Introduction
Condyloma acuminatum (CA) is a common sexually
transmitted disease that is caused by infection with human
papilloma virus (HPV) (1), and its
incidence is increasing annually. Clinically, it presents as
cauliflower-like or papillary growths in the genital area. Although
it is a benign hyperplasia, certain cases may transform into
malignant tumors (1). A minority
of cases may develop large CA due to excessive hyperplasia in a
short period of time. CA is very infectious (2), and has a negative impact on society
and the family of patients as it adversely affects the physical and
mental health of patients. High relapse rates are an issue
following CA treatment (3), and it
is extremely difficult to control the transmission and prevalence
of the disease (3). Therefore,
this disease has attracted attention in research.
Humans are the only natural host of HPV. Three types
of squamous epithelial cells on human skin, mucosa and metaplasia
are sensitive to HPV, which infects them via damaged squamous
epithelial cells (4). Studies have
reported that after human squamous epithelial cells are infected
with HPV, cells exhibit abnormal proliferation and apoptosis
(4,5). The specific mechanism underlying the
development of CA abnormal growths following HPV infection has not
previously been identified, however, increased cell division or
reduced cell death following HPV infection may be implicated
(6). Tumor necrosis factor-related
apoptosis-inducing ligand led to the apoptosis of tumor cells,
virus-infected cells and transformed cells (6). Caspase-3 is an established death
protease (6) that is a key protein
in the apoptosis pathway, and participates in cell apoptosis
induced by a variety of factors (4).
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway
is a typical signal transduction pathway that inhibits cell
apoptosis and promotes cell survival (7). In addition, the pathway has a key
role in the resistance of tumors to chemotherapy and radiotherapy,
the genesis and proliferation of tumor cells, and the invasiveness
and metastasis of tumor cells to other tissues (8). The PI3K/Akt signaling pathway is an
essential pathway in cells. Specifically, it has an important role
in the genesis and development of CA cells (8). Studies have demonstrated that the
PI3K/Akt pathway induces CA through various mechanisms: The pathway
inhibits the expression of protein p53, a tumor suppressor gene, in
the cell nucleus by promoting the anti-nuclear movement of Mdm2
proto-oncogene; therefore, excessive activation of the PI3K
signaling pathway leads to uncontrollable proliferation of CA
cells. Additionally, the pathway inhibits the cell apoptosis
process via the phosphorylation of various proteins, including
Bcl-2-associated agonist of cell death, caspase-9 and other
components of the apoptosis pathway, and also inhibits
conformational changes of certain apoptosis proteins, such as
Bcl-2-associated X (Bax) (8–10).
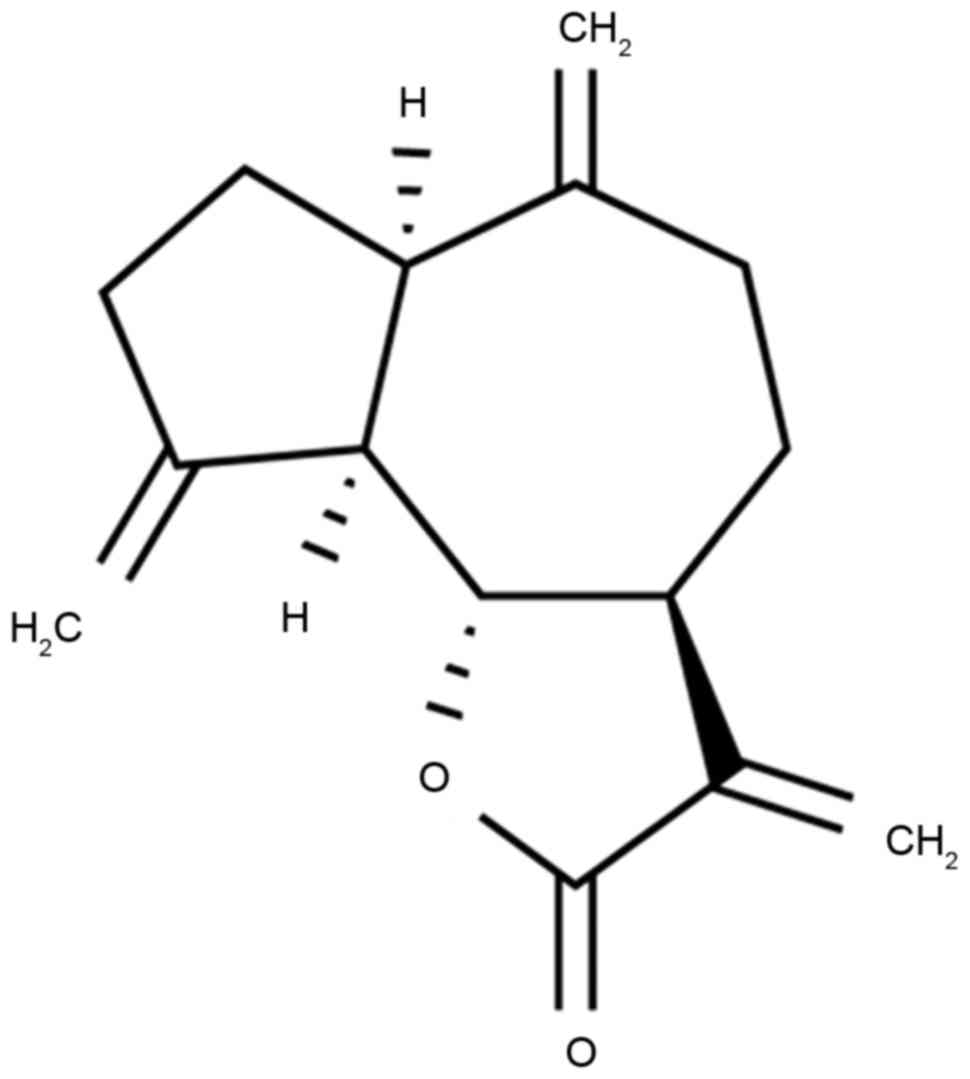
Dehydrocostus lactone, the structure of which is
presented in Fig. 1, is
extractable from dry Costus root. Costus originated
from India, and was introduced and cultivated in the province of
Yunnan in China (11,12). Dehydrocostus lactone is the major
ingredient of Costus root essential oil (13). It has been demonstrated through
modern pharmacological studies that Costus has certain
effects on the digestive, respiratory and cardiovascular systems
(14). Dehydrocostus lactone, as
the primary component of Costus, has been reported to
improve intestinal functions, promote gastric motility and
choleresis, and exhibit antidiarrheic, antihypertensive and
antibacterial effects (15). The
aim of the present study was to investigate the effects of
dehydrocostus lactone on the cell growth and apoptosis of
recombinant HPV-18 HaCaT cells, and to determine whether these
effects may occur via the PI3K/Akt signaling pathway.
Materials and methods
Cell lines and transfection of the
HPV-18 genome into HaCaT cells
The HaCaT human epithelial cell line was purchased
from the Shanghai Cell Bank of Chinese Academy of Sciences
(Shanghai, China), propagated in Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and then
supplemented with 10% heat-inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml of penicillin and 100
mg/ml of streptomycin at 37°C in an humidified atmosphere
containing 95% air and 5% CO2. A total of 200 ng of
HPV-18 expression plasmid was purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China) and transfected into cells (1×106 cell/well)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 4 h, the medium was replaced with fresh
DMEM medium supplemented with dehydrocostus lactone (Sigma-Aldrich;
Merck KGaA; Darmstadt, Germany). Following a 48 h incubation at
37°C, the Caspase-3 and caspase-9 expression levels were
investigated via western blot analysis and following a 72 h
incubation at 37°C, the MTT assay was performed.
MTT assay
Recombinant HPV-18 HaCaT cells (1×106 cells/well)
were seeded in 96-well plates and treated with either 2 µl of
dimethyl sulfoxide (DMSO) or 2 µl of dehydrocostus lactone (2.5, 5
and 10 µg/ml) for 0, 24, 48 and 72 h at 37°C. Following treatment,
MTT tetrazolium salt (0.5 mg/ml; Sigma-Aldrich; Merck KGaA) was
added for 4 h. The medium was then removed and 150 µl DMSO was
added per well to dissolve the formazan crystals. Absorbance was
measured using the SpectraMax 190 microplate reader (Molecular
Devices, LLC., Sunnyvale, CA, USA) at 490 nm.
Caspase-3 and caspase-9 activity
levels
Recombinant HPV-18 HaCaT cells (1×103 cell/well)
were seeded in 6-well plates and treated with 2 µl of DMSO or
dehydrocostus lactone (2.5, 5 and 10 µg/ml) for 48 h at 37°C. Cells
were subsequently washed with PBS and lysed using
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Nanjing, China). Cell extracts were
clarified by centrifugation at 10,000 × g for 15 min at 4°C and
protein concentration was measured by a BCA assay. Proteins (10 µg
per sample) were incubated with N-acetyl-Asp-Glu-Val-Asp
p-nitroanilide (also termed Ac-DEVD-pNA; Beyotime Institute of
Biotechnology) for 2 h at 37°C. The activity levels of caspase-3
and caspase-9 were subsequently determined using the SpectraMax 190
microplate reader at 405 nm.
Apoptosis rate
Recombinant HPV-18 HaCaT cells (1×103 cells/well)
were seeded into 6-well plates and treated with 2 µl of DMSO or
dehydrocostus lactone (2.5, 5 and 10 µg/ml) for 48 h at 37°C. Cells
were subsequently washed with PBS and fixed with 4%
paraformaldehyde for 15 min at room temperature. Cells were then
stained with 5 µl of Annexin V-fluorescein isothiocyanate (cat. no.
556570; BD Biosciences, San Jose, CA, USA) and 5 µl of propidium
iodide (cat. no. 556570; BD Biosciences) for 20 min in darkness at
room temperature. Cell apoptosis rate was then detected using a
flow cytometer (C6; BD Biosciences) and analyzed using FlowJo
software (version 7.6.1; FlowJo LLC, Ashland, OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from HaCaT cells
using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (500 ng) was reverse-transcribed using a
First-Strand cDNA Synthesis kit (GeneCopoeia, Inc., Rockville, MD,
USA) at 42°C for 2 min, 37°C for 30 min and 85°C for 5 sec.
Following this, qPCR was performed using SYBR Green PCR Master Mix
(cat. no. 303410; Takara Biotechnology Co., Ltd., Dalian, China) by
a LightCycler® 2.0 apparatus (Roche Applied Science,
Mannheim, Germany). The thermocycling conditions were as follows:
94°C for 5 min, followed by 40 cycles of 94°C for 30 sec, annealing
at 60°C for 30 sec, and a final extension of 72°C for 30 sec.
Primers used for amplification were as follows: HPV-18 forward,
5′-TACCTGTGTCACAAGCCGTT-3′ and reverse, 5′-CAGCAGTGTAAGCAACGACC-3′;
GAPDH forward, 5′-ACAGCAACAGGGTGGTGGAC-3′ and reverse,
5′-TTTGAGGGTGCACGAACTT-3′. The data were analyzed using the
2−ΔΔCq method (16).
Western blot analysis
Recombinant HPV-18 HaCaT cells (1×106 cell/well)
were seeded in 6-well plates and treated with either 2 µl of DMSO
or 2 µl of dehydrocostus lactone (2.5, 5 and 10 µg/ml) for 48 h at
37°C. Cells were washed with PBS and lysed using RIPA assay. Cell
extracts were clarified by centrifugation at 10,000 × g for 15 min
at 4°C and protein concentration was measured by a BCA assay.
Proteins (25 µg per sample) were analyzed on an 8–10% SDS-PAGE gel
and transferred onto polyvinylidene fluoride membranes (Merck
KGaA). Membranes were subsequently blocked with 5% skim milk powder
in TBS-0.1% Tween-20 for 1 h at 37°C and incubated with Bax (cat.
no. sc-6236; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), p53 (cat. no. sc-6243; 1:1,000; Santa Cruz Biotechnology,
Inc.), cyclin D1 (cat. no. sc-717; 1:500; Santa Cruz Biotechnology,
Inc.), phosphatase and tensin homology (PTEN; cat. no. sc-6817-R;
1:1,000; Santa Cruz Biotechnology, Inc.), PI3K (cat. no. sc-7174;
1:500; Santa Cruz Biotechnology, Inc.), Akt (cat. no. sc-8312;
1:500; Santa Cruz Biotechnology, Inc.), phosphorylated (p)-Akt
(cat. no. sc-16646-R; 1:500; Santa Cruz Biotechnology, Inc.) and
GAPDH (cat. no. sc-25778; 1:2,000; Santa Cruz Biotechnology, Inc.)
primary antibodies at 4°C overnight, which was followed by
incubation with goat anti-rabbit immunoglobulin G-horseradish
peroxidase (cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology,
Inc.) for 1 h at 37°C. The intensity of each band was detected
using BeyoECL Plus reagent (cat. no. P0018A; Beyotime Institute of
Biotechnology, Nanjing, China) and quantified using ImageJ software
6.0 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean + standard deviation
using SPSS v.20 (IBM Corp., Armonk, NY, USA). All experiments were
performed in triplicate. One-way analysis of variance followed by
Tukey post hoc test was performed to determine the significance of
differences among the experimental groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Dehydrocostus lactone reduces the
proliferation of recombinant HPV-18 HaCaT cells
The effect of dehydrocostus lactone on recombinant
HPV-18 HaCaT cell proliferation was determined using an MTT assay.
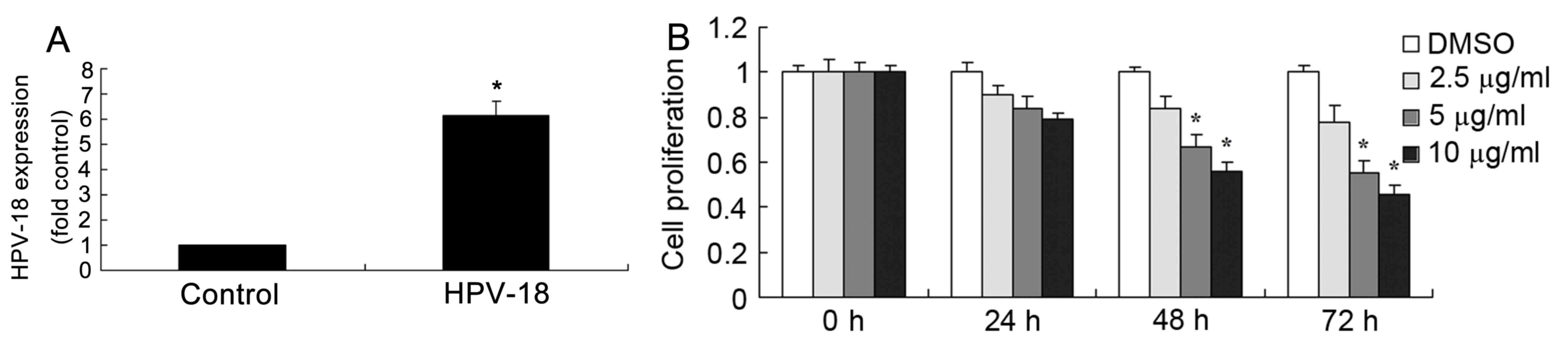
As revealed in Fig. 2A,
transfection with HPV-18 expression plasmid significantly increased
the expression of HPV-18 miRNA expression in HaCaT cells, compared
with the negative control group. As demonstrated in Fig. 2B, treatment with dehydrocostus
lactone reduced the proliferation of recombinant HPV-18 HaCaT cells
in dose- and time-dependent manner, compared with DMSO-treated
cells. In particular, 5 and 10 µg/ml dehydrocostus lactone for 48
or 72 h significantly reduced the proliferation of recombinant
HPV-18 HaCaT cells, compared with the DMSO control group at the
same time points (Fig. 2B).
Dehydrocostus lactone induces
apoptosis in HaCaT cells
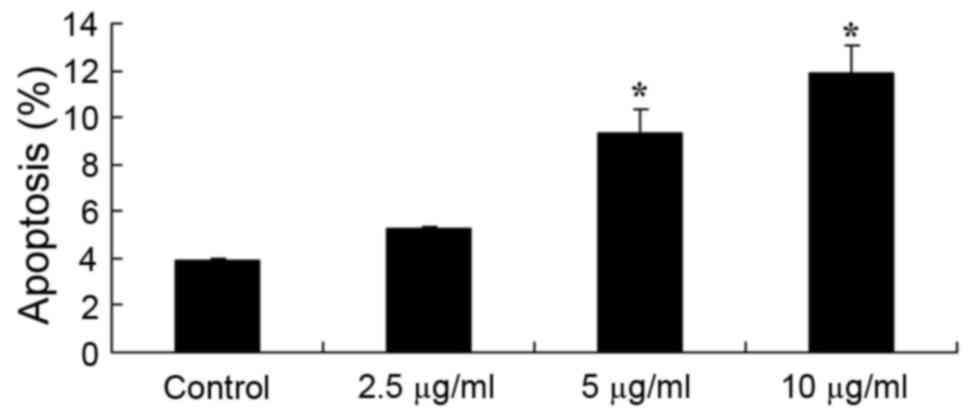
Furthermore, the present study also investigated the
effect of dehydrocostus lactone on apoptosis in recombinant HPV-18
HaCaT cells. The results demonstrated that the apoptosis rate of
recombinant HPV-18 HaCaT cells was significantly increased
following treatment for 48 h with 5 and 10 µg/ml dehydrocostus
lactone, compared with the DMSO control group (Fig. 3).
Dehydrocostus lactone promotes
caspase-3/9 activity in HaCaT cells
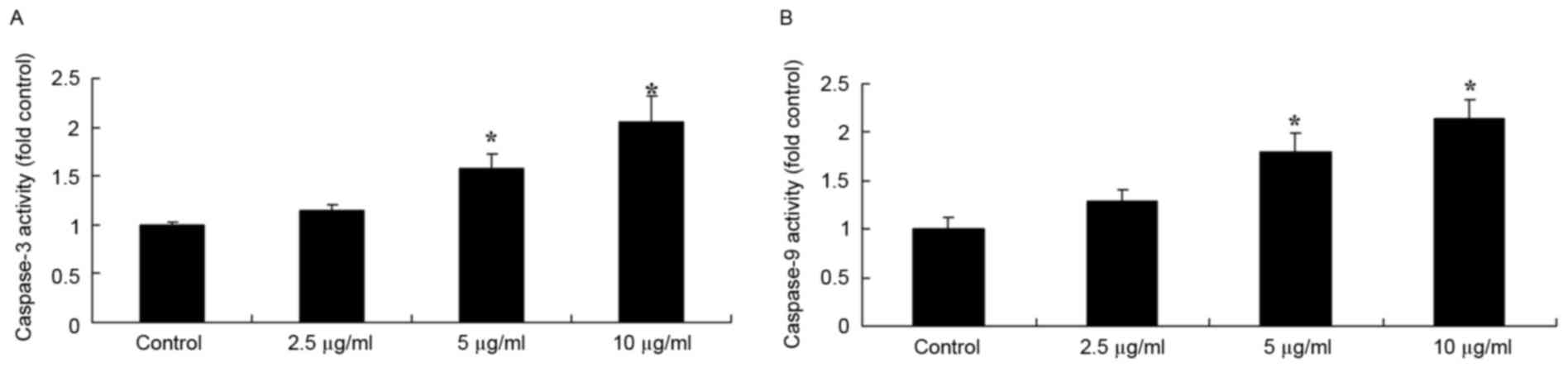
The activities of caspase-3/9 in DMSO- and
dehydrocostus lactone-treated recombinant HPV-18 HaCaT cells were
also investigated in the present study. As demonstrated in Fig. 4, dehydrocostus lactone treatment
for 48 h also led to significant increases in the caspase-3/9
activities of recombinant HPV-18 HaCaT cells at concentrations of 5
and 10 µg/ml, compared with the DMSO control group.
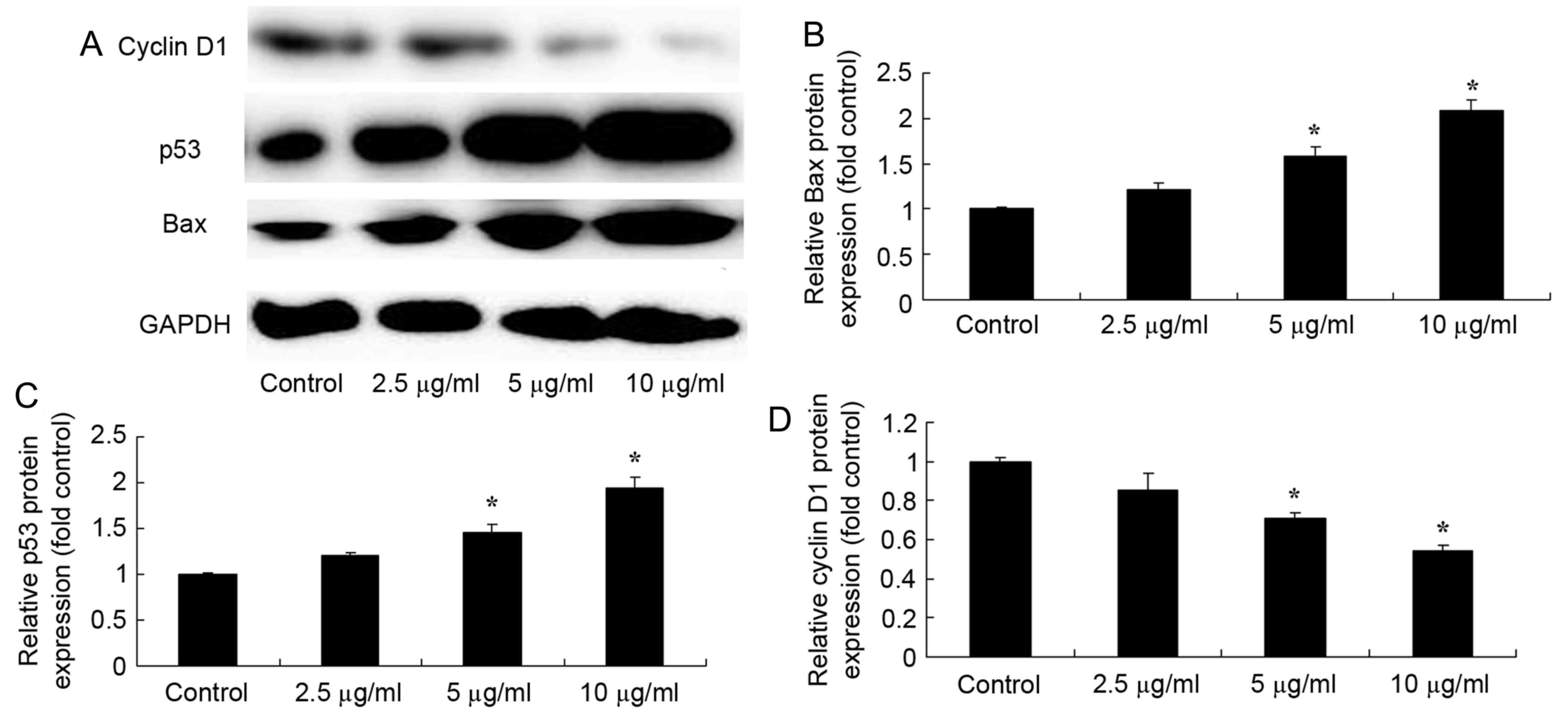
Dehydrocostus lactone promotes Bax and
p53 protein expression, and suppresses the protein expression of
cyclin D1 in HaCaT cells
To observe the effect of dehydrocostus lactone on
Bax, p53 and cyclin D1 protein expression in recombinant HPV-18
HaCaT cells, Bax and p53 protein expression was determined by
western blot analysis. Dehydrocostus lactone (5 and 10 µg/ml)
treatment for 48 h significantly increased Bax and p53 protein
expression, and suppressed cyclin D1 protein expression in
recombinant HPV-18 HaCaT cells, compared with the DMSO control
group (Fig. 5).
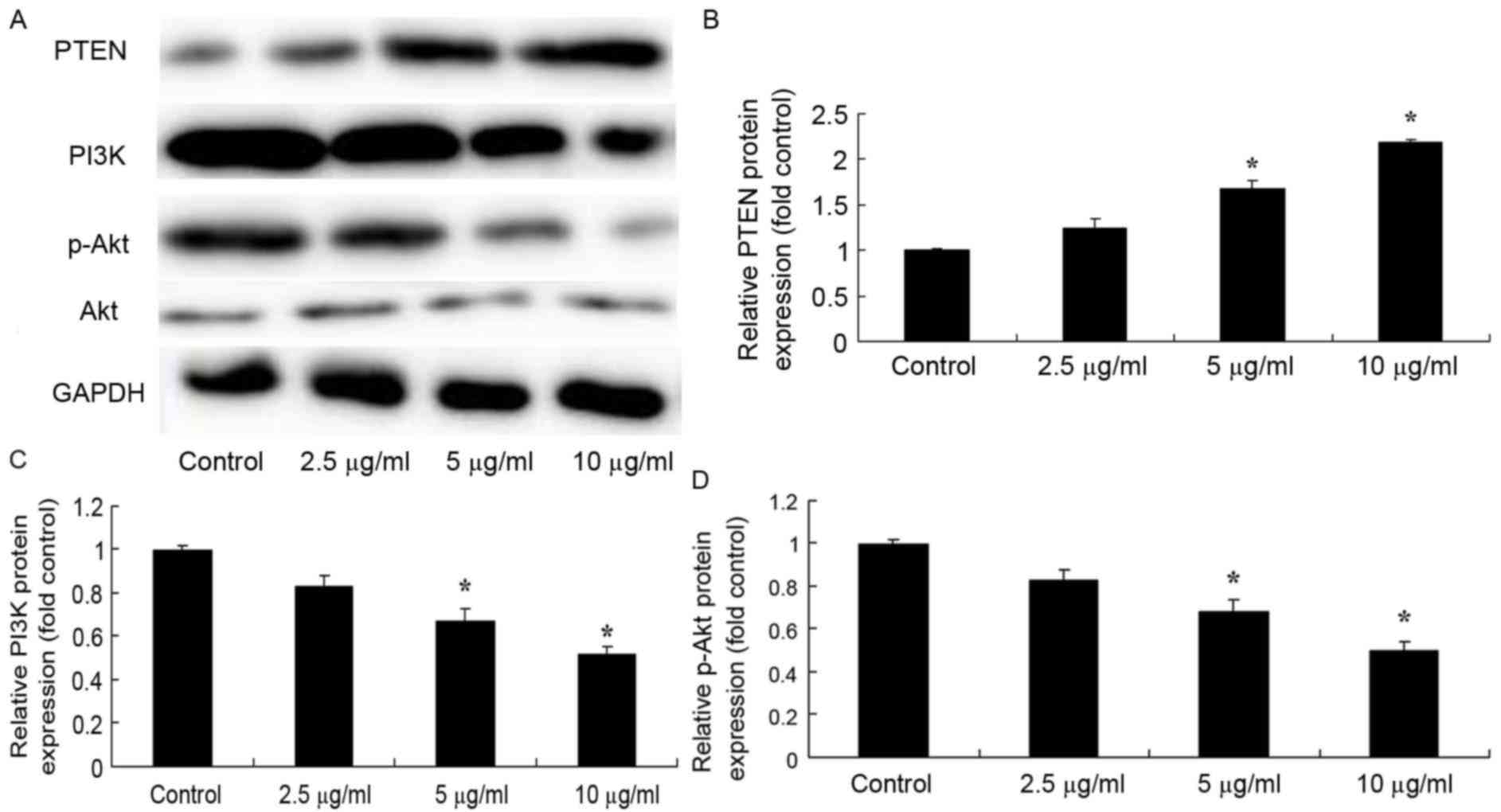
Dehydrocostus lactone increases PTEN
protein expression and downregulates the PI3K/Akt signaling pathway
in HaCaT cells
To determine the effect of dehydrocostus lactone on
PTEN protein expression and the PI3K/Akt signaling pathway in
recombinant HPV-18 HaCaT cells, western blot analysis was
performed. The results in Fig. 6
demonstrate that dehydrocostus lactone (5 and 10 µg/ml) treatment
for 48 h significantly increased PTEN protein expression, and
suppressed PI3K and p-Akt levels in recombinant HPV-18 HaCaT cells,
compared with the DMSO control group.
Discussion
A previous study on CA has indicated that human
immune responses, particularly cellular immune responses, are the
principal factor determining the outcome of CA (2). Patients with deficits in the cellular
immune response, including patients with acquired immune deficiency
syndrome, Hodgkin lymphoma, malignant lymphoma and chronic
lymphocytic leukemia, are at an increased risk of being affected by
HPV (6). Patients that are taking
immunosuppressive drugs, particularly those following renal
transplantation and heart transplants, are prone to broad and
persistent warts (6). The present
study demonstrated that dehydrocostus lactone significantly reduced
the proliferation of recombinant HPV-18 HaCaT cells, which
indicates that it may be useful for the prevention/treatment of
CA.
CA is a type of benign proliferative disease of the
skin membrane at the genitals, anus and perineum (17). It is a gynecological disease that
is commonly observed in the clinic. The primary methods of its
transmission between individuals are by sexual contact or through
indirect contact, and mother-to-child vertical transmission
(17). With the development of the
economy, and increased international and domestic exchanges, CA has
become a sexually transmitted disease with one of the highest
growth rates (18). A previous
study demonstrated that the genesis and development of CA involves
a complicated gene regulation process (18). Humans are the only natural host of
HPV (6); following infection of
human squamous epithelial cells by HPV, cells exhibit abnormal
proliferation and apoptosis. The results of the present study
demonstrated that dehydrocostus lactone significantly reduced
proliferation, and increased apoptosis, in recombinant HPV-18 HaCaT
cells. Furthermore, Sun et al (15) reported that dehydrocostus lactone
suppressed the proliferation of colorectal carcinoma cells via
downregulation of eukaryotic translation initiation factor 4E
expression.
Apoptosis is a type of programmed cell death and
abnormal apoptosis levels may lead to the excessive proliferation
of cells (19). Clinically, a
minority of patients with CA develop large CA due to excessive
hyperplasia in a short period of time (20). Additionally, CA may develop and
deteriorate further in certain patients, leading to the genesis of
malignant tumors (20). The
caspase-3 protein family and Bax have been demonstrated to enhance
apoptosis (21). However, few
studies have investigated their roles in hyperplastic diseases of
the skin. Caspase-3 and Bax have important roles in apoptosis
(21,22), therefore, the present study
investigated the activity/expression of these proteins, and the
results demonstrated that dehydrocostus lactone significantly
increased caspase-3/9 activities and induced Bax protein expression
in recombinant HPV-18 HaCaT cells.
A previous study demonstrated that the PI3K/Akt
signaling pathway is closely associated with CA (23). Additionally, studies concerning CA
have demonstrated that the PI3K/Akt signal transduction pathway is
involved in resisting Fas regulation and the apoptotic process
(23). Therefore, the activation
of the PI3K/Akt signal transduction pathway may lead to the genesis
of CA (23), and its mechanism of
action may involve the abnormal expression of PTEN (24). A further study regarding CA also
reported that PI3K/Akt signaling may increase the expression of
growth factor receptors to promote mitosis (23). Therefore, the PI3K/Akt signaling
pathway is closely associated with the genesis and development of
CA (24), and indicators of
PI3K/Akt signaling pathway activity in CA tissues may be used as an
indicator to evaluate CA malignant grade (24).
The PI3K/Akt signaling pathway is an important
signaling pathway in various biological processes. It is a signal
transduction pathway that has one of the strongest associations
with cell proliferation and apoptosis (23). A previous study demonstrated that
the PI3K/Akt signaling pathway is abnormally activated in various
tumor types (23). The activation
of the signal transduction pathway inhibits cell apoptosis induced
by various stimuli, and promotes cell cycle progression to promote
cell survival and proliferation (25). In addition, this signaling pathway
has important roles in angiopoiesis and tumor formation, and is
implicated in the invasion and metastasis of tumors (26). The results of the present study
demonstrated that dehydrocostus lactone significantly increased
PTEN protein expression, and downregulated PI3K and p-AKT protein
expression, in recombinant HPV-18 HaCaT cells. Furthermore, Jiang
et al (27) demonstrated
that dehydrocostus lactone inhibited the proliferation and invasion
of cervical cancer cells via the PI3K/Akt signaling pathway.
In conclusion, the results of the present study
indicate that dehydrocostus lactone may suppress cell growth and
induce apoptosis in recombinant HPV-18 HaCaT cells via the PI3K/Akt
signaling pathway, and suggests that dehydrocostus lactone may
exhibit anti-condyloma acuminatam effects. This novel target of
dehydrocostus lactone may provide a potential prognostic marker and
therapeutic target for CA patients. Future studies should
investigate the effects of dehydrocostus lactone using animal
models and investigate its effect on genital warts in a clinical
setting.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
WL designed the study. YBM, YQM and TL performed the
experiments. WL and YBM analysed the data. WL wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang X, Ha T, Hu Y, Lu C, Liu L, Zhang X,
Kao R, Kalbfleisch J, Williams D and Li C: MicroRNA-214 protects
against hypoxia/reoxygenation induced cell damage and myocardial
ischemia/reperfusion injury via suppression of PTEN and Bim1
expression. Oncotarget. 7:86926–86936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D, Liu J, Guo B, Liang C, Dang L, Lu C,
He X, Cheung HY, Xu L, Lu C, et al: Osteoclast-derived exosomal
miR-214-3p inhibits osteoblastic bone formation. Nat Commun.
7:108722016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu Q, Sun Y, Cui J and Xu T: Inducible
microRNA-214 contributes to the suppression of NF-κB-mediated
inflammatory response via targeting myd88 gene in fish. J Biol
Chem. 292:5282–5290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song X, Wang CT and Geng XH: MicroRNA-29a
promotes apoptosis of monocytes by targeting STAT3 during sepsis.
Genet Mol Res. 14:13746–13753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hyun J, Choi SS, Diehl AM and Jung Y:
Potential role of Hedgehog signaling and microRNA-29 in liver
fibrosis of IKKβ-deficient mouse. J Mol Histol. 45:103–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZH, Zhang JL, Duan YL, Zhang QS, Li
GF and Zheng DL: MicroRNA-214 participates in the neuroprotective
effect of Resveratrol via inhibiting α-synuclein expression in
MPTP-induced Parkinson's disease mouse. Biomed Pharmacother.
74:252–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Kong M, Jiang D, Qian J, Duan Q and
Dong A: MicroRNA-150 aggravates H2O2-induced cardiac myocyte injury
by down-regulating c-myb gene. Acta Biochim Biophys Sin (Shanghai).
45:734–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bagge A, Clausen TR, Larsen S, Ladefoged
M, Rosenstierne MW, Larsen L, Vang O, Nielsen JH and Dalgaard LT:
MicroRNA-29a is up-regulated in beta-cells by glucose and decreases
glucose-stimulated insulin secretion. Biochem Biophys Res Commun.
426:266–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang K, Cao W, Hao X, Xue X, Zhao J, Liu
J, Zhao Y, Meng J, Sun B, Zhang J and Liang XJ: Metallofullerene
nanoparticles promote osteogenic differentiation of bone marrow
stromal cells through BMP signaling pathway. Nanoscale.
5:1205–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Lu CM, Song Z, Xu KK, Wu SB and Li
ZJ: Expression and regulation of microRNA-29a and microRNA-29c in
early diabetic rat cataract formation. Int J Ophthalmol.
9:1719–1724. 2016.PubMed/NCBI
|
|
11
|
Dippel DW, Majoie CB, Roos YB, van der
Lugt A, van Oostenbrugge RJ, van Zwam WH, Lingsma HF, Koudstaal PJ,
Treurniet KM, van den Berg LA, et al: Influence of device choice on
the effect of intra-arterial treatment for acute ischemic stroke in
MR CLEAN (multicenter randomized clinical trial of endovascular
treatment for acute ischemic stroke in the Netherlands). Stroke.
47:2574–2581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawakami S, Tahara Y, Noguchi T, Yagi N,
Kataoka Y, Asaumi Y, Nakanishi M, Goto Y, Yokoyama H, Nonogi H, et
al: Time to reperfusion in ST-segment elevation myocardial
infarction patients with vs. Without pre-hospital mobile
telemedicine 12-lead electrocardiogram transmission. Circ J.
80:1624–1633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hill MD, Demchuk AM, Goyal M, Jovin TG,
Foster LD, Tomsick TA, von Kummer R, Yeatts SD, Palesch YY and
Broderick JP: IMS3 Investigators: Alberta stroke program early
computed tomography score to select patients for endovascular
treatment: Interventional management of stroke (IMS)-III trial.
Stroke. 45:444–449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang
G, Dong Y, Xu X, Liu Q, Huang D and Shi FD: Combination of the
immune modulator fingolimod with alteplase in acute ischemic
stroke: A pilot trial. Circulation. 132:1104–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun X, Kang H, Yao Y, et al: Dehydrocostus
lactone suppressed the proliferation, migration, and invasion of
colorectal carcinoma through the downregulation of eIF4E
expression. Anticancer Drugs. 26:641–648. 2015.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li R, Liu J, Li Q, Chen G and Yu X:
miR-29a suppresses growth and metastasis in papillary thyroid
carcinoma by targeting AKT3. Tumour Biol. 37:3987–3996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou H, Guo W, Zhao Y, Wang Y, Zha R, Ding
J, Liang L, Yang G, Chen Z, Ma B and Yin B: MicroRNA-135a acts as a
putative tumor suppressor by directly targeting very low density
lipoprotein receptor in human gallbladder cancer. Cancer Sci.
105:956–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang Y, Shen H, Li H, Cao Y, Qin R, Long
L, Zhu X, Xie C and Xu W: miR-106a confers cisplatin resistance by
regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim
Biophys Sin (Shanghai). 45:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Xu H, Shen H and Li H: microRNA-106a
modulates cisplatin sensitivity by targeting PDCD4 in human ovarian
cancer cells. Oncol Lett. 7:183–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rothschild SI, Gautschi O, Batliner J,
Gugger M, Fey MF and Tschan MP: MicroRNA-106a targets autophagy and
enhances sensitivity of lung cancer cells to Src inhibitors. Lung
Cancer. 107:73–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Zhang F, Sheng XG, Zhang SQ, Chen
YT and Liu BW: MicroRNA-106a regulates phosphatase and tensin
homologue expression and promotes the proliferation and invasion of
ovarian cancer cells. Oncol Rep. 36:2135–2141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ku SK and Bae JS: Antithrombotic
activities of wogonin and wogonoside via inhibiting platelet
aggregation. Fitoterapia. 98:27–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge X, Leung TM, Arriazu E, Lu Y, Urtasun
R, Christensen B, Fiel MI, Mochida S, Sørensen ES and Nieto N:
Osteopontin binding to lipopolysaccharide lowers tumor necrosis
factor-α and prevents early alcohol-induced liver injury in mice.
Hepatology. 59:1600–1616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang SD, Ma L, Yang DL and Ding WY:
Combined effect of 17β-estradiol and resveratrol against apoptosis
induced by interleukin-1β in rat nucleus pulposus cells via
PI3K/Akt/caspase-3 pathway. PeerJ. 4:e16402016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen C, Guo D and Lu G: Wogonin protects
human retinal pigment epithelium cells from LPS-induced barrier
dysfunction and inflammatory responses by regulating the TLR4/NF-κB
signaling pathway. Mol Med Rep. 15:2289–2295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang E, Sun X, Kang H, et al:
Dehydrocostus Lactone Inhibits Proliferation, Antiapoptosis, and
Invasion of Cervical Cancer Cells Through PI3K/Akt Signaling
Pathway. Int J Gynecol Cancer. 25:1179–1186. 2015. View Article : Google Scholar : PubMed/NCBI
|