Introduction
Osteosarcoma is a malignant tumor that cells occur
in bone, which has been reported to exhibit aberrant growth and
migration in osseous tissues (1,2). In
recent years, new strategies have been proposed, but the overall
survival of patients with osteosarcoma remained limited improvement
for a stubborn resistance of osteosarcoma cells (3,4).
However, malignant osteosarcoma cancer is a typical systemic
malignant disease, which causes common symptoms including bone and
joint pain, and fatigue in patients (5,6).
Although multiple reports demonstrated that treatment advances and
neoadjuvant chemotherapy lead to improved 5-year survival in
patients with osteosarcoma, the overall survival has had minimal
improvement since the introduction of neoadjuvant chemotherapy,
radiotherapy and surgery (7,8).
Currently, tumor apoptosis and necrosis is an
important predictor of patient prognosis during the treatment of
osteosarcoma (9). Resistance to
drug-induced apoptosis for osteosarcoma cells contributes to growth
and invasion of tumor cells in patients (10–12).
Although the emergence of adjuvant and neoadjuvant chemotherapy has
been greatly improved the survival rate of patients with
osteosarcoma, the morbidity and mortality rate of osteosarcoma is
still steadily increasing (13).
p53 has a key role in mediating cellular response to various stress
signals, mainly by inducing or repressing a number of proteins that
are involved in cancer cell cycle progression and apoptosis
(14). Clinically, apoptotic
resistance has become one of the greatest challenges in cancer
therapy due to fierce resistance of tumor cells through various
molecular mechanisms (15,16). Thus, underlying molecular mechanism
of apoptotic resistance and exploring more efficacy target
therapies are urgently required to improve the overall survival
rate for patients with osteosarcoma.
MicroRNAs (miRNA) are a subgroup of small non-coding
RNAs of 18–25 nucleotides, which have been identified as regulators
of disease initiation (17).
Reports have suggested that miRNAs have a crucial role in
tumorigenesis and progression by regulating the expression of
oncogenes (18,19). In addition, it has been reported
that miRNA may be associated with anti-cancer treatments, which
have been identified as promoters for tumor apoptosis through
upregulated expression of pro-apoptosis genes (20,21).
Furthermore, a report has demonstrated that cancer-associated miRNA
expression was highly associated with apoptosis in gastric tumors
(22). Furthermore, miRNAs are
reported to be crucial in the tumorigenesis, development,
apoptosis, treatment and prognosis of osteosarcoma cancer (23). Therefore, understanding of the
pathogenesis of osteosarcoma may identify candidate miRNAs that are
promising biomarkers for osteosarcoma diagnosis and treatment
(24).
In this study, the role of miRNA-124 (miR-124) in
progression and metastasis of osteosarcoma was investigated in
vitro and in vivo. The role of miRNA in the progression
of osteosarcoma and chondrosarcoma has been investigated (25); however, the molecular mechanism of
transforming growth factor-β (TGF-β)-mediated AKT serine threonine
kinase (AKT)/glycogen synthase kinase-3β (GSK-3β)/snail family
transcriptional repressor 1 (SNAIL-1) signaling pathway regulated
by miR-124-mediated integrin down-regulation has not been well
established in osteosarcoma cells. The efficacy of miR-124
transfection in inhibiting tumor growth, proliferation,
aggressiveness and promoting apoptosis was determined in
osteosarcoma-bearing mice.
Materials and methods
Ethics statement
This study was performed out in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of Ethics Committee (26). The protocol was approved by the
Ethics Committee of Renji Hospital Shanghai Jiao Tong University
School of Medicine. All surgery and euthanasia were performed under
sodium pentobarbital anesthesia, and all efforts were made to
minimize suffering.
Cell culture
Mg63 cells were purchased from American Type Culture
Collection (ATCC; Manassas, VA, USA) and cells were cultured in
RPMI 1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.). Normal bone cell line
MC3T3-E1 was cultured in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated FBS. All cells were cultured at 37°C and 5%
CO2. No mycoplasma contamination was detected.
TGF-β overexpression
Mg63 cells (1×106) were cultured in
six-well plate until 85% confluence and the media was then removed
from culture plate followed three PBS washes. The TGF-β
complementary DNA (cDNA) sequence was amplified from total RNA with
the following primers: Forward, 5′-CATTGGAGAGAAAGGAAAGTGTG-3′ and
reverse, 5′-GCTTGCATGTACGAAGAGGAT-3′. The TGF-β lentiviral vector
was co-transfected with 1,000 ng/µl packaging plasmid (Invitrogen;
Thermo Fisher Scientific, Inc.) and 2.0 µg envelope plasmid into
Mg63 cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to manufacturer's protocols. A
total of 10 µl TGF-β fresh vector supernatants in 3 ml RPMI 1640
medium were mixed with Mg63 cells in the presence of 8 µg/ml
polybrene (Invitrogen; Thermo Fisher Scientific, Inc.). The cells
were cultured for 24 h at 37°C in DMEM + 20% FBS medium.
TGF-β-overexpressing-Mg63 cells were transfected with miR-124.
Cells were analyzed for further analysis after 48 h
transfection.
Transfection of miRNA
All miRNAs were synthesized by Invitrogen (Thermo
Fisher Scientific, Inc.) including miR-124
(3′-CCGUAAGUGGCGCACGGAAU-5′) and siR-vector
(3′-UUAAUUCCCCUGUUG-5′). Mg63 cells (1×106) were
transfected with 100 pmol miR-12 or siR-vector mimics (Control;
Ambion; Thermo Fisher Scientific, Inc.) using a Cell Line
Nucleofector kit L (Lonza Group, Ltd., Basel, Switzerland) and a
Nucleofector I electroporation device (Lonza Group, Ltd.). All
procedures were performed according to the manufacturer's
instructions. Subsequent experiments were performed after 48 h
transfection.
MTT cytotoxicity assays
Mg63 cells were transfected with miR-124 with
miR-vector as control and cultured in 96-well plates for 72 h at
37°C. A total of 20 µl MTT (5 mg/ml) in PBS solution was added to
each well, the plate was further incubated for 4 h. The majority of
the medium was removed and 100 µl dimethyl sulfoxide was added into
the wells to solubilize the crystals. The optical density was
measured by a Bio-Rad (ELISA) reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from Mg63 cells using
RNAeasy Mini kit (Qiagen, Inc., Valencia, CA, USA). Total RNA (1
µg) was transcribed to cDNA by using an RT kit (Qiagen, Inc.) and
quality was confirmed by electrophoresis. The cDNA (10 ng) was
subjected to qPCR (Bio-Rad Laboratories, Inc.) using SYBR-Green
Master Mix system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). All the forward and reverse primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.; miR-124 forward,
5′-ACGGGATCCTCTTATTCCATCTTCTACCC-3′ and reverse,
5′-CGGAATTCCTGGCTCGGTCGGTCGCTC-3′; β-catenin forward,
5′-GCAGTTCGCCTTCACTATGGA-3′ and reverse,
5′-GCAGTTCGCCTTCACTATGGA-3′; E-cadherin forward,
5′-TCATGAGTGTCCCCCGGTAT-3′ and reverse,
5′-TCAAACACGAGCAGAGAATCATAAG-3′; α-SMA forward,
5′-GAGTTACGAGTTGCCTGATG-3′ and reverse,
5′-GGTCCTTCCTGATGTCAATATC-3′; Vimentin forward,
5′-GGCTCAGATTCAGGAACAGC-3′ and reverse, 5′-AGCCTCAGAGAGGTCAGCAA-3′;
and GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′. The thermocycling conditions were as
follows: 95°C for 30 sec, followed by 45 cycles of 95°C for 5 sec
and 60°C for 40 sec. Relative mRNA expression changes were
calculated by 2−ΔΔCq (27). The results are expressed as the
n-fold way compared with the control.
Flow cytometry
Mg63 cells were cultured until 90% confluence was
reached. Apoptosis was assessed by incubation these cells with
tunicamycin (2 mg/ml) for 24 h. Cells were trypsinized, collected
and then washed in cold PBS, adjusted to 1×106 cells/ml
with PBS, labeled with Annexin V-fluorescein isothiocyanate (FITC)
and propidium iodide (Annexin V-FITC kit; BD Biosciences, San Jose,
CA, USA), and analyzed with a FACScan flow cytometer (BD
Biosciences) using CellQuest Pro software (v5.1, BD
Biosciences).
Colony formation assay
Mg63 or miR-124-transfected cells were seeded into
6-well plates (1×103/cells). After culturing for 12
days, proliferating colonies were stained with 1% crystal violet
for 15 min at room temperature (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), and colonies containing ≥50 cells were
counted. All experiments were performed in triplicate.
Cells invasion and migration
assays
Mg63 cells were transfected with miR-124 and used to
analyze aggressiveness. Mg63 cells were cultured in a 24-well
culture plate with chamber inserts (BD Biosciences). For migration
assays, 1×104 cells/well were placed into the upper
chamber with the non-coated membrane. For invasion assays, cells
(1×104 cells/well) were placed into the upper chamber
with a Matrigel-coated membrane. In both assays, the cells were
transfected with miR-124 or miR-vector and subjected to the tops of
BD BioCoat Matrigel Invasion Chambers (BD Biosciences) for 24 h at
37°C according to the manufacturer's instructions. The lower
chamber of the Transwell plates were filled with 500 µl RPMI-1640
medium containing 10% FBS (Gibco; Thermo Fischer Scientific, Inc.)
Cells were fixed in 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA)
for 30 min at room temperature and stained with 0.1% crystal violet
(Sigma-Aldrich; Merck KGaA) to quantify cell migration and invasion
for 15 min at 37°C. The tumor cells invasion and migration were
counted in at least three randomly stained microscope fields for
every membrane.
Western blot analysis
Mg63 cells were transfected with miR-124 and
harvested by scraping and lysed in radioimmunoprecipitation assay
buffer (Sigma-Aldrich; Merck KGaA) followed by homogenization at
4°C for 10 min. Protein concentration was measured with a
Bicinchoninic Acid protein assay kit (Thermo Scientific, Pittsburgh
PA, USA). Protein (20 µg) were separated by 12% SDS-PAGE followed
transfer to polyvinylidene fluoride membranes (Merck KGaA) and
incubated with primary rabbit anti-human antibodies: Integrin
(ab150361), β-catenin (ab32572), Vimentin (ab92547), α-SMA
(ab108424), E-cadherin (ab11512), TGF-β (92486), Apaf-1 (ab2001),
Bad (ab220116), Bcl-2 (ab59348), P53 (ab32049), AKT (ab8805), pAKT
(ab38449), GSK-3β (ab93926), pGSK-3β (ab131097), SNAIL-1 (ab82846)
and β-actin (ab8227; all 1:500 dilution; Abcam, Shanghai, China)
for 12 h at 4°C post-blocking (5% skimmed milk) for 1 h at 37°C.
Subsequently, proteins were incubated with the corresponding rabbit
horseradish peroxidase-labeled Immunoglobulin G (ab6728; 1:2,000;
Abcam) for 12 h at 4°C. The proteins expression levels were
detected using a chemi-luminescence detection system with Quantity
One software (v3.0; Sigma-Aldrich; Merck KGaA).
Animal study
Female specific pathogen-free nude mice (6–8 weeks
old; n=80, body weight, 32–38 g) were purchased from Shanghai Slack
Experimental Animals Co., Ltd. (Shanghai, China). All mice were
housed at 22–24°C under a 12-h light-dark cycle and fed ad
libitum at 40–70% humidity. Mice were subcutaneously implanted
with miR-124-transfected or miR-vector-transfected Mg63 tumor cells
(1×107/ml) in 2 ml. Mice were divided into two groups
(20 per group). The tumor volumes were calculated according to a
previous study (28): Length ×
width2 × 0.52. On day 24, mice (n=20 in each group) were
sacrificed under 1.5% pentobarbital sodium (100 mg/kg) for further
analysis. Remaining mice (n=20 in each group) continued to be
housed in order to analyze the long-term survival rate (considered
to be 100 days). In the survival rate experiments, the largest
tumor size was ~2,000 mm3. Mice were sacrificed with
1.5% pentobarbital sodium (100 mg/kg) by intravenous injection when
tumor diameter reached 12 mm.
Immunohistochemistry
Tumors from Mg63-bearing xenograft mice were fixed
using 10% formaldehyde for 12 h at 25°C, embedded in paraffin wax,
and cut into serial sections of 4-µm thickness. Tumor samples were
prepared to tumor sections and antigen retrieval (Tris-EDTA buffer
solution, pH 9.0; cat. no. SRE0063; Sigma-Aldrich; Merck KGaA) was
also performed in tumor sections for 60 min at 65°C. Tumor sections
were incubated with primary antibodies at 4°C overnight: Integrin
(ab150361), AKT (ab8805), pAKT (ab38449), TGF-β (92486; all
1:1,000; Abcam) then with horseradish peroxidase (HRP)-labelled
streptavidin (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min and
3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA) and re-stained
with hematoxylin (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C, and
samples were visualized using a microscope (YS100; Nikon
Corporation, Tokyo, Japan). A Ventana Benchmark automated staining
system was used for observation of integrin.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) analysis
Tumor sections were permeabilized by immersing cells
slides in 0.2% Triton X-100 solution in PBS for 30 min at 4°C.
Subsequently, sections were incubated with equilibration buffer for
30 min at 4°C. The sections were then incubated with 50 µl of the
reaction mixture at 37°C for 60 min and washed three times with
PBS. The TUNEL Apoptosis assay kit (cat. no. 8088; ScienCell,
Carlsbad, CA, USA) were used to detect TUNEL-positive cells.
Finally, images were captured with a ZEISS LSM 510 confocal
microscope at 488 nm.
Statistical analysis
All data are presented as the mean ± standard error
in triplicate. Unpaired data was analyzed using Student's t-test
and comparisons of data between multiple groups were analyzed by
one-way analysis of variance followed by Tukey honest significant
difference test. Kaplan-Meier analysis was used to estimate the
risk of relapse and re-treatment during the 100-day treatment.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Evaluation of integrin expression in
miR-124-transfected osteosarcoma cells
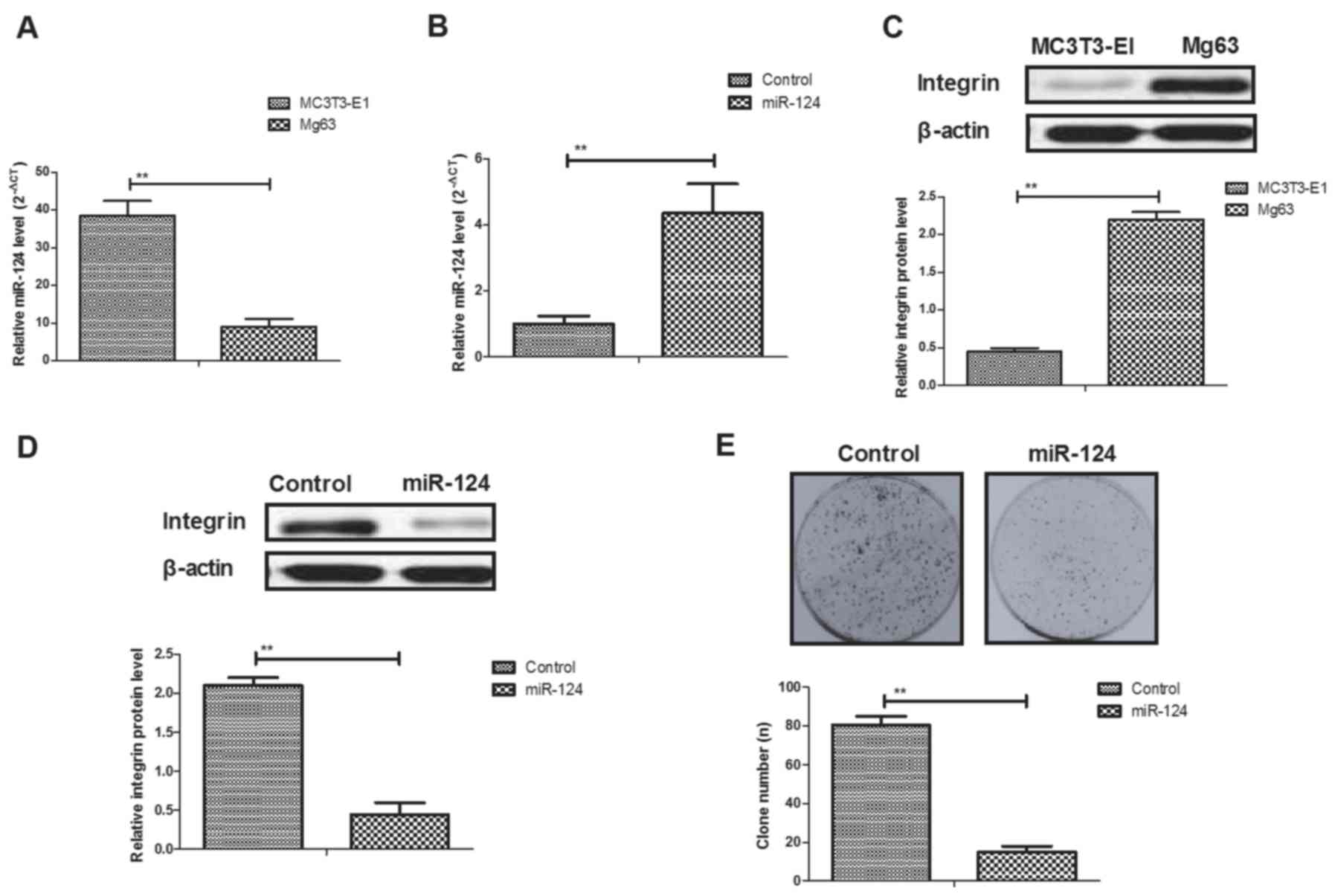
miR-124 expression was determined in Mg63 and
MC3T3-E1 cells. As shown in Fig.
1A, miR-124 expression levels were downregulated in Mg63
osteosarcoma cells compared with the MC3T3-E1 bone normal cell
line. Transfection of miR-124-increased miR-124 gene expression in
Mg63 cells (Fig. 1B). The results
also showed that integrin expression was upregulated in Mg63 cells
compared with MC3T3-E1 cells (Fig.
1C). miR-124 transfection significantly decreased integrin
(integrin-α5) expression in Mg63 cells (Fig. 1D). Results also demonstrated that
miR-124 transfection inhibited proliferation of Mg63 cells
(Fig. 1E).
Evaluation of cytotoxicity, migration
and invasion in osteosarcoma cells after transfected with
miR-124
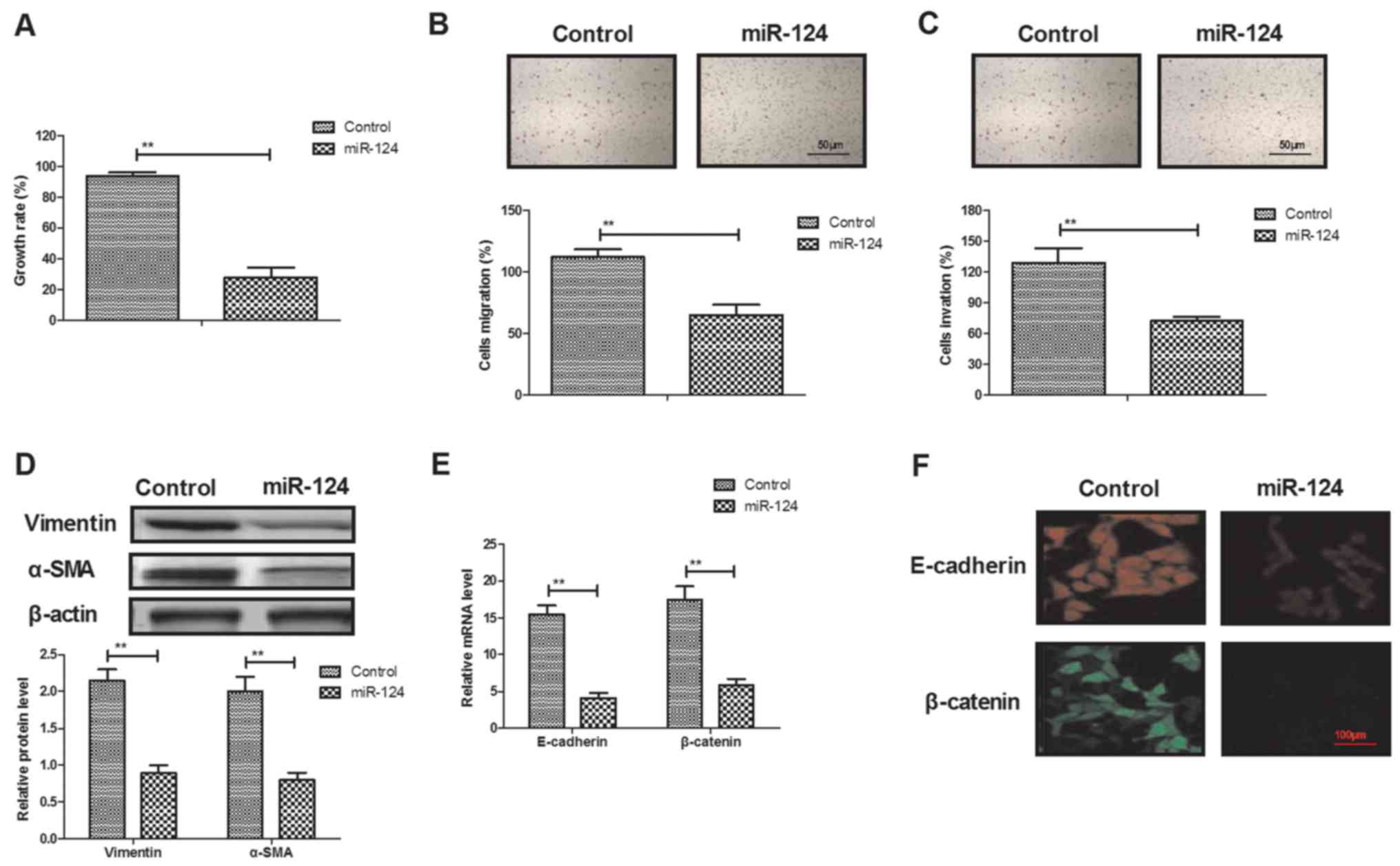
As shown in Fig.
2A, miR-124 transfection significantly inhibited Mg63 cell
growth compared with the control group. Transfection of miR-124
also suppressed migration and invasion of Mg63 cells after 24 h of
incubation (Fig. 2B and C).
Western blot demonstrated that expression levels of vimentin and
α-smooth muscle actin were downregulated by miR-124 transfection
(Fig. 2D). RT-qPCR and
immunofluorescence revealed that miR-124 transfection significantly
inhibited E-cadherin and β-catenin expression in Mg63 cells
(Fig. 2E and F).
Effects of miR-124 transfection on
TGF-β expression and apoptosis of osteosarcoma cells
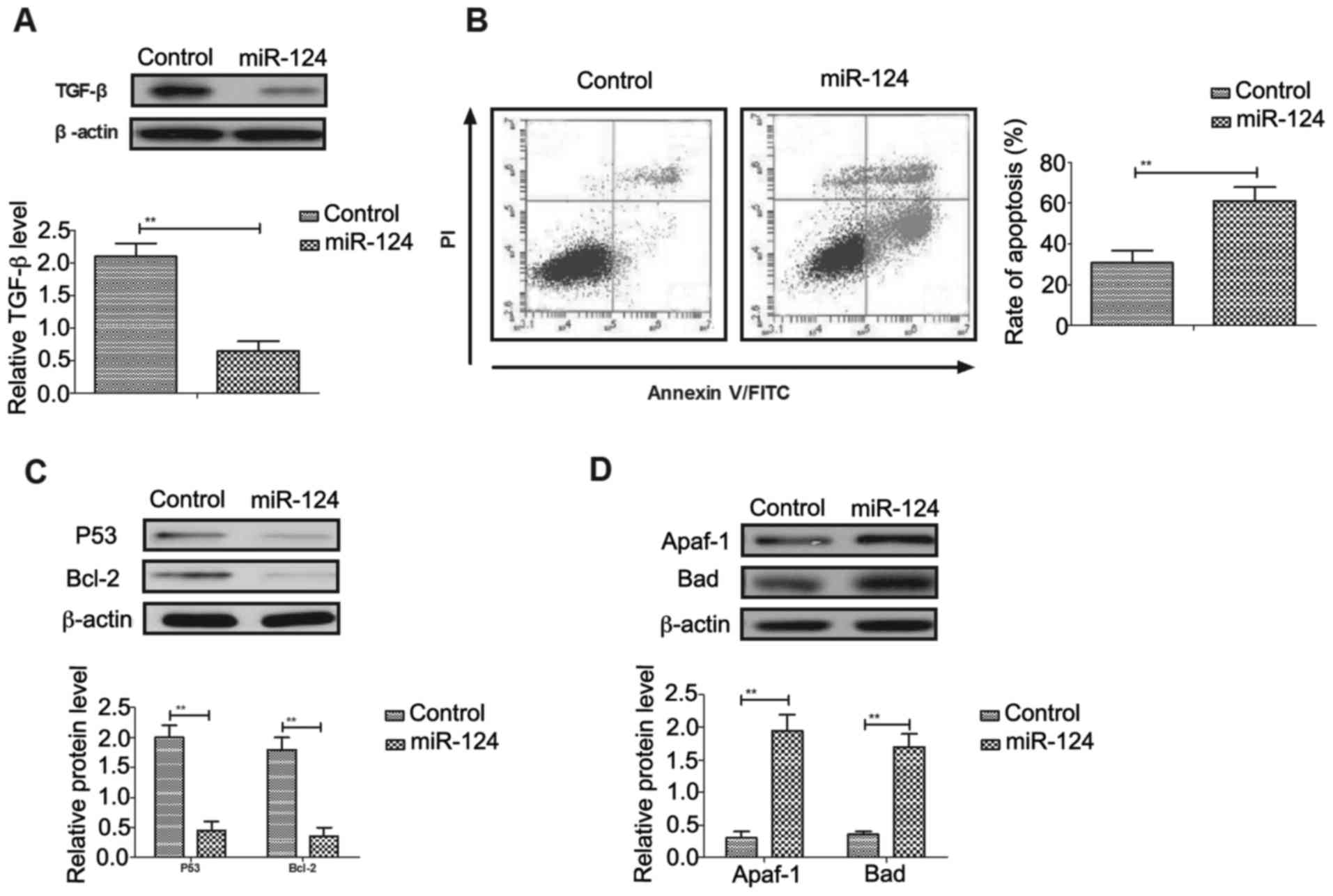
As shown in Fig.
3A, miR-124 transfection inhibited TGF-β expression in Mg63
cells. Flow cytometry analysis demonstrated that
tunicamycin-induced apoptosis was promoted by miR-124 in Mg63 cells
(Fig. 3B). Western blot analysis
demonstrated that P53 and Bcl-2 apoptosis regulator (Bcl-2)
expression levels were downregulated, and apoptotic peptidase
activating factor 1 (Apaf-1) and Bcl-2 associated agonist of cell
death (Bad) expression levels were upregulated in
miR-124-transfected Mg63 cells compared with control cells
(Fig. 3C and D). Transfection of
pTGF-β significantly increased TGF-β expression in Mg63 cells
(Fig. 3E). Results demonstrated
that TGF-β overexpression blocked miR-124-mediated apoptotic
sensitivity to tunicamycin in Mg63 cells (Fig. 3F). Upregulation of P53 and Bcl-2
expression levels induced by miR-124 transfection was also reversed
by TGF-β overexpression in Mg63 cells (Fig. 3G).
Evaluation of AKT/GSK-3β/SNAIL-1
signaling pathway in osteosarcoma cells following miR-124
transfection
In order to analyze the potential mechanisms of
miR-124-meidated inhibition of osteosarcoma cells,
AKT/GSK-3β/SNAIL-1 signaling was investigated in Mg63 cells. As
shown in Fig. 4A, miR-124
transfection inhibited AKT, GSK-3β and SNAIL-1 expression in Mg63
cells. However, miR-124 transfection did not change phosphorylation
levels of AKT and GSK-3β in Mg63 cells (Fig. 4B). The results demonstrated that
TGF-β overexpression promoted AKT, GSK-3β and SNAIL-1 expression in
Mg63 cells (Fig. 4C). Migration
and invasion assays demonstrated that TGF-β overexpression canceled
miR-124-inhibited aggressiveness of Mg63 cells (Fig. 4D and E). TGF-β overexpression
improved viability of Mg63 cells transfected by miR-124 (Fig. 4F).
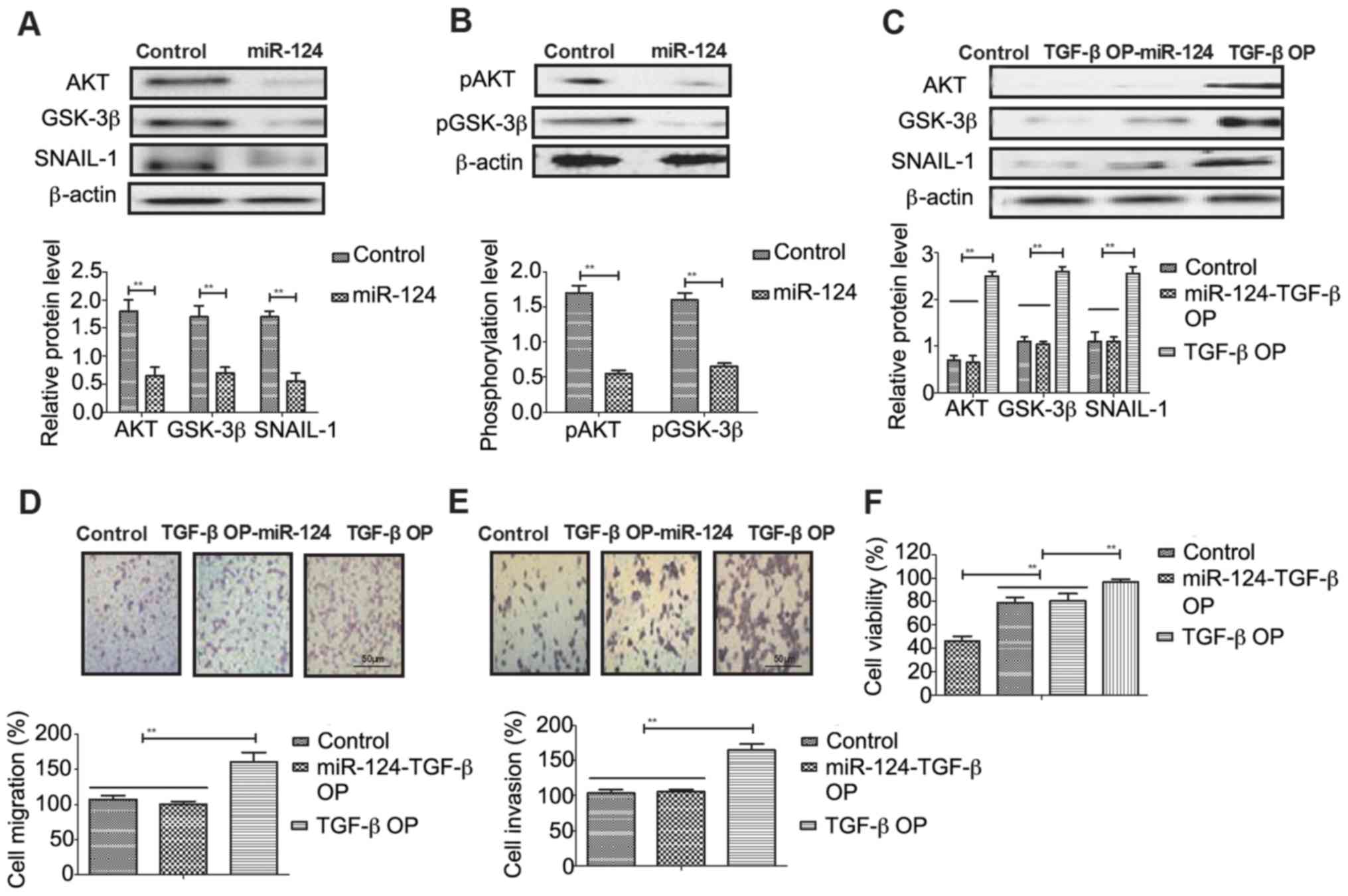 | Figure 4.miR-124 transfection regulates Mg63
cell migration/invasion and downregulates AKT/GSK-3β/SNAIL-1
signaling. (A) miR-124 transfection inhibits AKT, GSK-3β and
SNAIL-1 expression levels in Mg63 cells. (B) Effects of miR-124
transfection on pAKT and pGSK-3β in Mg63 cells. (C) Effects of
TGF-βOP on AKT, GSK-3β and SNAIL-1 expression levels in Mg63 cells.
Effects of TGF-βOP on (D) migration and (E) invasion in Mg63 cells
(magnification, ×400). (F) Effects of TGF-βOP on viability of Mg63
cells. Control, pvector; miR-124, microRNA-124; AKT, AKT
serine/threonine kinase; GSK-3β, glycogen synthase kinase-3β;
SNAIL-1, snail family transcriptional repressor 1; p,
phosphorylated; TGF-βOP, transforming growth factor-β
overexpression. Data represent the mean ± standard error in
triplicate of triplicate samples (n=3). **P<0.01. |
Effects of miR-124 transfection on
tumor growth and survival rate in osteosarcoma-bearing mice
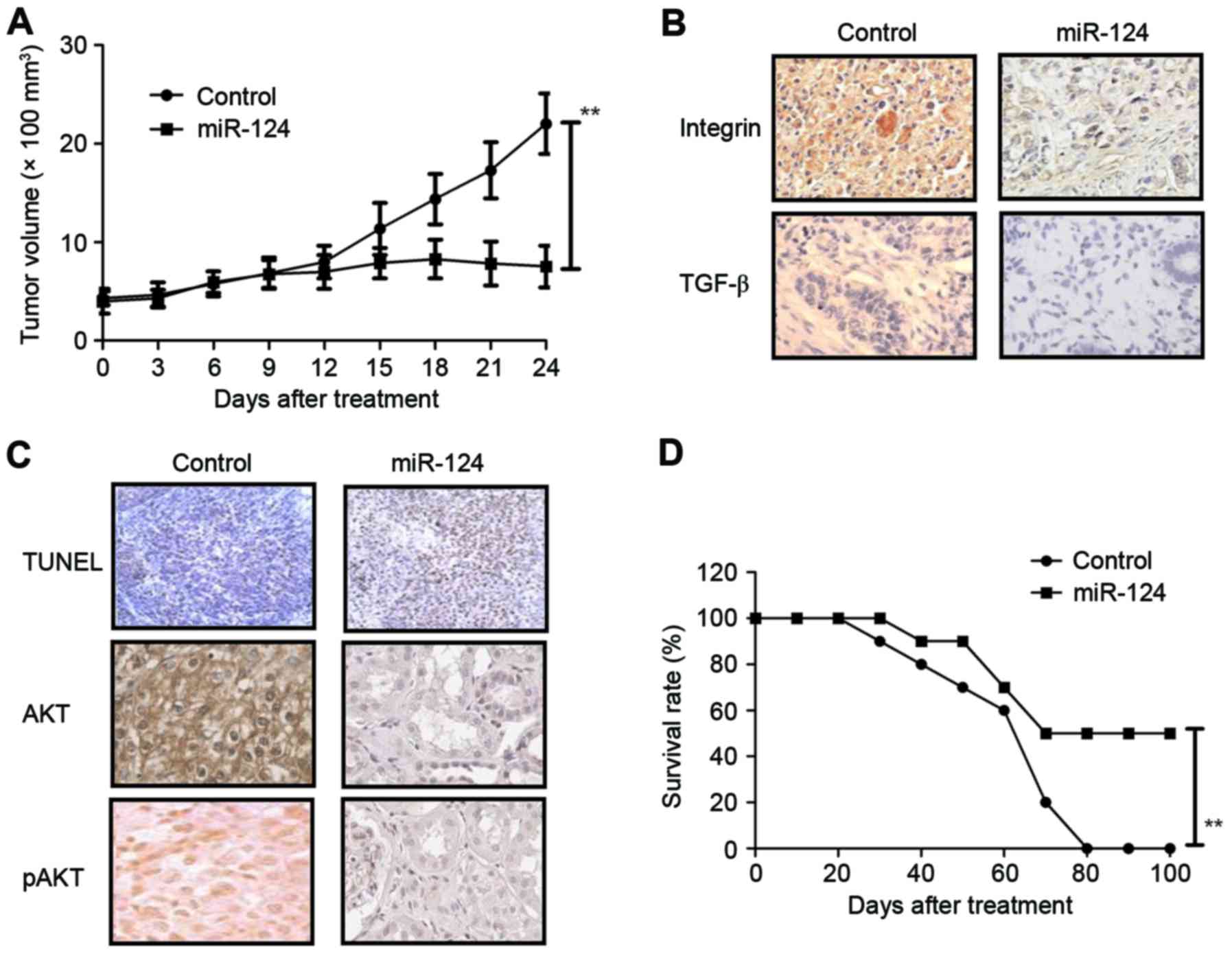
As shown in Fig.
5A, miR-124 transfection markedly inhibited tumor growth in
Mg63-bearing mice compared with the miR-vector group.
Immunohistochemistry indicated that integrin and TGF-β expression
levels were decreased in tumors generated from miR-124-transfected
Mg63 cells compared with untransfected cells (Fig. 5B). The number of apoptotic tumor
cells was increased, and AKT expression and phosphorylation levels
were decreased in miR-124-transfected tumors compared with the
control tumors (Fig. 5C). The
survival of tumor-bearing mice was prolonged in mice with tumors
overexpressing miR-124 compared with control tumor mice (Fig. 5D). These results suggest that
miR-124 transfection can inhibit tumor growth and prolong survival
of tumor-bearing mice, potentially by increasing the apoptosis of
tumor cells.
Discussion
miRNAs are small noncoding RNAs, which have a
complex role in post-transcriptional gene expression regulation,
and can theoretically be used as a diagnostic, therapeutic or
prognostic tool in tumor biology (29–31).
Reports have indicated that the expression levels of certain miRNAs
are associated with growth, proliferation, aggressive phenotypes
and apoptosis in human osteosarcoma (32,33).
The aim of the present study was to examine the inhibitory effects
of miR-124 in osteosarcoma tumorigenesis and development. The
results indicated that miR-124 transfection inhibits growth,
migration and invasion, and also promotes apoptosis of osteosarcoma
cells. Mechanism analysis demonstrated that miR-124 transfection
may inhibit tumor growth and aggressiveness through effects on
AKT/GSK-3β/SNAIL-1 signaling in osteosarcoma cells. In vivo
experiments suggested that miR-124 transfection inhibited tumor
growth and prolonged the survival of tumor-bearing mice,
potentially by increasing the apoptosis of tumor cells. These
findings indicate that miR-124 may serve as potential molecular
therapy for osteosarcoma.
Currently, increasing the apoptosis of osteosarcoma
tumor cells contributes to an efficient clinical regiment for
patients with cancer (34,35). Acquired resistance protects
osteosarcoma tumor cells against apoptosis induced by radiotherapy,
chemotherapy and combined therapy in the treatment of tumors
(36,37). Reducing the apoptotic resistance of
cancer cells and tumors tissues will contribute to the clinical
treatment for of with osteosarcoma who have undergone oncotherapy
and other comprehensive treatments (38,39).
In this study, the data indicated that miR-124 transfection
promoted apoptosis of osteosarcoma cells, potentially via
regulation of P53, Bcl-2, Apaf-1 and Bad expression. However, TGF-β
overexpression significantly blocked miR-124 transfection-mediated
tumor apoptosis in osteosarcoma cells.
A previous study suggested that miR-124 may be
associated with tumor growth and aggressiveness (40). Han et al (38) reported that miR-124 exerts tumor
suppressive functions on growth, proliferation, motility and
angiogenesis. In this study, the anti-tumor efficacy and potential
mechanism of miR-124 in osteosarcoma cells was analyzed. Research
has demonstrated that TGF-β-mediated AKT/GSK-3β/SNAIL-1 signaling
directly induces epithelial-mesenchymal transition in human
bronchial epithelial cells, which suggested these proteins as
potential novel targets in the development of therapeutic and
preventive approaches for human cancer (41). The findings of the present study
suggested that miR-124 inhibits growth and aggressiveness by
downregulation of integrin expression via TGF-β-mediated
AKT/GSK-3β/SNAIL-1 signaling in osteosarcoma cells. These results
indicate that miR-124 may have potential as an anti-osteosarcoma
agent.
In conclusion, the levels of miR-124 and its
potential molecular mechanisms in osteosarcoma cells were
investigated in the current study. Although previous studies have
reported the tumor suppressor role of miR-124, miR-124-regulated
integrin expression through TGF-β-mediated AKT/GSK-3β/SNAIL-1
signaling has not been reported in osteosarcoma cells (42,43).
The findings in the current study indicate miR-124 promotes
apoptotic sensitivity of osteosarcoma to tunicamycin, suppresses
growth and aggressiveness of osteosarcoma, and downregulates of
TGF-β-mediated AKT/GSK-3β/SNAIL-1 signaling, which provides a
potential mechanism tumor suppressor mechanism of miR-124 in
osteosarcoma cells and tumor tissues.
References
|
1
|
Berner K, Bjerkehagen B, Bruland ØS and
Berner A: Extra skeletal osteosarcoma in Norway, between 1975 and
2009, and a brief review of the literature. Anticancer Res.
35:2129–2140. 2015.PubMed/NCBI
|
|
2
|
Glass R, Asirvatham JR, Kahn L and Aziz M:
Beta-human chorionic gonadotropin producing osteosarcoma of the
sacrum in a 26-year-old woman: A case report and review of the
literature. Case Rep Pathol. 2015:8972302015.PubMed/NCBI
|
|
3
|
Dell'Amore A, Asadi N, Caroli G, Dolci G,
Bini A and Stella F: Recurrent primary cardiac osteosarcoma: A case
report and literature review. Gen Thorac Cardiovasc Surg.
62:175–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farcas N, Arzi B and Verstraete FJ: Oral
and maxillofacial osteosarcoma in dogs: A review. Vet Comp Oncol.
12:169–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubo T, Shimose S, Fujimori J, Furuta T
and Ochi M: Quantitative (201)thallium scintigraphy for prediction
of histological response to neoadjuvant chemotherapy in
osteosarcoma; systematic review and meta-analysis. Surg Oncol.
24:194–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabatabaei SH, Jahanshahi G and Dehghan
Marvasti F: Diagnostic challenges of low-grade central osteosarcoma
of jaw: A literature review. J Dent (Shiraz). 16:62–67.
2015.PubMed/NCBI
|
|
7
|
O'Kane GM, Cadoo KA, Walsh EM, Emerson R,
Dervan P, O'Keane C, Hurson B, O'Toole G, Dudeney S, Kavanagh E, et
al: Perioperative chemotherapy in the treatment of osteosarcoma: A
26-year single institution review. Clin Sarcoma Res. 5:172015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szewczyk M, Lechowski R and Zabielska K:
What do we know about canine osteosarcoma treatment? Review. Vet
Res Commun. 39:61–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friebele JC, Peck J, Pan X, Abdel-Rasoul M
and Mayerson JL: Osteosarcoma: A meta-analysis and review of the
literature. Am J Orthop (Belle Mead NJ). 44:547–553.
2015.PubMed/NCBI
|
|
10
|
Zhou Y, Zhao RH, Tseng KF, Li KP, Lu ZG,
Liu Y, Han K, Gan ZH, Lin SC, Hu HY and Min DL: Sirolimus induces
apoptosis and reverses multidrug resistance in human osteosarcoma
cells in vitro via increasing microRNA-34b expression. Acta
Pharmacol Sin. 37:519–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H, Peng C, Ruan G, Zhou J, Li Y and
Hai Y: Adenovirus-delivered PDCD5 counteracts adriamycin resistance
of osteosarcoma cells through enhancing apoptosis and inhibiting
Pgp. Int J Clin Exp Med. 7:5429–5436. 2014.PubMed/NCBI
|
|
12
|
Tsai HC, Huang CY, Su HL and Tang CH: CCN2
enhances resistance to cisplatin-mediating cell apoptosis in human
osteosarcoma. PLoS One. 9:e901592014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma G, Rana NK, Singh P, Dubey P,
Pandey DS and Koch B: p53 dependent apoptosis and cell cycle delay
induced by heteroleptic complexes in human cervical cancer cells.
Biomed Pharmacother. 88:218–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Locklin RM, Federici E, Espina B, Hulley
PA, Russell RG and Edwards CM: Selective targeting of death
receptor 5 circumvents resistance of MG-63 osteosarcoma cells to
TRAIL-induced apoptosis. Mol Cancer Ther. 6:3219–3228. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vourvouhaki E, Carvalho C and Aguiar P:
Model for Osteosarcoma-9 as a potent factor in cell survival and
resistance to apoptosis. Phys Rev E Stat Nonlin Soft Matter Phys.
76:0119262007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X, Yang X, Xing C, Zhang S and Cao J:
miRNA: The nemesis of gastric cancer (Review). Oncol Lett.
6:631–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banerjee N, Kim H, Krenek K, Talcott ST
and Mertens-Talcott SU: Mango polyphenolics suppressed tumor growth
in breast cancer xenografts in mice: Role of the PI3K/AKT pathway
and associated microRNAs. Nutr Res. 35:744–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bi C, Chung TH, Huang G, Zhou J, Yan J,
Ahmann GJ, Fonseca R and Chng WJ: Genome-wide pharmacologic
unmasking identifies tumor suppressive microRNAs in multiple
myeloma. Oncotarget. 6:26508–26518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Igaz I and Igaz P: Are circulating
microRNAs involved in tumor surveillance? EXS. 106:269–280.
2015.PubMed/NCBI
|
|
21
|
Hou LJ and Zhai JJ: Aberrant expression
profile of translationally controlled tumor protein and
tumor-suppressive microRNAs in cervical cancer. J BUON.
20:1504–1509. 2015.PubMed/NCBI
|
|
22
|
Golestaneh AF, Atashi A, Langroudi L,
Shafiee A, Ghaemi N and Soleimani M: miRNAs expressed differently
in cancer stem cells and cancer cells of human gastric cancer cell
line MKN-45. Cell Biochem Funct. 30:411–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mongre RK, Sodhi SS, Ghosh M, Kim JH, Kim
N, Sharma N and Jeong DK: A new paradigm to mitigate osteosarcoma
by regulation of MicroRNAs and suppression of the NF-κB signaling
cascade. Dev Reprod. 18:197–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang L, Shrestha S, LaChaud G, Scott MA
and James AW: Review of microRNA in osteosarcoma and
chondrosarcoma. Med Oncol. 32:6132015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mason TJ and Matthews M: Aquatic
environment, housing, and management in the eighth edition of the
guide for the care and use of laboratory animals: Additional
considerations and recommendations. J Am Assoc Lab Anim Sci.
51:329–332. 2012.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhuang T, Djemil T, Qi P, Magnelli A,
Stephans K, Videtic G and Xia P: Dose calculation differences
between Monte Carlo and pencil beam depend on the tumor locations
and volumes for lung stereotactic body radiation therapy. J Appl
Clin Med Phys. 14:40112013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin Z, Ding H, He E, Chen J and Li M:
Up-regulation of microRNA-491-5p suppresses cell proliferation and
promotes apoptosis by targeting FOXP4 in human osteosarcoma. Cell
Prolif. 50:2017. View Article : Google Scholar
|
|
30
|
Zhou Y, Yang C, Wang K, Liu X and Liu Q:
MicroRNA-33b inhibits the proliferation and migration of
osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol
Res. 25:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhi X, Wu K, Yu D, Wang Y, Yu Y, Yan P and
Lv G: MicroRNA-494 inhibits proliferation and metastasis of
osteosarcoma through repressing insulin receptor substrate-1. Am J
Transl Res. 8:3439–3447. 2016.PubMed/NCBI
|
|
32
|
Azam AT, Bahador R, Hesarikia H, Shakeri M
and Yeganeh A: Retraction Note to: Downregulation of microRNA-217
and microRNA-646 acts as potential predictor biomarkers in
progression, metastasis, and unfavorable prognosis of human
osteosarcoma. Tumour Biol. Nov 5–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
33
|
Taheriazam A, Bahador R, Karbasy SH,
Jamshidi SM, Torkaman A, Yahaghi E and Shakeri M: Retraction note:
Down-regulation of microRNA-26a and up-regulation of microRNA-27a
contributes to aggressive progression of human osteosarcoma. Diagn
Pathol. 11:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kovács D, Igaz N, Keskeny C, Bélteky P,
Tóth T, Gáspár R, Madarász D, Rázga Z, Kónya Z, Boros IM and
Kiricsi M: Silver nanoparticles defeat p53-positive and
p53-negative osteosarcoma cells by triggering mitochondrial stress
and apoptosis. Sci Rep. 6:279022016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Zhang T, Sun W, Wang Z, Zuo D,
Zhou Z, Li S, Xu J, Yin F, Hua Y and Cai Z: Erianin induces
G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK
signaling pathway in human osteosarcoma cells in vitro and in vivo.
Cell Death Dis. 7:e22472016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chinchar E, Makey KL, Gibson J, Chen F,
Cole SA, Megason GC, Vijayakumar S, Miele L and Gu JW: Sunitinib
significantly suppresses the proliferation, migration, apoptosis
resistance, tumor angiogenesis and growth of triple-negative breast
cancers but increases breast cancer stem cells. Vasc Cell.
6:122014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guidicelli G, Chaigne-Delalande B,
Dilhuydy MS, Pinson B, Mahfouf W, Pasquet JM, Mahon FX, Pourquier
P, Moreau JF and Legembre P: The necrotic signal induced by
mycophenolic acid overcomes apoptosis-resistance in tumor cells.
PLoS One. 4:e54932009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han J, Tian R, Yong B, Luo C, Tan P, Shen
J and Peng T: Gas6/Axl mediates tumor cell apoptosis, migration and
invasion and predicts the clinical outcome of osteosarcoma
patients. Biochem Biophys Res Commun. 435:493–500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kushlinskii NE, Solov'ev YN, Babkina IV,
Abbasova SG, Kostanyan IA, Lipkin VM and Trapeznikov NN: Leptin and
apoptosis inhibitor soluble Fas antigen in the serum of patients
with osteosarcoma and neuroectodermal bone tumors. Bull Exp Biol
Med. 129:496–498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie C, Han Y, Liu Y, Han L and Liu J:
miRNA-124 down-regulates SOX8 expression and suppresses cell
proliferation in non-small cell lung cancer. Int J Clin Exp Pathol.
7:6534–6542. 2014.PubMed/NCBI
|
|
41
|
Polimeni M, Gulino GR, Gazzano E, Kopecka
J, Marucco A, Fenoglio I, Cesano F, Campagnolo L, Magrini A,
Pietroiusti A, et al: Multi-walled carbon nanotubes directly induce
epithelial-mesenchymal transition in human bronchial epithelial
cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway.
Part Fibre Toxicol. 13:272016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Kang FB, Sun N, Wang J, Chen W, Li
D and Shan BE: The tumor suppressor miR-124 inhibits cell
proliferation and invasion by targeting B7-H3 in osteosarcoma.
Tumour Biol. 37:14939–14947. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Geng S, Zhang X, Chen J, Liu X, Zhang H,
Xu X, Ma Y, Li B, Zhang Y, Bi Z and Yang C: The tumor suppressor
role of miR-124 in osteosarcoma. PLoS One. 9:e915662014. View Article : Google Scholar : PubMed/NCBI
|