Introduction
Ovarian cancer (OC) presents as the most fatal of
the gynecological malignancies and is the leading cause of
mortality among women worldwide (1). Although improvements and advancements
have been made in current treatments of OC by combining surgery,
chemotherapy, radiotherapy and immunotherapy, the survival rate of
OC patients has not sufficiently improved (2). Therefore, it is necessary to identify
novel molecular biomarkers and therapeutic strategies for the
treatment of patients suffering from OC.
MicroRNAs (miRNAs) are recognized as small,
endogenous RNAs (~23 nucleotides), which act as tumor suppressors
or oncogenes and exhibit various important roles in carcinogenesis
(3–6). It is reported that the dysregulation
of miRNAs is frequently associated with cancer progression,
including ovarian cancer (7–9). It
is reported that miR-509-3p strongly attenuates multi-cellular
spheroids and cell metastasis of ovarian cancer (10). Zhang et al (11) suggests that miR-520 g represses
death associated protein kinase 2 and then promotes epithelial OC
progression and chemo-resistance. Wu et al (12) noted that miR-572 promotes cell
proliferation of human ovarian cancer cells by repressing protein
phosphatase 2 regulatory subunit B α (PPP2R2A) expression. miR-381
is reported to suppress cell proliferation, migration and invasion
of epithelial OC via suppression of YY1 transcription factor
expression (13). However, the
biological roles and underlying mechanisms of miR-614 during the
pathogenesis of OC have not yet been clearly elucidated. In the
present study, it was demonstrated that miR-614 was upregulated in
OC clinical tissues and cell lines. Ectopic overexpression of
miR-614 promoted the cell proliferation and colony-forming
abilities, and decreased the apoptotic rate of A2780 cells in
vitro. Furthermore, it was identified that PPP2R2A is a direct
target of miR-614. Overall, the findings indicated that miR-614
regulated OC cell proliferation, colony formation abilities and
cell apoptosis, by directly modulating PPP2R2A.
Materials and methods
Tissue samples and cell culture
Written informed consent was obtained from all
patients (age range, 32–55) at the Department of Traditional
Chinese Medicine Gynecology, Huang Huai University (Zhumadian,
China) that participated in the study, and the study was approved
by the Ethics Committee of Huang Huai University. None of the
patients had received chemoradiotherapy. A total of 8 pairs of
primary ovarian carcinoma tissues (T) and the matched adjacent
non-tumor normal tissues (ANT) were collected from patients having
undergone surgery. A total of two fresh normal ovarian tissues were
each collected from two patients. All specimens had been verified
with pathological diagnosis. Tissue samples were frozen and stored
in liquid nitrogen until their use in western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Human ovarian cancer cell lines COV362, SKOV3,
EFO27, COV644, A2780, OV90, CAOV3 and IGROV-1 were purchased from
the Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Human normal ovarian
epithelial cell line IOSE80 and ovary surface epithelial cells HOSE
were all purchased from Shanghai Huiying Biological Technology Co.,
Ltd. (Shanghai, China). Cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). All cell lines were maintained at 37°C in a
humidified incubator in an environment containing 5%
CO2.
miRNAs, small interfering (si)RNA and
transfection
The miR-614 mimic (HmiR0183-MR04; GeneCopoeia, Inc.,
Rockville, MD, USA), miR-614 inhibitor (miR-614-in;
HmiR-AN0718-AM02; GeneCopoeia, Inc.), the corresponding negative
controls (Vector and NC) and siRNA-PPP2R2A (PPP2R2A-siRNA#1,
HSH014174; PPP2R2A-siRNA#2 and HSH055016) were purchased from
GeneCopoeia, Inc., and A2780 cells (Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences,
Shanghai, China) were plated and cultured overnight and transfected
with 50 nM indicated RNA using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Cells were harvested 24 or 48 h after
transfection.
RNA extraction and RT-qPCR
Total RNA was extracted from clinical tissues and
cancer cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) and reverse transcribed to cDNA using a Reverse
Transcription kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. The expression levels of
miR-614, B cell lymphoma-2 associated agonist of cell death (BAD)
and Cyclin D1 were measured by RT-qPCR with SYBR-Green Mix Taq kit
(Takara Bio, Inc., Otsu, Shiga, Japan) in an ABI Prism7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following PCR primers were synthesized by GeneCopoeia,
Inc.: miR-614 (HmiRQP0718), BAD (HQP015538) and Cyclin D1
(HQP016204). U6 (HmiRQP9001) and GAPDH (HQP064347) were used as
internal controls for normalization. Relative expression levels
were calculated using the 2−ΔΔCq method (14).
MTT and 5-bromodeoxyuridine (BrdU)
incorporation assays
Cell proliferation was assessed using an MTT assay.
Following transfection, A2780 cells were tested using the MTT assay
at different time points. A total of 20 µl of 5 mg/ml MTT solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well and 150 µl dimethyl sulfoxide was added then incubated for 4 h
at 37°C. Following this, the absorbance was measured using a
microplate reader at a wavelength of 490 nm.
Cell growth was measured using a BrdU incorporation
assay (EMD Millipore, Billerica, MA, USA). Following transfection
and culturing for 24 h, A2780 were incubated with BrdU for 1 h, and
stained with BrdU antibodies (cat. no. 61273; 1:500; Upstate
Biotechnology, Inc., Lake Placid, NY, USA) at 4°C overnight, and
then incubated with horseradish peroxidase-modified secondary
antibodies (ab6741; 1:5,000; Abcam, Cambridge, UK) at 37°C for 2 h.
Gray level images were measured under a laser-scanning microscope
(Axioskop 2 plus; Carl Zeiss Co., Ltd., Jena, Germany).
Anchorage-independent growth ability
assay
For the anchorage-independent growth ability assay,
a 1.5-ml basal layer of 0.8% agar (Sigma-Aldrich; Merck KGaA) was
poured into a 6-well culture plate. A total of 500 cells
(quantified using a Z2 Coulter Counter Analyzer; Beckman Coulter,
Inc., Brea, CA, USA) were trypsinized and suspended in 2 ml
complete medium plus 0.3% agar and then added to each well, and
incubated at 37°C in an environment containing 5% CO2,
in a humidified incubator for 2 weeks. Colonies greater than 0.1 mm
in diameter were counted manually.
Cell apoptosis assay
Following transfection A2780 cells
(2×105) were plated into 6-well plates. After 48 h,
cells were digested with 0.25% trypsin, centrifuged, and the
supernatants discarded. The cell pellets were washed three times
with cold PBS and the supernatants discarded. The cells were then
incubated with 5 µl Annexin V-FITC (C1062; Beyotime Institute of
Biotechnology, Haimen, China) and 5 µl propidium iodide (PI; C1062;
Beyotime Institute of Biotechnology) for 15–20 min in the dark at
25°C, and then the percentage of apoptotic cells was detected by a
BD FACSVerse flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and analyzed using the BD FACStation data management
system.
Bioinformatics analysis
To investigate the target gene of miR-614,
TargetScan version 7.1 (http://www.targetscan.org) was used to predict the
potential target gene of miR-614.
Luciferase assay
The wild type 3′-untranslated region (UTR) PPP2R2A
vectors were co-transfected with miR-614 or miR-614-mutated or
relative negative controls, into A2780 cells using
Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), following the manufacturer's protocol. Following
48 h, cells were lysed and the Luciferase reporter assays were
measured using a Dual-Luciferase Reporter Assay System (Promega,
Madison, WI, USA). The luciferase activity was normalized to
Renilla luciferase activity.
Western blotting
Protein samples were lysed from OC cells that
underwent transfection, using a radioimmunoprecipitation assay
lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA).
Protein samples were quantified with the Pierce BCA Protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.) and were then boiled
for 10 min in sodium dodecyl sulfate (SDS) sample buffer. Protein
samples (20 µg) were separated with 10% SDS-PAGE, and subsequently
transferred onto polyvinylidene membranes via electroblotting.
Following blocking with 5% dried milk for 2 h at room temperature,
membranes were then incubated with anti-PPP2R2A, anti-Cyclin D1
(cat. no. 2978; 1:1,000), anti-BAD, anti-phosphorylated (p)-Rb
(cat. no. 8516; 1:1,000), anti-Rb (cat. no. 9313; 1:1,000) and
anti-α-tubulin antibodies (cat. no. 2144; 1:1,000; all from Cell
Signaling Technology, Inc.) overnight at 4°C, and then incubated
with corresponding horseradish peroxidase-conjugated secondary
antibodies (cat. no. 7074; 1:5,000, Cell Signaling Technology,
Inc.) for 2 h at room temperature. The protein bands were detection
by an enhanced chemiluminescence western blotting substrate
(Pierce; Thermo Fisher Scientific, Inc.) and quantified using
ImageJ software version 1.48 (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All statistical analyses were presented as the mean
± standard deviation and were conducted using SPSS software,
version 19.0 (IBM SPSS, Armonk, NY, USA). Comparisons between
groups were evaluated by a Student's t-test or one-way analysis of
variance followed by a post hoc Tukey test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-614 expression is upregulated in
OC tissues and cell lines
RT-qPCR analysis was firstly performed to examine
the expression of miR-614 in primary ovarian carcinoma tissues (T)
and the matched adjacent non-tumor normal tissues (ANT), and the
results revealed that miR-614 levels in primary ovarian carcinoma
tissues were significantly increased compared with adjacent
non-tumor normal tissues (Fig.
1A). miR-614 expression levels were then further detected in
eight ovarian cancer cell lines (COV362, SKOV3, EFO27, COV644,
A2780, OV90, CAOV3 and IGROV1), human normal ovarian epithelial
cell line IOSE80 and the ovary surface epithelial cells HOSE, and
the data indicated that miR-614 was markedly upregulated in ovarian
cancer cell lines (Fig. 1B),
providing evidence that miR-614 may be associated with ovarian
cancer progression.
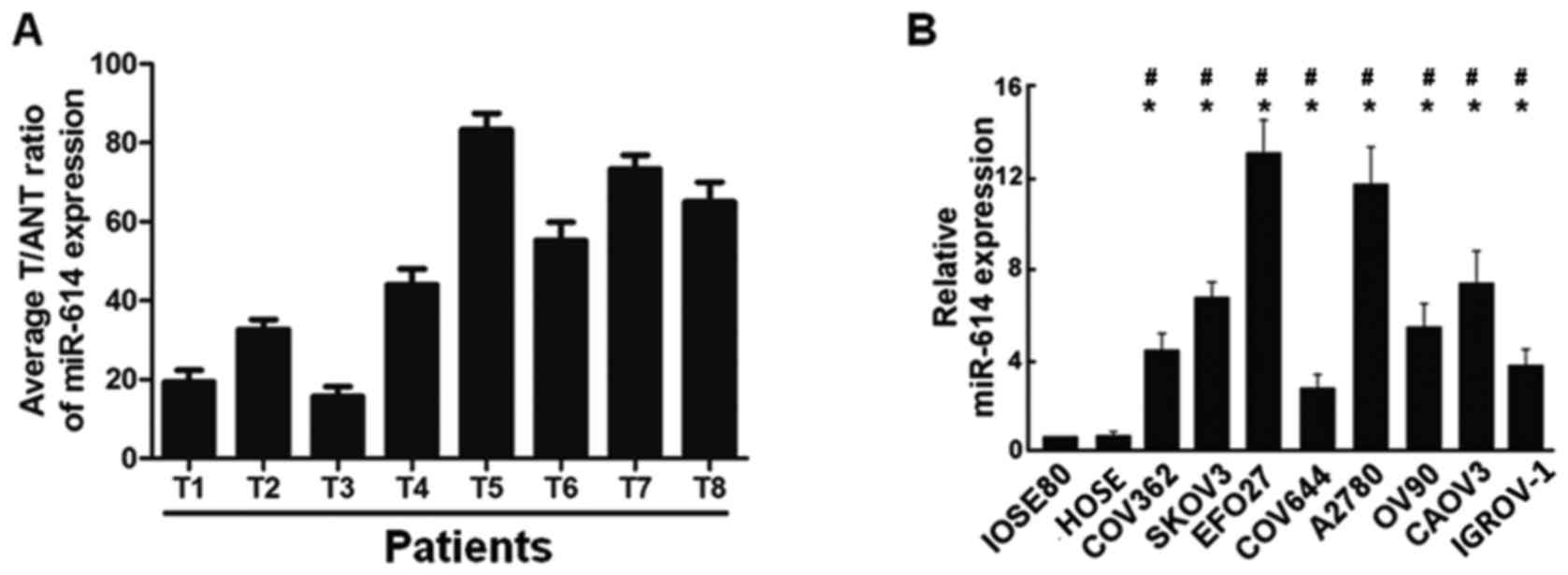 | Figure 1.Expression of miR-614 in human OC
tissues and cell lines. (A) Relative miR-614 mRNA expression levels
in 8 paired primary OC tissues (T) and the matched adjacent
non-tumor normal tissues (ANT) from the same patient were detected
by RT-qPCR analysis. (B) RT-qPCR analysis of miR-614 expression in
human normal ovarian epithelial cell line IOSE80, ovary surface
epithelial cells HOSE and OC cell lines, including COV362, SKOV3,
EFO27, COV644, A2780, OV90, CAOV3 and IGROV-1. *P<0.05 vs.
IOSE80; #P<0.05 vs. HOSE. miRNA, microRNA; OC,
ovarian cancer; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
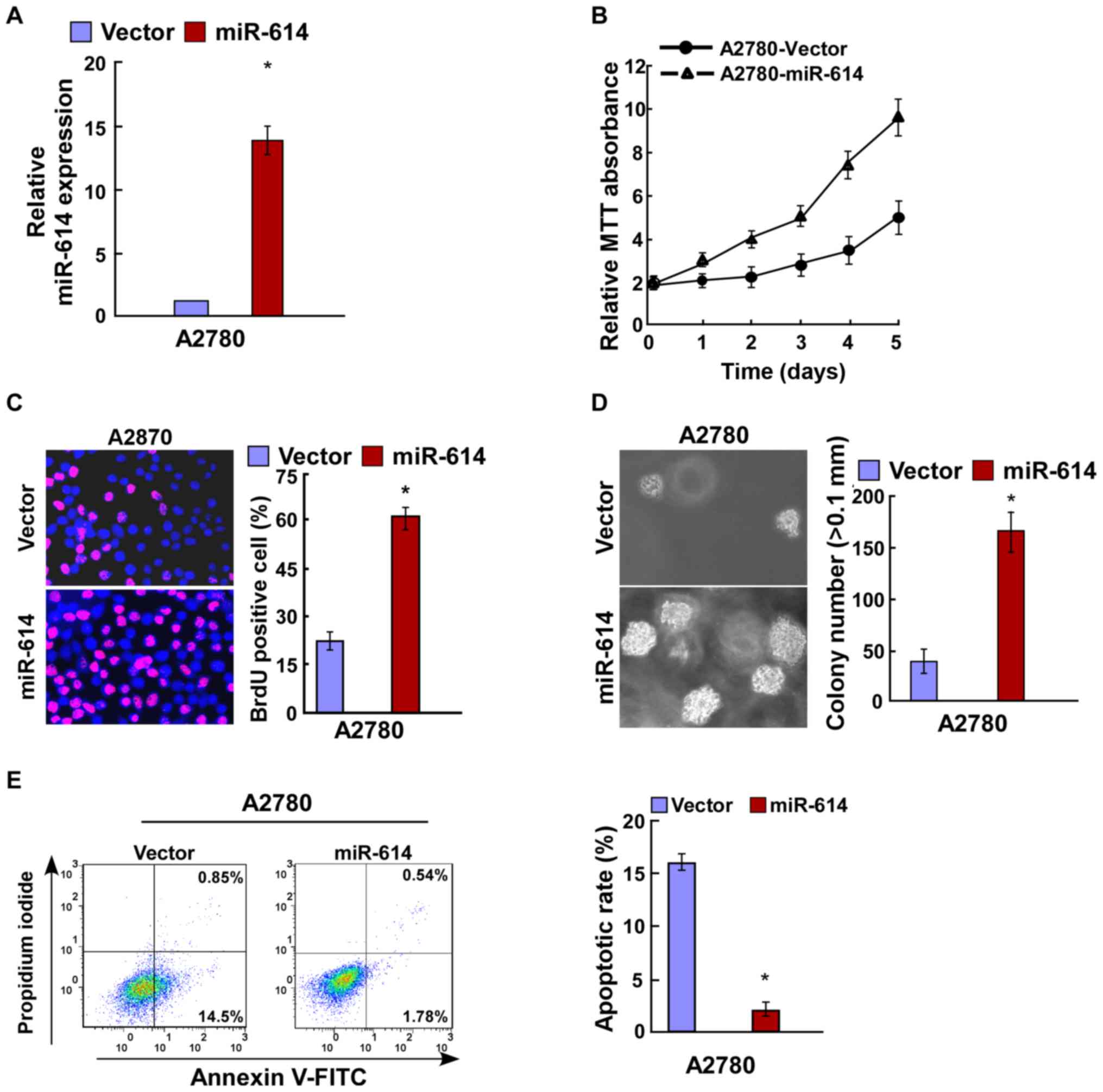
miR-614 promotes cell proliferation of
OC
Due to the upregulated expression of miR-614 in OC
clinical tissues and cell lines, the present study next
investigated its effects on OC cell lines. miR-614 mimic and the
respective controls were transfected into A2780 OC cells, and then
the transfection efficiency was verified by RT-qPCR (Fig. 2A). Results of the MTT and BrdU
assays revealed that miR-614 overexpression significantly increased
cell proliferation of A2780 cells (Fig. 2B and C), and this was further
verified by the anchorage-independent growth ability assay
(Fig. 2D). Cell apoptosis analysis
indicated that the apoptotic rate was significantly decreased in
A2780 cells following transfection with miR-614 in comparison with
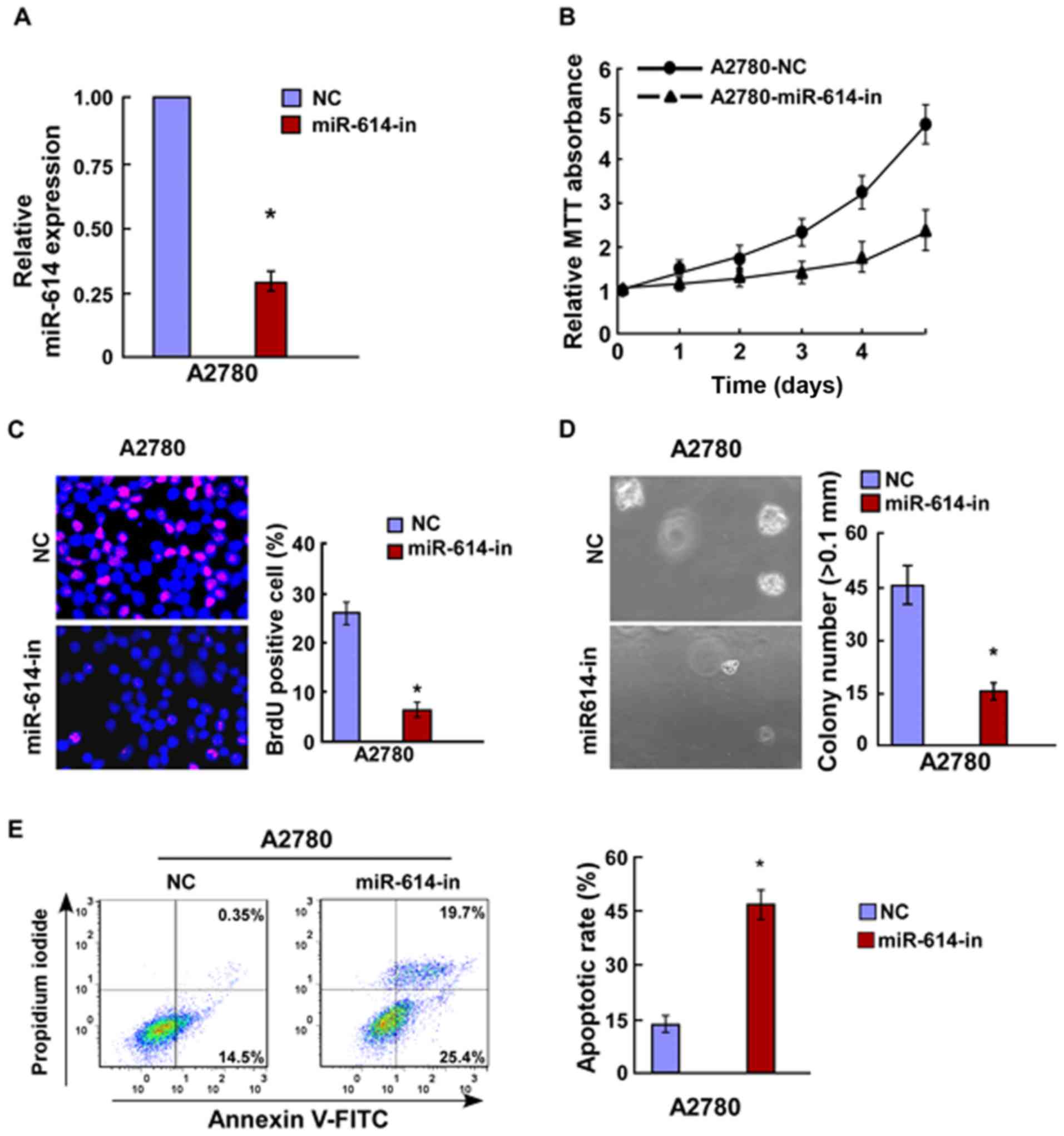
the controls (Fig. 2E). miR-614
inhibitor and the respective controls were transfected into A2780
OC cells, and the transfection efficiency was verified by RT-qPCR
(Fig. 3A). Results of the MTT and
BrdU assays indicated that miR-614-in significantly decreased cell
proliferation of A2780 cells (Fig. 3B
and C), and this was further verified by the
anchorage-independent growth ability assay (Fig. 3D). Cell apoptosis analysis revealed
that miR-614-in promoted cell apoptosis rate in A2780 cells
(Fig. 3E). Overall, miR-614 is
capable of promoting cell proliferation of OC which is associated
with the regulation of cell apoptosis.
miR-614 directly targets PPP2R2A by
binding to its 3′-UTR, and alters levels of proteins associated
with proliferation in OC
To explore the regulatory mechanism of miR-614 in OC
cell proliferation, the present study used algorithm programs
(Target Scan software; http://www.targetscan.org/vert_50/) and identified
that PPP2R2A 3′-UTR contained a predicted binding site of miR-614
(Fig. 4A). The western blotting
assay revealed that miR-614 overexpression suppressed PPP2R2A
expression, whereas miR-614-in increased its expression (Fig. 4B). The Luciferase activity assay
indicated that co-transfection of miR-614 and pGL3-PPP2R2A 3′-UTR
resulted in a significant reduction in luciferase activity, whereas
the luciferase activity was significantly increased with
co-transfection of miR-614-in and pGL3-PPP2R2A 3′-UTR. However,
there was no alteration in luciferase activity when co-transfection
with miR-614-mut and the luciferase reporter vector occurred.
Together, the results suggested that miR-614 directly binds to the
3′UTR of PPP2R2A (Fig. 4C). Given
that the results revealed that miR-614 regulated cell proliferation
and cell apoptosis of OC, the effects on the expression level of
PPP2R2A downstream genes were then investigated, including Cyclin
D1, BAD, Rb and p-Rb. As the results of the RT-qPCR indicated,
miR-614 significantly decreased the mRNA expression level of the
apoptotic gene BAD and increased expression of the cell
proliferation associated gene Cyclin D1, whereas miR-614-in
revealed the opposite effect, and miR-614-mut had no effect on
their mRNA expression levels (Fig.
4D). Western blot assays revealed that overexpression of
miR-614 enhanced the protein expression levels of Cyclin D1 and
p-Rb and decreased the BAD protein expression, whereas miR-614-in
significantly reduced the protein expression levels of Cyclin D1
and p-Rb and increased the BAD protein expression (Fig. 4E). Collectively, these results
demonstrated that miR-614 regulated OC cell growth and cell
apoptosis by targeting PPP2R2A.
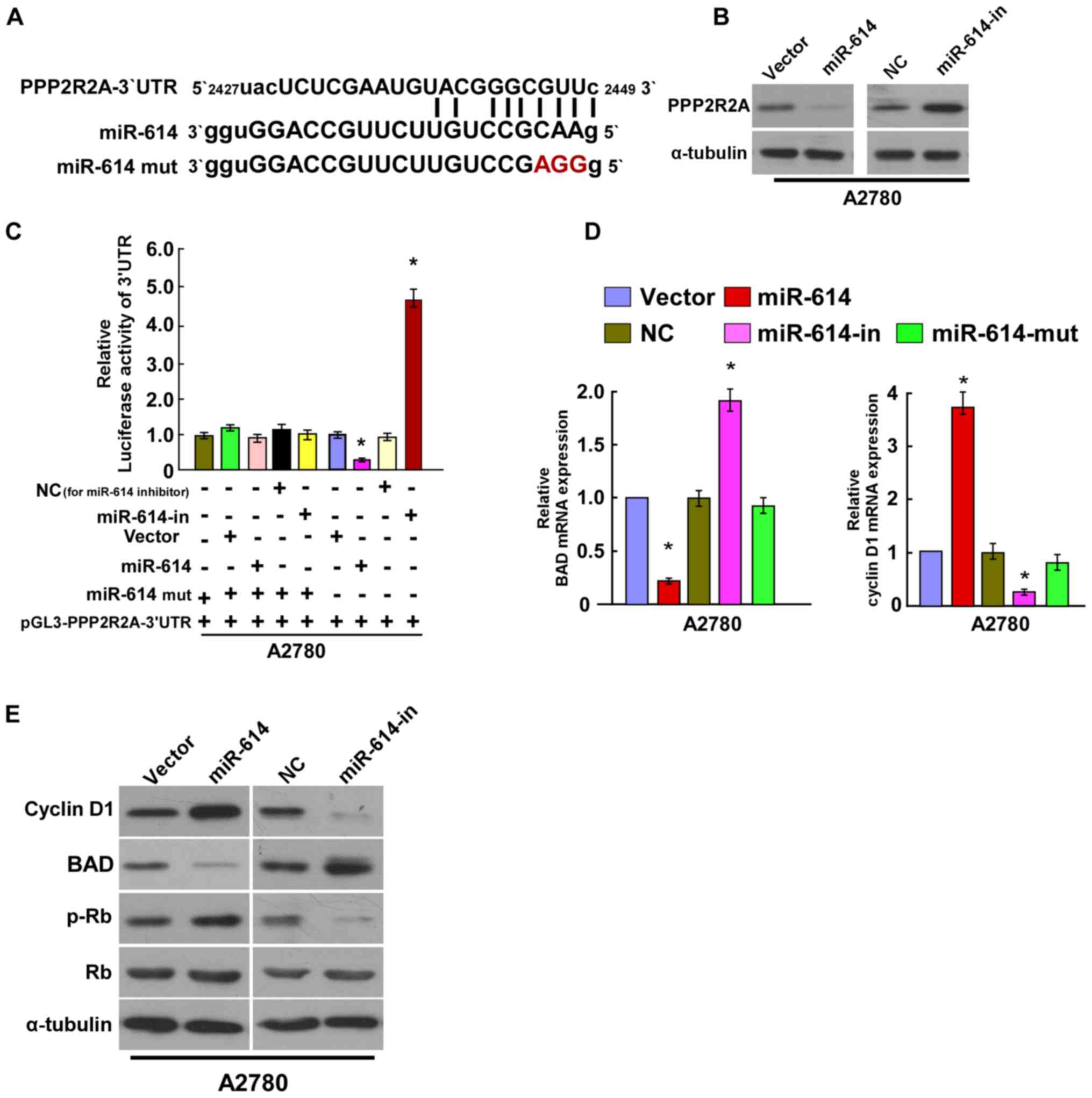 | Figure 4.miR-614 suppresses PPP2R2A expression
by directly targeting the PPP2R2A 3′-UTR and altered levels of
proteins associated with cell proliferation and cell apoptosis in
A2780 cells. (A) Predicted miR-614c target sequence in the 3′-UTR
of PPP2R2A (PPP2R2A-3′-UTR) and positions of three mutated
nucleotides (red) in the 3′-UTR of miR-614 (miR-614-mut). (B)
Expression of PPP2R2A in A2780 cells transfected with miR-614 or
the miR-614-in were detected by western blotting analysis.
α-tubulin served as the loading control. (C) Luciferase reporter
assay of A2780 cells transfected with the pGL3-PPP2R2A-3′-UTR
reporter and miR-614 or miR-614-in or miR-614-mut oligonucleotides.
(D) Reverse transcription-quantitative polymerase chain reaction
analysis of expression of BAD and Cyclin D1 in A2780 cells. (E)
Western blot analysis of protein expression of Cyclin D1, BAD, p-Rb
and Rb in A2780 cells. α-tubulin served as the loading control.
*P<0.05 vs. Control. BAD, B cell lymphoma-2 associated agonist
of cell death; miRNA, microRNA; in, inhibitor; UTR, untranslated
region; PPP2R2A, protein phosphatase 2 regulatory subunit B α; NC,
negative control; p, phosphorylated; mut, mutated. |
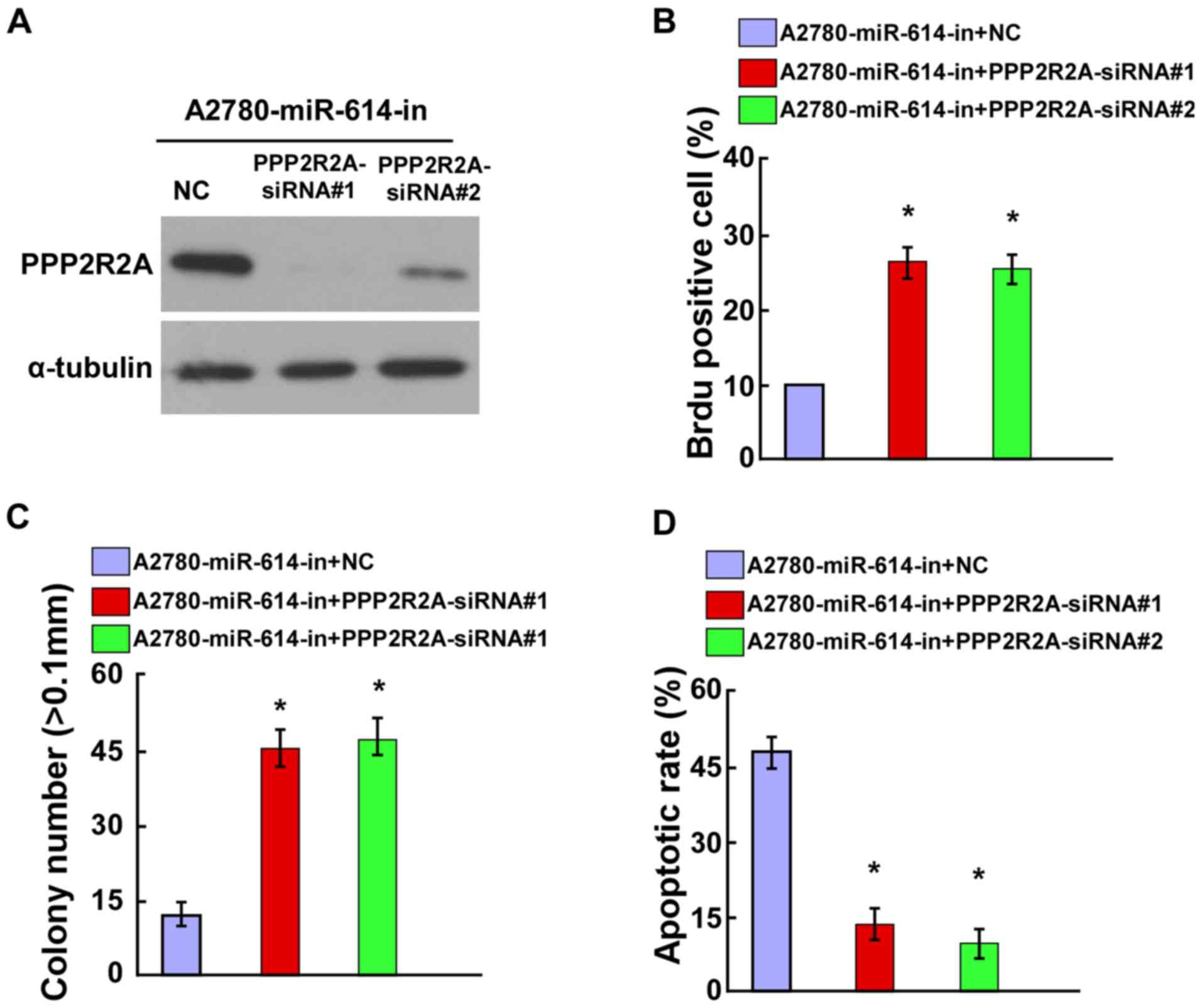
Knockdown of PPP2R2A counteracted the
effect of miR-614-in on OC cell proliferation and cell
apoptosis
To verify the role of PPP2R2A in OC cell
proliferation, it was investigated whether the knockdown of PPP2R2A
could counteract the proliferation arrest induced by miR-614-in.
The siRNA interference efficiency of PPP2R2A-siRNAs was evaluated
by western blot analysis (Fig.
5A). The BrdU positive rate of miR-614-in transfected A2780
cells was increased following transfection with PPP2R2A-siRNAs
(Fig. 5B). Additionally, the
anchorage-independent growth ability assay demonstrated that the
anchorage-dependent proliferation induced by miR-614-in could be
abolished by knockdown of PPP2R2A (Fig. 5C). Furthermore, the results of the
cell apoptosis assay indicated that miR-614-in increased the
apoptotic rate, which was then attenuated by knockdown of PPP2R2A
in A2780 cells (Fig. 5D).
Discussion
The present study, to the best of the author's
knowledge, demonstrated for the first time, that miR-614 was
upregulated in OC clinical tissues and cells. Increased expression
of miR-614 led to the promotion of cell proliferation and
colony-forming abilities, and conversely, decreased the apoptotic
rate of OC cells. Additionally, PPP2R2A may act as a novel target
of miR-614. The present study indicated that miR-614 may act as a
novel tumor promoter in OC by targeting PPP2R2A.
Accumulating evidence suggests that miRNAs exhibit
an essential role in human cancer pathological proceedings via
controlling different target genes, including those involved in
cell proliferation, migration, invasion, cycle and apoptosis
(15–18). Dysregulation of miRNA frequently
occurs in novel types of cancers, including ovarian cancer.
miR-21-3p inhibits cell proliferation and invasion of ovarian
cancer by targeting RNA binding protein with multiple splicing,
RCC1 and BTB domain containing protein 1 and Zinc finger protein
608 (19). Fu et al
suggests that miR-222-3p suppresses epithelial OC cell growth by
regulating G protein subunit α I2 (17). miR-203 promotes cell growth and
migration of OC by targeting pyruvate dehydrogenase (Lipoamide) β
(20). However, the functions of
miR-614 in OC have not been fully elucidated. The data of the
present study indicated that miR-614 expression was upregulated in
OC clinical tissues and cell lines. Overexpression of miR-614
enhanced OC cell proliferation and decreased cell apoptosis rate,
suggesting miR-614 exhibits an important role in OC
progression.
PPP2R2A, a regulatory subunit of phosphatase, acts
as a well-recognized regulator in the control of the AKT
serine/threonine kinase signaling pathway associated with tumor
growth (21–23). miR-136 promotes cell proliferation
of human non-small cell lung cancer cells by targeting PPP2R2A
(24). miR-31 acts as an oncogenic
microRNA in human lung cancer cells by repressing PPP2R2A (25). Liang et al (26) indicates that miR-892a promotes cell
proliferation of human colorectal cancer cells by regulating
PPP2R2A expression. Wong et al (27) reports that miR-222 is overexpressed
in hepatocellular carcinoma and promotes cell motility by targeting
PPP2R2A. The present study identified that PPP2R2A was a potential
target of miR-614 by using a bioinformatics search. Subsequently,
western blotting and luciferase reporter assays demonstrated that
miR-614 targeted PPP2R2A and suppressed its expression. Further
experiments to investigate the mechanism underlying the PPP2R2C
mediated cancer cell proliferation and cell apoptosis are required.
Results from RT-qPCR and western blotting analysis indicated that
BAD (mRNA and protein) levels were downregulated whereas Cyclin D1
(mRNA and protein) levels were upregulated in miR-614-transfected
A2780 cells, whereas miR-614-in demonstrated the opposite effect.
Furthermore, knockdown of PPP2R2A counteracted the effect of
miR-614-in on OC cell proliferation and cell apoptosis.
In conclusion, the findings suggested that miR-614
expression was upregulated in OC clinical tissues and cells.
Overexpression of miR-614 promoted cell proliferation and regulated
cell apoptosis through inhibition of PPP2R2A. The findings
suggested that miR-614 may act as a potential therapeutic target
for the treatment of OC in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and DG designed and performed this study. HZ
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients (age range, 32–55) at the Department of Traditional
Chinese Medicine Gynecology, Huang Huai University (Zhumadian,
China) that participated in the study, and the study was approved
by the Ethics Committee of Huang Huai University.
Consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krishnan V, Berek JS and Dorigo O:
Immunotherapy in ovarian cancer. Curr Probl Cancer. 41:48–63. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie F, Yuan Y, Xie L, Ran P, Xiang X,
Huang Q, Qi G, Guo X, Xiao C and Zheng S: miRNA-320a inhibits tumor
proliferation and invasion by targeting c-Myc in human
hepatocellular carcinoma. Onco Targets Ther. 10:885–894. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Y, Xie H, Liu Y, Liu W, Liu M and Tang
H: miR-484 suppresses proliferation and epithelial-mesenchymal
transition by targeting ZEB1 and SMAD2 in cervical cancer cells.
Cancer Cell Int. 17:362017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Y, Lin L, Li T, Yang J and Wei Y: The
role of miRNA-223 in cancer: Function, diagnosis and therapy. Gene.
616:1–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arechaga-Ocampo E, Lopez-Camarillo C,
Villegas-Sepulveda N, Gonzalez-De la Rosa CH, Perez-Añorve IX,
Roldan-Perez R, Flores-Perez A, Peña-Curiel O, Angeles-Zaragoza O,
Rangel Corona R, et al: Tumor suppressor miR-29c regulates
radioresistance in lung cancer cells. Tumour Biol.
39:10104283176950102017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Yang J, Xiang YY and Pi J:
Overexpression of Hsa-miR-320 Is associated with invasion and
metastasis of ovarian cancer. J Cell Biochem. 118:3654–3661. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Jiang N, Shi H, Zhao S, Yao S and
Shen H: miR-28-5p promotes the development and progression of
ovarian cancer through inhibition of N4BP1. Int J Oncol. Mar
16–2017.(Epub ahead of print). View Article : Google Scholar
|
|
9
|
Bai L, Wang H, Wang AH, Zhang LY and Bai
J: MicroRNA-532 and microRNA-3064 inhibit cell proliferation and
invasion by acting as direct regulators of human telomerase reverse
transcriptase in ovarian cancer. PLoS One. 12:e01739122017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan Y, Robertson G, Pedersen L, Lim E,
Hernandez-Herrera A, Rowat AC, Patil SL, Chan CK, Wen Y, Zhang X,
et al: miR-509-3p is clinically significant and strongly attenuates
cellular migration and multi-cellular spheroids in ovarian cancer.
Oncotarget. 7:25930–25948. 2016.PubMed/NCBI
|
|
11
|
Zhang J, Liu L, Sun Y, Xiang J, Zhou D,
Wang L, Xu H, Yang X, Du N, Zhang M, et al: MicroRNA-520 g promotes
epithelial ovarian cancer progression and chemoresistance via DAPK2
repression. Oncotarget. 7:26516–26534. 2016.PubMed/NCBI
|
|
12
|
Wu AH, Huang YL, Zhang LZ, Tian G, Liao QZ
and Chen SL: MiR-572 prompted cell proliferation of human ovarian
cancer cells by suppressing PPP2R2C expression. Biomed
Pharmacother. 77:92–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia B, Li H, Yang S, Liu T and Lou G:
MiR-381 inhibits epithelial ovarian cancer malignancy via YY1
suppression. Tumour Biol. 37:9157–9167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao C, Sun D, Zhang L and Song L: miR-186
affects the proliferation, invasion and migration of human gastric
cancer by inhibition of Twist1. Oncotarget. 7:79956–79963. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei ST, Shen F, Chen JW, Feng JH, Cai WS,
Shen L, Hu ZW and Xu B: MiR-639 promoted cell proliferation and
cell cycle in human thyroid cancer by suppressing CDKN1A
expression. Biomed Pharmacother. 84:1834–1840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu X, Li Y, Alvero A, Li J, Wu Q, Xiao Q,
Peng Y, Hu Y, Li X, Yan W, et al: MicroRNA-222-3p/GNAI2/AKT axis
inhibits epithelial ovarian cancer cell growth and associates with
good overall survival. Oncotarget. 7:80633–80654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen Z, Zhao S, Liu S, Liu Y, Li X and Li
S: MicroRNA-148a inhibits migration and invasion of ovarian cancer
cells via targeting sphingosine-1-phosphate receptor 1. Mol Med
Rep. 12:3775–3780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baez-Vega PM, Echevarria Vargas IM,
Valiyeva F, Encarnación-Rosado J, Roman A, Flores J,
Marcos-Martínez MJ and Vivas-Mejía PE: Targeting miR-21-3p inhibits
proliferation and invasion of ovarian cancer cells. Oncotarget.
7:36321–36337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiaohong Z, Lichun F, Na X, Kejian Z,
Xiaolan X and Shaosheng W: MiR-203 promotes the growth and
migration of ovarian cancer cells by enhancing glycolytic pathway.
Tumour Biol. 37:14989–14997. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng LP, Hu ZM, Li K and Xia K: miR-222
attenuates cisplatin-induced cell death by targeting the
PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J Cell Mol Med.
20:559–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng LP, Hu ZM, Li K and Xia K: miR-222
attenuates cisplatin-induced cell death by targeting the
PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J Cell Mol Med.
20:559–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hein AL, Seshacharyulu P, Rachagani S,
Sheinin YM, Ouellette MM, Ponnusamy MP, Mumby MC, Batra SK and Yan
Y: PR55α subunit of protein phosphatase 2A supports the tumorigenic
and metastatic potential of pancreatic cancer cells by sustaining
hyperactive oncogenic signaling. Cancer Res. 76:2243–2253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Ma T, Yang S, Xia M, Xu J, An H,
Yang Y and Li S: High-mobility group A1 proteins enhance the
expression of the oncogenic miR-222 in lung cancer cells. Mol Cell
Biochem. 357:363–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen S, Yue H, Li Y, Qin J, Li K, Liu Y
and Wang J: Upregulation of miR-136 in human non-small cell lung
cancer cells promotes Erk1/2 activation by targeting PPP2R2A.
Tumour Biol. 35:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Sempere LF, Ouyang H, Memoli VA,
Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, et al:
MicroRNA-31 functions as an oncogenic microRNA in mouse and human
lung cancer cells by repressing specific tumor suppressors. J Clin
Invest. 120:1298–1309. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang WL, Cao J, Xu B, Yang P, Shen F, Sun
Z, Li WL, Wang Q and Liu F: miR-892a regulated PPP2R2A expression
and promoted cell proliferation of human colorectal cancer cells.
Biomed Pharmacother. 72:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong QW, Ching AK, Chan AW, Choy KW, To
KF, Lai PB and Wong N: MiR-222 overexpression confers cell
migratory advantages in hepatocellular carcinoma through enhancing
AKT signaling. Clin Cancer Res. 16:867–875. 2010. View Article : Google Scholar : PubMed/NCBI
|