Introduction
Comprehensive studies on acute respiratory distress
syndrome (ARDS) have revealed that ARDS is the most severe stage of
the continuous pathological process of acute lung injury (ALI); ALI
is observed in all cases of ARDS, however ALI does necessarily
develop into ARDS. It is also established that ALI is implicated in
system inflammatory response syndrome (SIRS) (1,2). The
lung is vulnerable to damage and a role in SIRS (3). ALI commonly leads to severe
hypoxemia, thereby leading to the dysfunction of other organs and
potentially multiple organ failure (4). Therefore, ALI is the initial stage in
the whole pathological process of SIRS, which results in organ
dysfunction and subsequent multiple organ failure.
Newborns, particularly premature children, are prone
to ALI, which may develop into ARDS. In severe cases,
bronchopulmonary dysplasia impairs lung development, which is
closely associated with the development of lung maturity (5). ALI may initially develop in
utero and continue following birth. Pulmonary inflammation can
cause lung damage and lead to ALI (5). The lung of a newborn is in cyst and
alveolar period, which is immature (6). Pulmonary surfactant is observed after
28 weeks gestation, meaning that premature children are vulnerable
to ALI and the further development and maturation are affected. A
lower gestational age is associated with an increased risk of lung
damage (7,8).
Allicin is a type of volatile, oily matter that is
extracted from spherical garlic bulbs and is the major component of
garlic biological activity. Allicin is reported to exhibit various
biological functions and physiological effects, including
anti-inflammation, anti-cancer, cholesterol-lowering, anti-platelet
aggregation and liver-protection effects, and also functions in
preventing heart and vascular diseases and lowering blood pressure
(9–14). The aim of the current study was to
investigate whether allicin attenuates lipopolysaccharide
(LPS)-induced ALI in neonatal rats and whether these beneficial
actions may be mediated by ameliorating oxidative stress,
inflammation and apoptosis.
Materials and methods
Animals and ALI model
Male Sprague-Dawley neonatal rats (weight, 5–30 g, 1
week old) were provided by the Experimental Animal Center of Xuzhou
Medical University (Xuzhou, China) and kept at a temperature of
23±1°C, 55–60% humidity, 0.038% CO2, on a 12 h
light/dark cycle with food and water available ad libitum.
The present study was approved by the Institutional Animal Care and
Use Committee of The Second Affiliated Hospital of Xuzhou Medical
University. A total of 60 Sprague-Dawley rats were randomly divided
into three groups as follows: Sham group (n=20), model group (n=20)
and model + allicin group (n=20). All rat was anesthetizated using
5 mg/kg pentobarbital tail intravenous injection. In the sham
groups, rats only received normal saline.
ALI model rats received 5 mg/kg LPS (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). In the model + allicin treatment
group, rats received 80 mg/kg allicin (Sigma-Aldrich; Merck KGaA)
via tail intravenous injection for 8 days (15) after the ALI model was established.
Following induction of the ALI model and confirmation using
histological examination, the number of rats in each group was
recorded as NO of LPS at 6, 12, 18, 24, 30, 36, 42 and 48 h.
Mortality rate was calculated as follows: [(total number - NO of
LPS)/total number] × 100, where total number refers to the number
of rats in each group at the start of the experiment (n=20). The
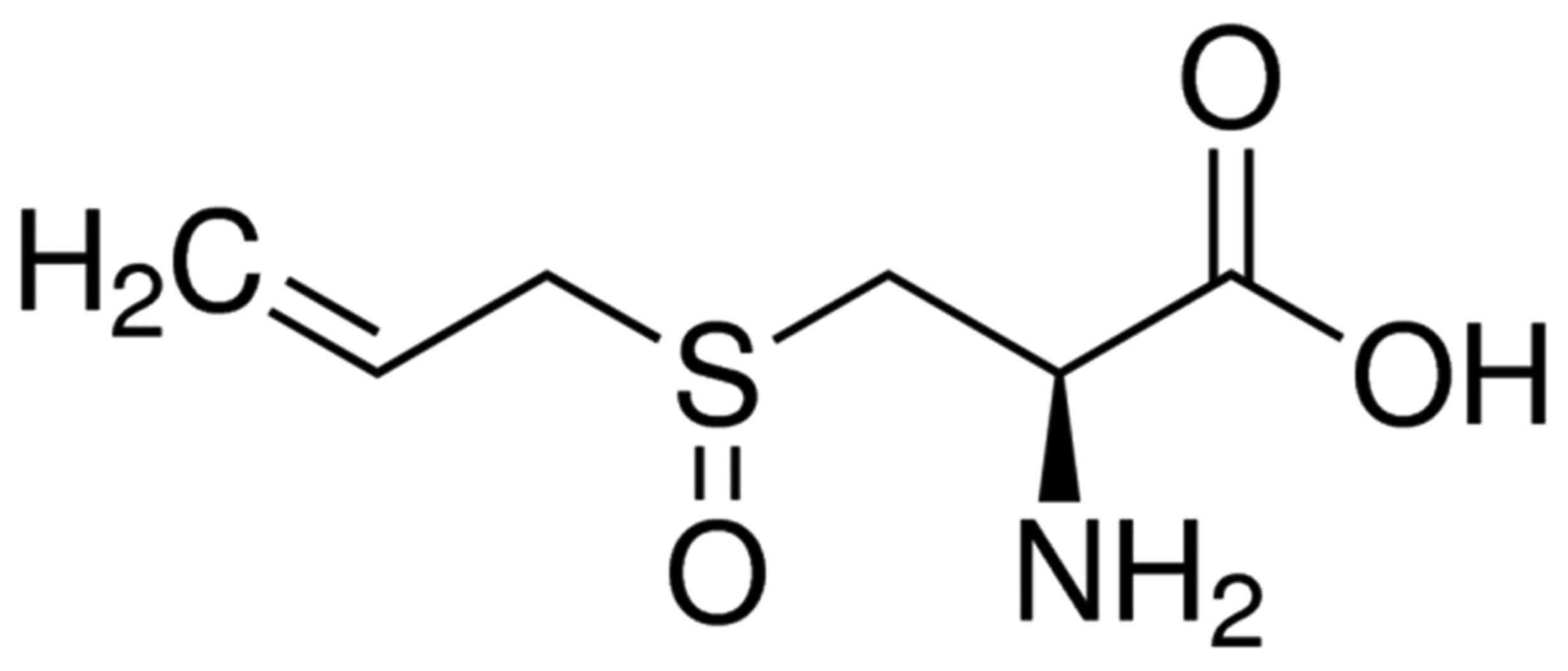
chemical structure of allicin is presented in Fig. 1.
Lung wet/dry (W/D) ratio of
LPS-treated rats and the lung concentration of proteins
The rats were euthanized using intravenous injection
of 35 mg/kg pentobarbital and sacrificed using decollation. The
thorax was opened and the whole lungs were immediately removed. The
superior lobe of the right lung was harvested, weighed and dried at
80°C for 48 h, and subsequently reweighed to calculate the W/D
weight ratio of the lung tissue. Bronchoalveolar lavage fluid
(BALF) was collected to determine the lung concentration of
proteins via the BCA method (Wuhan Boster Biological Technology,
Co., Ltd., Wuhan, China.)
Histological examination
Histological examination was used to determine
whether the induction of the model was successful. The rats were
euthanized using intravenous injection of 35 mg/kg pentobarbital
and decapitation. Right lung tissue was immersed in 10% neutral
phosphate-buffered formaldehyde fixative for 24 h at room
temperature and embedded in paraffin. Tissue was cut into 4 µm
sections and stained with hematoxylin and eosin (HE) for 15 min at
room temperature. Tissue sections were subsequently observed using
a Nikon SMZ 1500 light microscope (magnification, ×40; Nikon
Corporation, Tokyo, Japan).
Oxidative stress and inflammation
The left lung of each rat was harvested and washed
with ice-cold PBS. The volume of BALF was similar in each group and
centrifuged at 1,200 × g for 10 min at 4°C. The supernatant was
collected to determine the levels of interferon (IFN)-γ, caspase-3
activity, caspase-9 activity, glutathione (GSH), glutathione
peroxidase (GSH-PX), malondialdehyde (MDA), tumor necrosis factor
(TNF)-α and interleukin (IL)-6, −1β and −10 levels, and superoxide
dismutase (SOD) activity, using rat IFN-γ (cat. no. H025),
caspase-3 activity (cat. no. G015), caspase-9 activity (cat. no.
G018), GSH (cat. no. A006-2), GSH-PX (cat. no. A005), MDA (cat. no.
A003-1), TNF-α (cat. no. H052), IL-1β (cat. no. H002), IL-10 (cat.
no. H009), IL-6 (cat. no. H007) and SOD (cat. no. A001-1-1) ELISA
kits (Wuhan Boster Biological Technology, Co., Ltd.). The
concentration of proteins was measured using the BCA method.
Western blotting
BALF was collected following centrifugation at
10,000 × g for 10 min at 4°C to determine the concentration of
proteins via the BCA method. An equal amount of protein (50 µg) was
separated by 10–12% SDS-PAGE, and transferred to and immobilized on
a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The nitrocellulose membrane was blocked by incubation
with PBS containing 5% non-fat dried milk for 2 h at room
temperature and subsequently incubated with primary antibodies
against COX-2 (cat. no. 12282; 1:2,000; Cell Signaling Technology,
Inc), NF-κB (cat. no. 8242; 1:2,000; Cell Signaling Technology,
Inc), PI3K (cat. no. 4249; 1:2,000; Cell Signaling Technology,
Inc), Akt (cat. no. 4691; 1:2,000; Cell Signaling Technology, Inc),
p-Akt (cat. no. 4060; 1:2,000; Cell Signaling Technology, Inc),
GAPDH (cat. no. D110016; 1:5,000; Sangon Biotech Co., Ltd.,
Shanghai, China) overnight at 4°C. The nitrocellulose membrane was
washed three times with TBS containing 0.1% Tween-20 and incubated
with horseradish peroxidase-conjugated anti-rabbit or anti-mouse
secondary antibody (cat. nos. sc-2004 and sc-2005 respectively,
both 1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for
2 h at room temperature. Subsequently, the membrane was incubated
with chemiluminescence reagent (ECL Plus Western Blotting Detection
System; GE Healthcare, Chicago, IL, USA). Proteins were
quantitatively analyzed using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 19 (IBM Corp., Armonk, NY, USA). All
data were analyzed by one-way analysis of variance followed by
Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of allicin on the mortality
rate of LPS-treated neonatal rats
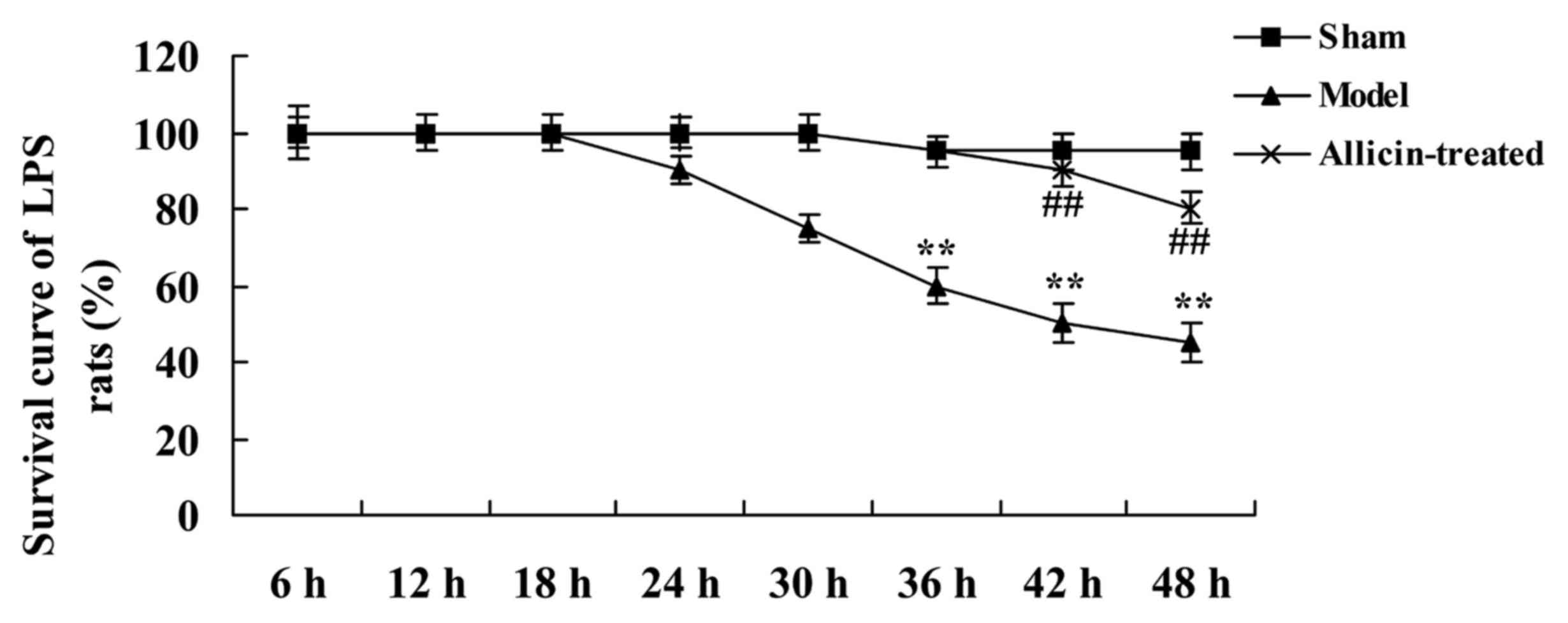
The survival of rats in the ALI model group began to
decrease at 24 h after treatment with allicin, compared with the
sham group (Fig. 2). Allicin
inhibited the mortality rate of LPS-treated rats at various
time-points, compared with the ALI model group (Fig. 2).
Effect of allicin on histological
examination of LPS-treated neonatal rats
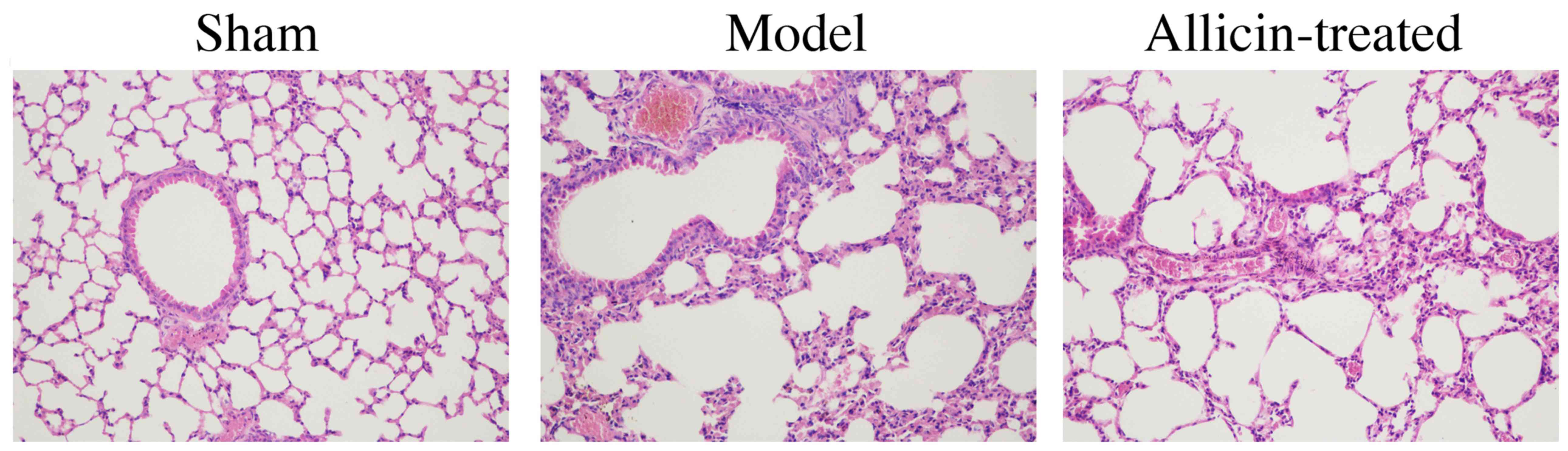
As demonstrated in Fig.
3, alveolar edema, hemorrhage, wall thickening and
hyperinflation, and inflammatory cell infiltration into the
alveolar and interstitial spaces, of the model group were higher
compared with the sham group. However, treatment with allicin
reduced the LPS-induced alveolar edema, hemorrhage, wall thickening
and hyperinflation, and inflammatory cell infiltration into the
alveolar and interstitial spaces, in ALI rats (Fig. 3).
Effect of allicin on lung W/D ratio
and protein concentration in LPS-induced ALI neonatal rats
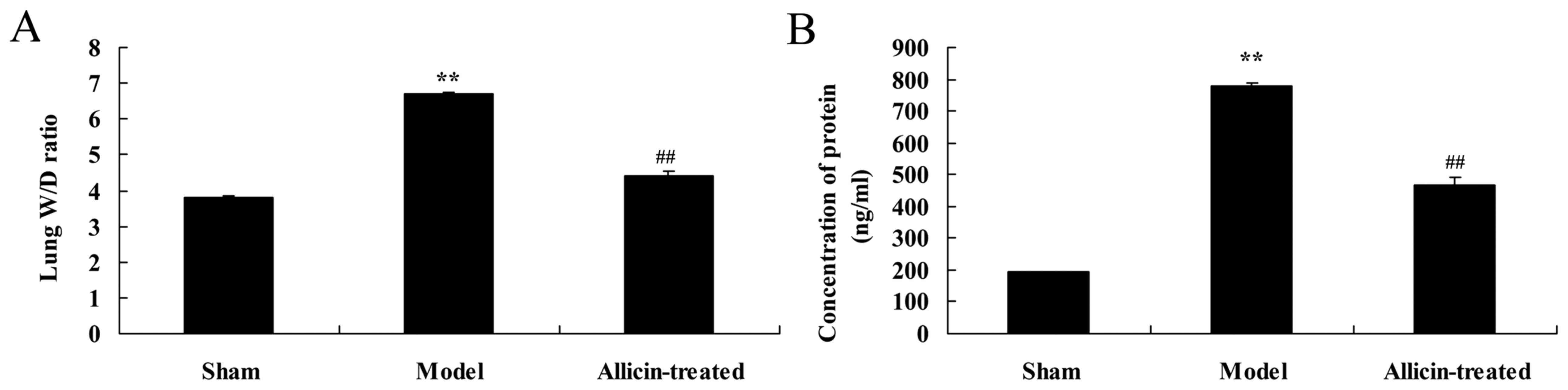
As demonstrated in Fig.
4, the lung W/D ratio and lung protein concentration in the ALI
model group were higher compared with the sham group (Fig. 4). However, allicin administration
significantly decreased the elevations in lung W/D ratio and lung
protein concentration, compared with the ALI model group (Fig. 4).
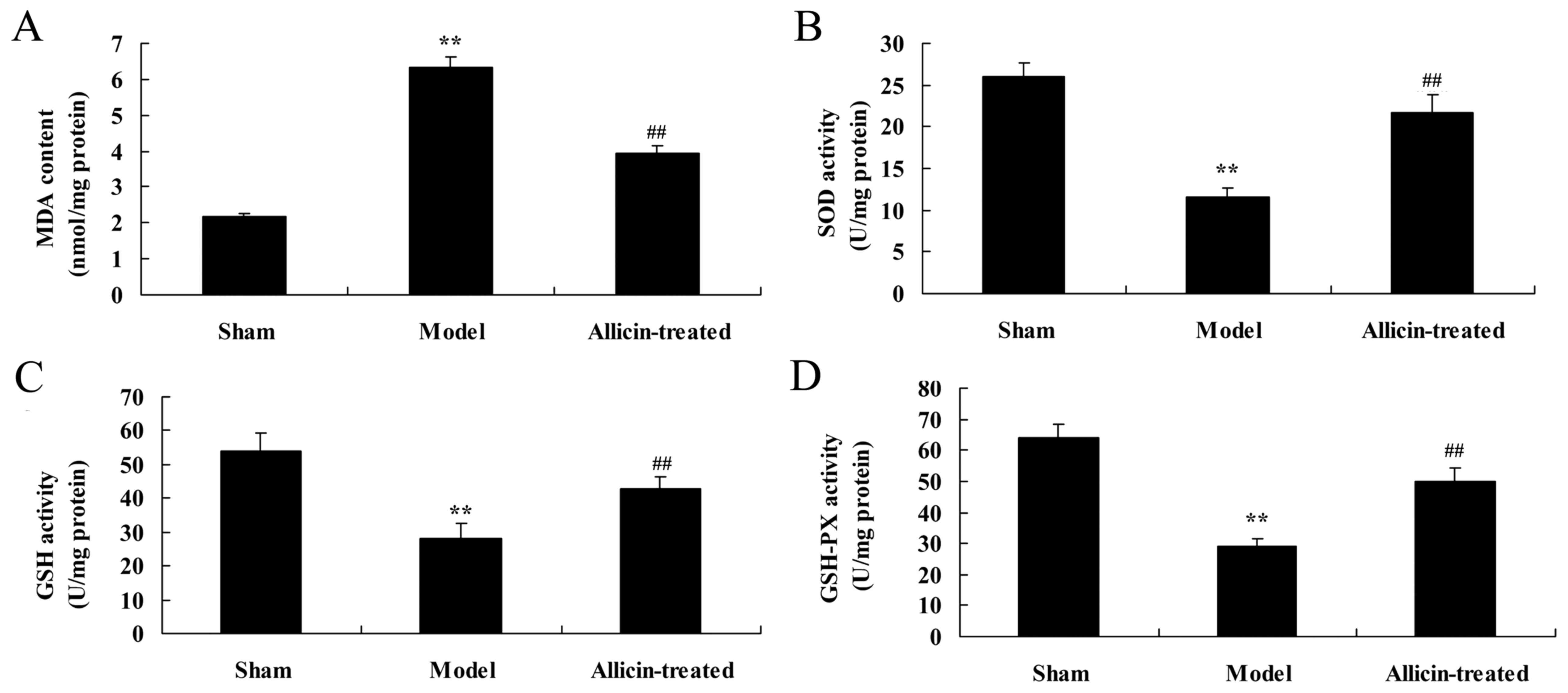
Effect of allicin on the levels of
MDA, and activity of SOD, glutathione (GSH) and GSH peroxidase
(GSH-PX), in the BALF of LPS-treated neonatal rats
Compared with the sham group, the levels of MDA were
significantly increased, and SOD, GSH and GSH-PX activity was
significantly inhibited, in the ALI model group (Fig. 5). However, allicin treatment
significantly reversed the effects of LPS on MDA levels, and SOD,
GSH and GSH-PX activity, compared with the ALI model group
(Fig. 5).
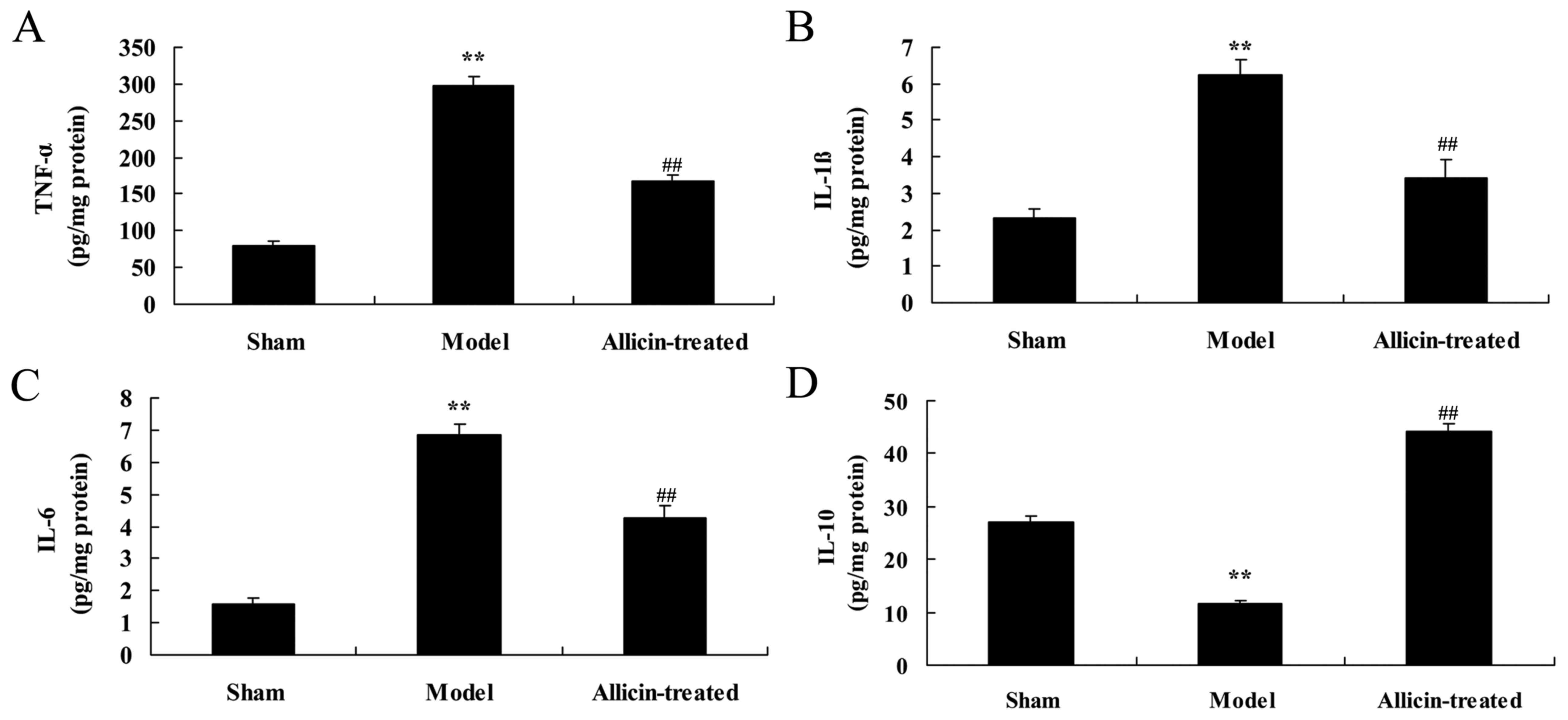
Effect of allicin on inflammation in
the BALF of LPS-treated neonatal rats
As demonstrated in Fig.
6A-C, the levels of TNF-α, IL-1β and IL-6 in the BALF of ALI
model rats were significantly increased, compared with the sham
group. However, TNF-α, IL-1β and IL-6 levels were significantly
suppressed by treatment with allicin, compared with the ALI model
group (Fig. 6A-C). In addition,
the levels of IL-10 in the BALF of ALI model rats were lower
compared with the sham group (Fig.
6D). Treatment with allicin significantly increased IL-10
levels in ALI rats, compared with the ALI model group (Fig. 6D).
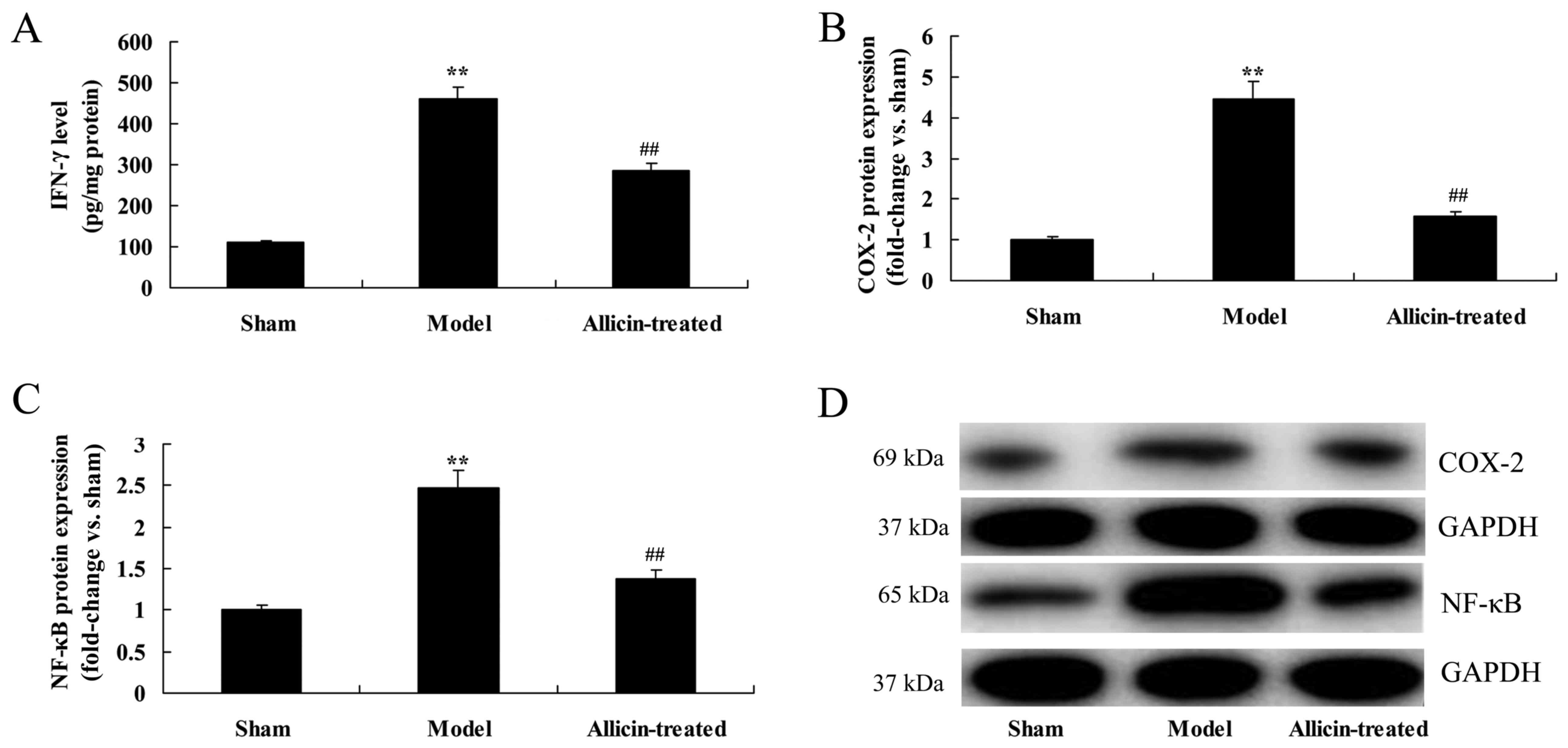
Effect of allicin on inflammatory
pathways
The levels of interferon (IFN)-γ, and the protein
expression of cyclooxygenase (COX)-2 and nuclear factor-κB (NF-κB),
in the BALF were significantly promoted in ALI model rats, compared
with the sham group (Fig. 7).
However, allicin treatment significantly reduced IFN-γ levels, and
COX-2 and NF-κB protein expression, in ALI rats, compared with the
ALI model group (Fig. 7).
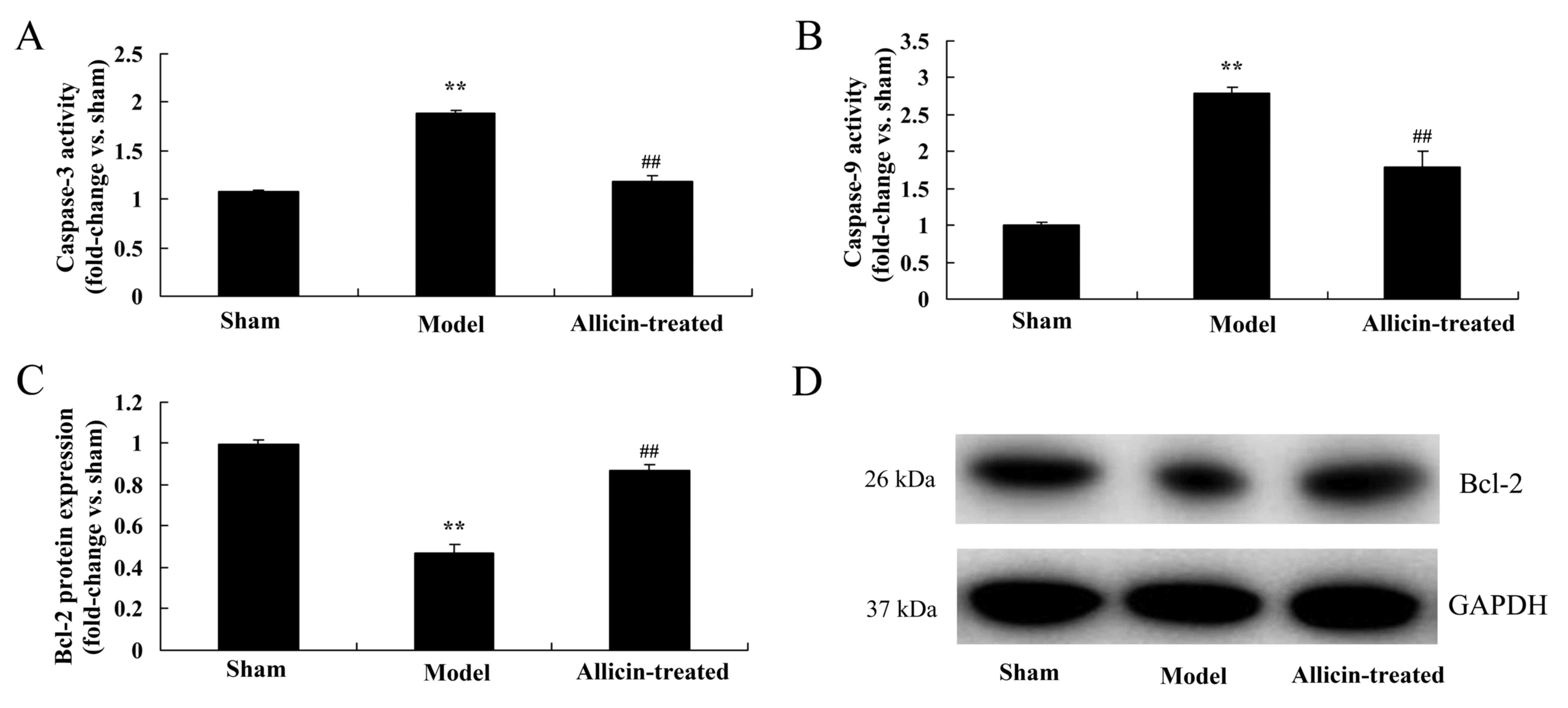
Effect of allicin on anti-apoptotic
protein of Bcl-2 and caspase-3/-9 pathway
The results of ELISA and western blotting also
demonstrated that upregulation of caspase-3/-9 activity and the
inhibition of Bcl-2 protein expression, respectively, in the ALI
model group compared with the sham group (Fig. 8). However, upregulation of
caspase-3/-9 activity and the inhibition of Bcl-2 protein
expression were induced by allicin administration, compared with
the ALI model group (Fig. 8).
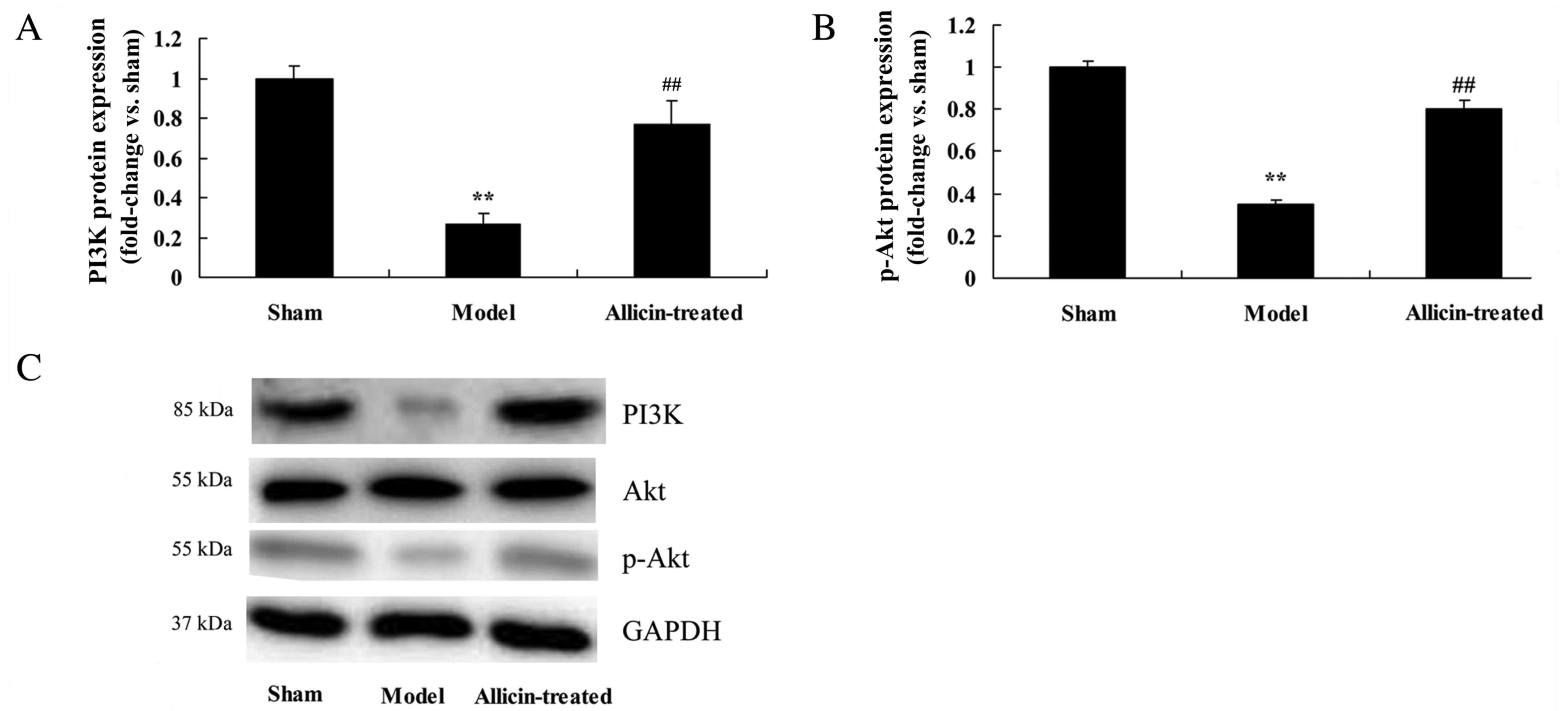
Effect of allicin on the
phosphatidylinositol 3-kinase (PI3K) and phosphorylated (p)-Akt
pathway
The protein expression of PI3K and p-Akt was
significantly reduced in the ALI model group, compared with the
sham group (Fig. 9). However, PI3K
and p-Akt protein expression levels were significantly increased by
treatment with allicin, compared with the ALI model group (Fig. 9).
Discussion
Oxidative stress is an important mechanism in the
pathogenesis of ALI (16). A large
number of oxygen free radicals (OFR) are produced during the ALI
process by various cell types within the vessel wall, including
endothelial cells, vascular smooth muscle cells and cells of the
outer membrane (17). The present
study demonstrated that allicin reduced increases in the lung W/D
ratio that were observed in the ALI model group, and identified
that it may have potential as a drug for the treatment of ALI.
Oxidative stress leads to increases in cell
permeability, cell edema and potentially lytic necrosis (18). The targets of OFR include nucleic
acids, proteins and membrane lipids, and lipid peroxidation is one
of the primary mechanisms of free radical damage (19). MDA is employed as a marker for
lipid peroxidation to reflect the degree of oxidative stress injury
following ALI. To combat oxidative stress injury, various
endogenous cytoprotective substances are generated in the lung
tissue, such as SOD, which may be used as an indicator of
antioxidative activity (20). A
dynamic equilibrium exists between oxidation and antioxidant
systems in vivo; when these systems become imbalanced,
tissue damage results and the lung is one of the most vulnerable
target organs (21). A previous
study demonstrated that MDA content was increased significantly in
ALI rat lung homogenates, while SOD level was decreased
significantly, which indicates that an imbalance between the
oxidation and antioxidant system may be involved in the
pathogenesis of ALI (22).
Furthermore, the results of the current study demonstrated that
allicin treatment significantly reversed the effects of ALI model
induction on MDA, SOD, GSH and GSH-PX levels in ALI neonatal
rats.
The development of ALI may be initiated by alveolar
inflammation under the perinatal asphyxia or infection, as well as
additional internal and external factors (23). The number and activation of
neutrophils is increased, which increases the release of various
inflammatory mediators, and epithelial and endothelial cells
functions are subsequently damaged, which is a major mechanism in
the pathogenesis of ALI (23). It
is reported that IL-6 combines with heparin sulfate glycoprotein on
the vascular endothelial cell surface, which has a potent
chemotactic effect and causes endothelial cells to adhere to and
activate neutrophils (24).
Therefore, the release of IL-6 is considered to be an important
factor in the pathogenesis of ALI (25). In addition, TNF-α also has an
important role in the inflammatory lesion of tissue and promotes
the release of other inflammatory mediators (26). The results of the present study
demonstrated that allicin significantly suppressed TNF-α, IL-1β,
IL-6 and IFN-γ levels, and COX-2 and NF-κB protein expression, and
increased IL-10 levels, in ALI neonatal rats.
Apoptosis is a type of programmed cell death that is
important in the development and injury of multicellular organisms
in various tissues and organs (27). Caspases and the Bcl-2 protein
family are closely associated with apoptosis (28). Apoptosis-associated genes are
divided into apoptosis-promoting genes and apoptosis-inhibiting
genes (29). Caspase-3 activation
leads to the induction of cell apoptosis, and has an important role
in the initiation and implementation of early apoptosis. Caspase-3
activation is a biochemical indicator of early apoptosis. Bcl-xl is
an anti-apoptotic gene that exerts anti-apoptosis effects by
inhibiting the activation of caspase proteases (30). Proapoptotic genes are dominant
during early apoptosis, during which Bcl-xl expression is
decreased, and as apoptosis develops, the internal anti-apoptotic
mechanism is activated (31). The
present study demonstrated that allicin significantly increased the
expression of the Bcl-2 anti-apoptotic protein and inhibited
caspase-3/-9 activity in ALI model neonatal rats, which may occur
via the PI3K/Akt pathway. Ding et al reported that allicin
inhibited oxidative stress-induced mitochondrial dysfunction and
apoptosis of osteoblast cells through PI3K/Akt signaling (32). The present study only analyzed the
effects of allicin on PI3K/Akt signaling in ALI, which is a
limitation of the study as allicin may also regulate additional
signaling pathways to exert effects on inflammation, oxidative
stress and apoptosis, which should be investigated in future
studies.
In conclusion, the present study demonstrated that
allicin treatment suppressed lung W/D ratio and the lung
concentration of proteins in ALI neonatal rats. These beneficial
effects may be due to its ability to inhibit oxidative stress and
inflammation, and to inhibit apoptosis, including Bcl-2 expression,
caspase-3/-9 activityin ALI neonatal rats, which may occur via the
PI3K/Akt pathway. Therefore, allicin may have potential as a novel
drug for the treatment of neonatal ALI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed the experiment. XW, CZ, CC, YG, XM and
CK performed experiments. XW analyzed the data and wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of The Second Affiliated Hospital of
Xuzhou Medical University (Xuzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reisinger MW, Moss M and Clark BJ:
National Heart, Lung, and Blood Institute Acute Respiratory
Distress Syndrome Network Investigators: Brief versus full alcohol
use disorders identification test in national heart, lung, and
blood institute acute respiratory distress syndrome network
clinical trials. Crit Care Med. 43:e382–e385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernard GR: Potential of N-acetylcysteine
as treatment for the adult respiratory distress syndrome. Eur
Respir J Suppl. 11:496s–498s. 1990.PubMed/NCBI
|
|
3
|
Braunschweig CA, Sheean PM, Peterson SJ,
Gomez Perez S, Freels S, Lateef O, Gurka D and Fantuzzi G:
Intensive nutrition in acute lung injury: A clinical trial
(INTACT). JPEN J Parenter Enteral Nutr. 39:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwameis R, Eder S, Pietschmann H,
Fischer B, Mascher H, Tzotzos S, Fischer H, Lucas R, Zeitlinger M
and Hermann R: A FIM study to assess safety and exposure of inhaled
single doses of AP301-A specific ENaC channel activator for the
treatment of acute lung injury. J Clin Pharmacol. 54:341–350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piva JP, Garcia PC and Fiori H: Mechanical
ventilation in children with acute respiratory distress syndrome: A
huge gap between what we know and our practice!*. Pediatr Crit Care
Med. 14:732–733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mulder HD, Augustijn QJ, van Woensel JB,
Bos AP, Juffermans NP and Wösten-van Asperen RM: Incidence, risk
factors, and outcome of transfusion-related acute lung injury in
critically ill children: A retrospective study. J Crit Care.
30:55–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenz RT and Cysewski GR: Commercial
potential for Haematococcus microalgae as a natural source
of astaxanthin. Trends Biotechnol. 18:160–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fitzgerald JC, Topjian AA, McInnes AD,
Mattei P, McCloskey JJ, Friess SH and Kilbaugh TJ: Bi-caval dual
lumen venovenous extracorporeal membrane oxygenation and
high-frequency percussive ventilatory support for postintubation
tracheal injury and acute respiratory distress syndrome. J Pediatr
Surg. 46:e11–e15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wallock-Richards D, Doherty CJ, Doherty L,
Clarke DJ, Place M, Govan JR and Campopiano DJ: Garlic revisited:
Antimicrobial activity of allicin-containing garlic extracts
against Burkholderia cepacia complex. PLoS One.
9:e1127262014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang S, Dai Y, Zhang Z, Hao W and Chen H:
Docosahexaenoic acid intake ameliorates ketamine-induced impairment
of spatial cognition and learning ability in ICR mice. Neurosci
Lett. 580:125–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Tang Y, Qian Y, Chen R, Zhang L,
Wo L and Chai H: Allicin prevents
H2O2-induced apoptosis of HUVECs by
inhibiting an oxidative stress pathway. BMC Complement Altern Med.
14:3212014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu YL, Ho CT, Chung JG, Raghu R, Lo YC
and Sheen LY: Allicin induces anti-human liver cancer cells through
the p53 gene modulating apoptosis and autophagy. J Agric Food Chem.
61:9839–9848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldberg RS, Chang SC, Kotik AN, Nadler M,
Neuwirth Z, Sundstrom DC and Thompson NH: In vitro mechanism of
inhibition of bacterial cell growth by allicin. Antimicrob Agents
Chemother. 32:1763–1768. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khodavandi A, Harmal NS, Alizadeh F,
Scully OJ, Sidik SM, Othman F, Sekawi Z, Ng KP and Chong PP:
Comparison between allicin and fluconazole in Candida
albicans biofilm inhibition and in suppression of HWP1
gene expression. Phytomedicine. 19:56–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elkayam A, Peleg E, Grossman E, Shabtay Z
and Sharabi Y: Effects of allicin on cardiovascular risk factors in
spontaneously hypertensive rats. Isr Med Assoc J. 15:170–173.
2013.PubMed/NCBI
|
|
16
|
Rice TW, Wheeler AP, Thompson BT,
deBoisblanc BP, Steingrub J and Rock P: NIH NHLBI Acute Respiratory
Distress Syndrome Network of Investigators: Enteral omega-3 fatty
acid, gamma-linolenic acid, and antioxidant supplementation in
acute lung injury. JAMA. 306:1574–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang TY, Tsai PS, Wang TY, Huang CL and
Huang CJ: Hyperbaric oxygen attenuation of
lipopolysaccharide-induced acute lung injury involves heme
oxygenase-1. Acta Anaesthesiol Scand. 49:1293–1301. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Wang HY, Liu ZW, Fu Y and Zhao B:
Effect of endogenous hydrogen sulfide on oxidative stress in oleic
acid-induced acute lung injury in rats. Chin Med J. 124:3476–3480.
2011.PubMed/NCBI
|
|
19
|
Yamaoka S, Kim HS, Ogihara T, Oue S,
Takitani K, Yoshida Y and Tamai H: Severe Vitamin E deficiency
exacerbates acute hyperoxic lung injury associated with increased
oxidative stress and inflammation. Free Radic Res. 42:602–612.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balyasnikova IV, Visintine DJ, Gunnerson
HB, Paisansathan C, Baughman VL, Minshall RD and Danilov SM:
Propofol attenuates lung endothelial injury induced by
ischemia-reperfusion and oxidative stress. Anesth Analg.
100:929–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lingappan K, Jiang W, Wang L, Wang G,
Couroucli XI, Shivanna B, Welty SE, Barrios R, Khan MF, Nebert DW,
et al: Mice deficient in the gene for cytochrome P450 (CYP)1A1 are
more susceptible than wild-type to hyperoxic lung injury: Evidence
for protective role of CYP1A1 against oxidative stress. Toxicol
Sci. 141:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sunil VR, Vayas KN, Massa CB, Gow AJ,
Laskin JD and Laskin DL: Ozone-induced injury and oxidative stress
in bronchiolar epithelium are associated with altered pulmonary
mechanics. Toxicol Sci. 133:309–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saini Y, Greenwood KK, Merrill C, Kim KY,
Patial S, Parameswaran N, Harkema JR and LaPres JJ: Acute
cobalt-induced lung injury and the role of hypoxia-inducible factor
1alpha in modulating inflammation. Toxicol Sci. 116:673–681. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lo Re S, Dumoutier L, Couillin I, Van Vyve
C, Yakoub Y, Uwambayinema F, Marien B, van den Brûle S, Van Snick
J, Uyttenhove C, et al: IL-17A-producing gammadelta T and Th17
lymphocytes mediate lung inflammation but not fibrosis in
experimental silicosis. J Immunol. 184:6367–6377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Misumi T, Tanaka T, Mikawa K, Nishina K,
Morikawa O and Obara H: Effects of sivelestat, a new elastase
inhibitor, on IL-8 and MCP-1 production from stimulated human
alveolar epithelial type II cells. J Anesth. 20:159–165. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Zhao L, He X, Zeng YJ and Dai SS:
Sinomenine protects against lipopolysaccharide-induced acute lung
injury in mice via adenosine A2A receptor signaling.
PLoS One. 8:e592572013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu W, Dong M, Bo L, Li C, Liu Q, Li Z and
Jin F: Epigallocatechin-3-gallate suppresses alveolar epithelial
cell apoptosis in seawater aspiration-induced acute lung injury via
inhibiting STAT1-caspase-3/p21 associated pathway. Mol Med Rep.
13:829–836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Damarla M, Parniani AR, Johnston L,
Maredia H, Serebreni L, Hamdan O, Sidhaye VK, Shimoda LA, Myers AC,
Crow MT, et al: Mitogen-activated protein kinase-activated protein
kinase 2 mediates apoptosis during lung vascular permeability by
regulating movement of cleaved caspase 3. Am J Respir Cell Mol
Biol. 50:932–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Halimah E, Diantini A, Destiani DP,
Pradipta IS, Sastramihardja HS, Lestari K, Subarnas A, Abdulah R
and Koyama H: Induction of caspase cascade pathway by
kaempferol-3-rhamnoside in LNCaP prostate cancer cell lines. Biomed
Rep. 3:115–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Q, Jin J, Yuan JX, Zeifman A, Chen J,
Shen B and Huang J: VEGF, Bcl-2 and Bad regulated by angiopoietin-1
in oleic acid induced acute lung injury. Biochem Biophys Res
Commun. 413:630–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiu X, Li H, Tang H, Jin Y, Li W, Yu Sun,
Ping Feng, Sun X and Xia Z: Hydrogen inhalation ameliorates
lipopolysaccharide-induced acute lung injury in mice. Int
Immunopharmacol. 11:2130–2137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mangiacapra F, Colaiori I, Ricottini E,
Balducci F, Creta A, Demartini C, Minotti G and Di Sciascio G:
Heart Rate reduction by IVabradine for improvement of ENDothELial
function in patients with coronary artery disease: The RIVENDEL
study. Clin Res Cardiol. 106:69–75. 2017. View Article : Google Scholar : PubMed/NCBI
|