Introduction
Neovascular glaucoma is a serious ophthalmic disease
that affects visual sense and leads to open-angle glaucoma and
angle closure glaucoma (1).
Neovascular glaucoma presents a potentially blinding secondary
glaucoma caused by the formation of abnormal new blood vessels on
iris, which can prevent normal drainage of aqueous liquid from the
anterior segment of the eye (2,3).
There are >40 different diseases that can lead to the cause of
neovascular glaucoma, which are almost extensive involvement in
posterior section of oxygen or localized front section of oxygen
(4). Studies have suggested that
central retinal vein occlusion, diabetic retinopathy and other
diseases are the most common inducer of neovascular glaucoma
(5,6). Mechanisms of the initiation and
progression of neovascular glaucoma have been widely investigated
and evidence indicates that imbalance of new vessels in iris area
contributes to the initiation of neovascular glaucoma (7). Therefore, many theories have been
proposed for explaining the neovascularization in the progression
of neovascular glaucoma.
Currently, various therapeutic schedules for
neovascular glaucoma have been put forward including physical and
drug therapies (8). Physical
treatment includes panrentinal photocoagulation, retina frozen and
iris-cornea-angle photocoagulation (9). Drug treatments include hormonal drugs
and antibody drugs (10,11). In previous years, a large numbers
of traditional Chinese medicine have been investigated for the
treatment of ophthalmic diseases and presented many benefits and
encouraging comprehensive effects on hypertension in eyes (12). This study has stimulated scientists
to investigate the mechanisms of traditional Chinese
medicine-mediated treatments for human diseases. Treatments of
neovascular glaucoma by using traditional Chinese medicine have
been widely accepted and achieved clinical outcomes (13). In the current study, the authors
investigated the efficacy of puerarin for the treatment of
neovascular glaucoma in a mice model.
Puerarin, a natural flavonoid, is a major
isoflavonoid compound extracted from Pueraria and exhibits
potential therapeutic effects for many human diseases (14,15).
Puerarin has been reported to have many benefits and medicinal
properties (16). In addition,
puerarin can attenuate amyloid-β-induced cognitive impairment
through suppression of apoptosis in the rat hippocampus in
vivo (17). A previous study
reported that puerarin can prevent the rat liver against oxidative
stress-mediated DNA damage and apoptosis induced by lead (18).
Furthermore, the synthesis of puerarin derivatives
and their protective effect on the myocardial ischemia and
reperfusion injury also have been examined in animal experiments
(19). The present study design
investigated the therapeutic effects of puerarin for the treatment
of neovascular glaucoma in a mouse model and results indicated that
puerarin significantly improved symptoms of neovascular glaucoma in
a 30-day treatment period.
In the present study, the purpose was to research
the efficacy of puerarin for neovascular glaucoma and potential
mechanisms of puerarin-mediated signaling pathway in the process of
neovascularization in the progression of neovascular glaucoma. In
addition, the authors analyzed inflammatory responses and oxidative
stress in mice with neovascular glaucoma. Notably, the authors also
studied the NF-κB signaling pathway in vascular endothelial cells
located on iris area in mice with neovascular glaucoma. The authors
aimed to explain the mechanism by which puerarin contributes to
prevention of neovascularization induced by hypoxia through
inhibition of the pigment epithelium-derived growth factor-induced
NF-κB signaling pathway.
Materials and methods
Ethical statement
The current study was approved by the ethics
committee of Tianjin Medical University College of Optometry
(Tianjin, China). All surgery and euthanasia were made to minimize
suffering.
Cells culture and regents
Vascular endothelial cells on iris area were
isolated from experimental mice and cultured in minimum essential
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented
with 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Vascular endothelial cells were cultured in a 37°C
humidified atmosphere of 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total mRNA was obtained from vascular endothelial
cells on iris area by using RNAeasy Mini kit (Qiagen, Inc.,
Valencia, CA, USA). Expression levels of nNOS and iNOS in vascular
endothelial cells were determined by qRT-PCR using TaqPath™ 1-Step
RT-qPCR Master Mix, CG (Applied Biosystems™) according to
manufacturer's instructions (20).
All the forward and reverse primers were synthesized by Invitrogen;
Thermo Fisher Scientific, Inc. (nNOS forward,
5′-CGGCTGTGCTTTAATGGAGAT-3′ and reverse,
5′-GAGGAGACGCTGTTGAATCG-3′; iNOS forward, 5′-AAGGATGGGAAGTACTGG-3′
and reverse, 5′-CAGTGTTTTCCTCAGAAAGAG-3′; ROS forward,
5′-GAGCCTTCTGGTGAAAATCAA-3′ and reverse,
5′-ACGATTCAGCTCATCCTCCA-3′; β-actin forward,
5′-AGAGCTACGAGCTGCCTGA-3′ and reverse, 5′-AGCACTGTGTTGGCGTACAG-3′).
The PCR conditions were 95°C for 15 min, and 95°C for 10 sec,
followed by 40 cycles by 54°C for 20 sec and 72°C for 20 sec.
Relative mRNA expression level changes were calculated by
2−ΔΔCq (21). The
results are expressed as the n-fold way compared to control.
Western blotting
Vascular endothelial cells were on iris area
isolated from experimental mice and homogenized in lysate buffer
containing protease-inhibitor (Sigma-Aldrich; Merck KGaA) and were
centrifuged at 8,000 × g at 4°C for 10 min. The supernatant of the
mixture were used for analysis of the protein. 12.5% SDS-PAGE
assays were performed as previously described (22). Proteins were transferred onto a
polyvinylidene fluoride (PVDF) membrane (Merck Millipore). For
western blotting, primary goat anti-mouse antibodies: p65 (1:1,000,
ab32536), IKK-β (1:1,000, ab124957), IκBα (1:1,000, ab109300),
vascular endothelial cell growth factor (VEGF; 1:1,000, ab32152),
TGF-β (1:1,000, ab31013), angiogenin (1:1,000, ab139947),
Neurotrophin 3 (1:1,000, ab53685) VSF (1:1,000, ab9695), IL-1β
(1:1,000, ab200478), IL-17 (1:1,000, ab79056), IFN-γ (1:1,000,
ab32152), SOD (1:1,000, ab137037), malondialdehyde (1:1,000,
ab6463) and β-actin (1:1,000, ab8227) (all from Abcam, Cambridge,
UK) were added following blocking (5% skimmed milk) for 60 min at
37°C. Following washing with PBS three times, the secondary rabbit
anti-goat antibodies goat anti-rabbit IgG mAb (1:5,000, PV-6001,
ZSGB-BIO; OriGene Technologies, Beijing, China) were used to detect
purpose protein for 2 h at 37°C. The results were visualized by
using enhanced chemiluminescence substrate ECL Select™ Ventana
Benchmark automated staining system (Roche Diagnostics, Basel,
Switzerland).
Animal study
A total of 20 female C57BL/6 mice (8 weeks, 25–30 g
body weight) were purchased from The Jackson Laboratory (Bar
Harbor, ME, USA) and housed with a 12 h light-dark artificial
cycle. All mice were free to access food and water. To detect the
efficacy of puerarin on neovascular glaucoma, mice model of
neovascular glaucoma was established by mutation in collagen 8A2
according to a previous study (23). Mice with neovascular glaucoma were
divided into two groups (n=10/group) and received treatment with
puerarin (0.2 mg/kg) with PBS as negative control.
Immunohistochemical staining
Immunohistochemical staining was performed by an
avidin-biotin-peroxidase technique. Paraffin-embedded tumor tissue
sections were prepared and epitope retrieval was performed for
further analysis. The paraffin sections were subjected with
hydrogen peroxide (3%) for 10–15 min, which subsequently were
blocked by a regular blocking solution (5% skimmed milk) for 10–15
min at 37°C. Finally, the sections were incubated in anti-p65
(1:1,000, ab32536), anti-IKK-β (1:1,000, ab124957), anti-IκBα
(1:1,000, ab109300), anti-VEGF (1:1,000, ab32152), anti-TGF-β
(1:1,000, ab31013) and anti-angiogenin (1:1,000, ab139947) (all
from Abcam), respectively, at 4°C for 12 h following blocking. All
sections were washed three times and incubated with secondary
antibodies for 1 h at 37°C and were observed by six random fields
of view under the microscope.
Activity of NF-κB
Activity of NF-κB in vascular endothelial cells from
healthy mice and mice with neovascular glaucoma were analyzed
following treatment with puerarin. The activity of NF-κB was
conducted according to previous study (24).
Inflammatory response analysis
Inflammatory responses in serum of mice with
neovascular glaucoma were analyzed after treatment with puerarin.
Percentage of numbers of lymphocytes, monocytes and neutrophils
were examined by flow cytometry according to a previous study
(25).
Statistical analysis
All data are presented as mean ± standard error of
the mean, are conducted in triplicate and performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical
differences between experimental groups were analyzed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Puerarin inhibits aberrant growth of
vascular endothelial cells induced by vaso-stimulating factor in
hypoxia
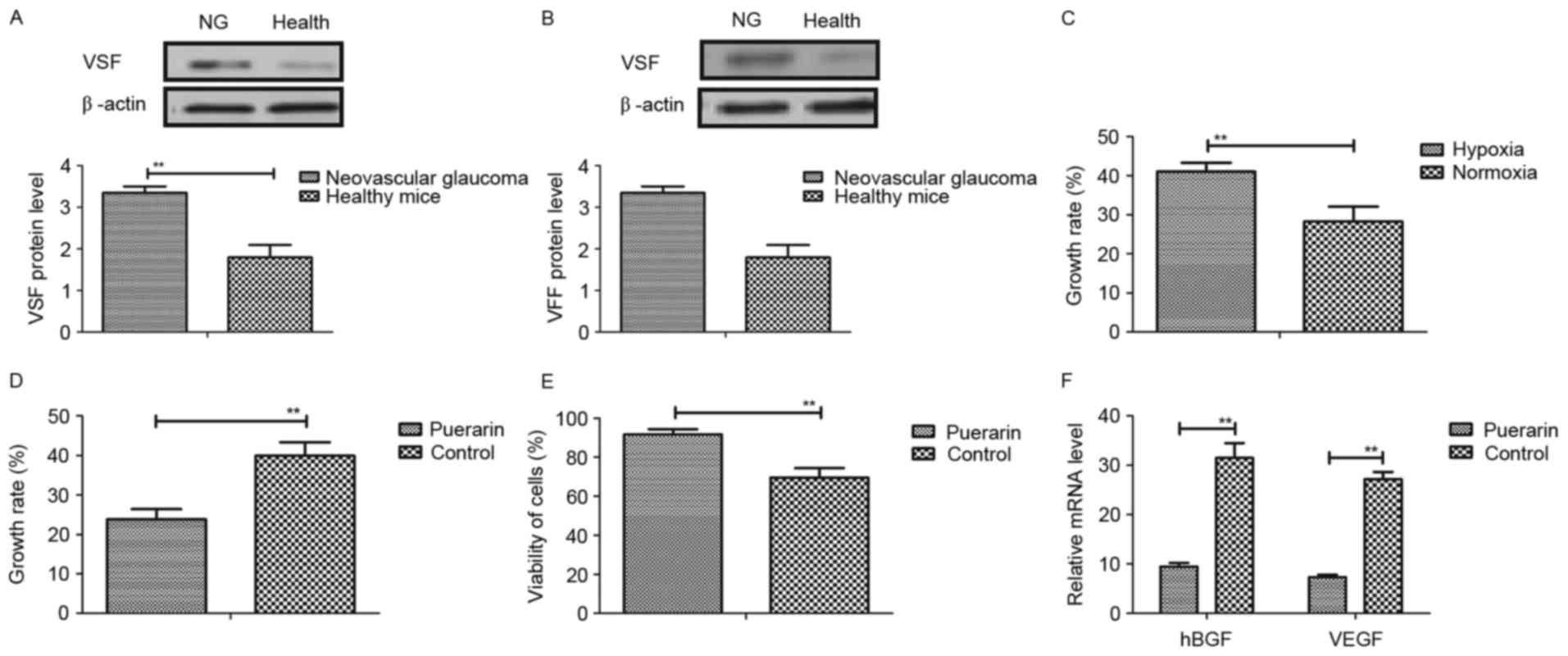
The authors first detected the effects of puerarin
on vascular endothelial cells in vitro. The expression
levels of vaso-stimulating factor (VSF) and vaso-formative factor
(VFF) were upregulated in mice with neovascular glaucoma compared
to healthy mice (Fig. 1A and B).
In addition, as presented in Fig.
1C, VSF could induce vascular endothelial cells growth under
hypoxic conditions, compared to normoxia. However, the authors
found that puerarin significantly inhibited growth of vascular
endothelial cells induced by VSF in hypoxia (Fig. 1D). Furthermore, it was observed
that puerarin improved viability of vascular endothelial cells in
normoxia (Fig. 1E). Moreover,
results suggested that heparin-binding growth factors and VEGF mRNA
expression levels were inhibited in vascular endothelial cells
induced VSF (Fig. 1F). Taken
together, these results suggested that puerarin can inhibit
aberrant growth of vascular endothelial cells induced by VSF in
hypoxia, which may be beneficial for neovascular glaucoma.
Puerarin suppresses expression levels
inflammatory responses and factors in mice with neovascular
glaucoma
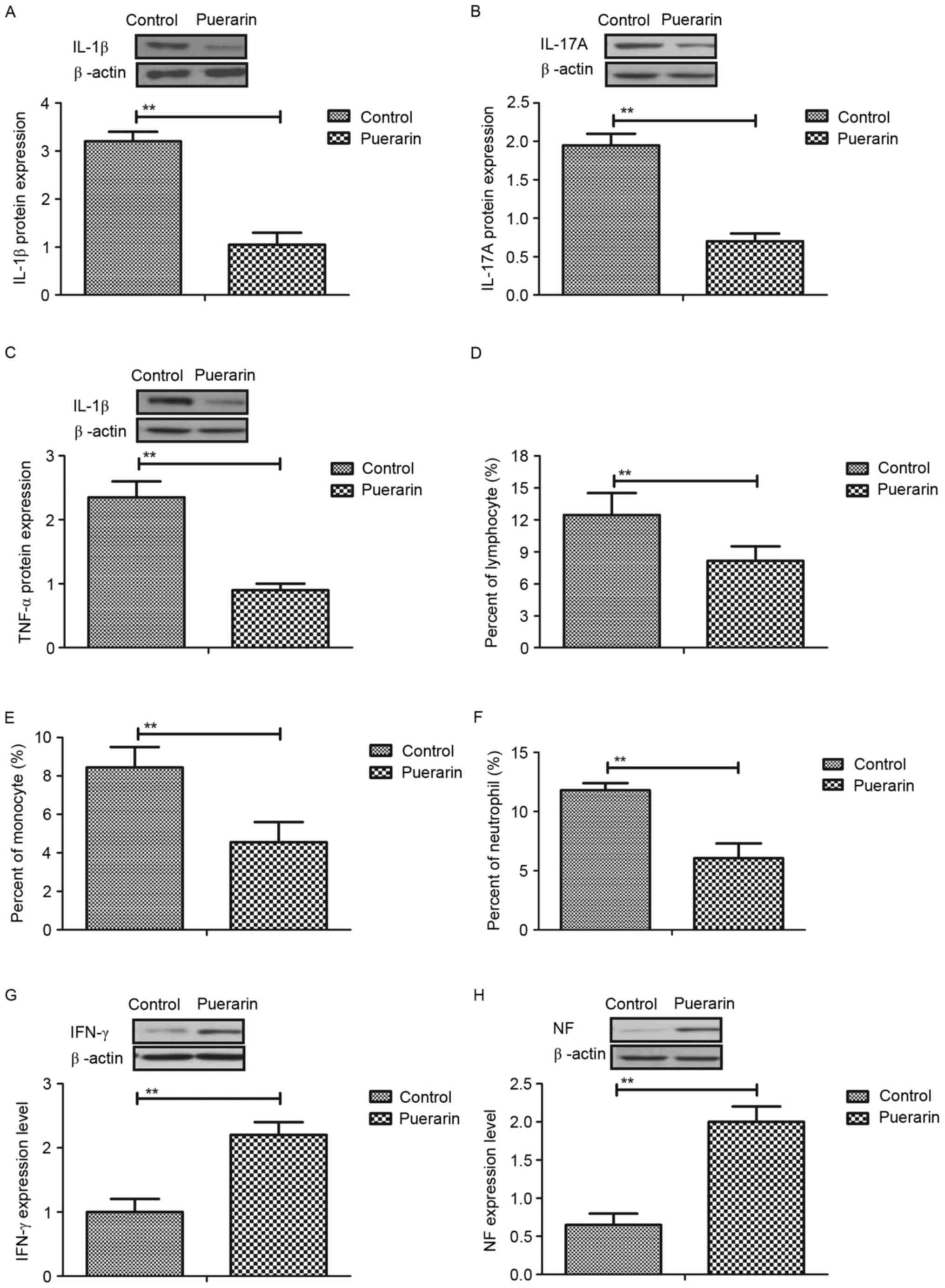
The authors next analyzed the anti-inflammatory
effects of puerarin in mice with neovascular glaucoma in
vivo. Interleukin (IL)-1β, IL-17A and tumor necrosis factor
(TNF)-α expression levels were increased in vascular endothelial
cells induced by neovascular glaucoma, whereas puerarin treatment
may significantly decreased upregulation of IL-1β, IL-17A and TNF-α
in vitro (Fig. 2A-C). In
addition, the authors observed that numbers of lymphocytes,
monocytes and neutrophils were upregulated and puerarin could
become downregulated in serum of mice with neovascular glaucoma
(Fig. 2D-F). Furthermore, it was
observed that interferon-γ and neurotrophic factor expression
levels were increased in vascular endothelial cells in mice with
neovascular glaucoma following treatment with puerarin (Fig. 2G and H). Collectively, the results
suggested that puerarin can suppress expression levels inflammatory
responses and factors in mice with neovascular glaucoma both in
vitro and in vivo.
Puerarin attenuates oxidative stress
in vascular endothelial cells and in mice with neovascular
glaucoma
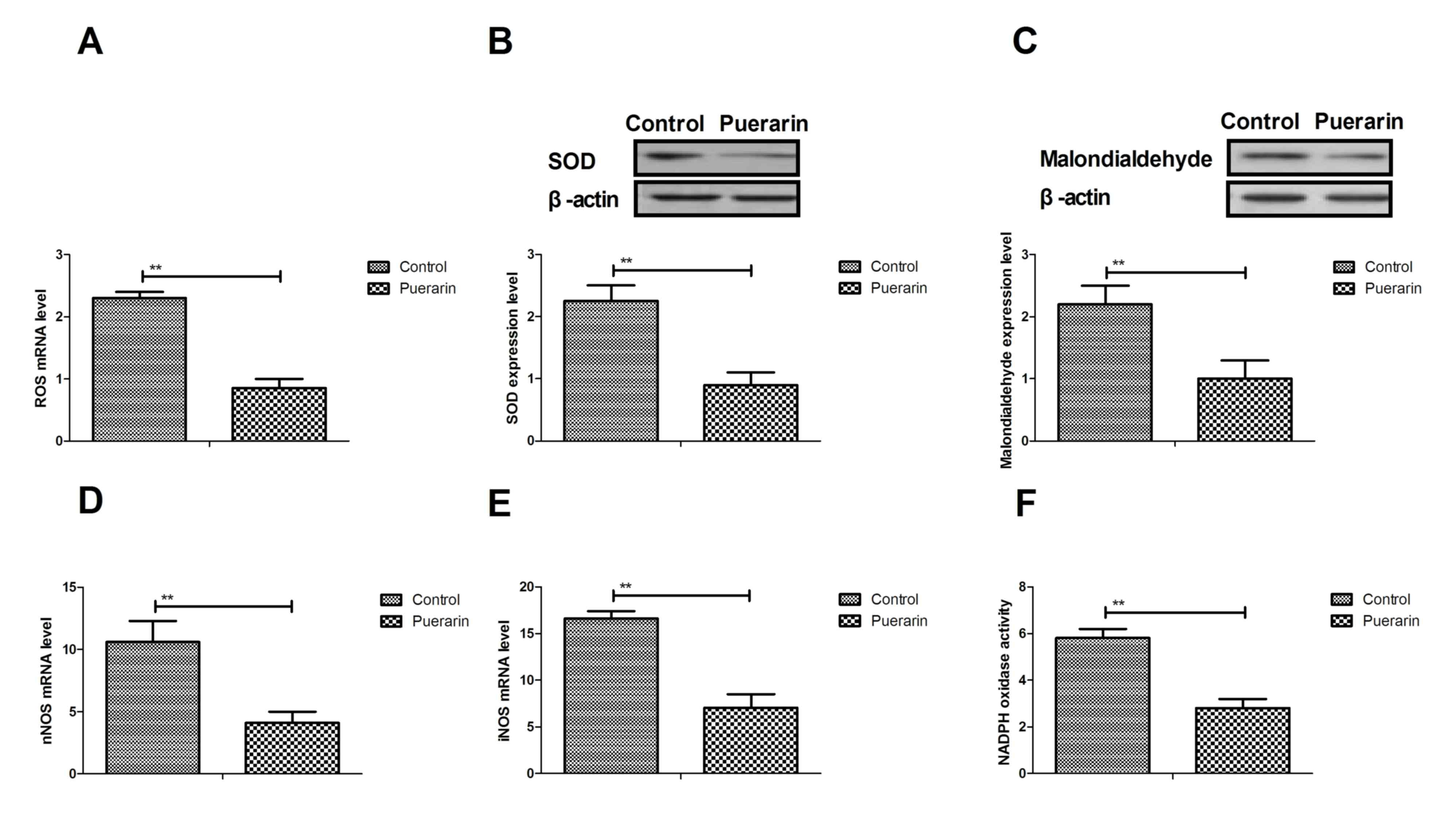
A previous study suggested that neovascular glaucoma
is associated with oxidative stress (26). Therefore, the authors analyzed the
effects of puerarin on changes of oxidative stress in vascular
endothelial cells and in mice with neovascular glaucoma. As
illustrated in Fig. 3A-C, puerarin
significantly decreased oxidative stress levels by reducing
reactive oxygen species (ROS), superoxide dismutase (SOD) and
malondialdehyde levels in vascular endothelial cells in
vitro. In addition, neuronal nitric oxide synthase (NOS) and
inducible NOS mRNA expression levels were markedly decreased by
puerarin in vascular endothelial cells (Fig. 3D and E). Furthermore, NADPH oxidase
activity was improved by puerarin in vascular endothelial cells in
mice with neovascular glaucoma compared to the control (Fig. 3F). Collectively, the results
suggested that puerarin can attenuate oxidative stress in vascular
endothelial cells and in mice with neovascular glaucoma.
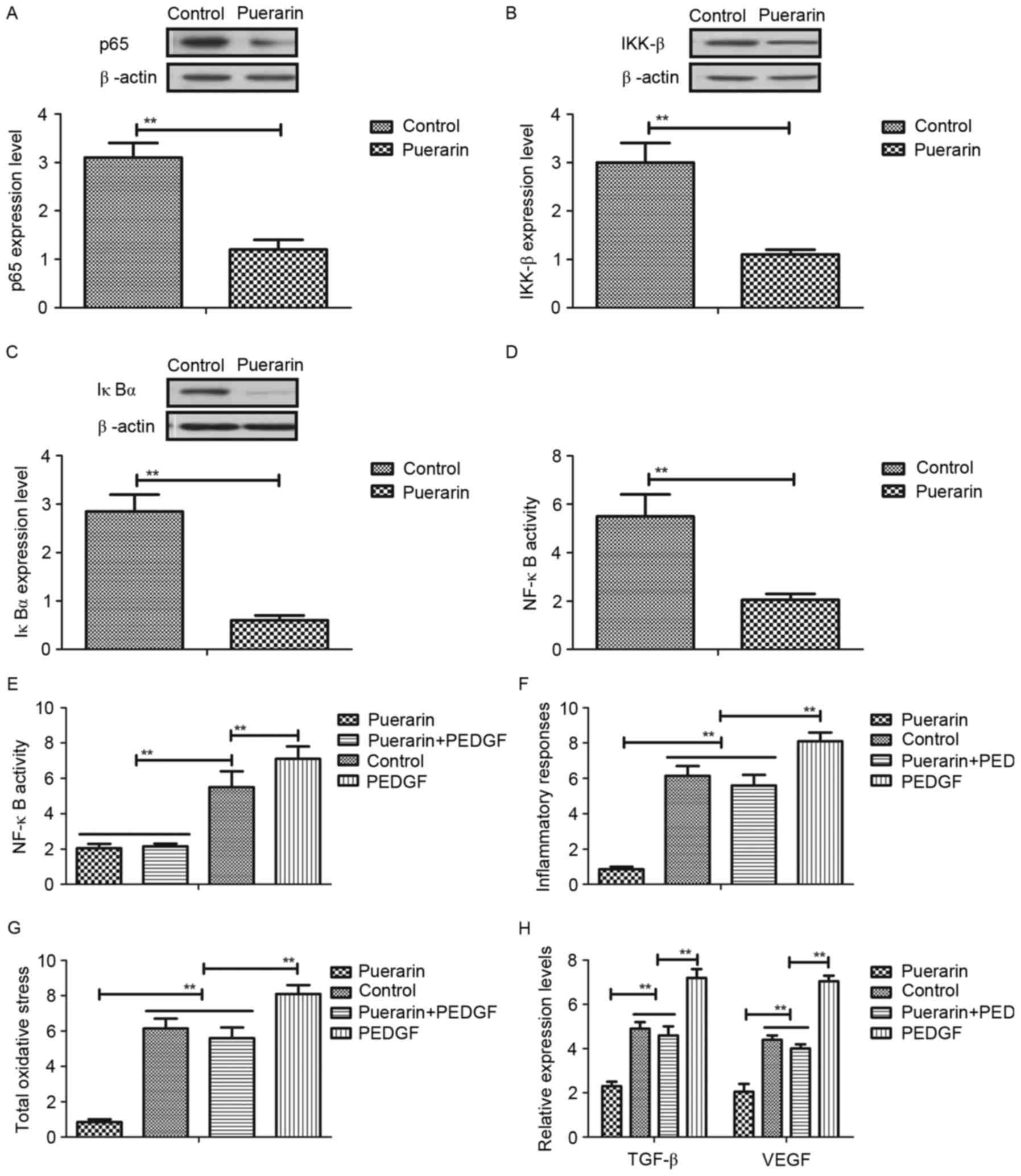
Puerarin regulates aberrant growth of vascular
endothelial cells through platelet derived growth factor
(PDGF)-induced NF-κB signaling pathway. In order to
identify the mechanism of puerarin-mediated neovascular glaucoma,
the authors analyzed the NF-κB signaling pathway and the target
proteins in vascular endothelial cells. As presented in Fig. 4A-C, puerarin markedly inhibited
expression levels of p65, inhibitor of NF-κB kinase subunit β
(IKK-β) and inhibitor of NF-κB kinase subunit α (IκBα) in vascular
endothelial cells. In addition, NF-κB activity was inhibited by
puerarin treatment in vascular endothelial cells (Fig. 4D). Puerarin decreased PDGF
expression, and restoration of PDGF expression abolished
puerarin-inhibited NF-κB activity (Fig. 4E). In addition, PDGF restoration
abolished the beneficial effects of puerarin on inflammatory
responses and oxidative stress (Fig.
4F and G). Furthermore, PDGF restoration also promoted TGF-β
and VEGF expression levels (Fig.
4H). These results suggested that puerarin can regulate
aberrant growth of vascular endothelial cells through the
PDGF-induced NF-κB signaling pathway.
Puerarin shows inhibition of aberrant
neovascularization in mice with neovascular glaucoma
To analyze the therapeutic effects of puerarin for
neovascular glaucoma, a mouse model of neovascular glaucoma was
established by mutation in collagen 8A2 (23). Intraocular pressure of experimental
mice was measured for examine the therapeutic efficacy of puerarin.
As demonstrated in Fig. 5A,
intraocular pressure was significantly improved by puerarin
treatment. It was also observed aberrant vascular proliferation was
inhibited in mice with neovascular glaucoma treated by puerarin
(Fig. 5B). In addition,
fluorescence staining showed that p65, IKK-β and IκBα expression
levels were significantly downregulated by puerarin treatment in
vascular endothelial cells (Fig.
5C). Furthermore, the results indicated that expression levels
of VEGF, TGF-β and angiogenin were downregulated in vascular
endothelial cells in puerarin-treat mice (Fig. 5D). Histological analysis indicated
that neovascularization and retinal degeneration were improved in
mice with neovascular glaucoma following treatment with puerarin
(Fig. 5E and F). Importantly,
visual function and retinal whole-mount analysis suggested that
puerarin could markedly improve visual function and pathological
hemorrhages in mice with neovascular glaucoma (Fig. 5G and H). Taken together, these
results puerarin presented benefits for the inhibition of aberrant
neovascularization and improves pathological symptoms in mice with
neovascular glaucoma.
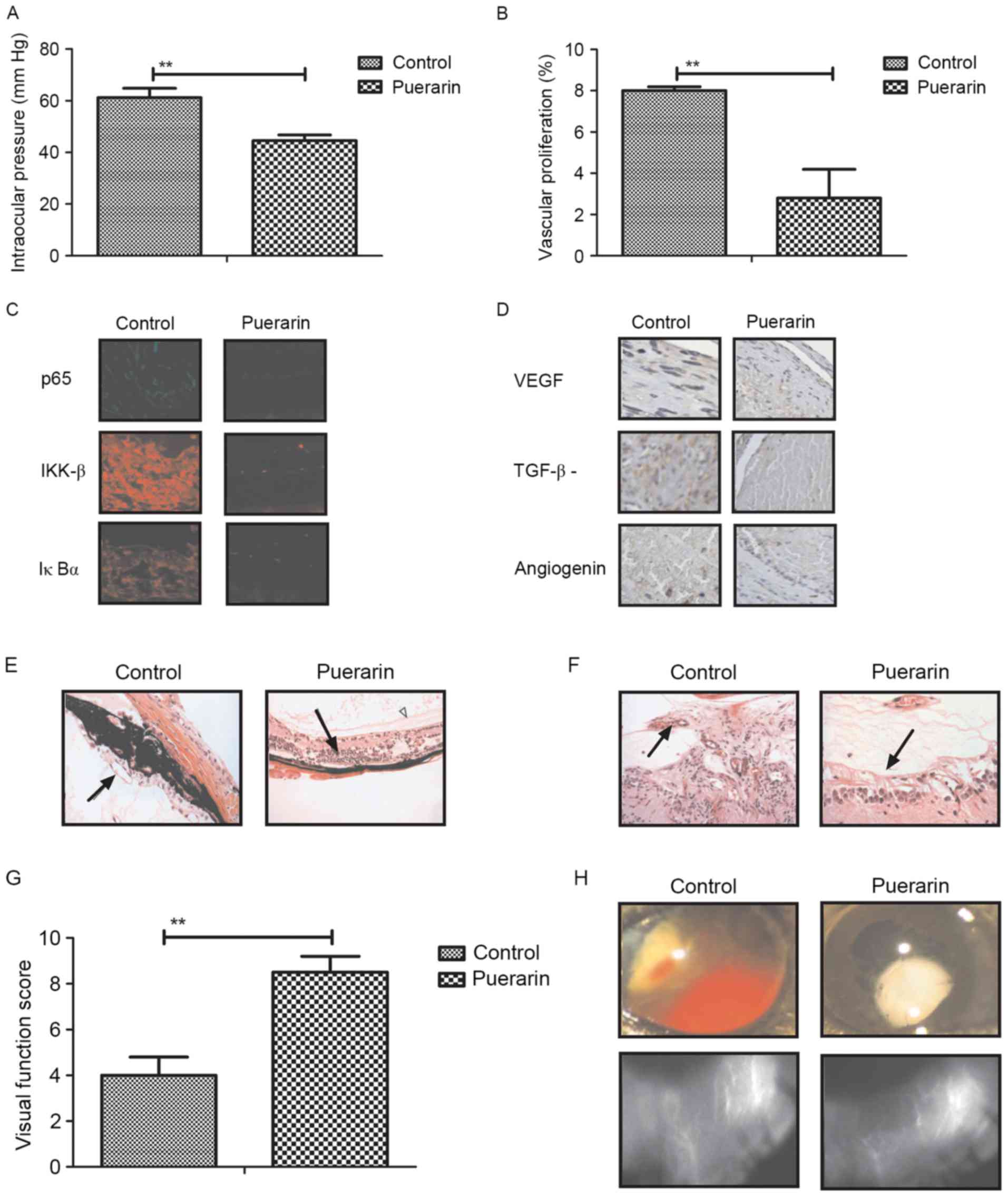 | Figure 5.In vivo effects of puerarin on
aberrant neovascularization in mice with neovascular glaucoma. (A)
Evaluation of intraocular pressure in mice with neovascular
glaucoma. (B) Vascular proliferation in mice with neovascular
glaucoma following treatment with puerarin. (C) Expression levels
of p65, IKK-β and IκBα in vascular endothelial cells determined by
immunostaining. Magnification, ×20. (D) Expression levels of VEGF,
TGF-β and angiogenin in vascular endothelial cells determined by
histological staining. Magnification, ×20. (E) Neovascularization
and (F) retinal degeneration was analyzed in iris area in mice with
neovascular glaucoma. Magnification, ×40. (G and H) Improvement of
visual function and pathological hemorrhages were examined in mice
with neovascular glaucoma. Magnification, ×5. One-way analysis of
variance or two-tailed Student's t-test revealed a significant
effect. Data are expressed as the mean ± standard error of the
mean. **P<0.01 vs. control. IKKβ, inhibitor of NF-κB kinase
subunit β; IκBα, inhibitor of NF-κB kinase subunit α; VEGF,
vascular endothelial growth factor; TGF-β, tumor necrosis
factor-β. |
Discussion
Neovascular glaucoma is a disease that new fiber
vascular membrane is overgrowth of the iris and trabecular, which
leads to fibrous thickening of the filtration membrane in the
operation area and the peripheral anterior synechia of the iris
area (27). Neovascular glaucoma
is also known as hemorrhagic glaucoma due to new blood vessels
rupture and recurrent anterior chamber bleeding, which has been
considered as intractable ophthalmic diseases (28). Therefore, treatments for
neovascular glaucoma has become a nightmare for patients in
clinical. Although many treatments have proposed for patients with
neovascular glaucoma, the efficacy is limited for patients and the
recurrence rate is higher following patients receiving clinical
treatments (29). In the present
study, the authors introduced an efficient drug of puerarin for the
treatment of neovascular glaucoma in an animal model. Not only have
the therapeutic effects of neovascular glaucoma been investigated,
but also elaborated the molecular mechanism of puerarin-mediated
improvement of neovascular glaucoma. Our results suggested that
puerarin can inhibit aberrant growth of vascular endothelial cells,
suppress inflammatory responses and factors and oxidative stress in
vascular endothelial cells and in mice with neovascular glaucoma.
Importantly, the findings indicated that puerarin regulates growth
of vascular endothelial cells, neovascularization and retinal
degeneration through PDGF-induced NF-κB signaling pathway in mice
with neovascular glaucoma.
Aberrant growth of vascular endothelial cells
contributes to the deterioration of vascular proliferation and
visual function for patients with neovascular glaucoma (30). As is known, inhibition of aberrant
growth of vascular endothelial cells is a potential treatment for
patients with neovascular glaucoma (9). In previous years, anti-vascular
endothelial growth factor for neovascular glaucoma therapy has been
prevalent and achieves efficacy for patients with neovascular
glaucoma (31). However, long-term
treatment of anti-vascular endothelial growth factor obviously
reduces pharmacodynamics of the antibody (32). The outcomes suggested that puerarin
can inhibit the aberrant growth of vascular endothelial cells and
avoid the defects of anti-vascular endothelial growth factor
antibody, which presents an ideal agent in anti-glaucoma
therapy.
Inflammatory responses have been identified
associated with the initiation and progression of glaucoma
(33). Chang et al
(34) suggested increasing mast
cell numbers as a possible indicator of preoperative glaucoma
surgery inflammation was observed in the conjunctiva of glaucoma
patients. In addition, Furtado et al (35) have showed that inflammation in
patients under topical glaucoma is an indicator following treatment
with indication to surgery. Vohra et al (36) have indicated that the role of
inflammation in the pathogenesis of glaucoma. Furthermore,
evidences have indicated that other factors such as genetics,
excitotoxicity and blood flow can act as potential causal factors
for progressive of glaucoma (37).
The current results demonstrated that puerarin could inhibit
inflammatory responses in mice with neovascular glaucoma. These
inhibitory effects of IL-1β, IL-17A, TNF-α, lymphocytes, monocytes
and neutrophils on experimental mice are beneficial for the
recovery of neovascular glaucoma. Interestingly, the authors also
observed that oxidative stress is downregulated by puerarin
treatment.
Previous studies have reported that
oxidative-antioxidative balance is associated with progression of
neovascular glaucoma and that the resulting oxidative stress
constitutes a major contribution involved in the pathophysiology of
neovascular glaucoma (38).
Research has indicated that retinal pigment epithelial damage
induces more production of PDGF and breaks the blood-retinal
barrier, and retinal inflammation in dogs with primary glaucoma
(39). Oxidative stress can
regulate the activity of complement in human glaucoma (40). These results simultaneously
suggested that decreasing of oxidative stress in vascular
endothelial cells may be contributed to the inhibition of aberrant
growth of vascular endothelial cells. The results revealed that
puerarin can markedly suppress ROS, SOD and malondialdehyde levels
in vascular endothelial cells in experimental mice. Downregulation
of oxidative stress contributes to the decreasing of intraocular
pressure. Notably, this downregulation is regulated by the NF-κB
signaling pathway in mice with neovascular glaucoma.
The NF-κB signaling pathway involves in various
processes of neovascular glaucoma. In the present study, the
authors investigated the relationships between the NF-κB pathway
and neovascular glaucoma in a mouse model following treatment with
puerarin. A previous study has suggested that the NF-κB pathway can
regulate the activation of NLRP3 and caspase-8 inflammasomes in
acute glaucoma (41). In addition,
NF-κB-mediated inflammatory stress response may be predictor of the
glaucoma marker SELE in trabecular meshwork cells (42). In the present study, puerarin
treatment significantly inhibits NF-κB expression and activity in
vascular endothelial cells in experimental mice. Stimulation of
NF-κB activity canceled the inhibitory effects of puerarin for
inflammatory responses and oxidative stress.
In conclusion, the study investigated the efficacy
and potential mechanism of puerarin-mediated improvement of
neovascular glaucoma in a mice model. Puerarin treatment not only
inhibits aberrant growth of vascular endothelial cells,
inflammatory responses and oxidative stress, but also improves
neovascularization, retinal degeneration and visual function in
mice with neovascular glaucoma. Most importantly, the NF-κB pathway
is involved in puerarin-inhibited inflammatory responses and
oxidative stress is observed in vitro and in vivo.
These outcomes suggested that puerarin is beneficial for the
treatment of neovascular glaucoma through regulation of the
PDGF-induced NF-κB signaling pathway.
References
|
1
|
Ng E, Choong AM and Walker PJ: Neovascular
glaucoma post carotid endarterectomy: A case report and review of
the literature. Ann Vasc Dis. 8:103–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiddee W, Tantisarasart T and
Wangsupadilok B: Neovascular glaucoma: A retrospective review of
5-year experience in Songklanagarind Hospital. J Med Assoc Thai. 95
Suppl 4:S36–S42. 2012.PubMed/NCBI
|
|
3
|
Martinez-Carpio PA, Bonafonte-Márquez E,
Heredia-Garcia CD and Bonafonte-Royo S: Efficacy and safety of
intravitreal injection of bevacizumab in the treatment of
neovascular glaucoma: Systematic review. Arch Soc Esp Oftalmol.
83:579–588. 2008.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takihara Y, Inatani M, Fukushima M, Iwao
K, Iwao M and Tanihara H: Trabeculectomy with mitomycin C for
neovascular glaucoma: Prognostic factors for surgical failure. Am J
Ophthalmol. 147(912–918): e12009.
|
|
5
|
Mishra KK, Daftari IK, Weinberg V, Cole T,
Quivey JM, Castro JR, Phillips TL and Char DH: Risk factors for
neovascular glaucoma after proton beam therapy of uveal melanoma: A
detailed analysis of tumor and dose-volume parameters. Int J Radiat
Oncol Biol Phys. 87:330–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goto A, Inatani M, Inoue T, Awai-Kasaoka
N, Takihara Y, Ito Y, Fukushima M and Tanihara H: Frequency and
risk factors for neovascular glaucoma after vitrectomy in eyes with
proliferative diabetic retinopathy. J Glaucoma. 22:572–576. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calugaru D and Calugaru M: Neovascular
glaucoma-etipathogeny and diagnosis. Oftalmologia. 56:3–14.
2012.(In Romanian). PubMed/NCBI
|
|
8
|
Rachitskaya AV, Lee RK, Dubovy SR and
Schiff ER: Combined central retinal vein and central retinal artery
occlusions and neovascular glaucoma associated with interferon
treatment. Eur J Ophthalmol. 22:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altintas AG, Arifoglu HB, Tutar E, Koklu G
and Ozcan PY: Effect on anterior chamber bevacizumab injection
combined with seton implantation in treatment of rubeosis iridis in
neovascular glaucoma. Cutan Ocul Toxicol. 31:124–127. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caujolle JP, Maschi C, Freton A, Pages G
and Gastaud P: Treatment of neovascular glaucoma after proton
therapy for uveal melanomas with ranibizumab injection: Preliminary
results. Ophthalmic Res. 47:57–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Călugăru D and Călugăru M: Treatment of
neovascular glaucoma. Oftalmologia. 56:20–39. 2012.(In
Romanian).
|
|
12
|
Zhou WY and Li YH: A survey on treatment
of dry eye by traditional chinese medicine and integrative chinese
and Western medicine. Chin J Integr Med. 12:154–159. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mi XS, Zhong JX, Chang RC and So KF:
Research advances on the usage of traditional Chinese medicine for
neuroprotection in glaucoma. J Integr Med. 11:233–240. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang HY, Yi XN, Liu YH, Lao ML and Zhang
XF: The protective effect of puerarin on Abeta(25–35)-induced PC12
cell injury. Zhong Yao Cai. 33:763–767. 2010.(In Chinese).
PubMed/NCBI
|
|
15
|
Zhang W, Liu CQ, Wang PW, Sun SY, Su WJ,
Zhang HJ, Li XJ and Yang SY: Puerarin improves insulin resistance
and modulates adipokine expression in rats fed a high-fat diet. Eur
J Pharmacol. 649:398–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L and Zhao Y: Effect of decoction
method on pharmacokinetics of puerarin in dogs. Zhong Yao Cai.
33:1442–1444. 2010.(In Chinese). PubMed/NCBI
|
|
17
|
Li J, Wang G, Liu J, Zhou L, Dong M, Wang
R, Li X, Li X, Lin C and Niu Y: Puerarin attenuates
amyloid-beta-induced cognitive impairment through suppression of
apoptosis in rat hippocampus in vivo. Eur J Pharmacol. 649:195–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CM, Ma JQ and Sun YZ: Puerarin
protects the rat liver against oxidative stress-mediated DNA damage
and apoptosis induced by lead. Exp Toxicol Pathol. 64:575–582.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng ZQ, Wang YY, Guo ZR, Chu FM and Sun
PY: The synthesis of puerarin derivatives and their protective
effect on the myocardial ischemia and reperfusion injury. J Asian
Nat Prod Res. 12:843–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: A diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steinhart MR, Cone FE, Nguyen C, Nguyen
TD, Pease ME, Puk O, Graw J, Oglesby EN and Quigley HA: Mice with
an induced mutation in collagen 8A2 develop larger eyes and are
resistant to retinal ganglion cell damage in an experimental
glaucoma model. Mol Vis. 18:1093–1106. 2012.PubMed/NCBI
|
|
24
|
Tanaka T, Imamura T, Yoneda M, Irie A, Ogi
H, Nagata M, Yoshida R, Fukuma D, Kawahara K, Shinohara M and
Nakayama H: Enhancement of active MMP release and invasive activity
of lymph node metastatic tongue cancer cells by elevated signaling
via the TNF-α-TNFR1-NF-kB pathway and a possible involvement of
angiopoietin-like 4 in lung metastasis. Int J Oncol. 49:1377–1384.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ao X, Sellati TJ and Stenken JA: Enhanced
microdialysis relative recovery of inflammatory cytokines using
antibody-coated microspheres analyzed by flow cytometry. Anal Chem.
76:3777–3784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Awodele O, Oreagba IA, Olayemi SO, Oladipo
I, Iruegbukpe CO, Balogun BG, Balogun MM and Adedokun AO:
Evaluation and comparison of the indices of systemic oxidative
stress among Black-africans with Age-related cataracts or primary
glaucoma. Middle East Afr J Ophthalmol. 22:489–494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeon S, Lee NY and Park CK: Neovascular
glaucoma following stereotactic radiosurgery for an optic nerve
glioma: A case report. Korean J Ophthalmol. 24:252–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Widder RA, Lemmen KD and Dietlein TS:
Neovascular glaucoma. Klin Monbl Augenheilkd. 227:R15–R27. 2010.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toyama S, Tsuji H, Mizoguchi N, Nomiya T,
Kamada T, Tokumaru S, Mizota A, Ohnishi Y and Tsujii H: Working
Group for Ophthalmologic Tumors: Long-term results of carbon ion
radiation therapy for locally advanced or unfavorably located
choroidal melanoma: Usefulness of CT-based 2-port orthogonal
therapy for reducing the incidence of neovascular glaucoma. Int J
Radiat Oncol Biol Phys. 86:270–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wittström E, Holmberg H, Hvarfner C and
Andréasson S: Clinical and electrophysiologic outcome in patients
with neovascular glaucoma treated with and without bevacizumab. Eur
J Ophthalmol. 22:563–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laplace O: Surgical session: Neovascular
glaucoma and anti-vascular endothelial growth factor treatment. J
Fr Ophtalmol. 32:230–235. 2009.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simha A, Braganza A, Abraham L, Samuel P
and Lindsley K: Anti-vascular endothelial growth factor for
neovascular glaucoma. Cochrane Database Syst Rev: CD007920. 2013.
View Article : Google Scholar
|
|
33
|
Reilly CM, Morris R and Dubielzig RR:
Canine goniodysgenesis-related glaucoma: A morphologic review of
100 cases looking at inflammation and pigment dispersion. Vet
Ophthalmol. 8:253–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang L, Wong T, Ohbayashi M, Bunce C,
Barton K, Ono SJ and Khaw PT: Increased mast cell numbers in the
conjunctiva of glaucoma patients: A possible indicator of
preoperative glaucoma surgery inflammation. Eye (Lond).
23:1859–1865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Furtado JM, Paula JS, Soares EG, Dhegaide
NH, Rocha EM, Donadi E and Mde Rodrigues L: Conjunctival
inflammation in patients under topical glaucoma treatment with
indication to surgery. Acta Cir Bras. 27:732–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vohra R, Tsai JC and Kolko M: The role of
inflammation in the pathogenesis of glaucoma. Surv Ophthalmol.
58:311–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ha Y, Liu H, Xu Z, Yokota H, Narayanan SP,
Lemtalsi T, Smith SB, Caldwell RW, Caldwell RB and Zhang W:
Endoplasmic reticulum stress-regulated CXCR3 pathway mediates
inflammation and neuronal injury in acute glaucoma. Cell Death Dis.
6:e19002015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schlötzer-Schrehardt U: Oxidative stress
and pseudoexfoliation glaucoma. Klin Monbl Augenheilkd.
227:108–113. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mangan BG, Al-Yahya K, Chen CT, Gionfriddo
JR, Powell CC, Dubielzig RR, Ehrhart EJ and Madl JE: Retinal
pigment epithelial damage, breakdown of the blood-retinal barrier,
and retinal inflammation in dogs with primary glaucoma. Vet
Ophthalmol. 10 Suppl 1:S117–S124. 2007. View Article : Google Scholar
|
|
40
|
Tezel G, Yang X, Luo C, Kain AD, Powell
DW, Kuehn MH and Kaplan HJ: Oxidative stress and the regulation of
complement activation in human glaucoma. Invest Ophthalmol Vis Sci.
51:5071–5082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chi W, Chen H, Li F, Zhu Y, Yin W and Zhuo
Y: HMGB1 promotes the activation of NLRP3 and caspase-8
inflammasomes via NF-kB pathway in acute glaucoma. J
Neuroinflammation. 12:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Itakura T, Peters DM and Fini ME:
Glaucomatous MYOC mutations activate the IL-1/NF-kB inflammatory
stress response and the glaucoma marker SELE in trabecular meshwork
cells. Mol Vis. 21:1071–1084. 2015.PubMed/NCBI
|