Introduction
Vanadium pentoxide (V2O5) is
the most common form of vanadium, a grey metal existing in
different states of oxidation (−1, 0, +2, +3, +4, and +5). The
majority of vanadium compounds are considered to be toxic.
The Occupational Safety and Health Administration
(OSHA) fixed a workplace threshold for V2O5
in the air of 0.05 mg/m3 for the dust and 0.1
mg/m3 for fumes (when considering an 8 h workday/40 h
work week) (1). Exposure to a 35
mg/m3 dose of vanadium it is considered dangerous and
may provoke serious and perpetual health issues that can lead up to
death [as established by The National Institute for Occupational
Safety and Health (NIOSH)] (1).
The toxic effects of vanadium mainly impact the respiratory system;
effects on the gastrointestinal system are less relevant due to the
minimal gut absorption rate of the substance (2–4).
However, due to insufficient data, it is not possible to determine
the reference range of a subchronic or chronic inhaled dose at
present.
Previous studies have evaluated the effects of oral
or inhaled vanadium exposures on serum parameters (5,6), on
liver (7), and nervous system
(8), and other tissues (9), in rat models.
It has been recently suggested that volcanic
pollution exerts a carcinogenic effect. Indeed, the incidence of
thyroid cancer registered in the Mount Etna volcanic area was found
to be higher with respect to a control area (18.5 and 9.6/105
inhabitants, respectively). Many trace elements are increased in
volcanic areas compared with control areas, in both drinking water
and lichens, indicating both water and atmospheric pollution; among
all the increased elements, vanadium was 8 times higher, suggesting
that it is a major determinant in the carcinogenic effect of
volcanic pollution on the thyroid (10,11).
To date, however, no in vivo or in vitro studies have
evaluated thyroid/endocrine disruption in humans and/or animals
following vanadium exposure.
Recently, it has been investigated whether the
secretion of chemokine (C-X-C motif) ligand (CXCL)8 and CXCL10 by
normal human thyrocytes is dependent upon specific proinflammatory
stimuli. The secretion of CXCL8 but not of CXCL10 has been detected
under basal conditions from thyrocytes. Furthermore, the two
chemokines show differences in their response to proinflammatory
cytokines. Indeed, significant secretion of CXCL10 was induced by
interferon (IFN)-gamma and not by tumor necrosis factor
(TNF)-alpha, whereas CXCL8 was secreted in response to TNF-alpha,
while being inhibited by IFN-gamma; meanwhile, a combination of
TNF-alpha plus IFN-gamma synergistically increased the
IFN-gamma-induced CXCL10 secretion, while reducing the
TNF-alpha-induced CXCL8 secretion (12).
Moreover, it has been recently shown that CXCL10
mRNA and protein levels were increased by
V2O5 (13),
and V2O5 exposure is a cause of occupational
bronchitis, as a study evaluated gene expression profiles in human
lung fibroblasts (in cultures) after V2O5
exposure, showing that among the 10 genes overexpressed by
V2O5, also CXCL8, CXCL9 and
CXCL10 were induced (14).
For these reasons, we evaluate the effect of
V2O5 on the secretion of CXCL8 and CXCL11
chemokines from normal thyrocytes.
Materials and methods
Thyroid follicular cells
We obtained samples of thyroid tissue from 10
euthyroid patients (5 females and 5 males, mean age, 41 years; age
range, 24–61), of whom 8 had undergone prior parathyroidectomy, and
2 had undergone laryngeal intervention. Written informed consent
for the study was obtained from all patients, and the study was
approved by the local Ethical Committee of the University of Pisa
(Pisa, Italy).
Thyrocytes were prepared from the tissue samples, as
reported previously (15–17). In brief, the tissue samples were
digested with collagenase (Roche, Mannheim, Germany; 1 mg/ml) with
RPMI 1640 medium (Whittaker Bioproducts, Inc., Walkersville, MD,
USA) for 1 h at temperature of 37°C. Subsequently, semi-digested
follicles were removed and sedimented for 2 min; they were then
washed and cultured with RPMI 1640 containing 10% fetal bovine
serum (FBS) (Seromed, Berlin, Germany), 50 mg/ml
penicillin/streptomycin and 2 mM glutamine with 5% CO2
at 37°C. The cells were all used at the 4th passage.
Cell viability and proliferation
assay
We assessed cell viability and proliferation with a
WST-1 assay (Roche Diagnostics, Almere, The Netherlands), which is
based on the use of
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide of the
MTT assay (17–19). We seeded thyroid follicular cells
(TFC) in each well of a 96-well plate at a concentration of 35,000
cells/ml in a final volume of 100 µl. To evaluate the effects of
V2O5 on TFC viability and proliferation, we
treated the TFC for 24 h with increasing concentrations of
V2O5 (1, 10 and 100 nM). The experiments were
performed in triplicate for each cell preparation. The plated cells
were treated for 24 h with V2O5 or its
vehicle alone.
Proliferation assay: Cell
counting
As the cell viability and proliferation WST-1 assay
may have limitations on evaluating cellular proliferation (20), we also performed cell number
counting to evaluate the proliferation of the TFCs [see above
(18,19,21–23)]. In the cell counting method, cells
were seeded at a density of 13,000 cells per well in 24-well tissue
culture plates in a medium supplemented with 10% v/v FBS, with
increasing concentrations of V2O5 (1, 10 and
100 nM). After 24 h in an atmosphere of 5% CO2 and 95%
air at 37°C, the cells were detached from the plates by incubation
with 500 ml phosphate-buffered saline (PBS) containing 100 mg
trypsin and 1 mmol/l ethylenediaminetetraacetic acid (EDTA). The
cells were then counted using a haemocytometer (18,19,21–23).
Chemokine secretion assay and
ELISA
To analyse CXCL8, CXCL11 and nuclear factor-κB
(NF-κB) secretion, cells were seeded in 96-well plates at a
concentration of 30,000 cells/ml in a final volume of 100 µl/well
in growth medium, which was removed after 24 h. The seeded cells
were washed in PBS, then incubated, for 24 h in phenol red and
serum-free medium with IFN-gamma (R&D Systems, Minneapolis, MN,
USA; 500; 1,000; 5,000 and 10,000 IU/ml), and 10 ng/ml TNF-alpha
(R&D Systems), alone or in combination (16). We performed preliminary experiments
to select the TNF-alpha concentration, in order to achieve maximal
responses (data not shown).
To evaluate how V2O5 may
affect chemokine secretion induced by IFN-gamma, we exposed the
cells for 24 h to increasing concentrations of
V2O5 (1, 10 and 100 nM) in the presence or
absence of IFN-gamma (1,000 IU/ml), and/or TNF-alpha (10
ng/ml).
An ELISA assay was used to evaluate CXCL8, CXCL11
and nuclear factor-κB (NF-κB) concentrations in the supernatants.
The experiments were carried out three times with each different
cell preparation.
CXCL11 and CXCL8 levels were measured in the culture
supernatants with commercially available kits (R&D Systems).
The mean minimum detectable dose was 2.9 pg/ml for CXCL8 and 3.4
pg/ml for CXCL11; the intra- and inter-assay coefficients of
variation were 3.4 and 6.2% for CXCL8, and 4.9 and 8.3% for CXCL11.
NF-κB levels were measured in the culture supernatants by ELISA
(Antibodies online; product no. ABIN 414848). The mean minimum
detectable dose was 0.15 ng/ml (detection range, 0.15–10
ng/ml).
Quality control pools of low, normal, and high
concentration for all parameters were included in each assay.
Statistical analysis
Values are presented as the mean ± standard
deviation for normally distributed variables (in text), or as the
mean ± standard error mean (in figures), or as the medians and
interquartile range. Mean group values were compared using one-way
analysis of variance for normally distributed variables, or the
Mann-Whitney U or Kruskal-Wallis test was performed. Proportions
were compared with the Chi-Square test. For multiple comparisons,
the post-hoc Bonferroni-Dunn test was applied for comparisons on
normally distributed variables.
Results
Cell viability and proliferation
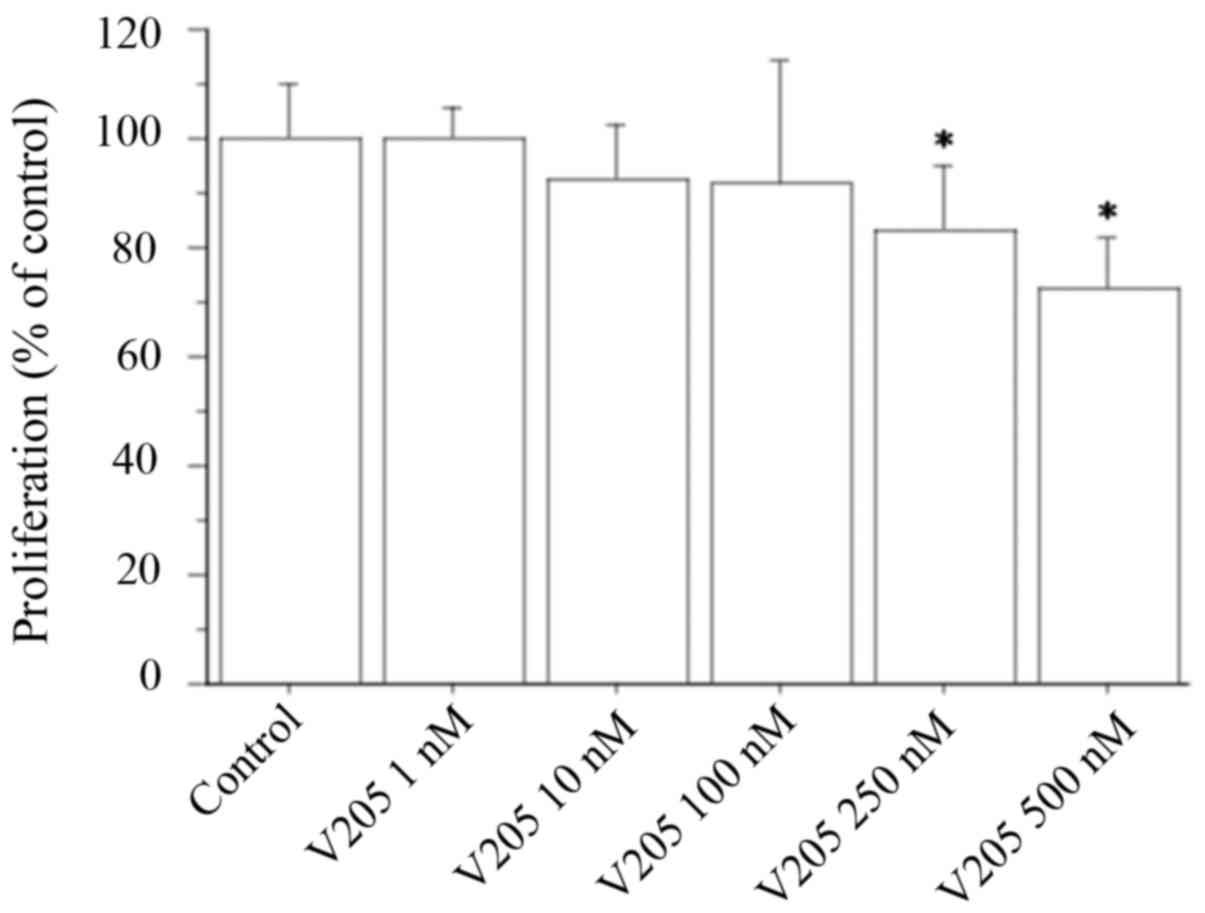
Cell counting showed that V2O5
(1, 10 or 100 nM) did not change the viability or proliferation
rate of TFCs (Fig. 1). These
results were confirmed by the WST-1 assay (data not shown).
Meanwhile, when treating TFC cells with V2O5
at 250 and 500 nM, viability and proliferation was inhibited by 17
and 28%, respectively (Fig.
1).
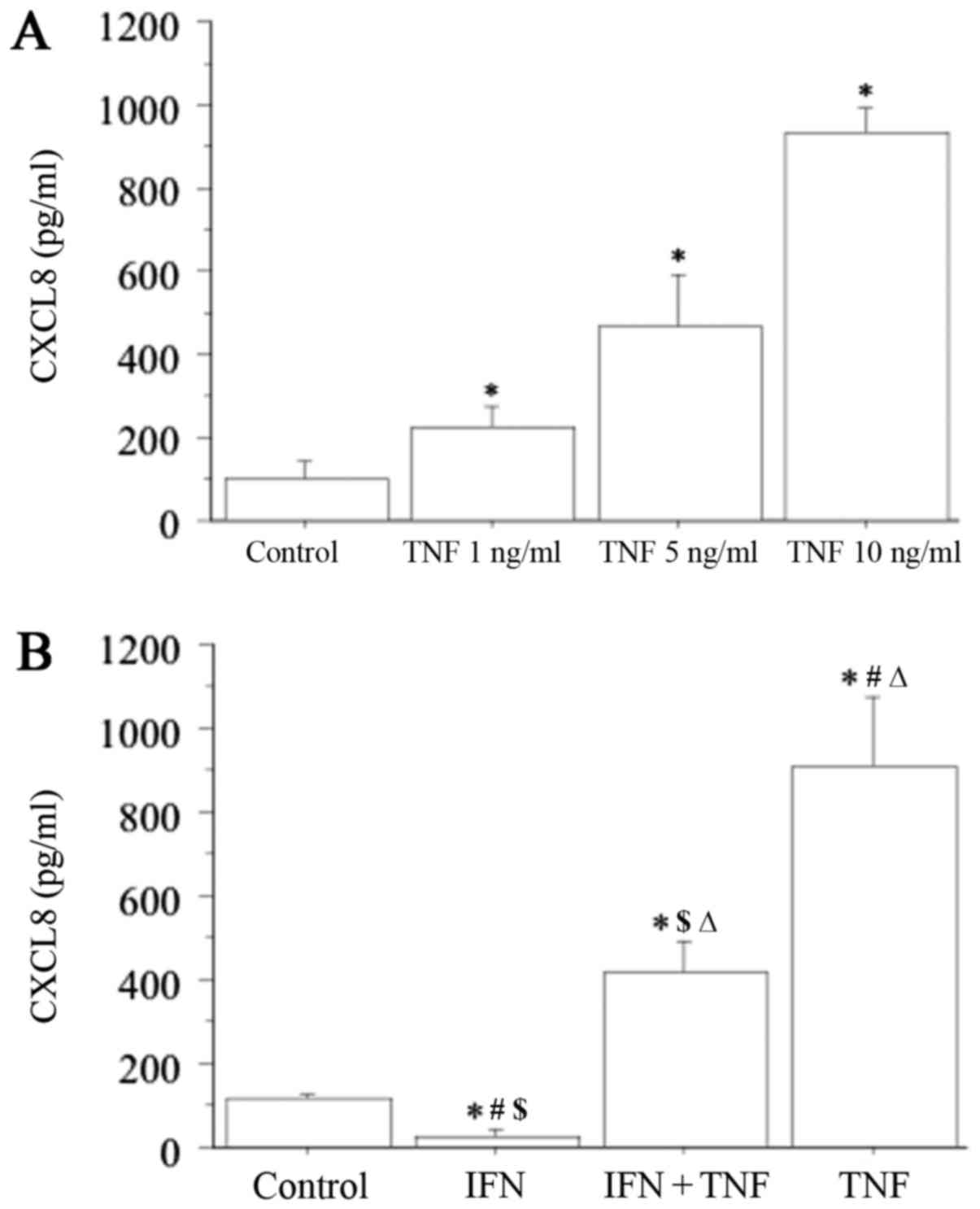
CXCL8 secretion from TFC cells
CXCL8 secretion was measured for all cultured
thyroid cell preparations, and under basal conditions, ranged from
37.8 to 173.5 pg/ml (Fig. 2A).
This secretion increased dose-dependently with increasing
concentrations of TNF-alpha (1, 5 and 10 ng/ml), with the highest
response obtained with 10 ng/ml TNF-alpha (basal 94±32 pg/ml vs.
TNF-alpha 931±257 pg/ml; P<0.01) (Fig. 2A).
By contrast, IFN-gamma (1,000 IU/ml) significantly
inhibited the basal CXCL8 secretion (IFN-gamma 28±16 pg/ml vs.
basal; P<0.05) (Fig. 2B).
Furthermore, the stimulatory effect of TNF-alpha was significantly
reversed after the addition of IFN-gamma (TNF-alpha+IFN-gamma
412±141 pg/ml vs. TNF-alpha 931±257 pg/ml; P<0.05) (Fig. 2B). However, IFN-gamma did not
completely reverse the stimulatory effect of TNF-alpha on the
secretion of CXCL8, as CXCL8 concentration remained significantly
higher with respect to the basal conditions (TNF-alpha+IFN-gamma
vs. basal; P<0.01).
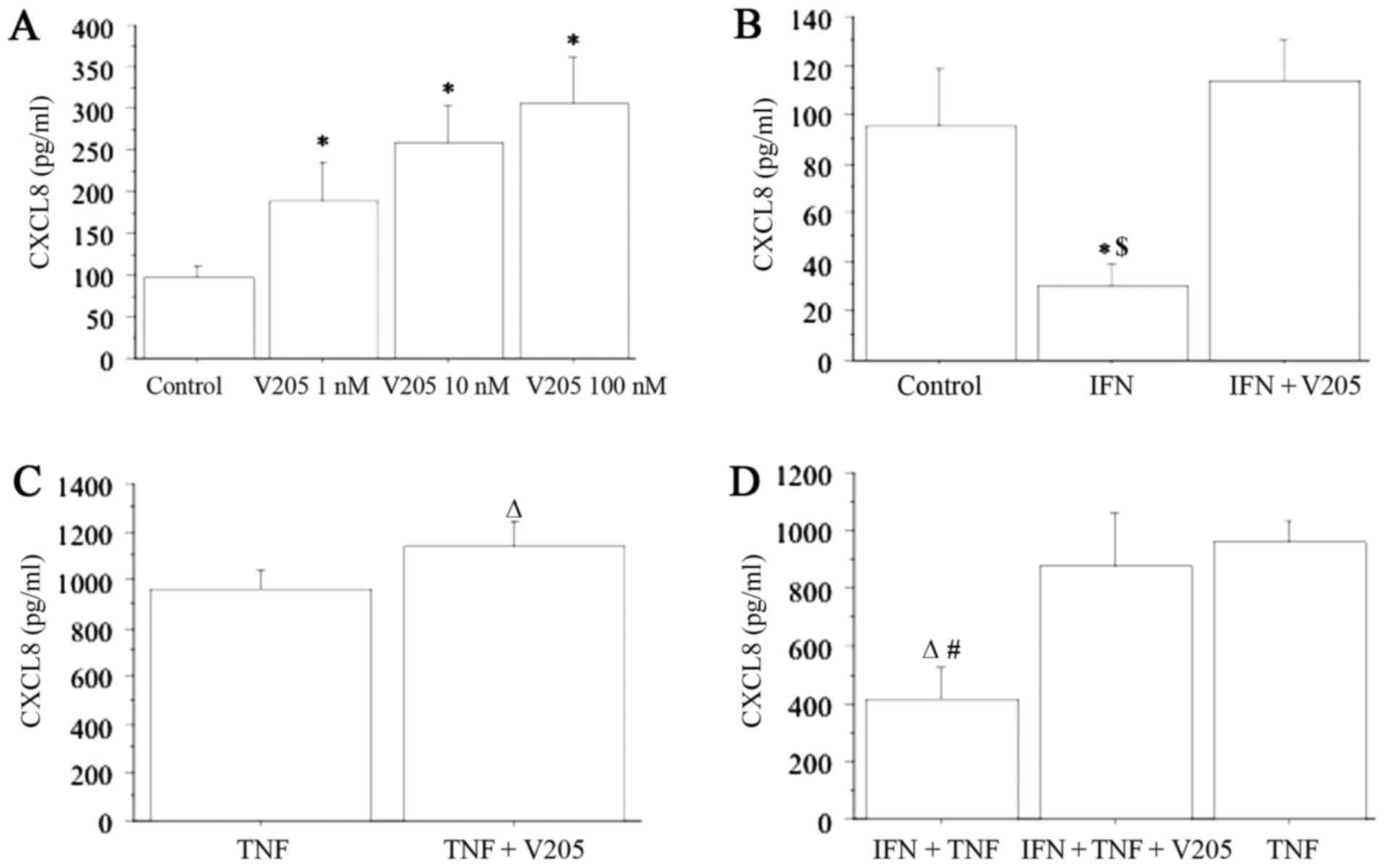
CXCL8 release was also dose-dependently stimulated
from thyrocytes following treatment with V2O5
(1, 10 and 100 nM) (ANOVA, P<0.0001) (Fig. 3A). When treating thyrocytes with
V2O5 (100 nM) together with IFN-gamma, CXCL8
release was not significantly changed with respect to the basal
level, and the inhibitory effect of IFN-gamma was not observed;
however the stimulatory effect of V2O5 was
abolished (Fig. 3B).
CXCL8 release was synergistically increased (ANOVA,
P<0.0001) when cells were treated with
V2O5 (100 nM) together with TNF-alpha,
compared to TNF-alpha alone (Fig.
3C). Additionally, when treating thyrocytes with
V2O5 (100 nM), plus IFN-gamma and
TNF-alpha, CXCL8 release was synergistically increased (ANOVA,
P<0.0001), and the inhibitory effect of IFN-gamma was abolished
(Fig. 3D).
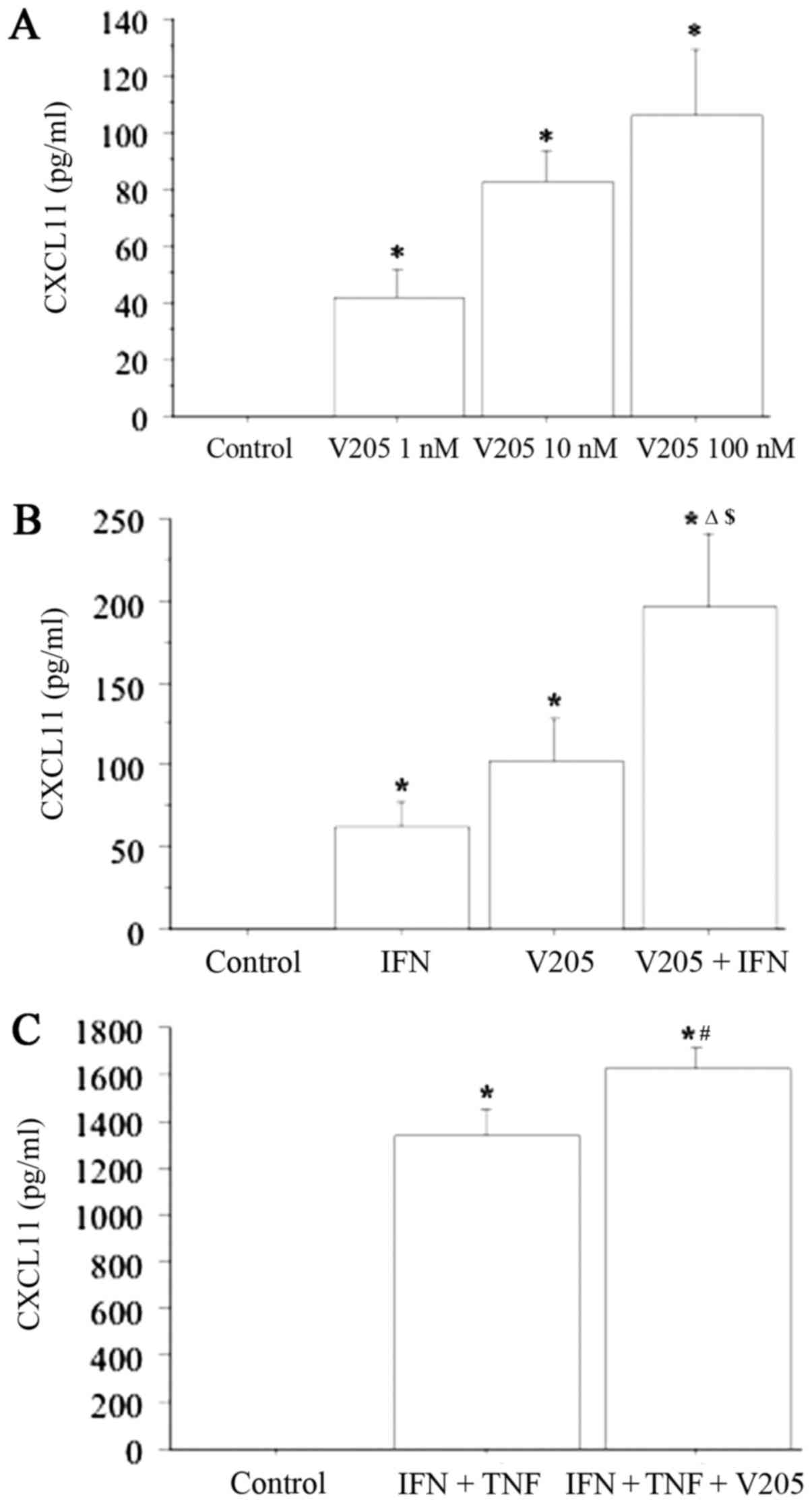
CXCL11 secretion from TFC cells
CXCL11 was not detectable in the supernatants
obtained from primary thyrocyte cultures. IFN-gamma
dose-dependently induced CXCL11 release (CXCL11 level: 0, 26±13,
65±25, 102±32, 145±48 pg/ml; respectively, with IFN-gamma at 0;
500; 1,000; 5,000 and 10,000 IU/ml; ANOVA, P<0.001), whereas
TNF-alpha alone had no effect (CXCL11 remained undetectable). The
combination of IFN-gamma and TNF-alpha had a significant
synergistic effect on CXCL11 secretion (CXCL11 level, 1,354±132 vs.
65±25 pg/ml with IFN-gamma alone, ANOVA, P<0.0001).
CXCL11 release was dose-dependently stimulated from
thyrocytes following treatment with V2O5 (1,
10 and 100 nM) (ANOVA, P<0.0001) (Fig. 4A). This CXCL11 release was not
significantly changed on treatment of the cells with
V2O5 (100 nM) together with TNF-alpha
compared with V2O5 treatment alone (data not
shown). Meanwhile, V2O5 (100 nM) together
with IFN-gamma synergistically increased CXCL11 release (ANOVA,
P<0.0001) (Fig. 4B). CXCL11
release was also synergistically increased (ANOVA, P<0.0001)
when thyrocytes were treated with V2O5 (100
nM) together with IFN-gamma and TNF-alpha (Fig. 4C).
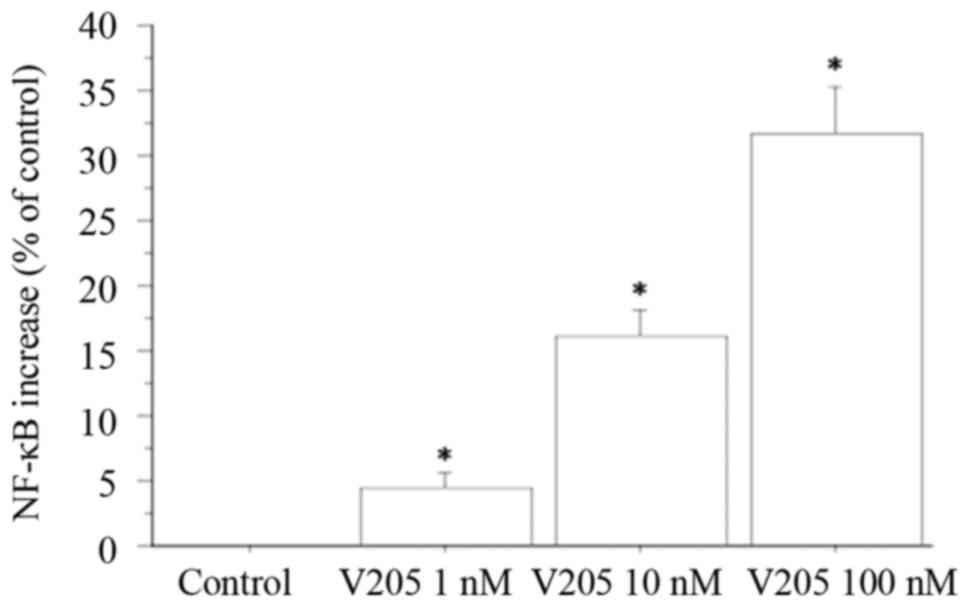
NF-κB secretion from TFC cells
NF-κB levels were measured in the supernatants
obtained from primary thyrocyte cultures. NF-κB release was
dose-dependently stimulated by treating thyrocytes with
V2O5 (1, 10 and 100 nM), with respect to
control treatment (ANOVA, P<0.005). NF-κB was increased by 4%
vs. the control with 1 nM V2O5; by 16% vs.
the control with 10 nM V2O5; and by 31% vs.
the control with 100 nM V2O5 (Fig. 5).
Discussion
Our results show that V2O5 can
promote the secretion of CXCL8 and IFN-gamma dependent CXCL11
secretion from TFCs, without altering the viability and
proliferation of the cells. Moreover, our study confirmed that
IFN-gamma and TNF-alpha stimulated CXCL11 secretion as expected
(17). Interestingly, CXCL11
secretion was increased by V2O5 combined with
IFN-gamma and TNF-alpha. Furthermore, a synergistic influence on
CXCL8 secretion was elicited by V2O5 combined
with TNF-alpha, which abolished the inhibitory effect of IFN-gamma.
Our results, on the whole, suggest that V2O5
can induce and perpetuate an inflammatory disorder in the thyroid
by inducing the secretion of inflammatory chemokines (18).
CXCL8, also called neutrophil chemotactic
factor, has two primary functions: it induces chemotaxis in
target cells, especially neutrophils but also other granulocytes,
causing them to migrate toward the site of infection; it is a
potent promoter of angiogenesis and it is often associated with
inflammation.
Whether the secretion of CXCL8 and CXCL10 by normal
human thyrocytes depends on specific proinflammatory stimuli has
been recently investigated. CXCL8 but not CXCL10 was detected in
basal conditions. The two chemokines showed differences in their
response to proinflammatory cytokines, as a significant secretion
of CXCL10 was induced by IFN-gamma and not TNF-alpha, while CXCL8
was secreted in response to TNF-alpha and inhibited by IFN-gamma.
The combination of TNF-alpha and IFN-gamma synergistically
increased the IFN-gamma-induced CXCL10 secretion, and reversed the
TNF-alpha-induced CXCL8 secretion (12).
It has recently been suggested that CXCL8 sustains a
first step toward a differentiation between autoimmune and
tumor-related inflammation in the thyroid (12). Therefore, it is feasible that the
induction of CXCL8 secretion from thyrocytes by
V2O5 could sustain a tumor-related
inflammatory response in the thyroid.
CXCL11 binds to the same chemokine receptor CXCR3 as
CXCL10 but with higher affinity and potency; its expression is
strongly increased in response to IFN-gamma and is involved in the
recruitment of T-helper 1 (Th1) lymphocytes to sites of
inflammation.
Furthermore, several types of normal mammalian cells
(such as thyrocytes, fibroblasts, colon epithelial cells, islet
cells and others) (15–19,21,24,25)
can produce IFN-gamma-inducible C-X-C chemokines. However, these
cells are not able to produce the C-X-C chemokines under basal
conditions, but only after stimulation by cytokines such as
IFN-gamma and TNF-alpha, which are generally secreted in a Th1 type
inflammatory sites, such as the thyroid at the beginning of Graves'
disease, by Th1-activated lymphocytes. This process has been
suggested to be involved in the initiation and the perpetuation of
the inflammation in several autoimmune diseases (15–19,21,24–27),
and on the basis of our results may be applicable to the
thyroid.
Moreover, our results showed a dose-dependent
increase in NF-κB release following the treatment of thyrocytes
with V2O5 with respect to control treatment.
NF-κB is a transcription factor found in almost all animal cell
types, that is involved in cellular responses to stimuli (such as
bacterial or viral antigens, stress, cytokines, heavy metals, free
radicals, ultraviolet irradiation, and others) and has a
determinant role in regulating the immune response. Its increase in
our study suggests that vanadium may act on the NF-κB pathway to
induce a final effect on chemokines, which is in agreement with
other studies reporting an association between CXCL8 or CXCL11
secretion and the NF-κB pathway (28–31).
The results of our study agree with those of other
research on different cell types. V2O5
exposure is a cause of occupational bronchitis, and a previous
study evaluated gene expression profiles in human lung fibroblasts
(in cultures) after in vitro exposure to
V2O5, aiming to identify genes that could
have a role in bronchial inflammation, repair, and fibrosis in the
pathogenesis of bronchitis. About twelve genes were overexpressed
in response to V2O5 among which were CXCL8
and the IFN-gamma-dependent chemokines CXCL9 and CXCL10 (14).
Interestingly, vanadium was able to increase
chemokine secretion over a dose range from 1 to 100 nM. It has been
reported that normal blood levels of vanadium range from 0.45 to
18.4 nM, and that 100 nM is a dose that may mimic an abnormally
high exposure (32).
Many studies conducted on mice have investigated the
mechanism through which V2O5 induces lung
cancer. In vivo and in vitro research suggests that
lung cancers are induced by secondary mechanisms, rather than
direct genotoxic pathways (33).
Therefore, it may also be hypothesized that the induction of an
inflammatory reaction in the thyroid may predispose for the
development of thyroid cancer, as recently seen for autoimmune
thyroiditis.
In conclusion, our study shows that
V2O5 is able to induce the secretion of the
chemokines CXCL8 and CXCL11 in the thyroid. Interestingly,
V2O5 synergistically increased the effect of
IFN-gamma on CXCL11 secretion. Moreover, V2O5
synergistically increased the effect of TNF-alpha on CXCL8
secretion, abolishing the inhibitory effect of IFN-gamma. On the
whole, these findings indicate that induction of CXCL8 and CXCL11
secretion may lead to the induction and perpetuation of an
inflammatory reaction in the thyroid. Further studies are now
necessary to investigate the mechanism of action at the basis of
this induction, and to evaluate thyroid function and nodule
development in occupationally and environmentally exposed subjects,
in order to clarify the relevance of these results in
vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PF, RF, AC, AA and SMF made substantial
contributions to the conception and design of the study, and to the
acquisition of data. GE, FR, AP, GF and SB analysed the data. PF,
AA and SMF drafted the manuscript, and AA revised it critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Written informed consent for the present study was
obtained from all patients, and the study was approved by the local
Ethical Committee of the University of Pisa (Pisa, Italy).
Consent for publication
Written informed consent was obtained from all
participating patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Occupational safety and health
administration: Occupational safety and health guidelines for
vanadium pentoxide. https://www.osha.gov/SLTC/healthguidelines/vanadiumpentoxidedust/recognition.htmlJanuary
29–2009
|
|
2
|
Sax NI: Dangerous properties of industrial
materials. 6th. Van Nostrand Reinhold Company; New York: pp.
2717–2720. 1984
|
|
3
|
Ress NB, Chou BJ, Renne RA, Dill JA,
Miller RA, Roycroft JH, Hailey JR, Haseman JK and Bucher JR:
Carcinogenicity of inhaled vanadium pentoxide in F344/N rats and
B6C3F1 mice. Toxicol Sci. 74:287–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wörle-Knirsch JM, Kern K, Schleh C,
Adelhelm C, Feldmann C and Krug HF: Nanoparticulate vanadium oxide
potentiated vanadium toxicity in human lung cells. Environ Sci
Technol. 41:331–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scibior A, Zaporowska H and Ostrowski J:
Selected haematological and biochemical parameters of blood in rats
after subchronic administration of vanadium and/or magnesium in
drinking water. Arch Environ Contam Toxicol. 51:287–295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
González-Villalva A, Fortoul TI,
Avila-Costa MR, Piñón-Zarate G, Rodriguez-Laraa V, Martínez-Levy G,
Rojas-Lemus M, Bizarro-Nevarez P, Díaz-Bech P, Mussali-Galante P
and Colin-Barenque L: Thrombocytosis induced in mice after subacute
and subchronic V2O5 inhalation. Toxicol Ind Health. 22:113–116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi K, Himeno S, Satoh M, Kuroda J,
Shibata N, Seko Y and Hasegawa T: Pentavalent vanadium induces
hepatic metallothionein through interleukin-6-dependent and
-independent mechanisms. Toxicology. 228:162–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soazo M and Garcia GB: Vanadium exposure
through lactation produces behavioral alterations and CNS myelin
deficit in neonatal rats. Neurotoxicol Teratol. 29:503–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barceloux DG: Vanadium. J Toxicol Clin
Toxicol. 37:265–278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malandrino P, Russo M, Ronchi A, Minoia C,
Cataldo D, Regalbuto C, Giordano C, Attard M, Squatrito S,
Trimarchi F and Vigneri R: Increased thyroid cancer incidence in a
basaltic volcanic area is associated with non-anthropogenic
pollution and biocontamination. Endocrine. 53:471–479. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrari SM, Fallahi P, Antonelli A and
Benvenga S: Environmental issues in thyroid diseases. Front
Endocrinol (Lausanne). 8:502017.PubMed/NCBI
|
|
12
|
Rotondi M, Coperchini F, Pignatti P,
Sideri R, Groppelli G, Leporati P, La Manna L, Magri F, Mariotti S
and Chiovato L: Interferon-γ and tumor necrosis factor-α sustain
secretion of specific CXC chemokines in human thyrocytes: A first
step toward a differentiation between autoimmune and tumor-related
inflammation? J Clin Endocrinol Metab. 98:308–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antao-Menezes A, Turpin EA, Bost PC,
Ryman-Rasmussen JP and Bonner JC: STAT-1 signaling in human lung
fibroblasts is induced by vanadium pentoxide through an IFN-beta
autocrine loop. J Immunol. 180:4200–4207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ingram JL, Antao-Menezes A, Turpin EA,
Wallace DG, Mangum JB, Pluta LJ, Thomas RS and Bonner JC: Genomic
analysis of human lung fibroblasts exposed to vanadium pentoxide to
identify candidate genes for occupational bronchitis. Respir Res.
8:342007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garcià-Lòpez MA, Sancho D, Sànchez-Madrid
F and Marazuela M: Thyrocytes from autoimmune thyroid disorders
produce the chemokines IP-10 and Mig and attract CXCR3+
lymphocytes. J Clin Endocrinol Metab. 86:5008–5016. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Antonelli A, Ferri C, Fallahi P, Ferrari
SM, Frascerra S, Sebastiani M, Franzoni F, Galetta F and Ferrannini
E: High values of CXCL10 serum levels in patients with hepatitis C
associated mixed cryoglobulinemia in presence or absence of
autoimmune thyroiditis. Cytokine. 42:137–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Antonelli A, Ferrari SM, Fallahi P,
Frascerra S, Santini E, Franceschini SS and Ferrannini E: Monokine
induced by interferon gamma (IFNgamma) (CXCL9) and IFNgamma
inducible T-cell alpha-chemoattractant (CXCL11) involvement in
Graves' disease and ophthalmopathy: Modulation by peroxisome
proliferator-activated receptor-gamma agonists. J Clin Endocrinol
Metab. 94:1803–1809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonelli A, Rotondi M, Fallahi P,
Romagnani P, Ferrari SM, Buonamano A, Ferrannini E and Serio M:
High levels of circulating CXC chemokine ligand 10 are associated
with chronic autoimmune thyroiditis and hypothyroidism. J Clin
Endocrinol Metab. 89:5496–5499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antonelli A, Ferrari SM, Frascerra S,
Pupilli C, Mancusi C, Metelli MR, Orlando C, Ferrannini E and
Fallahi P: CXCL9 and CXCL11 chemokines modulation by peroxisome
proliferator-activated receptor-alpha agonists secretion in Graves'
and normal thyrocytes. J Clin Endocrinol Metab. 95:E413–E420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quent VM, Loessner D, Friis T, Reichert JC
and Hutmacher DW: Discrepancies between metabolic activity and DNA
content as tool to assess cell proliferation in cancer research. J
Cell Mol Med. 14:1003–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kemp EH, Metcalfe RA, Smith KA, Woodroofe
MN, Watson PF and Weetman AP: Detection and localization of
chemokine gene expression in autoimmune thyroid disease. Clin
Endocrinol (Oxf). 59:207–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonelli A, Bocci G, La Motta C, Ferrari
SM, Fallahi P, Fioravanti A, Sartini S, Minuto M, Piaggi S, Corti
A, et al: Novel pyrazolopyrimidine derivatives as tyrosine kinase
inhibitors with antitumoral activity in vitro and in vivo in
papillary dedifferentiated thyroid cancer. J Clin Endocrinol Metab.
96:E288–E296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Barani L, Marchetti I, Ferrannini E and Miccoli P:
Primary cell cultures from anaplastic thyroid cancer obtained by
fine-needle aspiration used for chemosensitivity tests. Clin
Endocrinol (Oxf). 69:148–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonelli A, Ferrari SM, Corrado A,
Ferrannini E and Fallahi P: CXCR3, CXCL10 and type 1 diabetes.
Cytokine Growth Factor Rev. 25:57–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antonelli A, Ferrari SM, Giuggioli D,
Ferrannini E, Ferri C and Fallahi P: Chemokine (C-X-C motif) ligand
(CXCL)10 in autoimmune diseases. Autoimmun Rev. 13:272–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fallahi P, Ferrari SM, Ruffilli I, Elia G,
Biricotti M, Vita R, Benvenga S and Antonelli A: The association of
other autoimmune diseases in patients with autoimmune thyroiditis:
Review of the literature and report of a large series of patients.
Autoimmun Rev. 15:1125–1128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antonelli A, Fallahi P, Delle Sedie A,
Ferrari SM, Maccheroni M, Bombardieri S, Riente L and Ferrannini E:
High values of Th1 (CXCL10) and Th2 (CCL2) chemokines in patients
with psoriatic arthtritis. Clin Exp Rheumatol. 27:22–27.
2009.PubMed/NCBI
|
|
28
|
Jayaprakash K, Demirel I, Gunaltay S,
Khalaf H and Bengtsson T: PKC, ERK/p38 MAP kinases and NF-κB
targeted signalling play a role in the expression and release of
IL-1β and CXCL8 in Porphyromonas gingivalis-infected THP1 cells.
APMIS. 125:623–633. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen T, Yang Z, Cheng X, Xiao Y, Yu K, Cai
X, Xia C and Li Y: CXCL8 induces epithelial-mesenchymal transition
in colon cancer cells via the PI3K/Akt/NF-κB signaling pathway.
Oncol Rep. 37:2095–2100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crescioli C, Sottili M, Bonini P, Cosmi L,
Chiarugi P, Romagnani P, Vannelli GB, Colletti M, Isidori AM, Serio
M, et al: Inflammatory response in human skeletal muscle cells:
CXCL10 as a potential therapeutic target. Eur J Cell Biol.
91:139–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antonelli A, Ferrari SM, Fallahi P,
Frascerra S, Piaggi S, Gelmini S, Lupi C, Minuto M, Berti P,
Benvenga S, et al: Dysregulation of secretion of CXC
alpha-chemokine CXCL10 in papillary thyroid cancer: Modulation by
peroxisome proliferator-activated receptor-gamma agonists. Endocr
Relat Cancer. 16:1299–1311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sabbioni E, Kuèera J, Pietra R and
Vesterberg O: A critical review on normal concentrations of
vanadium in human blood, serum, and urine. Sci Total Environ.
188:49–58. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Assem FL and Levy LS: A review of current
toxicological concerns on vanadium pentoxide and other vanadium
compounds: Gaps in knowledge and directions for future research. J
Toxicol Environ Health B Crit Rev. 12:289–306. 2009. View Article : Google Scholar : PubMed/NCBI
|