Introduction
The ocean covers ~70% of the Earth's surface; it is
a rich source of natural resources and contains ~80% of all the
biodiversity on our planet. It is home to approximately one million
multicellular species and one billion unicellular species (1). During the past 30–40 years,
>22,000 novel natural products have been identified from marine
sources, whereas ~131,000 have been identified from terrestrial
sources. These marine products have various biological properties,
including anticancer and antiviral activities (2,3). The
study of anticancer marine natural products (MNPs) is an important
approach to global drug discovery. MNPs have featured significantly
in the development of anticancer drugs; furthermore, they are
generally free of deleterious side effects and are usually
inexpensive (4).
A large proportion of MNPs have been extracted from
marine invertebrates, including alkaloids, peptides, terpenes and
polyketides (5,6). Tegillarca granosa is a type of
marine invertebrate widely distributed in China, which has been a
traditional Chinese seafood for hundreds of years. Haishengsu (HSS)
is an extract isolated from T. granosa, and its major
chemical constituents are small molecular weight proteins that
rangebetween 15 and 23 kDa. Previous clinical and experimental
studies regarding HSS have investigated its anticancer properties,
and the possible mechanisms associated with its activity. Clinical
studies have indicated that HSS, used in combination with other
chemotherapeutic agents, may display significant activity against
human cancers, such as non-small-cell lung cancer and advanced
renal cell cancer; therefore, it may have the potential to improve
the quality of life for these patients (7,8).
Experimental studies have demonstrated that HSS may have an
inhibitory effect on several human cancer cells, including ovarian
cancer cell lines (SKOV-3 and OVCAR-3), lung carcinoma cell lines
(A549 and NCI-H292), leukemia cell lines (K562 and K562/ADM) and
renal carcinoma cell lines (OS-RC-2) (9–13).
Furthermore, murine studies have indicated that HSS may inhibit the
growth of Ehrlich ascites tumors and improve immunological
functions (14). Our previous
study demonstrated that HSS was able to induce the apoptosis of
human hepatocellular carcinoma (HCC) cells in a
concentration-dependent manner, through the Fas signaling pathway,
with caspase-8 and caspase-3 activation (15).
As a type of programmed cell death, apoptosis is a
genetically controlled suicide mechanism that enables organisms to
control cell number and eliminate cells that threaten survival
(16). Classical apoptosis can be
initiated via two major pathways: The intrinsic or
mitochondrial-mediated pathway, and the extrinsic or death
receptor-mediated pathway (17). A
study from our laboratory indicated that HSS induces apoptosis in
HCCBEL-7402 cells via the Fas signaling pathway (15). However, little is currently known
regarding the mechanisms responsible for the mitochondrial-mediated
apoptotic pathway of HSS against HCC cells. The present study
further investigated whether mitochondrial-mediated apoptotic
pathways are involved in the inhibitory effects of HSS on BEL-7402
cells, with the aim of providing further experimental evidence to
support the HSS-based treatment of HCC.
Materials and methods
HSS preparation
HSS was extracted from the shellfish Tegillarca
granosa as described previously (15), and dissolved in phosphate-buffered
saline (PBS) prior to use.
Cell culture
The human HCC cell line BEL-7402 was obtained from
the Shanghai Cell Bank at the Institute of Biochemistry and Cell
Biology (Chinese Academy of Sciences, Shanghai, China). Cells were
maintained in RPMI 1640 medium (GE Healthcare, Chicago, IL, USA)
supplemented with 10% (v/v) fetal bovine serum (GE Healthcare), 100
U/ml penicillin and 100 mg/ml streptomycin, under 5% CO2
at 37°C, in a humidified incubator.
DNA fragmentation assay
DNA fragmentation is a marker of cell apoptosis
(18). Briefly, BEL-7402 cells
were treated with various concentrations(0–100 µg/ml)of HSS for 48
h at 37°C. Subsequently the cells were centrifuged (500 × g for 5
min at 4°C) and washed with PBS, and the pellets were then
resuspended in 500 µl cell lysis buffer [150 mM NaCl, 10 mM
Tris-HCl (pH 7.5), 10 mM EDTA, 0.5% SDS, 500 mg/l proteinase K] and
incubated overnight at 50°C. Following incubation, the cell lysate
was extracted with phenol/chloroform/isopropyl alcohol (25:24:1
v/v), the DNA was precipitated with sodium acetate (50 µl; 3 M, pH
5.2) and ethanol (1 ml) at −20°C overnight, and subsequently washed
with 70% ethanol. The DNA pellet was dissolved in Tris-EDTA buffer
(10 mM Tris, 1 mM EDTA, pH 8.0) and incubated with RNase A (20
µg/ml) at 37°C for 30 min. Subsequently, DNA samples were separated
by horizontal electrophoresis on 1.5% agarose gels, stained with
ethidium bromide, and visualized under UV light.
Caspase activity assay
The activities of caspase-3 and −9 were detected
using a Caspase Colorimetric Assay kit (cat. nos. ab39401 and
ab65608; Abcam, Cambridge, UK), according to the manufacturer's
protocol. Caspase activity was measured via the cleavage of
chromogenic caspase substrates. Briefly, following treatment with
various concentrations (0–100 µg/ml) of HSS for 48 h at 37°C,
BEL-7402 cells were harvested and lysed in the supplied lysis
buffer. The lysed cells were centrifuged at 18,000 × g for 10 min,
and protein concentrations were determined using a bicinchoninic
acid assay. The proteins were mixed with caspase substrates at 37°C
for 2 h, and the p-nitroaniline light emission was quantified using
a microplate reader at 405 nm.
Western blot analysis
After treatment, cells were harvested and lysed with
ice-cold lysis buffer (Cell Signaling Technology, Inc., Danvers,
MA, USA) containing protease inhibitors (Sigma Aldrich, Merck KGaA,
Darmstadt, Germany). The mitochondrial and cytosolic proteins were
extracted using a Cell Mitochondria Isolation kit (Beyotime
Biotechnology, Haimen, China) according to the manufacturer's
protocol. Briefly, the cell pellet was then resuspended in the
extraction buffer which was enclosed in the isolation kit. After 30
min incubation on ice, cells were homogenized with a dounce
homogenizer, and the homogenate was centrifuged at 600 × g for 10
min at 4°C. The supernatant was spun at 11,000 × g for 10 min at
4°C. The supernatant (cytosolic fraction) was removed and
maintained at −80°C. The pellet containing mitochondria was
resolved in the lysis buffer which was enclosed in the isolation
kit. The protein concentration was determined using the
bicinchoninic acid assay (Beyotime Biotechnology). Western blotting
was performed as previously described (19). Extracted proteins (50 µg) in
lysates were separated by 10–12% SDS-PAGE and then transferred
electrophoretically onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with 5%
non-fat milk overnight at 4°C. Subsequently, membranes were stained
with primary antibodies against poly (ADP-ribose) polymerase (PARP;
1:1,000), caspase-9 (1:1,000), caspase-3 (1:100), B-cell lymphoma 2
(Bcl-2; 1:500), Bcl-2-associated X protein (Bax; 1:1,000),
cytochrome c, cytochromecoxidase (COX)-IV (1:100),
α-tubulin (1:5,000), (cat. nos. ab4830, ab32539, ab4051, ab59348,
ab32503, ab14744 and ab7291 respectively; Abcam). A rabbit
polyclonal antibody to GAPDH was used as a loading control (1:500;
cat. no. ab8245). Bands were detected with SuperSignal West Femto
Maximum Sensitivity substrate (Pierce; Thermo Fisher Scientific,
Inc.) and imaged with the ChemiDoc™ MP Imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Densitometry analysis was
performed using Quantity One analysis software (version 4.6.9;
Bio-Rad Laboratories, Inc.).
In vivo antitumor efficacy
Female BALB/c nude mice (6–8 weeks of age, weight
18–22 g) were provided by the Laboratory Animal Institute, Chinese
Academy of Medical Sciences (Beijing, China). Animal experiments
were performed in accordance with the Chinese Animal Protection
Network, and laboratory protocols were approved by the Animal Care
and Use Committee of Qingdao University (Qingdao, China). The mice
were housed in cages in a room with an artificial 12-h light/dark
cycle at a constant temperature range (24±2°C) and relative
humidity (55±10%). The mice were acclimated for one week prior to
the study, and fed standard chow and water ad libitum. In
particular, mice were maintained in
specific-pathogen-free-conditions. Animals were subjected to
surgical procedures in accordance with Institutional guidelines.
Tumors were established by subcutaneous injection of
2×106 BEL-7402 cells into the flank. Tumor volumes were
estimated according to the formula: Tumor volume (mm3) =
(length × width2)/2. When tumors reached a size of ~100
mm3, the mice were randomly assigned to 4 groups
(n=6/group): Control and 3 treatment groups. The mice in the
HSS-treatment groups were intragastrically administrated 0.5 ml HSS
daily, at doses of 62.5, 125 or 250 mg/kg in a solution containing
0.5% sodium carboxymethylcellulose. The vehicle treated control
mice received 0.5% sodium carboxymethylcellulose. The mice were
monitored closely and weighed twice a week. The mice were
sacrificed 26 days and then tumor xenografts, liver and kidney
samples were collected for the following experiments.
Transmission electron microscopy (TEM)
assay
Tumor tissues from HSS-treated mice were examined
using transmission electron microscopy. Briefly, 1 mm3
tumor tissue sections were fixed in 4% (w/v) glutaraldehyde
followed by 1% (w/v) osmic tetroxide, and then embedded in epoxy
resin. Serial 0.5 µm sections were sliced and stained with uranyl
acetate-lead citrate, and examined under an H-7650 transmission
electron microscope at 80 kV (Hitachi, Ltd., Tokyo, Japan).
Histological evaluations
Tissue sections (4 µm) were generated from the
paraffin-embedded tissue specimens, and were stained with
hematoxylin and eosin (H&E). Endogenous peroxidase activity was
blocked with 3% H2O2 in methanol at room
temperature for 15 min after deparaffinization and rehydration.
Antigen retrieval was performed by heating the slides in 0.01 M
sodium citrate buffer at 95°C for 3 min. After blocking with 10%
bovine serum albumin in PBS for 60 min, the slides were incubated
with a primary antibody against caspase-3 (1:10; cat. no. ab4051;
Abcam) at 4°C overnight. After three washes in PBS, the slides were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:200; cat. no. ab6728; Abcam). The immune complex was
visualized using 3,3′-diaminobenzidene-chromogen (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturer's protocol. Counterstaining was performed on
representative sections with hematoxylin, and then the slides were
observed under a light microscope (Olympus Corporation, Tokyo,
Japan). Quantitative analysis was performed using Image-Pro Plus
software (version 4.5; Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
Statistical analyses were performed with SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). All data were presented as the mean
± standard deviation. The differences between groups were evaluated
using one-way analysis of variance followed by Student Newman-Keuls
test. P<0.05 is considered to indicate a statistically
significant difference.
Results
HSS induces apoptosis of BEL-7402
cells
Our previous research demonstrated that HSS induces
apoptosis of BEL-7402 cells, as determined by flow cytometric
analysis and Hoechst 33258 nuclear staining (15). To further confirm these results, a
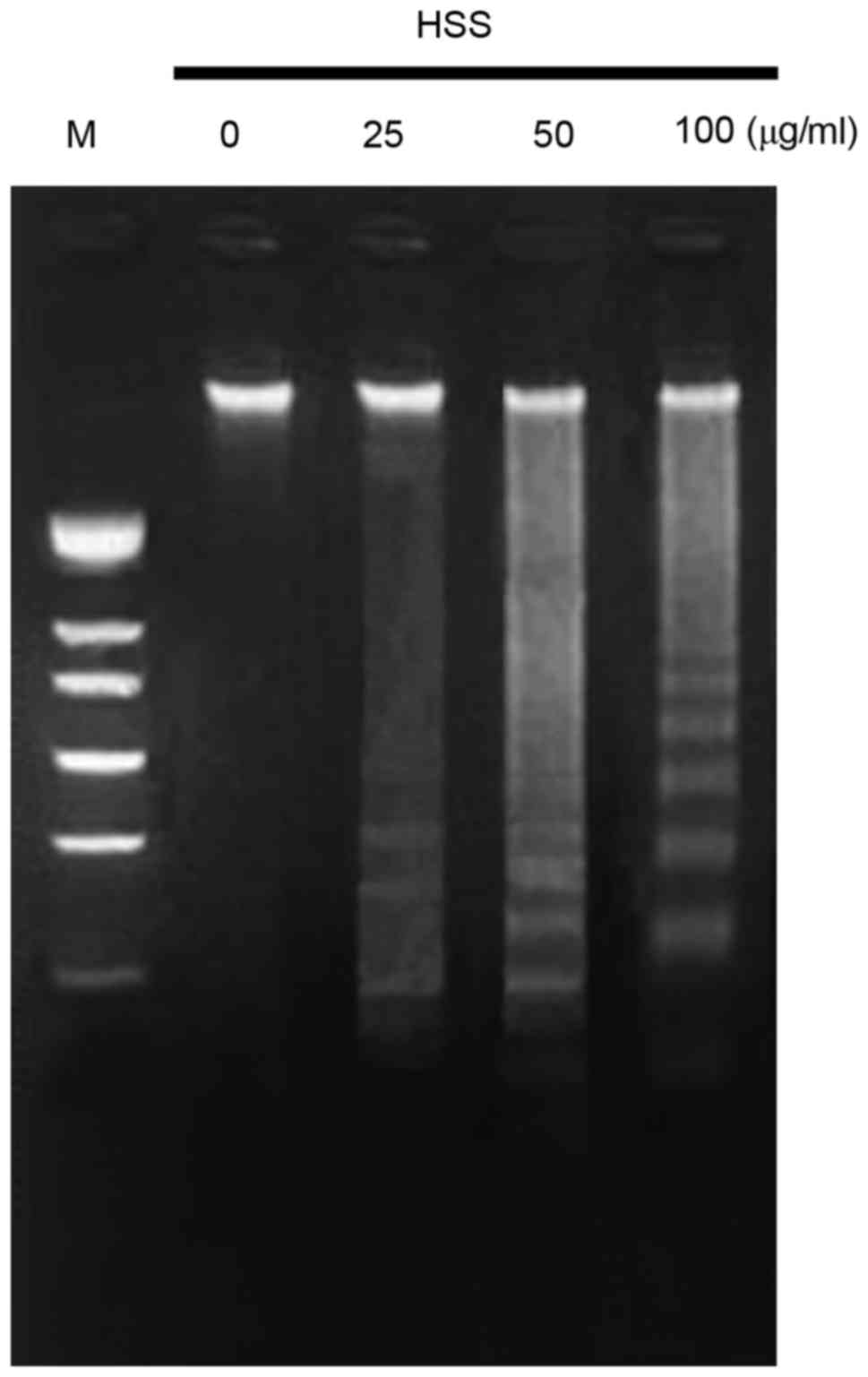
DNA fragmentation assay was conducted, since DNA fragmentation is a
classical hallmark of apoptosis. Apoptosis of BEL-7402 cells was
observed following treatment with HSS for 48 h at 25, 50 and 100
µg/ml. HSS treatment resulted in increased DNA fragmentation in
BEL-7402 cells, when treated with increasing HSS concentrations
(Fig. 1). These results indicated
that HSS exhibited a significant apoptosis-inducing effect on
BEL-7402 cells.
HSS induces caspase activation in
BEL-7402 cells
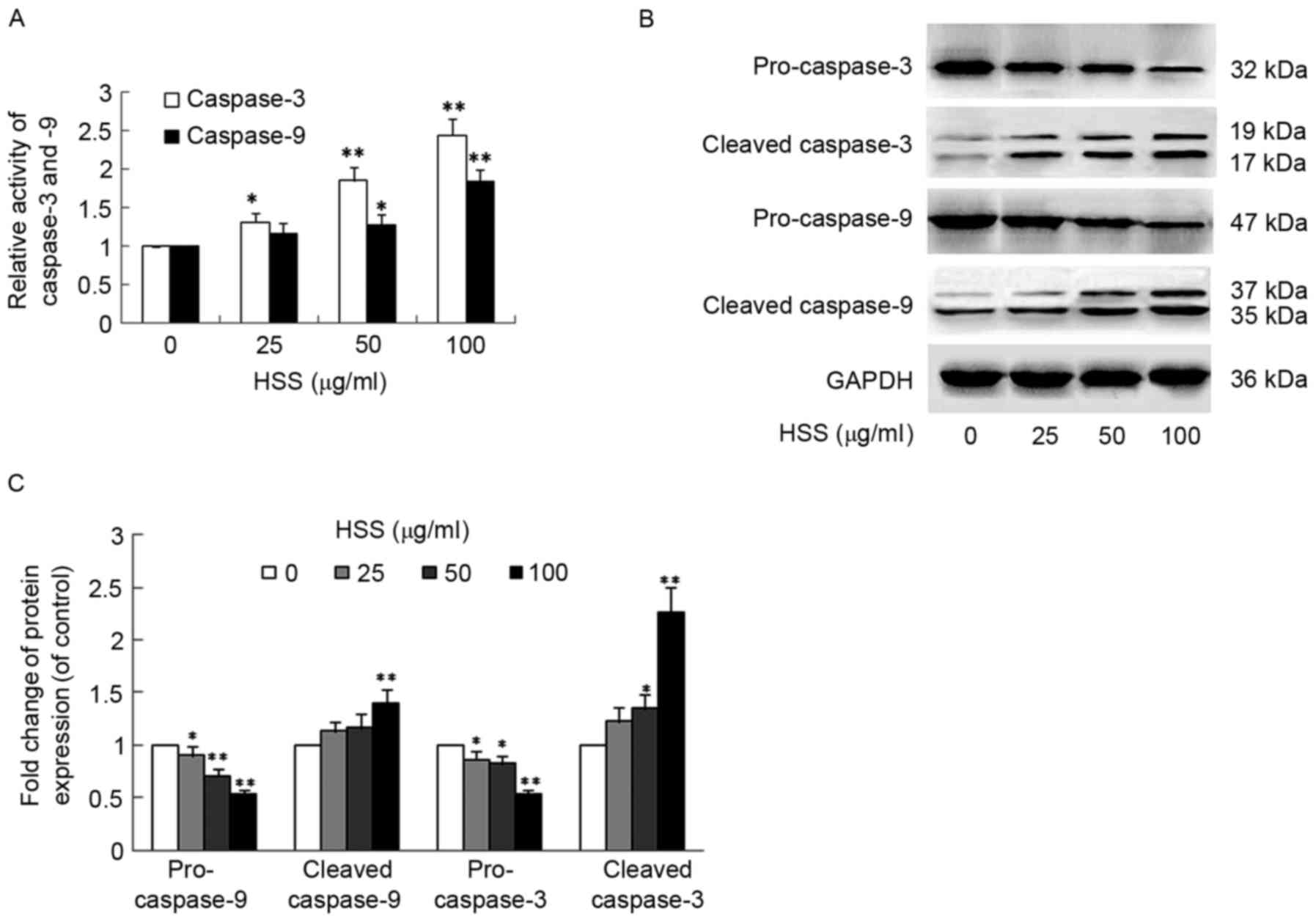
Activation of the caspase cascade is an important
mechanism in apoptotic cell death in most cell systems (20). The enzymatic activities of
caspase-3 and caspase-9 were detected using a Caspase Colorimetric
Assay kit. Following treatment with HSS, the enzymatic activities
of caspase-3 and caspase-9 were significantly increased (Fig. 2A). The relative activities of
caspase-3 and caspase-9 were increased to 2.438±0.219 (P<0.01)
and 1.834±0.163 (P<0.01), respectively, following treatment with
100 µg/ml HSS. To further confirm the role of caspase, western blot
analysis was performed to detect the protein levels of caspase-3
and caspase-9. In agreement with the caspase activity assay
results, caspase-9 activation accompanied the proteolytic
degradation of the inactive pro-enzyme (47 kDa) into the active
form (37/35 kDa) (Fig. 2B).
Furthermore, HSS exhibited a concentration-dependent effect on
caspase-9 activation (Fig. 2C).
Similarly, caspase-3 activation, via proteolytic degradation of the
32 kDa pro-enzyme into the 19/17 kDa activate form, was detected in
the presence of HSS in a concentration-dependent manner (Fig. 2B and C).
HSS regulates apoptosis-related
protein expression in BEL-7402 cells
To further investigate the involvement of the
HSS-induced apoptotic pathway in BEL-7402 cells, the expression of
apoptosis-related proteins: Bax, Bcl-2, cytochrome c, PARP
and cleaved PARP, was determined by western blot analysis (Fig. 3). BEL-7402 cells treated with HSS
for 48 h exhibited a concentration-dependent upregulation of the
proapoptotic protein Bax, as compared with the control group,
whereas the anti-apoptotic protein Bcl-2 was downregulated
(Fig. 3A and C). The ratio of
Bax/Bcl-2 was also significantly increased with HSS treatment
(Fig. 3D). Furthermore, cytochrome
c was released from the mitochondria to the cytosol; a
decrease in the relative density of mitochondrial cytochrome
c was observed, with an increase in the relative density of
cytosolic cytochrome c, at 25, 50 and 100 µg/ml HSS
(Fig. 3B and C). PARP is a
downstream target of active caspase-3 during the induction of the
apoptotic signaling pathway, and previous research has indicated
that itis cleaved into two fragments (21). HSS was also able to induce PARP
cleavage into its active form, as indicated by a reduction in the
full-length form (116 kDa) and an increase in the cleaved form (85
kDa) in a concentration-dependent manner (Fig. 3A and C).
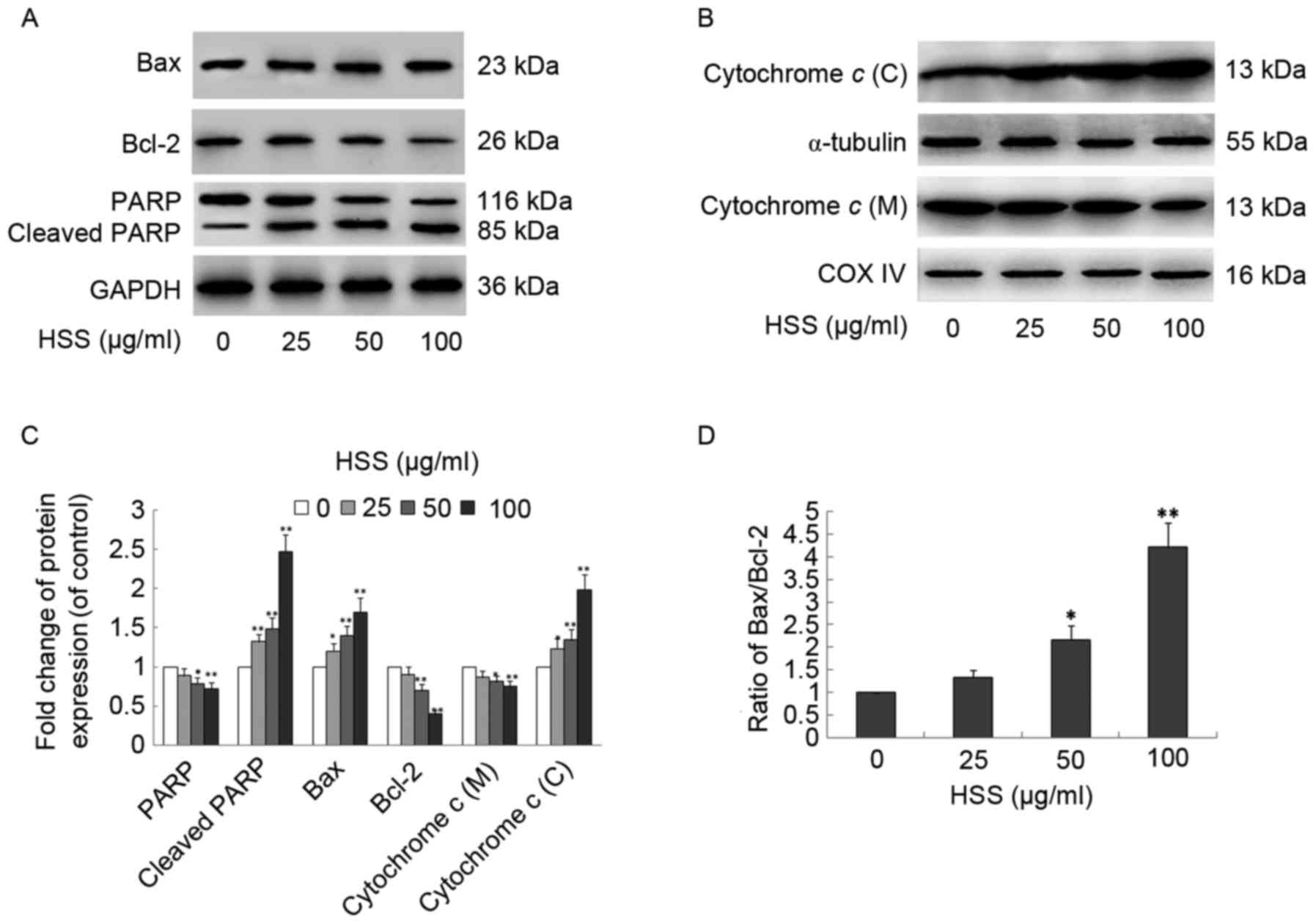 | Figure 3.HSS induces alterations in
apoptosis-related proteins in hepatocellular carcinoma-derived
BEL-7402 cells. BEL-7402 cells were treated with 0, 25, 50 or 100
µg/ml HSS for 48 h, and western blot analysis was performed on
whole cell lysates, and cytosolic and mitochondrial fractions. (A)
Expression of Bcl-2, Bax, PARP and cleaved-PARP was analyzed by
western blot analysis. GAPDH from the same sample served as the
loading control. (B) Cytochrome c localization assay. Cytochrome c
was measured in mitochondrial and cytosolic fractions by western
blot analysis. COX IV and α-tubulin were used as internal controls
for the mitochondria and cytosol, respectively. (C) Protein
expression levels of Bcl-2, Bax, PARP, cleaved PARP and cytochrome
c were quantified using Quantity One analysis software in BEL-7402
cells. (D) Bax/Bcl-2 ratios following treatment with various
concentrations of HSS were compared. Data are presented as the mean
± standard deviation from three independent experiments. *P<0.05
and **P<0.01, compared with the control group. HSS, Haishengsu;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; PARP,
poly (ADP-ribose) polymerase; COX IV, cytochrome c oxidase IV; C,
cytosolic; M, mitochondrial. |
HSS administration inhibits tumor
growth in nude mice
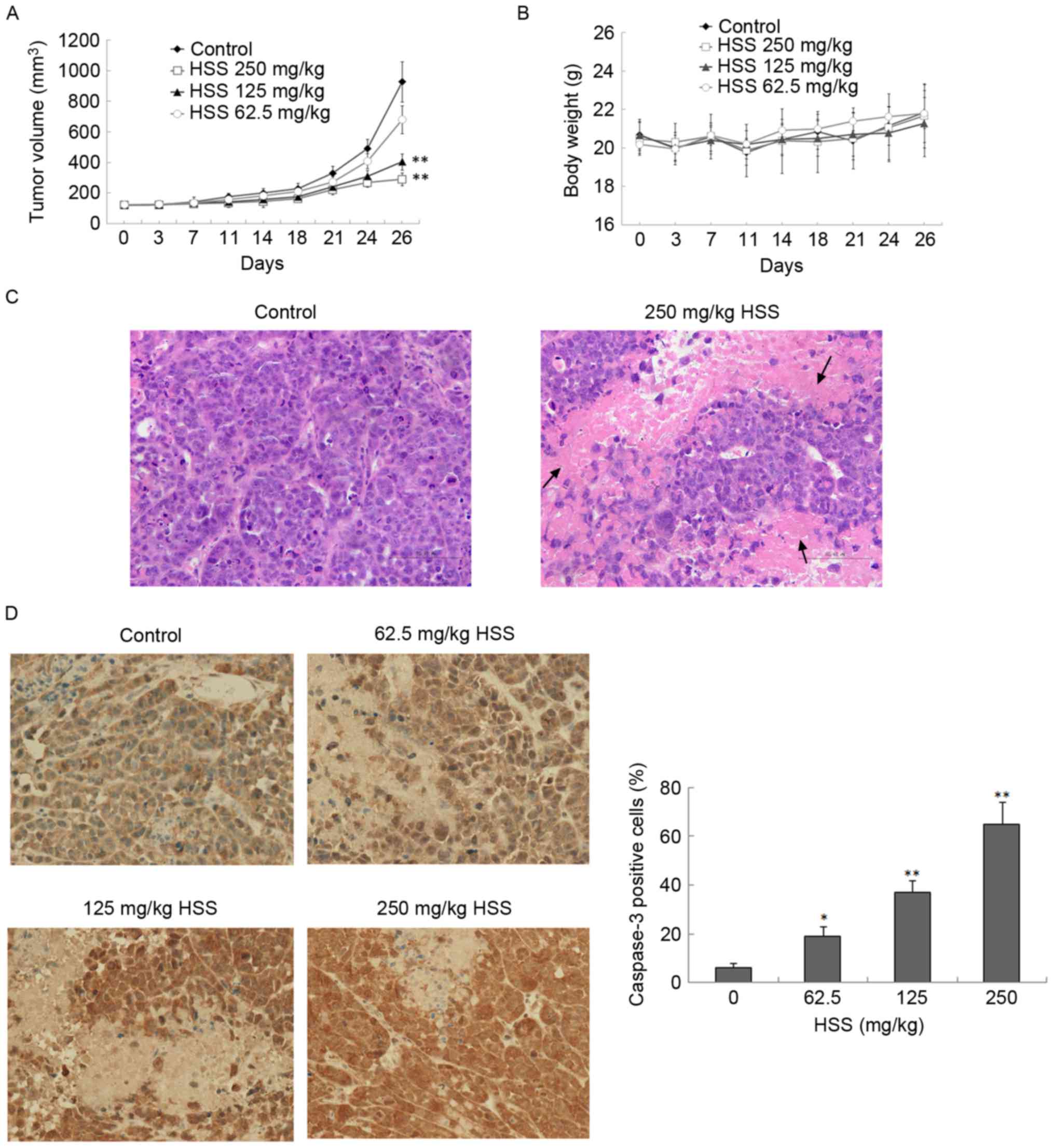
The effects of HSS on tumor growth were further
investigated using a xenograft model. BEL-7402 cells were implanted
into the flank of female BALB/c nude mice. The experiment was
terminated after 26 days and the tumor size was measured. Tumors in
the control group developed faster than the HSS treatment groups,
and were 928.9±131.4 mm3 in size 26 days post-injection
(Fig. 4A). HSS administration
inhibited tumor growth in a concentration-dependent manner in
xenograft nude mice (Fig. 4A).
There were no significant differences in overall body weight
between the control and the HSS-treated groups (Fig. 4B). Furthermore, H&E staining
revealed an increase in necrotic regions in the tumors of HSS
treatment groups, compared with the control (Fig. 4C). Histological analysis of liver
and kidney samples from both the control and HSS-treated mice
revealed no obvious pathological alterations (data not shown),
indicating that HSS did not exhibit any strong toxicity.
HSS administration increases apoptosis
in nude mice
Caspase-3 expression was investigated in xenograft
models by immunohistochemical staining. Treatment with HSS resulted
in a significant increase in the proportion of caspase-3-positive
cells, in a dose-responsive manner (Fig. 4D). HSS treatment significantly
increased the ratio of caspase-3-positive cells by 13, 31 and 59%,
at concentrations of 62.5, 125 and 250 mg/kg, respectively,
compared with the control (P<0.05).
To further evaluate the effects of HSS on apoptosis,
the ultrastructural morphology of xenografts was observed by TEM.
Irregular contours of cells and nuclei, nucleolar margination and
reduced presence of cytoplasm were observed in the control group
(Fig. 5A). Conversely, cells
treated with HSS exhibited apoptotic phenomena; the nucleus
decreased in size and demonstrated irregular indented edges and
variable shape. The karyotheca was crumpled, the karyoplasm was
dense and conglomerated, and the chromosomes were condensed and
bound to the karyotheca (Fig. 5B and
C). Furthermore, the mitochondria were swollen, and the
mitochondrial membrane was reduced in thickness or partially
ruptured (Fig. 5D). The
mitochondrial alterations indicated that the HSS-induced apoptosis
in BEL-7402 cells was associated with the mitochondrial-mediated
apoptotic pathway.
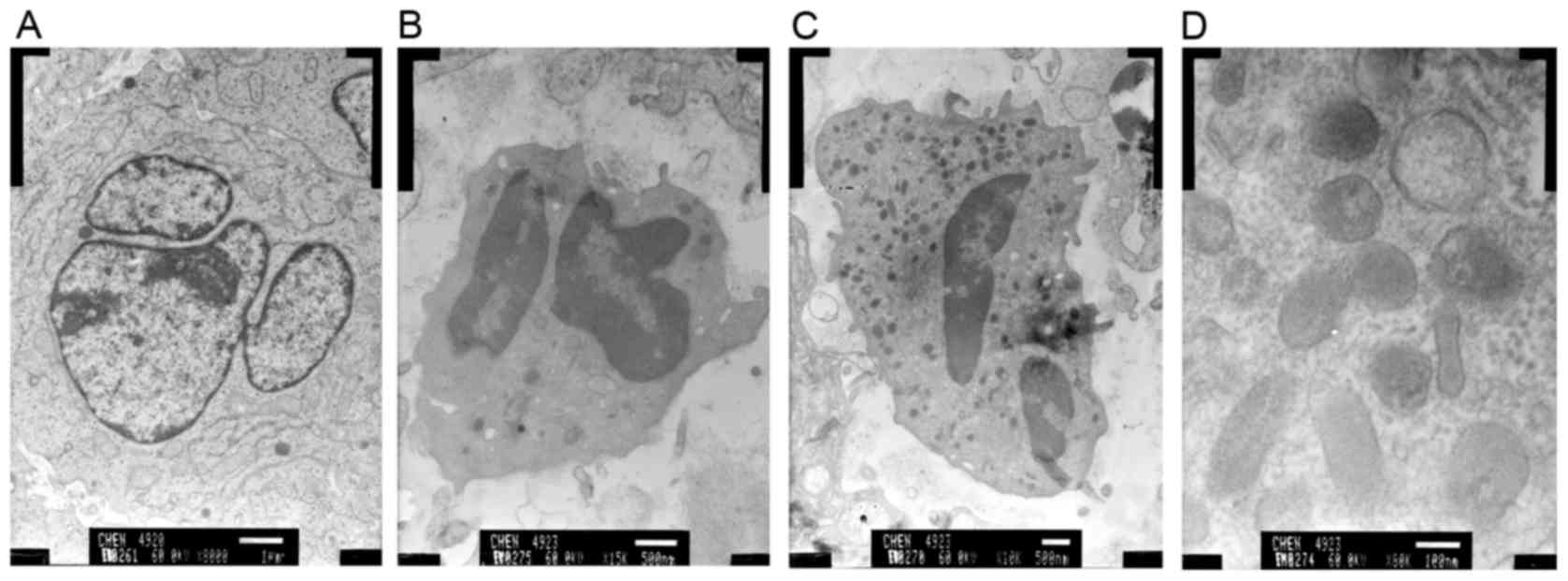 | Figure 5.HSS administration alters the cellular
ultrastructure in nude mice. Ultrastructural features of BEL-7402
cells induced by HSS were examined using a transmission electron
microscope. (A) Control group (magnification, ×8,000), (B) HSS
group (62.5 mg/kg; magnification, ×15,000), (C) HSS group (125
mg/kg; magnification, ×10,000), and (D) HSS group (250 mg/kg;
magnification, ×80,000). HSS, Haishengsu. |
Discussion
HCC is one of the most common malignancies
worldwide, with ~630,000 new cases reported annually (22). Late diagnosis, recurrence and
metastasis often result in a poor prognosis for patients with HCC,
and the 5-year survival rate of patients undergoing surgical
treatment is <5% (23).
Chemotherapy with conventional cytotoxic agents is often very toxic
and insufficiently efficacious (24). Therefore, novel chemotherapeutic
agents and more effective therapies for the treatment of HCC are
urgently required.
Natural products may provide promising sources of
novel anticancer therapies. The limited side effects of natural
products in anticancer therapies has already been recognized, and
>60% of drugs used to treat cancer are derived from natural
sources (25). HSS is a natural
extract from T. granosa, which has been used in clinical
cancer therapy in China. Our previous research indicated that HSS
may induce apoptosis of HCC cells in a concentration-dependent
manner, via the Fas signaling pathway (15); however, the mechanisms involved in
HSS-induced mitochondrial-mediated apoptosis in HCC cells remain
unclear. In the present study, it was observed that HSS effectively
induced the apoptosis of BEL-7402 cells via the
mitochondrial-mediated pathway in vitro, and inhibited tumor
xenograft growth in vivo.
Two major pathways are known to be involved in the
regulation of apoptosis, the death receptor (extrinsic) pathway and
the mitochondrial-mediated (intrinsic) pathway (26). Increasing evidence has indicated
that these two apoptotic pathways are not isolated; crosstalk
occurs between them. In the intrinsic pathway, mitochondria are the
critical mediators of apoptosis (27–29).
Certainstimuli, such as anticancer drugs, can induce
permeabilization of the outer mitochondrial membrane and activate
the mitochondrial pathway. The present study indicated that HSS
treatment may induce the apoptosis of BEL-7402 cells through the
intrinsic pathway. HSS treatment promoted the release of cytochrome
c into the cytosol, which in turn cleaved and activated
caspase-9. This resulted in the activation of caspase-3, as
evidenced by an increase in the levels of the cleaved caspase-3
protein, and a decrease in the pro-caspase-3 form. Following
caspase-3 activation, caspase-3 subsequently cleaves PARP into two
fragments, and this leads to DNA fragmentation and the induction of
apoptosis (29). The present study
also demonstrated that HSS was able to induce the cleavage of PARP
into its active form, as evidenced by a reduction in the
full-length p116 form, and an increase in the cleaved p85 form. In
agreement with the findings of the present study, Li et al
demonstrated that HSS treatment resulted incaspase-3 activation and
Bcl-2 downregulation in K562/ADM cells, demonstrating that HSS
treatment could induce apoptosis in these tumor cells (12). Furthermore, our previous study
reported that HSS treatment activated caspase-8 and upregulated Fas
protein and mRNA expression in HCC cells (15), thus suggesting that HSS acts
through the extrinsic and the intrinsic apoptotic pathways.
Bcl-2 family proteins regulate the activation of
caspases during mitochondrial-mediated apoptosis. The Bcl-2 family
of proteins consists of anti-apoptotic proteins, such as Bcl-2, and
a number of proapoptotic proteins, such as Bax; overexpression of
Bcl-2 blocks mitochondrial outer membrane permeabilization and
inhibits apoptosis (30). It has
been proposed that an agent that could enhance the expression of
proapoptotic proteins and/or inhibit the expression of
anti-apoptotic proteins may induce apoptosis in cancer cells.
Therefore, the balance between Bax and Bcl-2 serves a critical role
in the intrinsic pathway of apoptosis (31). Downregulation of Bcl-2 and
upregulation of Bax also stimulates the release of cytochrome
c from the mitochondria into the cytosol. The present study
indicated that HSS treatment upregulated the expression of Bax and
downregulated the expression of Bcl-2, leading to an increase in
the Bax/Bcl-2 protein ratio.
In conclusion, the results of the present study
indicated that HSS could induce the apoptosis of HCC-derived
BEL-7402 cells in vitro and in vivo, via intrinsic
apoptotic pathways. These findings may provide the basis for
further investigations into the potential clinical effects of T.
granosain HCC.
Acknowledgements
The present study was supported by the National
Science Foundation of China Grant (grant no. 81473384).
References
|
1
|
Burgess JG: New and emerging analytical
techniques for marine biotechnology. Curr Opin Biotechnol.
23:29–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin X, Liu M, Hu C and Liao DJ: Targeting
cellular proapoptotic molecules for developing anticancer agents
from marine sources. Curr Drug Targets. 11:708–715. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abraham I, El Sayed K, Chen ZS and Guo H:
Current status on marine products with reversal effect on cancer
multidrug resistance. Mar Drugs. 10:2312–2321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta SC, Kim JH, Prasad S and Aggarwal
BB: Regulation of survival, proliferation, invasion, angiogenesis,
and metastasis of tumor cells through modulation of inflammatory
pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leal MC, Madeira C, Brandão CA, Puga J and
Calado R: Bioprospecting of marine invertebrates for new natural
products-a chemical and zoogeographical perspective. Molecules.
17:9842–9854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu GP, Yuan J, Sun L, She ZG, Wu JH, Lan
XJ, Zhu X, Lin YC and Chen SP: Statistical research on marine
natural products based on data obtained between 1985 and 2008. Mar
Drugs. 9:514–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li GY, Yu XM, Zhang HW, Zhang B, Wang CB,
Xin YC, Yang CZ, Zhou RX and Wang LX: Haishengsu as an adjunct
therapy to conventional chemotherapy in patients with non-small
cell lung cancer: A pilot randomized and placebo-controlled
clinical trial. Complement Ther Med. 17:51–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu JZ, Chen SG, Zhang B, Wang CB, Zhao
XW, Li GY and Wang LX: Effect of haishengsu as an adjunct therapy
for patients with advanced renal cell cancer: A randomized and
placebo-controlled clinical trial. J Altern Complement Med.
15:1127–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao MQ, Han YT, Zhu L, Chen SG, Hong ZY
and Wang CB: Cytotoxicity of natural extract from Tegillarca
granosa on ovarian cancer cells is mediated by multiple
molecules. Clin Invest Med. 32:E368–E375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu HP, Gao ZH, Cui SX, Xue X, Hou CY,
Jiang ZM, Zhao CR, Wang CB, Chen SG and Qu XJ: Haishengsu, a
protein from shellfish Tegillarca L. granosa,
inhibits the growth and the activity of matrix metalloproteinases-2
and −9 in human lung carcinoma. Food Biophysics. 6:390–396. 2011.
View Article : Google Scholar
|
|
11
|
Li GY, Liu JZ, Zhang B, Yang M, Chen SG,
Hou M and Wang LX: Tegillarca granosa extract Haishengsu
(HSS) suppresses expression of mdr1, BCR/ABL and sorcin in
drug-resistant K562/ADM tumors in mice. Adv Med Sci. 58:112–117.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li GY, Liu JZ, Chen SG, Zhang B, Wang CB
and Wang LX: Tegillarca granosa extract Haishengsu inhibits
the expression of p-glycoprotein and induces apoptosis in
drug-resistant K562/ADM cells. Pharm Biol. 48:529–533. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Kong X, Jiang C, Liu X and Xu L: The
anti-tumor effect of a polypeptide extracted from Tegillarca
granosa Linnaeus on renal metastatic tumor OS-RC-2 cells. Arch
Med Sci. 11:849–855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li GY, Liu JZ, Chen SG, Wang CB, Bin Z and
Wang LX: Effect of a seashell protein Haishengsu on the
immunological function of mice with Ehrlich ascites tumor.
Immunopharmacol Immunotoxicol. 31:669–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Han Y, Zhan S, Wang C and Chen S:
Tegillarca granosa extract Haishengsu induces apoptosis in
human hepatocellular carcinoma cell line BEL-7402 via Fas-signaling
pathways. Cell Biochem Biophys. 71:837–844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hajra KM and Liu JR: Apoptosome
dysfunction in human cancer. Apoptosis. 9:691–704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khodarev NN, Sokolova IA and Vaughan ATM:
Mechanisms of induction of apoptotic DNA fragmentation. Int J
Radiat Biol. 73:455–467. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han YT, Chen XH, Gao H, Ye JL and Wang CB:
Physcion inhibits the metastatic potential of human colorectal
cancer SW620 cells in vitro by suppressing the transcription factor
SOX2. Acta pharmacol Sin. 37:264–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ice. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alves RC, Alves D, Guz B, Matos C, Viana
M, Harriz M, Terrabuio D, Kondo M, Gampel O and Polletti P:
Advanced hepatocellular carcinoma. Review of targeted molecular
drugs. Ann Hepatol. 10:21–27. 2011.PubMed/NCBI
|
|
23
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Newman DJ, Cragg GM and Snader KM: Natural
products as sources of new drugs over the period 1981–2002. J Nat
Prod. 66:1022–1037. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai JJ, Niu YF, Wu CF, Zhang SH and Zhang
DF: Both death receptor and mitochondria mediated apoptotic
pathways participated the occurrence of apoptosis in porcine
vitrified mii stage oocytes. Cryo Letters. 37:129–136.
2016.PubMed/NCBI
|
|
28
|
Yoo KH, Park JH, Lee DK, Fu YY, Baek NI
and Chung IS: Pomolic acid induces apoptosis in SK-OV-3 human
ovarian adenocarcinoma cells through the mitochondrial-mediated
intrinsic and death receptor-induced extrinsic pathways. Oncol
Lett. 5:386–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi F, Li A, Inagaki Y, Xu H, Wang D, Cui
X, Zhang L, Kokudo N, Du G and Tang W: Induction of apoptosis by
cinobufacini preparation through mitochondria- and Fas-mediated
caspase-dependent pathways in human hepatocellular carcinoma cells.
Food Chem Toxicol. 50:295–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elumalai P, Gunadharini DN, Senthilkumar
K, Banudevi S, Arunkumar R, Benson CS, Sharmila G and Arunakaran J:
Induction of apoptosis in human breast cancer cells by nimbolide
through extrinsic and intrinsic pathway. Toxicol Lett. 215:131–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li P, Tian W and Ma X: Alpha-mangostin
inhibits intracellular fatty acid synthase and induces apoptosis in
breast cancer cells. Mol Cancer. 13:1382014. View Article : Google Scholar : PubMed/NCBI
|