Introduction
Renal cell carcinoma (RCC), one of the most common
solid cancers found in adult kidneys, is an epithelial carcinoma
derived from the proximal tubules of nephrons (1). RCC accounts for ~90% of kidney
carcinomas and 3% of all adult malignancies (1). Due to its inherent insensitivity to
radiotherapy and chemotherapy, surgery remains the only curative
strategy for RCC (2). However,
approximately one-third of patients develop metastases after
surgery (3). Therefore, novel
therapeutic strategies are urgently needed.
In recent years, considerable attention has been
paid to natural products for preventing or treating cancers due to
their safety. Over the past 30 years, over 70% of all drugs
approved by the Food and Drug Administration (FDA) for cancer have
originated from natural products or traditional medicine (4). Dauricine, a bisbenzylisoquinoline
(BBIQ) alkaloid isolated from the rhizome of Menispermum dauricum
DC, has been found to yield various pharmacological results, such
as anti-arrhythmic and anti-inflammatory effects (5). Moreover, several studies have
indicated that dauricine has potent antitumor activities, including
inducing apoptosis, repressing viability and overcoming drug
resistance in tumor cells (6).
However, the effect of dauricine on RCC remains to be
elucidated.
In the present study, we investigated the biological
effects and mechanisms underlying dauricine action in RCC cells. We
found that dauricine inhibits viability in cells and induces cell
cycle arrest at the G0/G1 phase and apoptosis via the intrinsic
pathway in RCC cells. The mechanisms of dauricine's action included
the repression of anti-apoptotic Bcl-2 proteins and the PI3K/Akt
signaling pathway.
Materials and methods
Cell culture and reagents
The human RCC cell lines 786-O, Caki-1, A-498 and
ACHN were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum (both from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 1% (v/v)
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 37°C in a humidified atmosphere containing 5%
CO2. Dauricine was purchased from Solarbio Biotechnology
Co., Ltd. (Beijing, China).
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) and propidium iodide (PI) were purchased from Sigma-Aldrich
Chemicals (St. Louis, MO, USA). Primary antibodies against
caspase-8, caspase-3, caspase-9, cleaved PARP, Bcl-2, p21, and Bax
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Primary antibodies against cyclin D1, cyclin-dependent kinase
2 (CDK2), CDK4, p-Akt, Akt, p-PI3K, and PI3K were all purchased
from Abcam (Cambridge MA, USA), and primary antibodies against
GAPDH and donkey anti-rabbit and sheep anti-mouse immunoglobulin
were purchased from Sigma-Aldrich. All other chemicals not
specifically mentioned here were purchased from Sigma-Aldrich;
Merck KGaA.
Cell viability assay
Cell viability was evaluated by MTT assay. Briefly,
cells were seeded in a 96-well plate (5×103 cells/well)
and then treated with various doses of dauricine for 24 h. A total
of 50 µl of MTT solution (5 mg/ml) was added, and the cells were
incubated for another 4 h. The medium was then removed, and 200 µl
of DMSO was added to each well. The absorbance of the solutions was
measured on a BioTek microplate reader at 595 nm. The relative cell
viability was normalized to the control, which was treated with
0.1% DMSO.
Cell cycle analysis
Cells were harvested after treatment with dauricine
and fixed in ethanol. The cells were washed with PBS and stained
with propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA) for
30 min in PBS supplemented with RNase at room temperature in the
dark. The cell cycle distribution was then evaluated using
FACSVerseTM (Beckman Coulter Fullerton, CA, USA), and the data were
analyzed using FlowJo V10 (Tree Star, Inc., Ashland, OR, USA).
Nucleosome ELISA assay for the
detection of apoptosis
For apoptosis assays, cells were seeded at a density
of 1×104 cells/well into 96-well plates at 37°C
overnight and treated with various doses of dauricine for 24 h.
Cells were subsequently harvested and treated with the Nucleosome
ELISA kit (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
according to the manufacturer's instructions.
Caspase activity assay
The activity of caspases was measured using a
caspase activation kit (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer's instructions. Briefly, cell lysates
were prepared after treatment with various doses of dauricine and
incubated with the supplied reaction buffer and the colorimetric
substrates at 37°C for 2 h in the dark. Then, the absorbance of the
solutions was measured on a BioTek microplate reader (BioTek
Instruments, Winooski, VT, USA) at 405 nm.
Western blot analyses
Total protein was lysed in RIPA lysis buffer
(Invitrogen Life Technologies, Carlsbad, CA, USA) and then
quantified with a BCA kit. Equal amounts of proteins were resolved
on 12% SDS-PAGE gels and transferred onto PVDF membranes. Membranes
were incubated with primary antibodies overnight at 4°C followed by
HRP-conjugated secondary antibodies at room temperature for 1 h;
signals were visualized by ECL reagent (Pierce, Rockford, IL,
USA).
Statistical analyses
All the experiments were carried out at least three
times. The data were analyzed using GraphPad Prism 6.0 software.
The results are shown as the mean ± standard deviation (SD), and
the differences were measured using one-way analysis of variance
(ANOVA) followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Dauricine inhibited the viability of
renal carcinoma cells
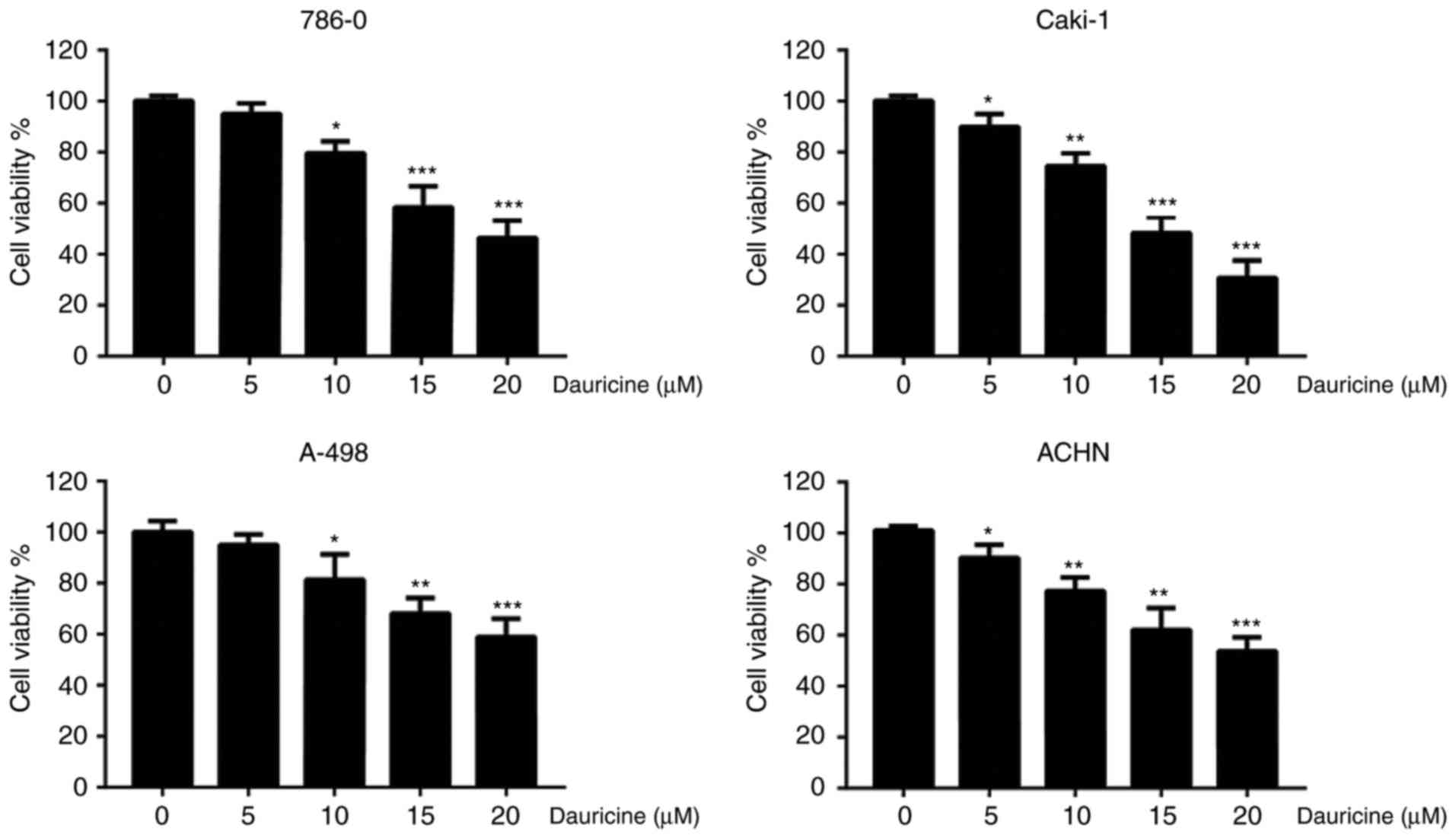
To investigate the cytotoxicity of dauricine, four
different renal carcinoma cell lines (786-O, Caki-1, A-498, and
ACHN) were treated with various concentrations of dauricine (0, 5,
10, 15, 20 µM) for 24 h, followed by MTT assay. As shown in
Fig. 1, dauricine significantly
decreased cell viability in a dose-dependent manner, and the
IC50 values of dauricine in different cell lines are
listed in Table I. Caki-1 and
A-498 cells were the most sensitive and the least sensitive to
dauricine, respectively. These two cell lines and 786-O cells were
used for the subsequent studies.
 | Table I.IC50 values of dauricine in
renal carcinoma cells. |
Table I.
IC50 values of dauricine in
renal carcinoma cells.
| Cell lines | IC50 |
|---|
| 786-0 |
13.42±2.1
µM |
| Caki-1 |
8.46±1.3
µM |
| A-498 |
18.28±0.6
µM |
| ACHN |
11.36±1.5
µM |
Dauricine induced cell cycle arrest at
the G0/G1 phase
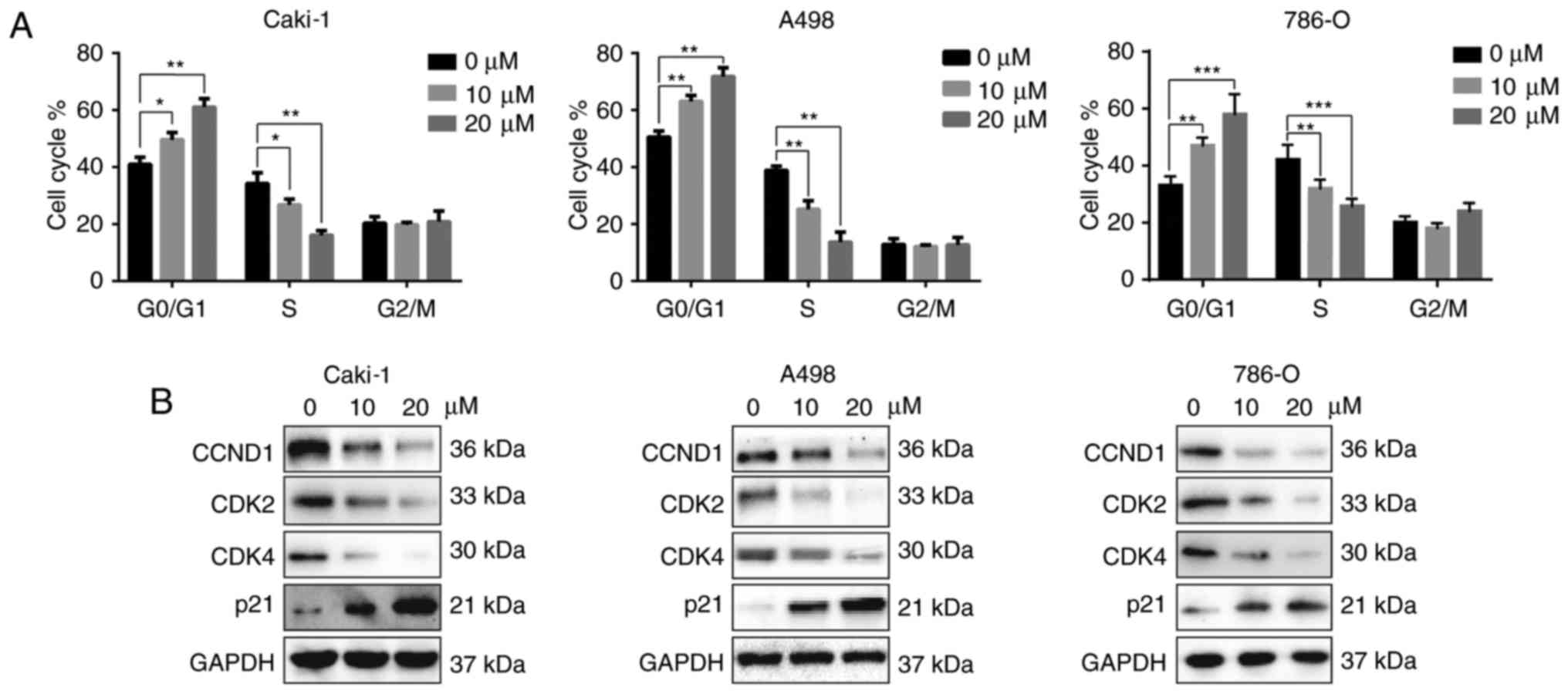
MTT assay revealed that dauricine inhibits the
viability of RCC cells. To better understand the underlying
mechanism, the effects of dauricine on cell cycle were analyzed
using flow cytometry. Caki-1, A-498 and 786-O cells were treated
with different doses of dauricine (10 and 20 µM) for 24 h. As shown
in Fig. 2A, incubation with
dauricine triggered cell cycle arrest at the G0/G1 phase as well as
a reduction in the S phase in a dose-dependent manner. Moreover,
cell cycle-related proteins, such as cyclin D1, CDK2, and CDK4,
were decreased, and p21 was increased after treatment with
dauricine (Fig. 2B).
Dauricine induced apoptosis via the
intrinsic pathway in renal carcinoma cells
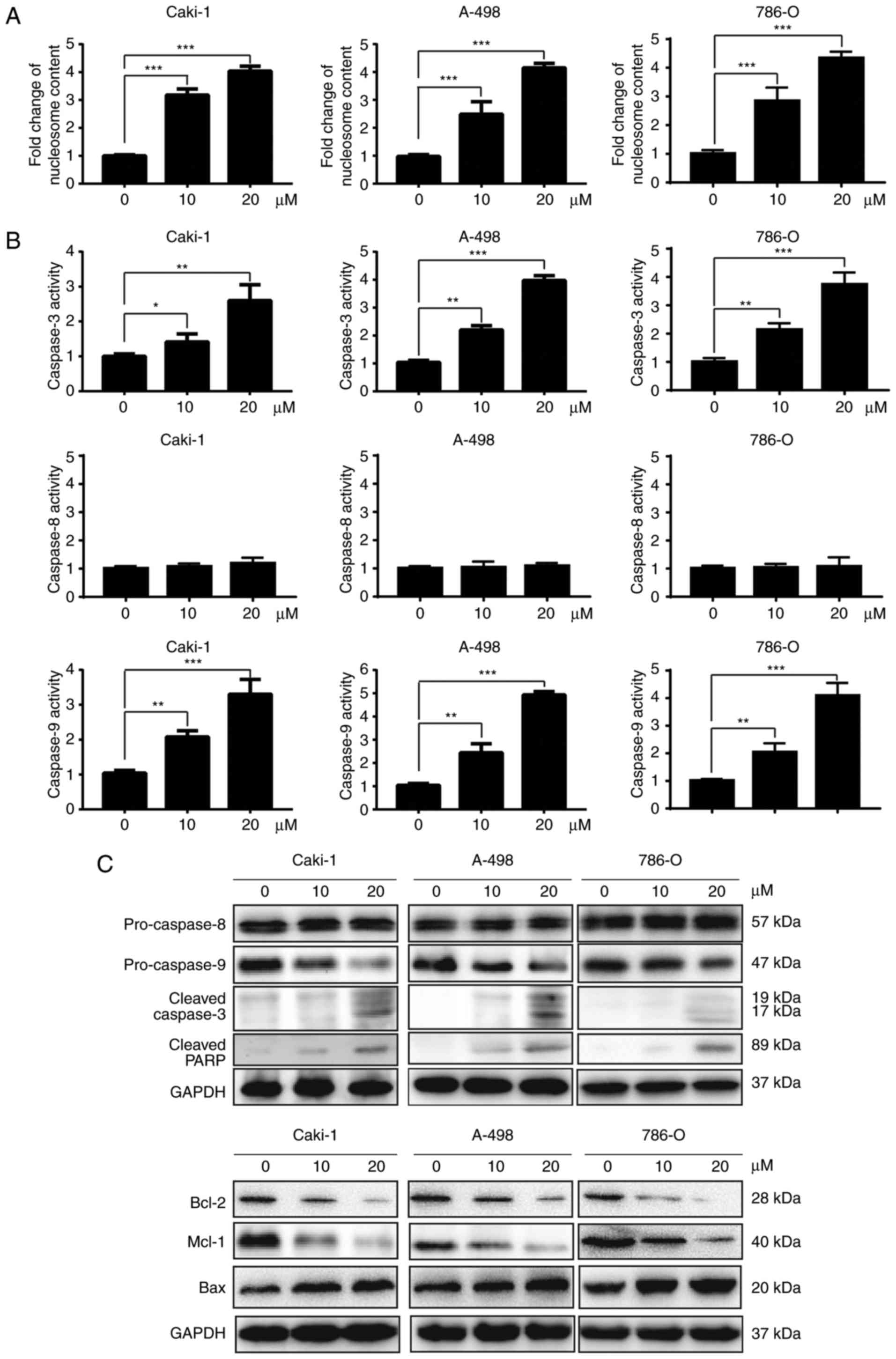
To measure apoptosis induced by dauricine,
nucleosome ELISA assays were used. As shown in Fig. 3A, dauricine treatment led to an
increase in apoptosis in a dose-dependent manner. The activation of
caspases plays a very important role in the process of apoptosis.
There are two pathways that lead to apoptosis, namely, the
extrinsic pathway and the intrinsic pathway. The extrinsic and
intrinsic pathways are initiated by caspase-8 and caspase-9,
respectively. Activation of both caspase-8 and caspase-9 can lead
to the activation of caspase-3, which finally leads to apoptosis.
Therefore, we investigated whether dauricine activated any of these
caspases using a colorimetric caspase activity assay and western
blot analyses. As shown in Fig. 3B and
C, activation of caspase-3 and caspase-9 but not caspase-8 was
observed after incubation with dauricine. Besides the cleavage of
caspase-3, we also observed the expression of pro-caspase-9 was
decreased which indicated the activation of caspase-9 (7,8)
(Fig. 3C). In addition, cleavage
of PARP, which is a substrate of caspase-3, was observed (Fig. 3C). The Bcl-2 family proteins are
well-known regulators of the intrinsic apoptotic pathway. We also
found that dauricine was able to downregulate the anti-apoptotic
Bcl-2 and Mcl-1 proteins in a dose-dependent manner (Fig. 3C). Moreover, the pro-apoptotic
protein Bax was increased by dauricine in a dose-dependent manner
(Fig. 3C). These data suggest that
dauricine induced apoptosis mainly through the intrinsic apoptotic
pathway in renal carcinoma cells.
Dauricine suppresses the PI3K/Akt
signaling pathway in renal carcinoma cells
The PI3K/Akt signaling pathway plays a critical role
in cell survival and protecting cancer cells from apoptosis
(9). Therefore, we investigated
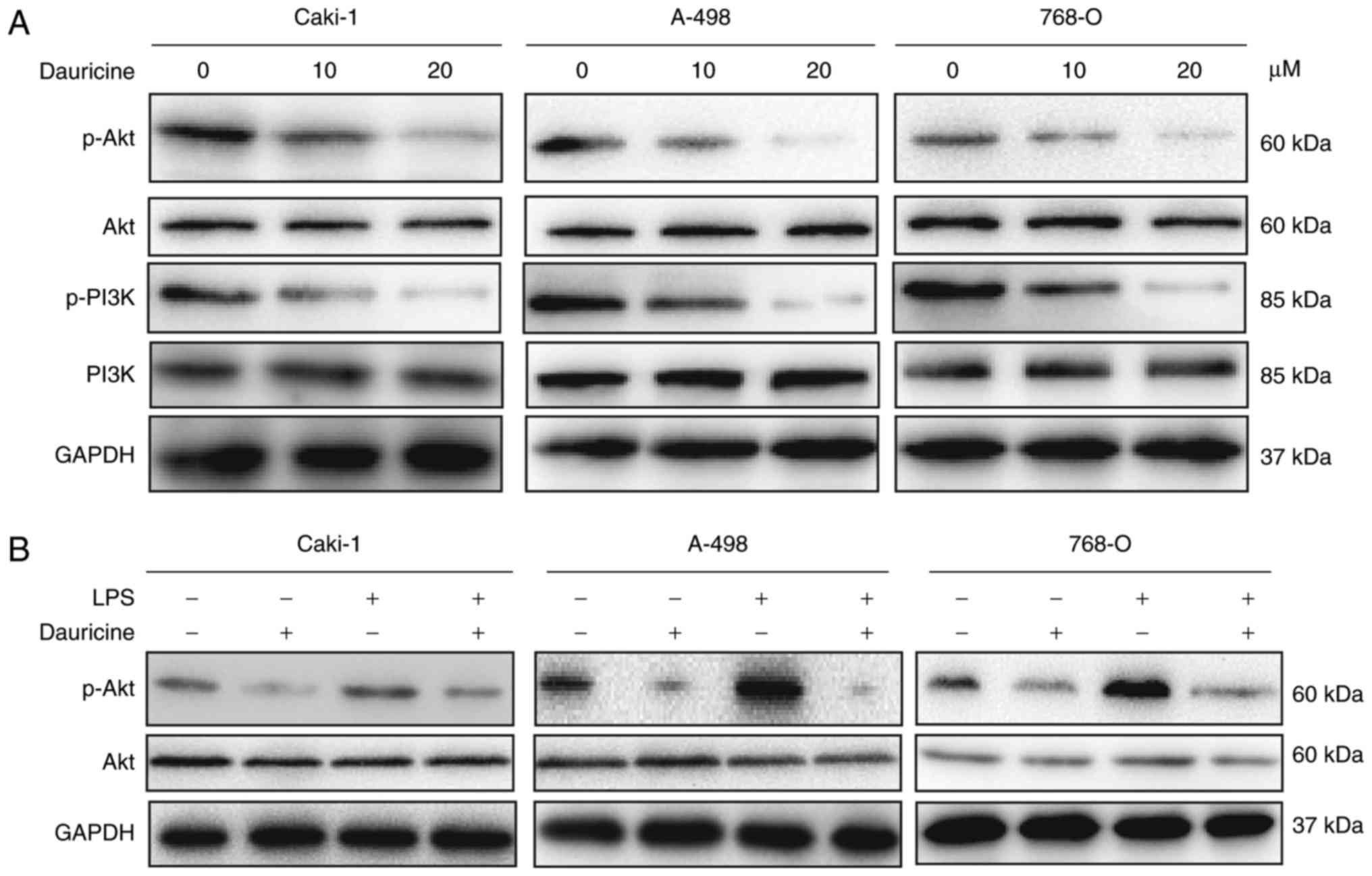
whether dauricine could affect the PI3K/Akt signaling pathway.
Caki-1, A-498 and 786-O cells were treated with various
concentrations of dauricine for 24 h; then, cellular lysates were
subjected to western blot analysis. As shown in Fig. 4A, the activation of PI3K and Akt
was suppressed by dauricine. To further investigate the role of
dauricine in PI3K inhibition, we examined its effect with or
without the endotoxin LPS, which is a potent activator of the
PI3K/Akt signaling pathway. The cells were incubated with 2 µg/ml
LPS or 20 µM dauricine for 24 h. As depicted in Fig. 4B, stimulation with LPS
significantly induced Akt activation, and dauricine could repress
the activation of Akt. These data suggest that dauricine may exert
antitumor effects through the inhibition of PI3K/Akt.
Discussion
Currently, the treatment of RCC includes
chemotherapy, radiotherapy, immunotherapy, targeted therapy and
surgery. Unfortunately, RCC is insensitive to radiotherapy and
chemotherapy. Surgery is mainly used during the early metastasis of
RCC, thereby limiting its application. Immunotherapy and targeted
therapy have not yielded reliable remission in RCC patients and
need to be further developed (10,11).
Therefore, novel therapeutic agents for treating RCC are urgently
needed.
BBIQs are a large and diverse family of natural
alkaloids that can be isolated from many plants. These alkaloids
have received considerable attention due to their diverse
pharmacologic activities, such as anti-inflammatory,
cardiovascular, and antitumor effects (12–14).
Dauricine is a BBIQ alkaloid isolated from the root of Menispermum
dauricum DC (15). Several studies
have found that dauricine possesses potent antitumor activities.
For example, dauricine could inhibit angiogenesis in breast cancer
cells (16). Dauricine could also
overcome doxorubicin resistance in human leukemia cells (12). However, the effects of dauricine in
RCC cells have not yet been studied.
In the present study, we investigated the effects of
dauricine on RCC cells and the molecular events underlying the
effects. Our results showed that treatment with dauricine
significantly reduced the viability of four RCC cell lines (786–0,
Caki-1, A-498, and ACHN) in a dose-dependent manner. Moreover,
dauricine was found to cause cell cycle arrest at the G0/G1 phase.
The cell cycle is a series of events leading to cell division and
replication and is strictly regulated by cyclins and CDKs. It has
been documented that cyclin D1 and CDKs are critical for cells to
cross the G1/S threshold (17).
Our data demonstrated that cyclin D1, CDK2 and CDK4 were
downregulated and that p21 was upregulated after treatment with
dauricine. These findings were similar to a previous study in which
dauricine induced cell cycle arrest at the G0/G1 phase in colon
cancer cells (18).
Apoptosis, also known as programmed cell death, is
considered as one of the most potent natural defenses against
cancer, and the ability to escape from apoptosis is a hallmark of
cancer (19). Apoptosis is
initiated by caspases, a family of cysteine aspartyl-specific
proteases. There are two pathways that lead to apoptosis, namely,
the extrinsic and intrinsic pathways, which are initiated by
caspase-8 and caspase-9, respectively (20). According to our findings, the
activation of caspase-3 and caspase-9 was detected, but that of
caspase-8 was not affected in the cells treated with dauricine.
Dauricine treatment also led to increased levels of cleaved PARP.
Furthermore, dauricine was found to decrease the expression of
Bcl-2 and Mcl-1 dose-dependently. These data suggest that dauricine
induces apoptosis via the intrinsic pathway in RCC cells.
Deregulation of the PI3K/Akt signaling pathway is
implicated in the initiation and development of various human
malignancies, such as NSCLC, bladder cancer, RCC, breast cancer and
colon cancer (21,22). Many studies have indicated that the
pro-apoptotic effects of some antitumor agents are tightly
associated with the repression of the PI3K/Akt signaling pathway
(9,21). In addition, aberrant activation of
the PI3K/Akt pathway is also related to insensitivity to
chemotherapeutic agents (23).
These findings suggest that targeting the PI3K/Akt signaling
pathway may serve as a promising strategy for the treatment of
cancers. In the present study, we investigated the possible role of
the PI3K/Akt pathway in dauricine-induced apoptosis in RCC cells
and found that phosphorylated PI3K and Akt were markedly decreased
after dauricine treatment, but the total levels of PI3K and Akt
were not changed. In addition, dauricine also inhibited the
activation of PI3K/Akt induced by LPS. These findings suggest that
dauricine exerts its antitumor effect via repressing the PI3K/Akt
signaling pathway in RCC cells.
In conclusion, we investigated the antitumor
potential of dauricine in RCC cells. We found that dauricine
reduced viability in RCC cells, induced cell cycle arrest at the
G0/G1 phase and induced apoptosis via the intrinsic pathway.
Dauricine-induced cell cycle arrest was associated with the
downregulation of cyclin D1, CDK2, and CDK4 and the upregulation of
p21. In addition, dauricine-induced apoptosis was associated with
the activation of caspase-9 and caspase-3, and the expression of
anti-apoptotic Bcl-2 proteins was downregulated. Furthermore,
dauricine also inhibited the activation of the PI3K/Akt signaling
pathway. These findings suggest that dauricine may be a promising
chemotherapeutic agent for treating RCC. Nevertheless, further
study is needed to completely elucidate the mechanisms of the
antitumor effects of dauricine.
References
|
1
|
Ridge CA, Pua BB and Madoff DC:
Epidemiology and staging of renal cell carcinoma. Semin Intervent
Radiol. 31:3–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow WH, Devesa SS, Warren JL and Fraumeni
JF Jr: Rising incidence of renal cell cancer in the United States.
JAMA. 281:1628–1631. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Escudier B, Porta C, Schmidinger M,
Rioux-Leclercq N, Bex A, Khoo V, Gruenvald V and Horwich A: ESMO
Guidelines Committee: Renal cell carcinoma: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
Suppl 5:v58–v68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins I and Workman P: New approaches to
molecular cancer therapeutics. Nat Chem Biol. 2:689–700. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang XY, Jiang SQ, Zhang L, Liu QN and
Gong PL: Inhibitory effect of dauricine on inflammatory process
following focal cerebral ischemia/reperfusion in rats. Am J Chin
Med. 35:477–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Law BY, Chan WK, Xu SW, Wang JR, Bai LP,
Liu L and Wong VK: Natural small-molecule enhancers of autophagy
induce autophagic cell death in apoptosis-defective cells. Sci Rep.
4:55102014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c
and dATP-dependent formation of Apaf-1/caspase-9 complex initiates
an apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Y, Benedict MA, Ding L and Núñez G:
Role of cytochrome c and dATP/ATP hydrolysis in
Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J.
18:3586–3595. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu R, Yu BX, Chen JF, Lv XY, Yan ZJ, Cheng
Y and Ma Q: Anti-tumor effects of atractylenolide I on bladder
cancer cells. J Exp Clin Cancer Res. 35:402016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanesvaran R and Tan MH: Targeted therapy
for renal cell carcinoma: The next lap. J Carcinog. 13:32014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Derosa L, Albiges L, Massard C, Loriot Y,
Fizazi K and Escudier B: Safety of available treatment options for
renal cell carcinoma. Expert Opin Drug Saf. 15:1097–1106. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He QY, Meng FH and Zhang HQ: Reduction of
doxorubicin resistance by tetrandrine and dauricine in
harringtonine-resistant human leukemia (HL60) cells. Zhongguo Yao
Li Xue Bao. 17:179–181. 1996.(In Chinese). PubMed/NCBI
|
|
13
|
Marshall SJ, Russell PF, Wright CW,
Anderson MM, Phillipson JD, Kirby GC, Warhurst DC and Schiff PL Jr:
In vitro antiplasmodial, antiamoebic, and cytotoxic activities of a
series of bisbenzylisoquinoline alkaloids. Antimicrob Agents
Chemother. 38:96–103. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian JQ: Cardiovascular pharmacological
effects of bisbenzylisoquinoline alkaloid derivatives. Acta
Pharmacol Sin. 23:1086–1092. 2002.PubMed/NCBI
|
|
15
|
Li YH and Gong PL: Neuroprotective effects
of dauricine against apoptosis induced by transient focal cerebral
ischaemia in rats via a mitochondrial pathway. Clin Exp Pharmacol
Physiol. 34:177–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang XD, Zhou X and Zhou KY: Dauricine
inhibits insulin-like growth factor-I-induced hypoxia inducible
factor 1alpha protein accumulation and vascular endothelial growth
factor expression in human breast cancer cells. Acta Pharmacol Sin.
30:605–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salazar-Roa M and Malumbres M: Fueling the
cell division cycle. Trends Cell Biol. 27:69–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Z, Li C, Wang X, Zhai C, Yi Z, Wang
L, Liu B, Du B, Wu H, Guo X, et al: Dauricine induces apoptosis,
inhibits proliferation and invasion through inhibiting NF-kappaB
signaling pathway in colon cancer cells. J Cell Physiol.
225:266–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brenner C, Galluzzi L, Kepp O and Kroemer
G: Decoding cell death signals in liver inflammation. J Hepatol.
59:583–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Wu J, Ling MT, Zhao L and Zhao
KN: The role of the PI3K/Akt/mTOR signalling pathway in human
cancers induced by infection with human papillomaviruses. Mol
Cancer. 14:872015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Porta C and Figlin RA:
Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney
cancer, and the therapeutic potential of
phosphatidylinositol-3-kinase/Akt inhibitors. J Urol.
182:2569–2577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Faes S and Dormond O: PI3K and AKT:
Unfaithful partners in cancer. Int J Mol Sci. 16:21138–21152. 2015.
View Article : Google Scholar : PubMed/NCBI
|