neuropharmacological study of ATP
receptors, especially in the relationship between Glia and Pain.
Yakugaku Zasshi. 137:563–569. 2017.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laursen JC, Cairns BE, Kumar U, Somvanshi
RK, Dong XD, Arendt-Nielsen L and Gazerani P: Nitric oxide release
from trigeminal satellite glial cells is attenuated by glial
modulators and glutamate. Int J Physiol Pathophysiol Pharmacol.
5:228–238. 2013.PubMed/NCBI
|
|
28
|
Gwak YS, Hulsebosch CE and Leem JW:
Neuronal-Glial interactions maintain chronic neuropathic pain after
spinal cord injury. Neural Plast. 2017:24806892017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung MK, Asgar J, Lee J, Shim MS, Dumler
C and Ro JY: The role of Trpm2 in hydrogen peroxide-induced
expression of inflammatory cytokine and chemokine in rat trigeminal
ganglia. Neuroscience. 297:160–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taft RJ, Pang KC, Mercer TR, Dinger M and
Mattick JS: Non-Coding RNAs: Regulators of disease. J Pathol.
220:126–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fitzgerald KA and Caffrey DR: Long
noncoding RNAs in innate and adaptive immunity. Curr Opin Immunol.
26:140–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lutz BM, Bekker A and Tao YX: Noncoding
RNAs: New players in chronic pain. Anesthesiology. 121:409–417.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu P, Zuo X, Deng H, Liu X, Liu L and Ji
A: Roles of long noncoding RNAs in brain development, functional
diversification and neurodegenerative diseases. Brain Res Bull.
97:69–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma B, Gao Z, Lou J, Zhang H, Yuan Z, Wu Q,
Li X and Zhang B: Long noncoding RNA MEG3 contributes to
cisplatininduced apoptosis via inhibition of autophagy in human
glioma cells. Mol Med Rep. 16:2946–2952. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kobayashi K, Yamanaka H and Noguchi K:
Expression of ATP receptors in the rat dorsal root ganglion and
spinal cord. Anat Sci Int. 88:10–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inoue K: P2 receptors and chronic pain.
Purinergic Signal. 3:135–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Skaper SD, Debetto P and Giusti P: The
P2×7 purinergic receptor: From physiology to neurological
disorders. FASEB J. 24:337–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sperlágh B, Vizi ES, Wirkner K and Illes
P: P2×7 receptors in the nervous system. Prog Neurobiol.
78:327–346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amaral PP, Clark MB, Gascoigne DK, Dinger
ME and Mattick JS: lncRNAdb: A reference database for long
noncoding RNAs. Nucleic Acids Res. 39(Database Issue): D146–D151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maass PG, Luft FC and Bähring S: Long
non-coding RNA in health and disease. J Mol Med (Berl). 92:337–346.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Simionescu-Bankston A and Kumar A:
Noncoding RNAs in the regulation of skeletal muscle biology in
health and disease. J Mol Med (Berl). 94:853–866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jarvis MF and Khakh BS: ATP-gated P2X
cation-channels. Neuropharmacology. 56:208–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Caseley EA, Muench SP, Fishwick CW and
Jiang LH: Structure-based identification and characterisation of
structurally novel human P2X7 receptor antagonists. Biochem
Pharmacol. 116:130–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Volonté C, Apolloni S, Skaper SD and
Burnstock G: P2X7 receptors: Channels, pores and more. CNS Neurol
Disord Drug Targets. 11:705–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei L, Caseley E, Li D and Jiang LH:
ATP-induced P2X receptor-dependent large pore formation: How much
do we know? Front Pharmacol. 7:52016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dubyak GR: Go it alone no more-P2X7 joins
the society of heteromeric ATP-gated receptor channels. Mol
Pharmacol. 72:1402–1405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu H, Yang L and Chen LL: The diversity of
long noncoding RNAs and their generation. Trends Genet. 33:540–552.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li
YJ, Tao ZF, Song YC, Chen Q and Jiang Q: lncRNA-MIAT regulates
microvascular dysfunction by functioning as a competing endogenous
RNA. Circ Res. 116:1143–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peiró C, Lorenzo Ó, Carraro R and
Sánchez-Ferrer CF: IL-1β inhibition in cardiovascular complications
associated to diabetes mellitus. Front Pharmacol. 8:3632017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kojima S, Ohshima Y, Nakatsukasa H and
Tsukimoto M: Role of ATP as a key signaling molecule mediating
radiation-induced biological effects. Dose Response.
15:15593258176906382017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee JH, Zhang Y, Zhao Z, Ye X, Zhang X,
Wang H and Ye J: Intracellular ATP in balance of pro- and
anti-inflammatory cytokines in adipose tissue with and without
tissue expansion. Int J Obes (Lond). 41:645–651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Blum E, Procacci P, Conte V and Hanani M:
Systemic inflammation alters satellite glial cell function and
structure. A possible contribution to pain. Neuroscience.
274:209–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mima A: Inflammation and oxidative stress
in diabetic nephropathy: New insights on its inhibition as new
therapeutic targets. J Diabetes Res. 2013:2485632013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu Y, Wang Z, Xie W, Gu Z, Xu Q and Su L:
Oxidative stress regulates mitogenactivated protein kinases and
c-Jun activation involved in heat stress and
lipopolysaccharideinduced intestinal epithelial cell apoptosis. Mol
Med Rep. 16:2579–2587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ji RR, Berta T and Nedergaard M: Glia and
pain: Is chronic pain a gliopathy? Pain. 154 Suppl 1:S10–S28. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ponnusamy M, Liu N, Gong R, Yan H and
Zhuang S: ERK pathway mediates P2×7 expression and cell death in
renal interstitial fibroblasts exposed to necrotic renal epithelial
cells. Am J Physiol Renal Physiol. 301:F650–F659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ji RR, Gereau RW IV, Malcangio M and
Strichartz GR: MAP kinase and pain. Brain Res Rev. 60:135–148.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Son Y, Kim S, Chung HT and Pae HO:
Reactive oxygen species in the activation of MAP kinases. Methods
Enzymol. 528:27–48. 2013. View Article : Google Scholar : PubMed/NCBI
|